Abstract
Neural transplantation has been investigated experimentally and clinically for the purpose of developing new treatment options for intractable epilepsy. In the present study we assessed the anticonvulsant efficacy and safety of bilateral allotransplantation of genetically engineered striatal GABAergic rat cell lines into the substantia nigra pars reticulata (SNr). Rats with previously-established seizures, induced by amygdala kindling, were used as a model of temporal lobe epilepsy. Three cell lines were transplanted: (1) immortalized GABAergic cells (M213-2O) derived from embryonic rat striatum; (2) M213-2O cells (CL4) transfected with human GAD67 cDNA to obtain higher GABA synthesis than the parent cell line; and (3) Control cells (121-1I), also derived from embryonic rat striatum, but which did not show GAD expression. A second control group received injections of medium alone. Transplantation of M213-2O cells into the SNr of kindled rats resulted in significant but transient anticonvulsant effects. Neither control cells nor medium induced anticonvulsant effects. Strong tissue reactions were, however, induced in the host brain of kindled but not of non-kindled rats, and only in animals that received grafts of genetically-modified CL4 cells. These tissue reactions included graft rejection, massive infiltration of inflammatory immune cells, and gliosis. The anticonvulsant effect of M213-2O cells emphasizes the feasibility of local manipulations of seizures by intranigral transplantation of GABA-producing cells. On the other hand, the present data suggest that kindling-induced activation of microglia in the SNr can enhance immune reactions to transplanted cells. In this case, under conditions of further immunological stimulation by CL4 cells, transfected with a human cDNA, substantial immune reactions occurred. Thus, it appears that the condition of the host brain and the production of foreign proteins by transplanted cells have to be considered in estimating the risks of rejection of transplants into the brain.
Keywords: Substantia nigra pars reticulata, basal ganglia, seizures, genetically engineered cells, gene therapy, neural transplantation, grafting, kindling
Introduction
Several attempts have been made to develop new treatment options for difficult-to-treat types of seizures, such as complex partial seizures associated with temporal lobe epilepsy. Neural transplantation of cells of various types is one approach which has been investigated in a variety of experimental seizure models (for review see Björklund and Lindvall, 2000; Boison, 2005; Löscher et al., 2008; Raedt et al., 2007; Turner and Shetty, 2003) and in a few epilepsy patients (Schachter et al., 1998). The aim of neural transplantation in intractable epilepsy is to correct a presumed imbalance between excitatory and inhibitory neurotransmission in the epileptic brain. Thus, seizures might be suppressed by transplanting cells that continuously release an inhibitory transmitter such as GABA into appropriate brain regions.
The pars reticulata of the substantia nigra (SNr) is believed to be an important brain site involved in the control of several types of experimental seizures, including electrically kindled seizures (Depaulis et al., 1994; Deransart and Depaulis, 2002; Iadarola and Gale, 1982; McNamara et al., 1986; Morimoto and Goddard, 1987; Velísková and Moshé, 2006). Kindled seizures induced by electrical stimulation of limbic structures in rats (Goddard et al., 1969) is one of the most widely used models of temporal lobe epilepsy (Bertram, 2007). In contrast to post-status epilepticus models of temporal lobe epilepsy, no loss of GABAergic neurons was detected in the SNr of kindled rats (Freichel et al., 2004). Nevertheless, abnormal GABAergic function in the SNr may be involved in the epileptic network underlying kindling (Gernert et al., 2004; Löscher and Schwark, 1985; Löscher and Schwark, 1987). Accordingly, profound modifications of neuronal activity in the SNr and related structures can be detected ictally and interictally in the kindling model (Bonhaus et al., 1986; Chassagnon et al., 2006; Chassagnon et al., 2005; Gernert et al., 2004; Löscher and Ebert, 1996; Löscher et al., 2006; Nolte et al., 2006b; Shi et al., 2007). These network changes provide a rationale for transplanting inhibitory cells into the SNr to alleviate seizures in this model.
In addition, anticonvulsant effects can be obtained in a wide variety of experimental seizure models, including electrical kindling, by enhancing GABAergic transmission within the SNr (Depaulis et al., 1994; Iadarola and Gale, 1982; Löscher et al., 1987; McNamara et al., 1984; Nolte et al., 2006a; Velísková and Moshé, 2006). Accordingly, several studies using GABA-releasing polymer matrices (Kokaia et al., 1994), fetal GABAergic cells (Fine et al., 1990; Löscher et al., 1998) or genetically engineered GABA-producing cell lines (Castillo et al., 2006; Thompson et al., 2000; Thompson and Suchomelova, 2004) have observed that local enhancement of GABAergic function in the SNr can produce anticonvulsant effects.
In our previous transplantation studies, we have allografted fetal GABAergic cells into the SNr of fully kindled rats (Löscher et al., 1998). These cells survived and had transient anticonvulsant effects. In clinical applications, the use of tissue originating from aborted fetuses is limited by ethical, infectious, regulatory, and practical concerns (Harrower and Barker, 2004; Holt et al., 1997), which has led to the search for alternative sources of cells, including immortalized cell lines. In more recent studies, an immortalized mouse cortical neuronal cell line genetically engineered to produce high amounts of GABA was investigated concerning its effect on kindling development after transplantation into the SNr (Thompson et al., 2000) or into limbic system structures (Gernert et al., 2002; Thompson, 2005). Despite strong astrogliosis around the xenogeneic cells transplanted from mouse to rat brain we did not observe a rejection of the graft (Gernert et al., 2002), although xenografts are known to be associated with a higher risk of immune-mediated rejection (Barker, 2000; Brevig et al., 2000; Takei et al., 1990). Therefore, in the present study we investigated allotransplantation of immortalized GABA-releasing cell lines originating from rat striatal cells in the SNr using a kindling model of temporal lobe epilepsy.
In order to be applicable for human clinical use, transplanted cells should have the capacity to suppress seizure activity in animals with previously-established seizures, rather than acting in a prophylactic manner (Björklund and Lindvall, 2000). We therefore transplanted the GABA-producing cells into the SNr of previously kindled rats. Thus, for the present study we aimed (1) to investigate whether immortalized GABAergic striatal cells allotransplanted into the SNr of previously kindled rats produce anticonvulsant effects and (2) to investigate if efficacy can be increased by using cells with enhanced GABA-production by additional transfection with the human form of the GABA-synthesizing enzyme glutamic acid decarboxylase (GAD) (Conejero-Goldberg et al., 2000). Some of the data have been presented in abstract form (Nolte et al., 2008).
Materials and Methods
Animals
Adult female Wistar rats (Harlan-Winkelmann; Borchen, Germany) were purchased at a body weight of 200–220 g. The rats were housed individually and kept under controlled environmental conditions with a 12/12 h light/dark cycle, lights on at 6:00 a.m., for at least one week before the experiments. Standard laboratory chow (Altromin 1324 standard diet) and tap water were allowed ad libitum. All experiments were done in compliance with the German Animal Welfare Act.
Kindling and seizure threshold testing
Rats were anesthetized with chloral hydrate (360 mg/kg i.p.) and a teflon-insulated bipolar stainless steel electrode was stereotaxically implanted for stimulation of the right basolateral amygdala and recording of afterdischarges. The following stereotaxic coordinates in mm relative to bregma according to the atlas of Paxinos and Watson (1998) were used: posterior (P) −2.2, lateral (L) −4.8, and ventral (V) −8.5 mm. The incisor bar was set at −3.3 mm. A stainless steel screw, placed above the left parietal cortex, served as the indifferent reference electrode. Bipolar and ground electrodes were connected to plugs. Additional skull screws and dental acrylic cement were used to anchor the headset. The skull above the SNr was kept free of acrylic cement for later cell transplantation surgery.
After surgery, the animals were allowed a recovery period of two weeks, after which all rats were electrically kindled via the electrode in the basolateral amygdala (see Fig. 1 for the study time line). Constant current stimulations (500 μA, 1 msec, monophasic square-wave pulses, 50/sec for 1 sec) were delivered once daily to the basolateral amygdala until at least 15 generalized kindled seizures classified as stage 5 (Racine, 1972) (see below) were elicited. One day before the kindling procedure was started, and again at the earliest four days after the kindling procedure was completed, the stimulation thresholds for eliciting afterdischarges in the electroencephalogram (EEG) recorded from the kindling electrode (ADT = afterdischarge threshold) were determined. An ascending staircase procedure was used, starting with an initial current intensity of 30 μA (1 sec train, 1 msec stimulus at 50 Hz), followed by increases in current intensity by about 20% of the previous current at intervals of 1 min until afterdischarges of at least 3 sec duration were elicited.
Fig. 1.
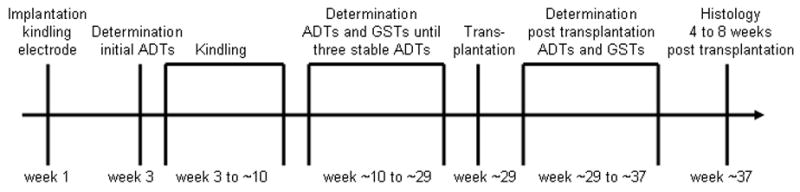
Time line representing the sequence of procedures conducted during the present study. Refer to text for details. ADT, afterdischarge threshold; GST, generalized seizure threshold.
Subsequently, in fully kindled rats the ADTs as well as the generalized seizure threshold (GST) (Gernert and Löscher, 2001) were determined once a week to establish a reliable baseline prior to transplantation. Again, the ascending staircase procedure was used. Here, we did not start with an initial current intensity of 30 μA but instead started individually with a current intensity which was three 20%-steps below the previous ADT. The determinations of ADTs and GSTs before and after transplantation were performed at the same day of the week to avoid interday variance and at the same time of the day (in the afternoon) to avoid intraday variance in seizure thresholds. Typically six to twenty test stimulations were necessary in the present study to achieve reliable seizure thresholds as well as reliable seizure parameters, described in the following.
Severity of seizures at ADT and GST was classified according to Racine (1972): stage 1, immobility and facial automatisms (eye closure, facial clonus); stage 2, head nodding, associated with more severe facial clonus; stage 3, unilateral forelimb clonus; stage 4, rearing and bilateral forelimb clonus; stage 5, rearing and falling accompanied by generalized tonic-clonic seizures. In addition to seizure severity, seizure duration and afterdischarge duration (ADD) were determined at ADT as well as at GST. Seizure duration was further subdivided into seizure duration-1 and seizure duration-2. Seizure duration-1 was the period of limbic (stage 1–3) and/or motor seizures (stages 4/5), while seizure duration-2 was seizure duration-1 plus the time of postictal immobility (often associated with signs of focal seizure activity) until the rats showed normal behavior and reactions. ADD was the total time of epileptiform spikes in the EEG, including the time of stimulation.
Transplanted cell lines
Three cell lines were used for the present transplantation study. M213-2O cells (GABAergic), M213-2O hGAD67 cells (CL4 cells, GABAergic; hGAD, human glutamic acid decarboxylase), and 121-1I cells (non-GABAergic; control cell line) (Conejero-Goldberg et al., 2000; Giordano et al., 1993; Giordano et al., 1996). The parent cell line M213-2O was derived from embryonic rat striatum, and was immortalized with the tsA58 allele of SV40 large T antigen. Among other neuronal characteristics, the parent cell line as well as the CL4 cell line express GAD constitutively and are GABAergic (Giordano et al., 1993; Giordano et al., 1996). The CL4 cell line was additionally transfected with the hGAD67 cDNA using an episomal, Epstein-Barr virus-based plasmid vector, resulting in higher GABA synthesis than the parent cell line (Conejero-Goldberg et al., 2000). The control cell line, 121-1I, was also derived from embryonic rat striatum. It has an epithelial-like morphology, shows no GAD expression and is positive for vimentin (Giordano et al., 1996).
Cells were grown at the permissive temperature of 33°C in DMEM/F12 1:1 with 10% fetal calf serum, 1% penicillin-streptomycin and, only for CL4, hygromycin B (200 μg/ml) (all Invitrogen, Karlsruhe, Germany). At least three days prior to transplantation, cells were cultured at a temperature of 37°C to allow differentiation (Conejero et al., 1999) and adaption to temperature conditions after grafting into host brain. All cell types responded similarly by forming processes. At the day of transplantation, cells were trypsinized, washed, resuspended in DMEM/F12 1:1 (without serum and antibiotics), and held on ice until transplantation at a concentration of 100,000 cells/μl. Viability of cells was determined to be at least 90% immediately after transplantation using the remaining cells held on ice, by the trypan blue exclusion assay.
In preliminary transplantation experiments using the three cell lines, an analysis period of one to three weeks after transplantation was used to investigate viability of cells after transplantation and also, in the case of M213-2O and CL4 cells, served as positive controls for immunohistological detection of GAD and EBNA-1. Each cell line was transplanted at least 10 times into different nigral locations (anterior or posterior) and in both hemispheres, so that the animal number for the preliminary experiments altogether was twenty rats. The rats used for these preliminary experiments were not kindled.
Microtransplantation of cells
Three to five days after three stable ADTs have been determined, kindled rats received microtransplants of either M213-2O cells (n=8), CL4 cells (n=8), 121-1I cells (n=8), or cell free medium (n=7). Transplantation was performed using a method adapted from Nikkhah et al. (1994) to ensure air tight connections between a Hamilton syringe, a rubber connector, and a glass capillary (Fig. 2). The glass capillary was pulled to a tip diameter of 50 to 70 μm by means of a horizontal micropipette puller (PC-84 Sachs-Flaming, Sutter Instruments, San Rafael, CA, USA).
Fig. 2.
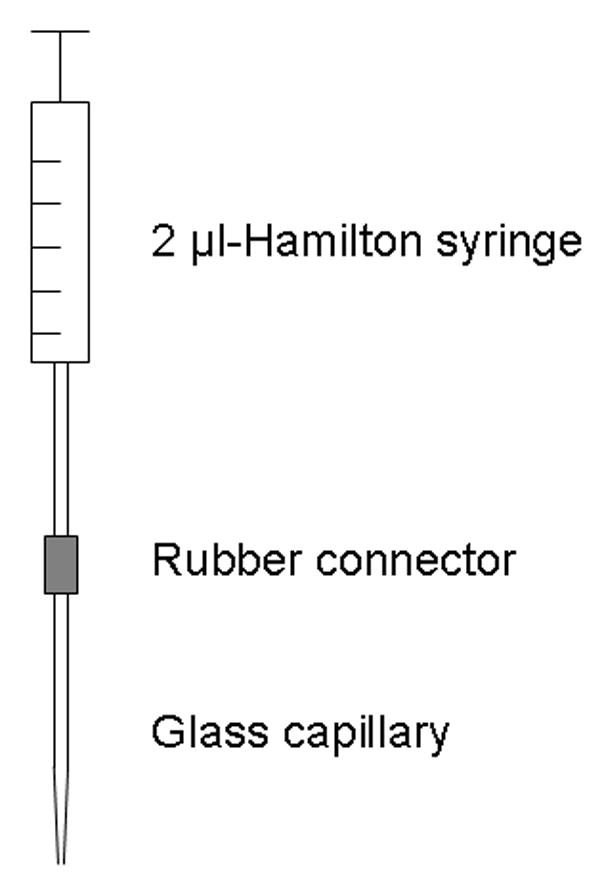
Microtransplantation equipment adapted from Nikkhah et al. (1994) in order to ensure air tight connections between a 2 μl Hamilton syringe (7002 N, Omnilab Laborzentrum, Gehrden, Germany), a rubber connector (Inner diameter 0.25 mm, Kleinfeld Labortechnik, Gehrden, Germany), and a glass capillary without filament (Outer diameter 1.09 mm, Inner diameter 0.8 mm; Süd-Laborbedarf, Gauting, Germany).
For transplantation, the kindled rats were again anesthetized with chloral hydrate (360 mg/kg i.p.) and placed into a stereotaxic frame according to Paxinos and Watson (1998). The part of the skull behind the dental acrylic cement of the kindling electrode connector was exposed and the micrografts were stereotaxically implanted into the SNr. According to a previous study by our group (Löscher et al., 1998), cells were implanted throughout the SNr. 500 nl deposits of cell suspension or medium, respectively, were bilaterally injected into eight locations (4 per hemisphere) using the following coordinates (in mm relative to Bregma): (1) P −5.1, L ±2.6, V −8.0; (2) P −5.1, L ±2.0, V −8.4; (3) P −5.8, L ±2.4, V −8.0; (4) P −5.8, L ±1.8, V −8.4. Finally, the trephine holes in the skull were covered with acrylic cement. After transplantation, no adverse neurological reactions were observed. The experimenter (M.W.N.) performing the rat experiments was blinded to the type of transplanted material throughout the study, i.e. at the time of transplantation and subsequently during determination of ADTs. Following a recovery period of 9 to 11 days, weekly determinations of ADTs and GSTs were resumed (Fig. 1). The determinations of ADTs and GSTs and seizure parameters at ADTs and GSTs, respectively, were continued for at least four weeks and up to eight weeks (i.e., as long as differences from control values could be detected).
Immunohistochemical analysis
After termination of the experiments, the rats were deeply anesthetized with chloral hydrate and transcardially perfused with 0.9% saline in 0.01M phosphate buffer (PBS, pH 7.6), followed by about 300 ml 4% paraformaldehyde/0.1% glutaraldehyde in 0.1M PBS (pH 7.6). The brains were removed and cryoprotected in 30% sucrose in 0.1M PBS (pH 7.6) and stored at 4°C. Two series of coronal sections were cut at 40 μm on a freezing microtome. One series was Nissl stained with thionine for verification of microtransplantation and stimulation sites, the other one was frozen at −20°C until processing free-floating for immunohistochemistry to detect either (1) GAD (isoforms 65 and 67), (2) Epstein-Barr nuclear antigen-1 (EBNA-1), or (3) glial fibrillary acidic protein (GFAP).
For immunohistochemistry the brain sections were treated as follows (between the following steps, sections were washed in 0.05 M Tris-buffered saline [TBS, pH 7.6]). Sections were incubated for 30 min in 0.5% hydrogen peroxide solution to block endogenous peroxidase activity and then preincubated in blocking solution for 60 min: TBS with 2% bovine serum albumin (BSA), 0.3% Triton X-100 and either (1) 10% normal rabbit serum or (2) 10% normal goat serum or (3) 10% normal swine serum. Thereafter, samples were directly transferred into “carrier”-solution (TBS with 1% BSA, 0.3% Triton X-100 and 1% serum of the animal species in which the secondary antibody was generated in) for ten min followed by a direct transfer into the primary antiserum at 4°C for 20 h for detection of GAD and GFAP, respectively. For the detection of EBNA-1, samples were incubated in primary antiserum for 60 min at 37°C prior to incubation at 4°C for 20 h. The primary antibodies were used at the following dilutions: (1) anti-GAD65/67 1440 (1:1000; a generous gift from Prof. Dr. W. H. Oertel [Dept. of Neurology, Philipps-University Marburg, Germany]; sheep polyclonal); (2) anti-EBNA-1 (1:50; Chemicon, Hofheim, Germany; mouse monoclonal); and (3) anti-bovine GFAP (1:1500; DAKO, Hamburg, Germany; rabbit polyclonal). The next day sections were again incubated in “carrier”-solution for ten min and then were directly exposed to the matching secondary antibody conjugated to biotin for 90 min at room temperature. The secondary antibodies were used at the following dilutions: (1) anti-goat (1:500; DAKO; rabbit); (2) anti-mouse (1:500; DAKO; goat); (3) anti-rabbit (1:500; DAKO; swine). Sections were then incubated in horseradish peroxidase-labeled streptavidin (1:375; DAKO) for 90 min at room temperature, followed by the nickel-intensified diaminobenzidine (DAB) reaction (0.05% 3,3-DAB and 0.6% ammonium nickel sulphate in TBS, both from Sigma-Aldrich, Munich, Germany) in the presence of 0.01% hydrogen peroxide for 15 min at room temperature. All antibodies were dissolved in “carrier”-solution. Finally, sections were mounted on gelatin-coated glass slides, air-dried, treated with toluol for one minute, and coverslipped with Entellan (Merck, Darmstadt, Germany) followed by microscopic analysis. Samples with omission of primary antibody served as negative controls.
Two randomly-chosen brains of the CL4 transplantation group were paraffinized after storage in 30% sucrose for further immunohistological analysis. Briefly, serial brain sections were cut at 6 μm on a rotatory microtome and were stained for haematoxylin-eosin and cresylviolet. In addition, immunohistology for the detection of reactive astrocytes, monocytes (activated microglia and macrophages), T-lymphocytes, proliferating cells and grafted cells was carried out using following primary antisera: (1) anti-GFAP antibody (DAKO, Hamburg, Germany; rabbit polyclonal; 1:1000 in PBS/1% BSA), (2) anti-ED1 antibody (Serotec, Eching, Germany; mouse monoclonal; 1:1000 in PBS/1% BSA), (3) anti-CD3 antibody (DAKO, Hamburg, Germany; rabbit polyclonal; 1:300 in PBS/1% BSA), (4) anti-Ki67 antibody (Dianova, Hamburg, Germany; mouse monoclonal; 1:100 in PBS/0.5% BSA), (5) anti-GAD65/67 antibody sheep polyclonal; 1:1000 in PBS/1% BSA) and (6) anti-EBNA-1 antibody (Chemicon, Hofheim, Germany; mouse monoclonal; 1:50 in PBS/1% BSA) and the avidin-biotin-complex method adapting previous protocols (Herden et al., 2005). Serial brain sections were deparaffinized in xylene and rehydrated through graded alcohols and endogenous peroxidase was quenched with 0.03% H202 diluted in methanol.
For Ki67 detection, microwave pretreatment (600 W microwave oven) was performed in citrate buffer (pH 6.0, 6 times for 5 min). To block nonspecific immunoglobin binding sites, sections were incubated for 20 min with 20% normal goat serum (polyclonal antibodies, monoclonal anti-ED1 antibody) or 20% normal horse serum (monoclonal antibodies). This was followed by exposure with the respective antisera overnight at 4°C. Primary polyclonal antibodies were detected using biotinylated antisera: (1) goat anti-rabbit, (2) rabbit anti-sheep for the GAD-antibody, (3) horse anti-mouse for the monoclonal antibodies, or (4) goat anti-mouse for the monoclonal anti-ED1 antibody (all: 1:200 in PBS, Vector, Burlingame, USA). The visualization of the antigen-antibody complex was carried out by the avidin-biotin-complex method (ABC, Vector, Burlingame, USA) with 3,3’-DAB as substrate. Brain sections were slightly counterstained with haemalaun. Between all steps, brain sections were extensively rinsed in PBS. Negative controls consisted of sections incubated with either rabbit preimmune sera (Sigma-Aldrich, Taufkirchen, Germany) or sheep preimmune sera (Clinic for Swine, Small Ruminants, Forensic Medicine, and Ambulatory Service, University of Veterinary Medicine, Hannover, Germany) instead of the primary polyclonal antibody or ascites from Balb/c mice (ABgene®, Hamburg, Germany) instead of the monoclonal antibody.
Statistical Analysis
Only data from rats with at least five microimplants within the SNr (at least two implants in each hemisphere) and the kindling electrode within the BLA were used for final evaluation. Data were evaluated using non-parametric tests. For comparisons between groups, Kruskal-Wallis analysis of variance (ANOVA) followed by the Mann-Whitney U-test was used, whereas for comparison of effects within groups Friedman ANOVA followed by the Wilcoxon test for paired data was used. The latter test was performed only for values obtained up to 4 weeks after transplantation due to the progressively decreasing group sizes thereafter. Applying a 4-sigma rule of statistics for GST and seizure duration-2, data of one animal of the medium group were excluded from further statistical analysis. To examine the significance of the association between the two variables pre-/post-transplantation and number of animals showing seizure/no seizure in a 2×2 contingency table the Fisher’s exact test was used. All tests were used two-tailed and an error probability of <5% was considered significant.
Results
Altogether, 26 out of 31 transplanted animals fulfilled the inclusion criteria described in methods (M213-2O, n=7; CL4, n=7; 121-1I, n=6; medium, n=6).
Kindling development before transplantation
For transplantation, fully-kindled rats were randomly assigned to four groups, i.e. for transplantation of M213-2O cells, CL4 cells, 121-1I cells, and medium. Kindling parameters of the rats used for final evaluation are shown in Table 1. As could be expected from a random assignment procedure, there were no significant differences between the four groups in pre-kindling and first post-kindling ADTs as well as in the kindling rate (number of stimulations until first stage 5 seizure and cumulative ADD until first stage 5 seizure, respectively). For all groups, ADTs decreased during progression of kindling (Table 1) resulting in lower post-kindling ADTs compared to pre-kindling values. This progression slowly continued until transplantation, so that the pre-transplantation ADTs were lower than the first post-kindling ADTs (Table 1 and Fig. 3B). An increased seizure susceptibility of the focus (i.e., the BLA) is one of the characteristics of the kindling phenomenon and is thought to eventually lead to the appearance of spontaneous seizures after a long period of repeated focal stimulations (Pinel, 1981; Sato et al., 1990).
Table 1. Kindling parameters before transplantation.
The afterdischarge thresholds (ADT) before (prekindling) and after (postkindling) acquisition of kindling, the number of stimulations, and the cumulative afterdischarge duration (ADD) to reach the first stage 5 seizure are shown. Data are means ± SEM. Statistical analysis did not reveal significant differences of kindling parameters between groups. In all groups, postkindling ADT tended to be lower than prekindling ADT. The difference was statistically significant in the M213-2O group.
| Cell line | Sample size | Prekindling ADT [μA] | Postkindling ADT [μA] | Number of stimulations until first stage 5 seizure | Cumulative ADD until first stage 5 seizure [sec] |
|---|---|---|---|---|---|
| M213-2O | 7 | 164±12 | 111±14* | 5.4±0.7 | 116±33 |
| CL4 | 7 | 145±22 | 134±27 | 6.0±0.7 | 152±35 |
| 121-1I | 6 | 131±18 | 95±7 | 5.0±0.9 | 88±32 |
| Medium | 6 | 150±6 | 122±10 | 4.7±0.4 | 69±15 |
p=0.0313, Wilcoxon test.
Fig. 3.
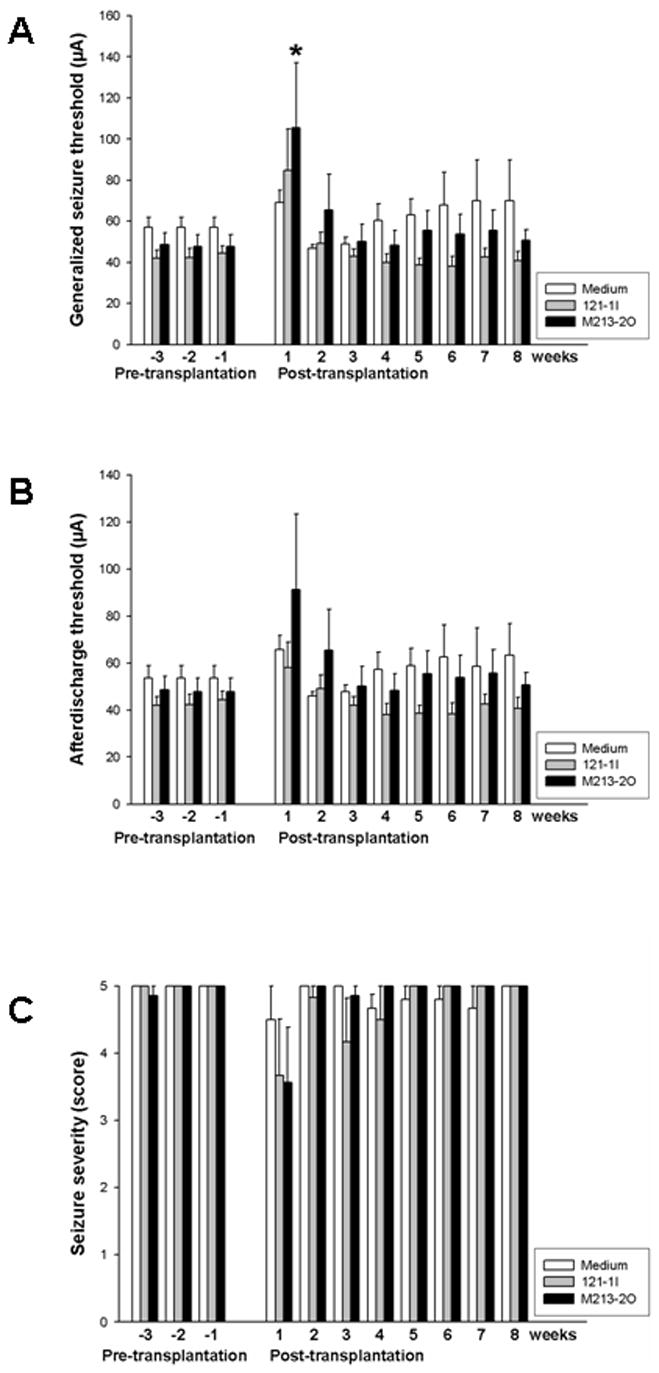
Means and SEM of (A) generalized seizure thresholds (GSTs), (B) afterdischarge thresholds (ADTs), and (C) seizure severities determined at individual ADT before and after microtransplantation of GABAergic M213-2O cells, non-GABAergic 121-1I cells, and medium in the SNr in fully kindled rats. GST significantly increased after transplantation of GABAergic M213-2O cells when compared with the last pre-transplantation value (Wilcoxon test *p=0.0313). There were no significant differences in ADTs and seizure severities between pre- and post-transplantation values.
Viability and location of M213-2O and 121-1I cells
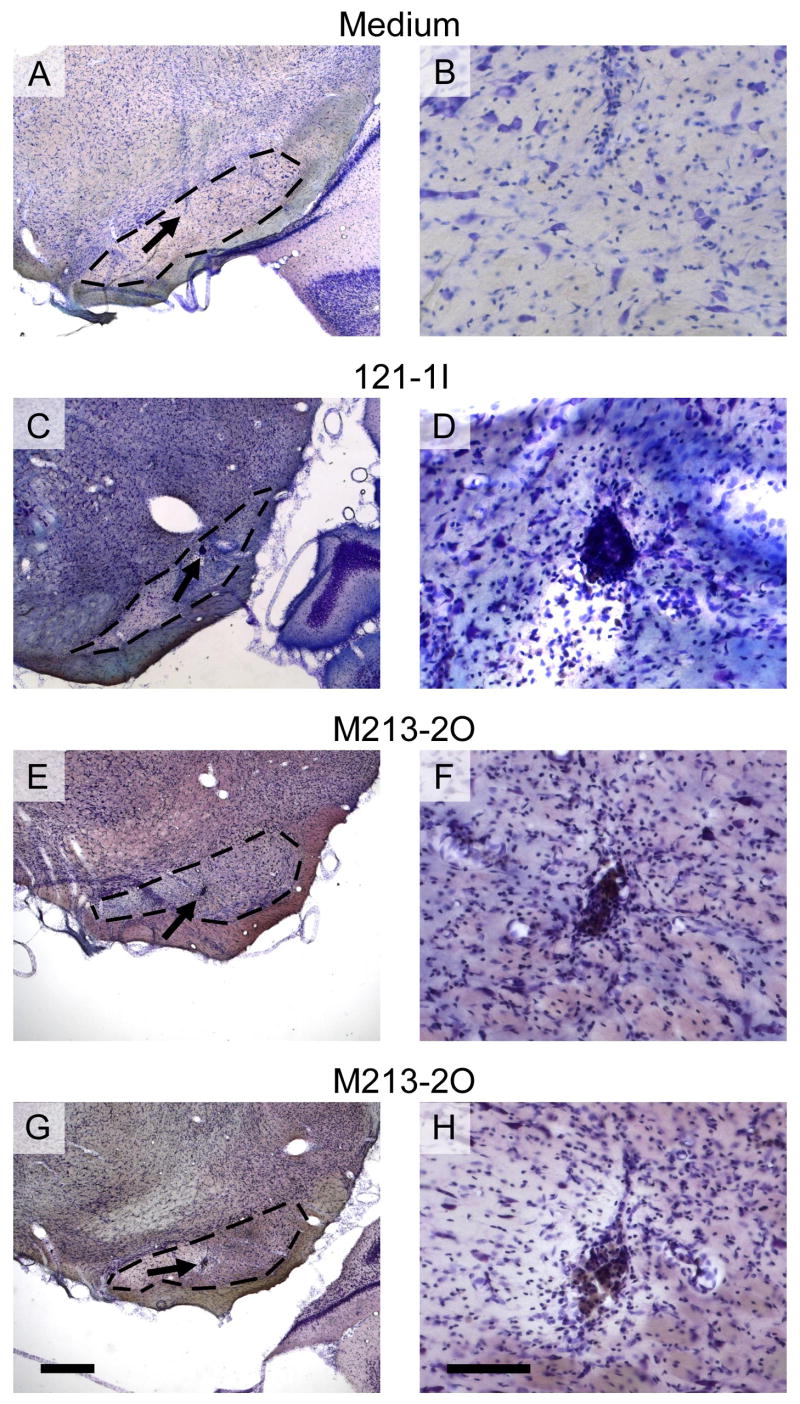
Viability and locations of transplants of M213-2O cells were analyzed at intervals of 4 to 8 weeks following implantation in individual rats, i.e. when individual seizure parameters regained pre-transplantation values. Animals which received 121-1I cells and medium, respectively, were processed for histological evaluation in a time-matched manner. Nissl-staining revealed areas containing implanted 121-1I and M213-2O cells, respectively, within the SNr (Fig. 4). Location of medium was determined by the needle tract (Fig. 4A). The borders of implants (arrow in Fig. 5A) as well as the needle tracts (Fig. 5B) were marked by moderate gliosis.
Fig. 4.
Nissl-stained coronal rat brain sections showing the substantia nigra pars reticulata (SNr) microinjected with medium (A and B), or microimplanted with a cell suspension of either non-GABAergic cells (121-1I, C and D), or GABAergic cells (M213-2O, E–H). Arrows on the left side depict areas which are shown in higher magnification on the right side. The shown pellets resemble the average pellet size. In the medium-injected rats, the gliotic cannula track was visible (A and B), whereas vital micrografts were visible within the 121-1I group (C and D) and the M213-2O group (E–H), surrounded by moderate gliosis. The dashed lines on the left side indicate the area of the SNr. Scale bars, 500 μm on the left side, and 100 μm on the right side.
Fig. 5.
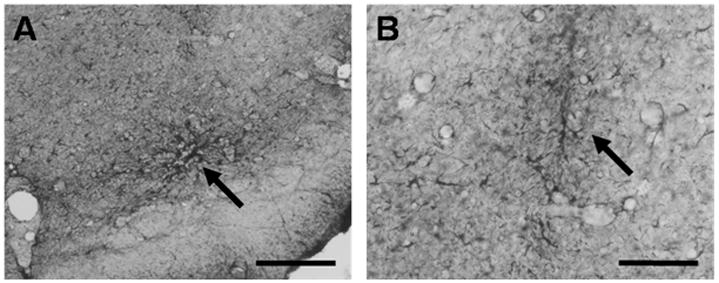
Immunohistological detection of glial fibrillary acid protein (GFAP) in coronal rat brain sections. Diaminobenzidine was used for chromogenic reaction. Shown are areas of the SNr microimplanted with 121-1I cells. Arrow in (A) depicts an area, where GFAP-positive cells border implanted 121-1I cells. Arrow in (B) indicates the cannula tract surrounded by moderate gliosis. Scale bars, 200 μm in (A) and 100 μm in (B).
Immunohistochemistry revealed GAD-positive transplants (cell bodies and processes) within the M213-2O group (Fig. 6C). Thus, the implanted M213-2O cells were viable at least to the end of the experiments. In the remaining groups (121-1I, medium), apart from a positive staining of host cells, no transplanted material was stained with the antibody against GAD (Figs. 6A and 6B). Control sections with omission of primary antibody (negative control) only showed background staining (not shown).
Fig. 6.
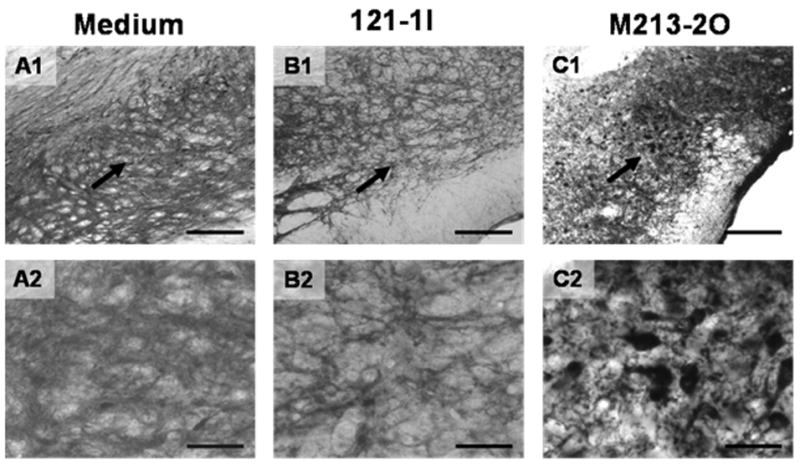
Immunohistological detection of glutamate decarboxylase protein (GAD) in coronal rat brain sections. Diaminobenzidine was used for chromogenic reaction. Shown are areas of the SNr either microinjected with medium (A1+2), or microimplanted with 121-1I cells (B1+2) or with M213-2O cells (C1+2). Arrows in the upper row (A1–C1) depict areas which are shown at higher magnification in the lower row (A2–C2). The tissues were treated identically and developed at the same time. Note the prominent positive staining for GAD in the GABAergic implants (M213-2O; C1+2), surrounded by immunopositive host cells, whereas in the other groups (A1+2; B1+2) only the positively stained host cells are visible. Scale bars 200 μm in A1–C1 and 50 μm in A2–C2.
Fig. 7 shows the locations of the microimplants within the SNr of the 121-1I group (Fig. 7B, left side) and the M213-2O group (Fig. 7B, right side). As mentioned above, only animals with at least five microimplants within the SNr (at least two microimplants per hemisphere) were selected for final evaluation of data. Some cell deposits were found in different locations outside the SNr and some were not found. We cannot exclude that in individual animals some of the deposits located outside the SNr might have influenced limbic and motor pathways outside the SNr. Fig. 7 only shows implants which were in the target area of the SNr. Note that microimplants were detected throughout the SNr.
Fig. 7.

Microimplants of 121-1I and M213-2O cells located within the SNr (adapted from Paxinos and Watson, 1998). (A) Scheme of a coronal section of a rat brain 5.3 mm posterior to bregma. The cutout on the right side shows a higher magnification of the SNr. (B) Summarized information about locations of the microtransplanted cells within the SNr (black marks; left: 121-1I group; right: M213-2O group). Only microimplants within the SNr are shown. The distance to bregma is given in the center line.
Effects of transplantation of M213-2O cells on kindled seizures
Within-group comparisons were made for pre- and post-transplantation values. Microtransplantation of the GABAergic cell line M213-2O into the SNr caused a significant increase in GST compared to the value obtained before transplantation (Fig. 3A; p=0.0313), whereas no such significant increase was seen in the control groups. The anticonvulsant effect of the GABAergic graft was transient. A significant increase of GST compared to the value obtained before transplantation of M213-2O cells was only seen at the first trial after transplantation (i.e. 9 to 11 days after transplantation; Fig. 3A). The ADT was not significantly raised after implantation of GABAergic M213-2O cells (p>0.05; Fig. 3B). No significant effect on seizure thresholds was observed with control cells, neither for ADT nor for GST (p>0.05 for both parameters; Fig. 3).
Before transplantation of GABAergic M213-2O cells into the SNr, all seven rats in this group showed electrographic as well as generalized behavioral seizure activity at their individual ADT. After transplantation, only two of the seven rats showed electrographic seizure activity when stimulated at the individual pre-transplantation ADT. From these two rats, one showed generalized behavioral seizure activity when stimulated at the individual pre-transplantation ADT and one did not show motor seizure at all. In other words, five out of seven rats were completely protected from electroencephalographic and behavioral seizures by the bilateral GABAergic transplants in the SNr when stimulated at the individual pre-transplantation ADT. The difference in the number of animals showing electrographic seizures before and after transplantation of GABAergic cells was significant (*p=0.021, Fisher’s exact test). No significant difference between pre- and post-transplantation was found for animals that received control cells (p>0.05). Although not significantly different from pre-transplantation, three weeks after transplantation of M213-2O cells two out of seven rats still were protected from electrographic or behavioral seizure activity when stimulated at the individual pre-transplantation ADT.
In contrast to this marked anticonvulsant effect seen when transplanted animals were stimulated at their individual pre-transplantation ADT, mean seizure severity observed at post-transplantation ADT was only slightly decreased after transplantation of M213-2O cells and was not significantly different from pre-transplantation values (Fig. 3C). Intranigral application of control cells or medium had no effect on seizure severity when compared to pre-transpantation values. At individual ADTs, neither transplantation of M213-2O cells, nor 121-1I cells, nor microinjection of medium significantly affected further seizure parameters, i.e., seizure duration-1, seizure duration-2, and ADD (not shown), when compared to pre-transplantation values.
The anticonvulsant effect of GABAergic M213-2O cells was not associated with any apparent adverse effects, i.e., the animals behaved normally after transplantation and at the time of test trials.
Effects of transplantation of hGAD67-transfected CL4 cells on kindled seizures
Comparisons were made within the CL4 group between pre- and post-transplantation values. Microtransplantation of CL4 cells into the SNr of kindled rats failed to cause significant changes in ADT and GST at different investigated time points after transplantation when compared to values before transplantation (p>0.05; not illustrated). Because all animals of the CL4 group showed generalized motor seizures at electrographic seizure threshold, the values for ADT and GST were the same. Mean values (± SEM) for ADT as well as for GST were 48.3 ± 7.2 μA before transplantation and 64.7 ± 16.9 μA at the first trial after transplantation. In addition, transplantation of CL4 cells did not significantly affect other seizure parameters, i.e., seizure duration-1, seizure duration-2, and ADD when compared to pre-transplantation values. This complete lack of effect is likely due to unexpected tissue reactions observed in kindled rats after transplantation of CL4 cells (see below).
Unexpected effects of hGAD67-transfected CL4 cells
(Immuno-)histochemical analysis of non-kindled rats transplanted with CL4 cells after one to three weeks confirmed viability of CL4 cells in non-kindled rats. In these animals, GAD-positive (not illustrated) and EBNA-1-positive cells were detected (Figs. 8A and 8B).
Fig. 8.
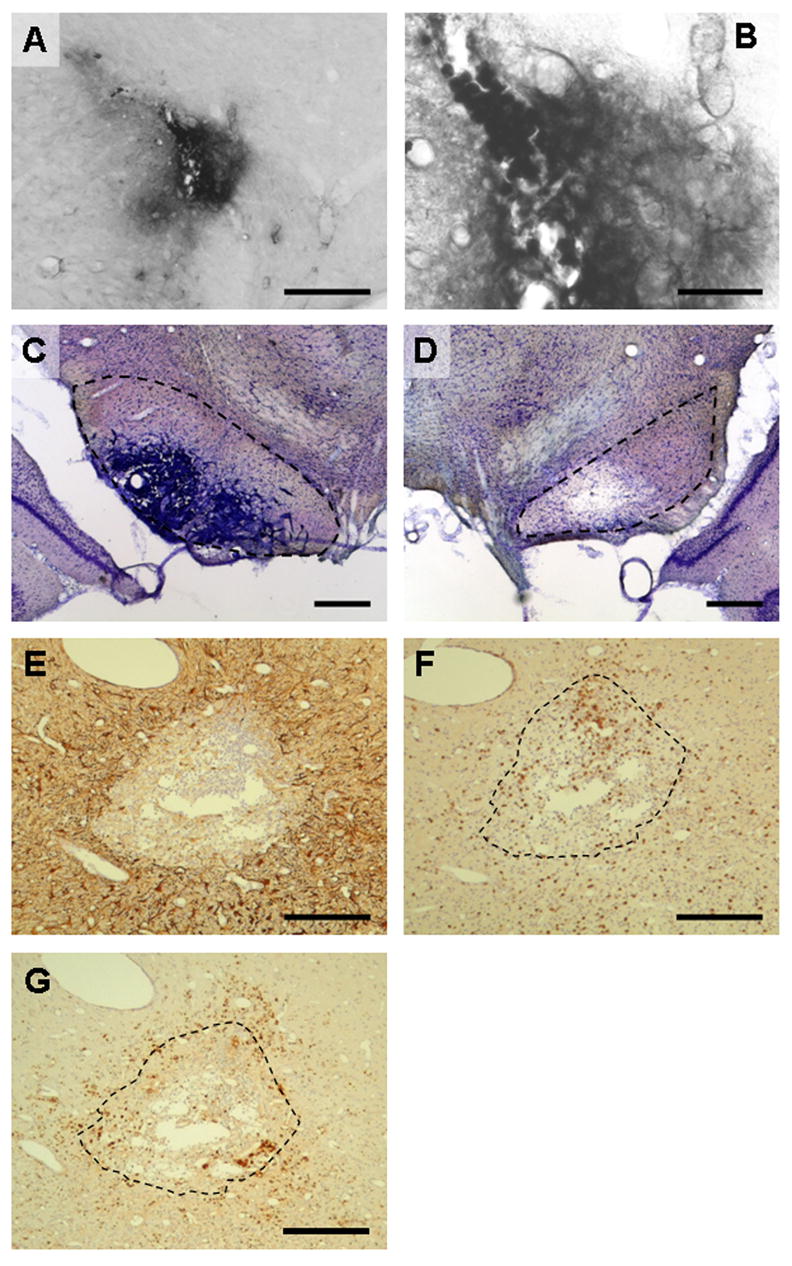
Immunohistological investigation of the SNr in coronal brain sections after microtransplantation of CL4 cells. (A) Immunohistological detection of Epstein-Barr Nuclear Antigen-1 (EBNA-1) in a non-kindled (naïve) rat which received microtransplantation of CL4 cells into the SNr. Diaminobenzidine was used for chromogenic reaction. EBNA-1 was detected in the cytoplasm of positive cells as observed before by Conejero-Goldberg et al. (2000). Immunopositive cells were detected within the transplantation area. Scale bar, 200 μm. (B) Higher magnification of detected cells in (A). Scale bar, 50 μm. (C) Nissl-stained section showing the SNr of a kindled rat microtransplanted with CL4 cells. At (and beyond) the site of microtransplantation, heavily-packed accumulations of diverse cells are visible within the SNr. Note the blood vessels at the border of the hypercellular region. Scale bar, 500 μm. (D) Same staining as shown in (C) for another kindled rat. A hypocellular region is visible at the site of microtransplantation. Transplanted CL4 cells were not detected. Scale bar, 500 μm. Dashed lines in (C) and (D) indicate the area of the SNr. (E) Immunohistological detection of glial fibrillary acid protein (GFAP) in a kindled rat which received microtransplantation of CL4 cells into the SNr. GFAP-positive cells enclose a hypercellular area. (F) Immunohistological detection of CD3-positive T-cells in a kindled rat which received microtransplantation of CL4 cells into the SNr. CD3-positive cells are enriched in the hypercellular area. (G) Immunohistological detection of ED1-positive activated microglia and macrophages in a kindled rat which received microtransplantation of CL4 cells into the SNr. ED1-positive cells were detected within as well as outside the hypercellular area. In (E), (F), and (G), diaminobenzidine was used for chromogenic reaction. Immunopositive cells appear brown. Sections were counterstained with haemalaun (blue cell nuclei). The dashed lines in (F) and (G) mark the enclosed area in (E). Scale bars, 200 μm in (E), (F), and (G).
On the other hand, immunohistochemical analysis of kindled rats which received transplanted CL4 cells revealed major cell infiltration, but no surviving transplanted cells. A densely-packed accumulation of infiltrating cells, including inflammatory cells, was observed in some cases (Figs. 8C, 8F, and 8G) while in other cases areas of neuronal loss could be observed in Nissl-stained sections (Fig. 8D).
Using Nissl-staining (Figs. 8C and 8D) and immunohistological detection of GAD and EBNA-1, in all but two animals of the CL4-group transplanted CL4 cells were not detected at any time point investigated. In some cases, tissue reactions were not restricted to the SNr but additionally involved parts of the brain stem. In these cases, tissue boundaries were completely lost and identification of brain regions was impossible. Interestingly, microtransplantation of CL4 cells was not associated with any adverse effects, i.e. as in the other groups, the animals behaved normally when observed after transplantation and shortly before determination of ADTs.
For a more detailed analysis, paraffin sections from two randomly-chosen rat brains of the CL4 transplanted group were stained either with haematoxylin-eosin and cresylviolet or were stained immunohistochemically for the detection of GAD, EBNA-1, GFAP, ED1, CD3, and Ki67. A more-or-less cell-rich area with features of malacia admixed with inflammatory cells (lymphocytes, T-cells, Fig. 8F), activated microglia and macrophages (Fig. 8G), and granulocytes (not shown) was detected. This area was bordered by gliosis (Figs. 8E and 8G). Transplanted CL4 cells could not be detected within this area by immunostaining for GAD or EBNA-1 (not shown). Finally, negative staining for Ki67 did not reveal enhanced cellular proliferation as an indication of tumor formation (not shown). Neither literature data (Castillo et al., 2006; Castillo et al., 2008; Ross et al., 2002) nor our own preliminary experiments mentioned above indicated similar tissue reactions under other conditions when CL4 cells have been intracerebrally transplanted.
Discussion
The two main findings were: (1) intranigral allotransplantation of immortalized GABAergic M213-2O cells, but not control cells or medium, caused transient anticonvulsant effects in previously amygdala-kindled rats, and (2) microtransplantation of hGAD67-transfected GABA-producing CL4 cells caused an unexpected strong tissue reaction within the SNr, but only in previously amygdala-kindled rats.
For clinical relevance, transplanted cells would be required to suppress seizure activity in animals with a previously-established epileptic syndrome, rather than acting prophylactically (Björklund and Lindvall, 2000). We therefore performed transplantations after progressive development of seizures, using previously kindled rats. This study therefore is different from previous studies in which genetically-engineered cells were transplanted before onset of kindling (Gernert et al., 2002; Thompson et al., 2000; Thompson, 2005). Thus, we now were able to show significant, although transient, anticonvulsant effects of genetically immortalized GABAergic M213-2O cells when transplanted into the SNr of already kindled rats. The observed anticonvulsant effect emphasizes the feasibility of local manipulations of seizure induction and propagation by intranigral GABAergic M213-2O grafts. A benefit associated with this strategy is that local manipulations of seizure activity may have fewer unwanted side effects as compared to systemic manipulations.
Anticonvulsant effects of intranigral transplantation of GABA-producing cells in rats with established epilepsy have been shown before, using fetal striatal cells in kindled rats (Löscher et al., 1998) and genetically engineered mouse cortical cells in a spontaneous rat seizure model (Thompson and Suchomelova, 2004). The advantage of the present study was to avoid using fetal cells, which are restricted from clinical use due to ethical and practical reasons (Harrower and Barker, 2004; Holt et al., 1997), and because of the use of cells from rats (allotransplantation) instead of mice (xenotransplantation) to minimize the risk of immune-mediated rejection or cross-species infections (Barker, 2000; Takei et al., 1990). M213-2O cells, which derived from rat striatum, did not cause graft rejection. This is in contrast to the host tissue reactions induced by CL4 cells (see below).
Although both fetal striatal cells (Löscher et al., 1998) and M213-2O cells (present study) caused anticonvulsant effects after intranigral transplantation in kindled rats, there are some differences concerning the quality of effects. Transplantation of fetal cells caused a significant increase of ADT and a significantly reduced seizure severity (Löscher et al. 1998). These parameters were similarly affected by M213-2O cells but failed to be significantly different from control values. On the other hand, transplanting M213-2O cells clearly influenced generalization of seizures. This is reflected by a significant increase of GST. In both studies, about 70 to 80 % of the rats did not exhibit any electrographic or behavioral seizure activity after transplantation when stimulated with their individual pre-transplantation ADT.
The positive effects on seizures we observed at early post-transplantation time-point are very unlikely to be linked to variable injury inflicted while grafting cells or medium because selective bilateral lesions of the SNr were shown not to affect fully kindled seizures in rats (Wahnschaffe & Löscher, 1990). Instead, the anticonvulsant effect we observed after intranigral transplantation of M213-2O cells in kindled rats was most likely attributed to an increased GABAergic inhibition of the SNr, most likely due to tonic, non-synaptic release of GABA. This is supported by studies, which showed that M213-2O cells produce GABA in vitro (Conejero-Goldberg et al., 2000; Giordano et al., 1993). However, we have no direct evidence that the effects of M213-2O cell grafts on seizures were linked to increased GABA levels in the SNr. One potential technique to address this important point would be intranigral microdialysis in transplanted rats.
We recently reported a kindling-induced hyperactivity of SNr neurons (Gernert et al., 2004). It is conceivable that the transplanted cells produce and locally release GABA and thereby normalize or reduce the increased SNr activity of kindled rats, comparable to the anticonvulsant effects observed after local microinjections of GABA-mimetic drugs (Depaulis et al., 1994; Iadarola and Gale, 1982; Löscher and Schwark, 1987; McNamara et al., 1984; Nolte et al., 2006a; Velísková and Moshé, 2006).
It should be noted that the SNr is not a uniform structure concerning its seizure modulating properties. Functionally different subregions (anterior and posterior SNr) have been described depending on age, sex and seizure type of the used model (Moshé et al., 1995; Shehab et al., 1996; Velísková and Moshé, 2006). Anterior-posterior differences were also described concerning kindling-induced changes in spontaneous SNr activity and concerning strength of response to systemic application of the antiepileptic drug valproic acid (Gernert et al., 2004). However, in the present study we did not differentiate between these SNr subregions but rather implanted the cell lines to several sites throughout the SNr in order to inhibit the SNr as extensively as possible.
In particular, the secondary generalization of seizures was significantly prevented at individual seizure thresholds in response to transplantation of GABAergic M213-2O cells. Indeed, the SNr has long been suggested as being a crucial site for seizure generalization in the kindling model of epilepsy (Morimoto and Goddard, 1987). This nigral influence has been suggested to be mediated by brain regions such as the dorsal midbrain (Gale et al., 1993; Garant and Gale, 1987) and the pedunculopontine nucleus (Nolte et al., 2006b). Furthermore, the SNr is thought to have a widespread influence on brain networks involved not only in propagation but also in initiation of seizure activity, perhaps explaining why some animals in the present study did not show a focal seizure after transplantation when stimulated at their individual pre-transplantation seizure thresholds.
According to Björklund and Lindvall (2000) and to Löscher et al. (2008), the main drawbacks of neural transplantation in experimental epilepsy include the difficulties in obtaining long-lasting effects and uncertainties concerning the possible risks to the host brain. Comparable to previous transplantation studies mentioned above we also observed only transient anticonvulsant effects (about ten days) despite long-term survival of GABA-producing M213-2O cells (up to eight weeks) transplanted into the SNr of kindled rats. Thereafter, non-significant seizure threshold variations from baseline were observed comparable to the non-significant variations induced by control cells and medium. It seems that concerning cell survival and duration of anticonvulsant effects allotransplantation may not have advantages over xenotransplanted or fetal cells.
Neither in the former studies nor in the present study was the survival of grafted cells measured at different time-points after transplantation to determine whether the number of surviving grafted cells decreases with time. In this respect it might be noted that a recent study demonstrated the possibility of increasing survival of hippocampal fetal cells, transplanted to the hippocampus of epileptic rats, by pretreating the grafts with neurotrophic factors and a caspase inhibitor (Rao et al., 2007). Accordingly, in upcoming studies we plan to investigate whether transplantation of cells together with neurotrophic factors or transplantation into other target regions will improve the beneficial effects of GABAergic cell lines on seizures. Furthermore, identification of the optimum cell suspension concentrations should be determined, since this parameter has been found to influence cell survival rate after transplantation (Terpstra et al., 2007). Independent from a putative loss of transplanted cells over time, it is conceivable that an altered synthesis and/or release of GABA by the grafted cells occurred in a time-dependent manner after transplantation. This latter issue should also be addressed in future studies.
Furthermore, there may be cell type-independent mechanisms that underlie the temporally restricted ability of transplantation in epilepsy, such as compensatory down-regulation of GABA-receptors in response to chronic exposure to GABA (Fenelon and Herbison, 1996). In order to circumvent this problem associated with the transplantation of GABA-producing cells, grafting of neural progenitor cells or stem cells may be beneficial providing an integration of the cells which then may lead to a physiological release of GABA rather than to a chronic and tonic release comparable to a cellular mini-pump.
On the other hand, it is conceivable that the amount of GABA released by the M213-2O cells declined over time and then was insufficient to cause long-lasting anticonvulsant effects. In this respect, the CL4 cells were expected to induce longer-lasting and perhaps more prominent anticonvulsant effects because they were transfected with hGAD67 to produce higher levels of GABA (Conejero-Goldberg et al., 2000). While the unexpected tissue reactions in response to CL4 cells in kindled rats in our study did not allow us to test this hypotheses, recent studies by Castillo et al. (2006, 2008) found CL4 cells to be able to modulate seizures induced by kainate administered systemically about two months after transplantation of CL4 cells into the SNr.
As mentioned above, Björklund and Lindvall (2000) suggested uncertainties concerning possible risks to the host brain as being one of the main drawbacks associated with neural transplantation in epilepsy. We indeed observed strong tissue alterations in and close to the SNr of the kindled host brain after transplantation of CL4 cells. These extensive tissue reactions did not induce changes in seizure parameters, which is in agreement with a previous study showing that selective bilateral lesion of the SNr did not affect kindled seizures (Wahnschaffe and Löscher, 1990).
Regarding risks of immune reactions to transplantation akin to those observed in the present study, two factors probably contribute to these risks: (1) the graft itself and (2) the condition of the host. Concerning the latter factor, it is important to note that the strong tissue alterations in response to transplantation of CL4 cells in kindled rats were not observed in our preliminary experiments using non-kindled (naïve) rats. Also, previous studies using CL4 cells transplanted either into the inferior colliculus to suppress audiogenic seizures (Ross et al., 2002) or into the SNr to modulate kainate-induced seizures (Castillo et al., 2006) also did not result in significant local tissue reactions. These data strongly suggest that the host condition, i.e. the kindled state of the brain, contributed to the observed tissue reactions. Although not sufficient to explain the present finding, it is interesting to note that a previous study showed a massive bilateral activation of microglia in response to kindling within several limbic brain regions (piriform cortex and dentate gyrus) and, most pronounced, within the SNr (Ebert et al., 1997). Currently, there is no indication that this microglia activation in the SNr of kindled rats is linked to neurodegeneration. This is because there was no degeneration of GABAergic nigral cells in fully kindled rats six weeks after a generalized kindled seizure (Freichel et al., 2004). It was suggested that this activation of resting microglia occurs during kindling and that it reflects an endogenous response of the brain to counteract limbic epileptogenesis (Ebert et al., 1999). Together with an immunological stimulus that may be presented by transfected CL4 cells (see below), this overactivation of microglia might result in the tissue reactions we observed after transplantation of CL4 cells but not M213-2O cells in the SNr of kindled rats. Indeed, overactivation of microglia is known to contribute to inflammatory and neurotoxic reactions (Block et al., 2007). It is also important to note that although the Ki67 staining was negative, we can not definitely exclude that tumorous alterations might have contributed to the strong tissue alterations observed after transplantation of CL4 cells.
A previous study, in which another GABA-producing cell line was transplanted into the SNr in the kindling model, did not indicate strong tissue reactions within the SNr (Thompson et al., 2000). Irrespective of several methodological differences between this previous study (transplantation prior to entorhinal cortex kindling) and the present study (transplantation subsequent to amygdala kindling), the question is raised of why in the present study CL4 cells but not the parent M213-2O cells caused strong tissue reactions in kindled rats. Although the brain has long been considered as being immunologically privileged, more recent observations showed that immune responses can occur (Barker and Widner, 2004). The CL4 cells differ from the parent M213-2O cell line in that they were transfected with the hGAD67 cDNA using an episomal, Epstein-Barr virus-based plasmid vector (Conejero-Goldberg et al., 2000). These cells therefore produce several proteins, i.e., the EBNA1 antigen, hGAD67, and the hygromycin selection marker, which could contribute to immune reactions.
In a related study (Castillo et al., 2008), the same CL4 cell line used in our study was transplanted into the SN in a seizure model induced by kindling with systemic injections of kainic acid, resulting in a long-term alleviation of seizures and mortality. The transplanted cells survived for extended periods without a substantial immune reaction to the CL4 grafts. This reinforces our conclusion that the combination of amygdala kindling and genetically-modified cell transplants was necessary to produce the immune reactions to CL4 cells that we observed. Thus, it is possible that under circumstances where there is no additional immunological stimulation or physical stress of the brain, genetically modified cells akin to CL4 would be functionally effective. Nevertheless, for any potential human application, the possibility of immune stimulation of the brain would always exist. Therefore, if any human use of genetically-modified cells were to be considered, it would be essential to identify the cellular components which are capable of producing immune reactions in the seizure-activated brain, and eliminate these components from any transplanted genetically-modified cells. In the present study, the human GAD67 enzyme, the EBNA protein, and the hygromycin-resistance gene product are likely candidates (Burbelo et al., 2008).
Two conclusions can be drawn from the present study in the light of previous studies. (1) Significant, although transient, anticonvulsant effects can be observed after transplantation of different cell types. (2) The host condition as well as the type of transplanted cells must both be considered in estimate the risks of immune reactions to intracerebrally transplanted cells, as indicated by our observation that intranigral transplantation of CL4 cells caused strong tissues alterations in previously kindled rats but not in non-kindled rats.
Acknowledgments
We are grateful to Michael Weißing for excellent help in cell culture. We thank Holger Volk for crucial help in immunohistology. Horst Briese did a wonderful job in adapting a probe holder needed during the stereotaxic transplantation procedure. The assistance of Christiane Bartling is gratefully acknowledged. We thank Prof. Dr. Claudia Grothe and Marco Timmer (both Dept. of Neuroanatomy, Medical School Hannover, Germany) for methodological advice with the microtransplantation technique. The research was supported in part by a grant of the German National Academic Foundation to MWN and in part by the NIDA IRP, NIH, DHHS.
References
- Barker RA. Porcine neural xenografts: what are the issues? Novartis Found Symp. 2000;231:184–96. doi: 10.1002/0470870834.ch12. discussion 196-201, 302-6. [DOI] [PubMed] [Google Scholar]
- Barker RA, Widner H. Immune problems in central nervous system cell therapy. NeuroRx. 2004;1:472–81. doi: 10.1602/neurorx.1.4.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram E. The relevance of kindling for human epilepsy. Epilepsia. 2007;48(Suppl 2):65–74. doi: 10.1111/j.1528-1167.2007.01068.x. [DOI] [PubMed] [Google Scholar]
- Björklund A, Lindvall O. Cell replacement therapies for central nervous system disorders. Nat Neurosci. 2000;3:537–44. doi: 10.1038/75705. [DOI] [PubMed] [Google Scholar]
- Block ML, et al. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- Boison D. Adenosine and epilepsy: from therapeutic rationale to new therapeutic strategies. Neuroscientist. 2005;11:25–36. doi: 10.1177/1073858404269112. [DOI] [PubMed] [Google Scholar]
- Bonhaus DW, et al. Activation of substantia nigra neurons: role in the propagation of seizures in kindled rats. J Neurosci. 1986;6:3024–30. doi: 10.1523/JNEUROSCI.06-10-03024.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brevig T, et al. Xenotransplantation for CNS repair: immunological barriers and strategies to overcome them. Trends Neurosci. 2000;23:337–44. doi: 10.1016/s0166-2236(00)01605-2. [DOI] [PubMed] [Google Scholar]
- Burbelo PD, et al. High definition profiling of autoantibodies to glutamic acid decarboxylases GAD65/GAD67 in stiff-person syndrome. Biochem. Biophys. Res. Commun. 2008;366:1–7. doi: 10.1016/j.bbrc.2007.11.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo CG, et al. Intranigral transplants of immortalized GABAergic cells decrease the expression of kainic acid-induced seizures in the rat. Behav Brain Res. 2006;171:109–15. doi: 10.1016/j.bbr.2006.03.025. [DOI] [PubMed] [Google Scholar]
- Castillo CG, et al. Intranigral transplants of a GABAergic cell line produce long-term alleviation of established motor seizures. Behav Brain Res. 2008 doi: 10.1016/j.bbr.2008.04.023. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassagnon S, et al. Optimal window for ictal blood flow mapping. Insight from the study of discrete temporo-limbic seizures in rats. Epilepsy Res. 2006;69:100–18. doi: 10.1016/j.eplepsyres.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Chassagnon S, et al. Time course and mapping of cerebral perfusion during amygdala secondarily generalized seizures. Epilepsia. 2005;46:1178–87. doi: 10.1111/j.1528-1167.2005.07505.x. [DOI] [PubMed] [Google Scholar]
- Conejero C, et al. Glutamate and antimitotic agents induce differentiation, p53 activation, and apoptosis in rodent neostriatal cell lines immortalized with the tsA58 allele of SV40 large T antigen. Exp Neurol. 1999;158:109–20. doi: 10.1006/exnr.1999.7083. [DOI] [PubMed] [Google Scholar]
- Conejero-Goldberg C, et al. Transduction of human GAD67 cDNA into immortalized striatal cell lines using an Epstein-Barr virus-based plasmid vector increases GABA content. Exp Neurol. 2000;161:453–61. doi: 10.1006/exnr.1999.7258. [DOI] [PubMed] [Google Scholar]
- Depaulis A, et al. Endogenous control of epilepsy: the nigral inhibitory system. Prog Neurobiol. 1994;42:33–52. doi: 10.1016/0301-0082(94)90020-5. [DOI] [PubMed] [Google Scholar]
- Deransart C, Depaulis A. The control of seizures by the basal ganglia? A review of experimental data. Epileptic Disord. 2002;4(Suppl 3):S61–72. [PubMed] [Google Scholar]
- Ebert U, et al. Increase of microglial markers in the limbic system and substantia nigra of amygdala-kindled rats. Soc Neurosci Abstr. 1997;23:2161. [Google Scholar]
- Ebert U, et al. Glia cells influence limbic epileptogenesis in the piriform cortex of the rat. In: Elsner N, Eysel U, editors. Göttingen Neurobiology Report. Thieme; Stuttgart: 1999. p. 772. 1999. [Google Scholar]
- Fenelon VS, Herbison AE. In vivo regulation of specific GABAA receptor subunit messenger RNAs by increased GABA concentrations in rat brain. Neuroscience. 1996;71:661–70. doi: 10.1016/0306-4522(95)00492-0. [DOI] [PubMed] [Google Scholar]
- Fine A, et al. Modulation of experimentally induced epilepsy by intracerebral grafts of fetal GABAergic neurons. Neuropsychologia. 1990;28:627–34. doi: 10.1016/0028-3932(90)90038-p. [DOI] [PubMed] [Google Scholar]
- Freichel C, et al. Amygdala-kindling does not induce a persistent loss of GABA neurons in the substantia nigra pars reticulata of rats. Brain Res. 2004;1025:203–9. doi: 10.1016/j.brainres.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Gale K, et al. Blockade of GABA receptors in superior colliculus protects against focally evoked limbic motor seizures. Brain Res. 1993;603:279–83. doi: 10.1016/0006-8993(93)91248-q. [DOI] [PubMed] [Google Scholar]
- Garant DS, Gale K. Substantia nigra-mediated anticonvulsant actions: role of nigral output pathways. Exp Neurol. 1987;97:143–59. doi: 10.1016/0014-4886(87)90289-5. [DOI] [PubMed] [Google Scholar]
- Gernert M, et al. Subregional changes in discharge rate, pattern, and drug sensitivity of putative GABAergic nigral neurons in the kindling model of epilepsy. Eur J Neurosci. 2004;20:2377–86. doi: 10.1111/j.1460-9568.2004.03699.x. [DOI] [PubMed] [Google Scholar]
- Gernert M, Löscher W. Lack of robust anticonvulsant effects of muscimol microinfusions in the anterior substantia nigra of kindled rats. Eur J Pharmacol. 2001;432:35–41. doi: 10.1016/s0014-2999(01)01458-3. [DOI] [PubMed] [Google Scholar]
- Gernert M, et al. Genetically engineered GABA-producing cells demonstrate anticonvulsant effects and long-term transgene expression when transplanted into the central piriform cortex of rats. Exp Neurol. 2002;176:183–92. doi: 10.1006/exnr.2002.7914. [DOI] [PubMed] [Google Scholar]
- Giordano M, et al. Immortalized GABAergic cell lines derived from rat striatum using a temperature-sensitive allele of the SV40 large T antigen. Exp Neurol. 1993;124:395–400. doi: 10.1006/exnr.1993.1213. [DOI] [PubMed] [Google Scholar]
- Giordano M, et al. Constitutive expression of glutamic acid decarboxylase (GAD) by striatal cell lines immortalized using the tsA58 allele of the SV40 large T antigen. Cell Transplant. 1996;5:563–75. doi: 10.1177/096368979600500506. [DOI] [PubMed] [Google Scholar]
- Goddard GV, et al. A permanent change in brain function resulting from daily electrical stimulation. Exp Neurol. 1969;25:295–330. doi: 10.1016/0014-4886(69)90128-9. [DOI] [PubMed] [Google Scholar]
- Harrower TP, Barker RA. The emerging technologies of neural xenografting and stem cell transplantation for treating neurodegenerative disorders. Drugs Today (Barc) 2004;40:171–89. doi: 10.1358/dot.2004.40.2.799428. [DOI] [PubMed] [Google Scholar]
- Herden C, et al. Expression of allograft inflammatory factor-1 and haeme oxygenase-1 in brains of rats infected with the neurotropic Borna disease virus. Neuropathol Appl Neurobiol. 2005;31:512–21. doi: 10.1111/j.1365-2990.2005.00668.x. [DOI] [PubMed] [Google Scholar]
- Holt DA, et al. Infectious issues in human fetal neural transplantation. Cell Transplant. 1997;6:553–6. doi: 10.1177/096368979700600605. [DOI] [PubMed] [Google Scholar]
- Iadarola MJ, Gale K. Substantia nigra: site of anticonvulsant activity mediated by gamma-aminobutyric acid. Science. 1982;218:1237–40. doi: 10.1126/science.7146907. [DOI] [PubMed] [Google Scholar]
- Kokaia M, et al. Seizure suppression in kindling epilepsy by intracerebral implants of GABA- but not by noradrenaline-releasing polymer matrices. Exp Brain Res. 1994;100:385–94. doi: 10.1007/BF02738399. [DOI] [PubMed] [Google Scholar]
- Löscher W, et al. Effect of microinjections of gamma-vinyl GABA or isoniazid into substantia nigra on the development of amygdala kindling in rats. Exp Neurol. 1987;95:622–38. doi: 10.1016/0014-4886(87)90304-9. [DOI] [PubMed] [Google Scholar]
- Löscher W, Ebert U. Basic mechanisms of seizure propagation: targets for rational drug design and rational polypharmacy. Epilepsy Res Suppl. 1996;11:17–43. [PubMed] [Google Scholar]
- Löscher W, et al. Seizure suppression in kindling epilepsy by grafts of fetal GABAergic neurons in rat substantia nigra. J Neurosci Res. 1998;51:196–209. doi: 10.1002/(SICI)1097-4547(19980115)51:2<196::AID-JNR8>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Löscher W, et al. Distribution of GABAergic neurons in the striatum of amygdala-kindled rats: an immunohistochemical and in situ hybridization study. Brain Res. 2006;1083:50–60. doi: 10.1016/j.brainres.2006.01.096. [DOI] [PubMed] [Google Scholar]
- Löscher, et al. Cell and gene therapies in epilepsy - promising avenues or blind alleys? TINS. 2008;31:62–73. doi: 10.1016/j.tins.2007.11.012. [DOI] [PubMed] [Google Scholar]
- Löscher W, Schwark WS. Evidence for impaired GABAergic activity in the substantia nigra of amygdaloid kindled rats. Brain Res. 1985;339:146–50. doi: 10.1016/0006-8993(85)90634-1. [DOI] [PubMed] [Google Scholar]
- Löscher W, Schwark WS. Further evidence for abnormal GABAergic circuits in amygdala-kindled rats. Brain Res. 1987;420:385–90. doi: 10.1016/0006-8993(87)91262-5. [DOI] [PubMed] [Google Scholar]
- McNamara JO, et al. Role of the substantia nigra in the kindling model of limbic epilepsy. Adv Exp Med Biol. 1986;203:139–46. doi: 10.1007/978-1-4684-7971-3_10. [DOI] [PubMed] [Google Scholar]
- McNamara JO, et al. Evidence implicating substantia nigra in regulation of kindled seizure threshold. J Neurosci. 1984;4:2410–7. doi: 10.1523/JNEUROSCI.04-09-02410.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto K, Goddard GV. The substantia nigra is an important site for the containment of seizure generalization in the kindling model of epilepsy. Epilepsia. 1987;28:1–10. doi: 10.1111/j.1528-1157.1987.tb03613.x. [DOI] [PubMed] [Google Scholar]
- Moshé SL, et al. Ontogeny and topography of seizure regulation by the substantia nigra. Brain Dev. 1995;17(Suppl):61–72. doi: 10.1016/0387-7604(95)90074-8. [DOI] [PubMed] [Google Scholar]
- Nikkhah G, et al. A microtransplantation approach for cell suspension grafting in the rat Parkinson model: a detailed account of the methodology. Neuroscience. 1994;63:57–72. doi: 10.1016/0306-4522(94)90007-8. [DOI] [PubMed] [Google Scholar]
- Nolte MW, et al. Microinfusion of gamma-vinyl-GABA but not muscimol into the anterior substantia nigra pars reticulata exerts robust anticonvulsant effects in male kindled rats. 7th European Congress on Epileptology. Abstracts from the 7th European Congress on Epileptology (7th ECE Proceedings); Helsinki, Finland. 2006a. p. 84. [Google Scholar]
- Nolte MW, et al. Pedunculopontine neurons are involved in network changes in the kindling model of temporal lobe epilepsy. Neurobiol Dis. 2006b;23:206–18. doi: 10.1016/j.nbd.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Nolte MW, et al. Benefits and risks of intranigral microtransplantation of immortalized GABA-producing cell lines in the kindling model of temporal lobe epilepsy. Naunyn-Schmiedeberg’s Arch Pharmacol. 2008;377(Suppl 1):51. [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; Sydney: 1998. [DOI] [PubMed] [Google Scholar]
- Pinel JPJ. Spontaneous kindled motor seizures in rats. In: Wada JA, editor. Kindling. Vol. 2. Raven Press; New York: 1981. pp. 179–187. [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–94. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Raedt R, et al. Cell therapy in models for temporal lobe epilepsy. Seizure. 2007;16:565–78. doi: 10.1016/j.seizure.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Rao MS, et al. Strategies for promoting anti-seizure effects of hippocampal fetal cells grafted into the hippocampus of rats exhibiting chronic temporal lobe epilepsy. Neurobiol Dis. 2007;27:117–32. doi: 10.1016/j.nbd.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross KC, et al. Transplantation of M213-2O cells with enhanced GAD67 expression into the inferior colliculus alters audiogenic seizures. Exp Neurol. 2002;177:338–40. doi: 10.1006/exnr.2002.7987. [DOI] [PubMed] [Google Scholar]
- Sato M, et al. Kindling: basic mechanisms and clinical validity. Electroencephalogr Clin Neurophysiol. 1990;76:459–72. doi: 10.1016/0013-4694(90)90099-6. [DOI] [PubMed] [Google Scholar]
- Schachter SC, et al. Porcine fetal GABA-producing neural cell transplants for human partial onset seizures: safety and feasibility. Epilepsia. 1998;39:67. [Google Scholar]
- Shehab S, et al. Regional distribution of the anticonvulsant and behavioural effects of muscimol injected into the substantia nigra of rats. Eur J Neurosci. 1996;8:749–757. doi: 10.1111/j.1460-9568.1996.tb01260.x. [DOI] [PubMed] [Google Scholar]
- Shi LH, et al. Temporal sequence of ictal discharges propagation in the corticolimbic basal ganglia system during amygdala kindled seizures in freely moving rats. Epilepsy Res. 2007;73:85–97. doi: 10.1016/j.eplepsyres.2006.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takei K, et al. Immunological rejection of grafted tissue in xenogeneic neural transplantation. Prog Brain Res. 1990;82:103–9. doi: 10.1016/s0079-6123(08)62596-0. [DOI] [PubMed] [Google Scholar]
- Terpstra BT, et al. Increased cell suspension concentration augments the survival rate of grafted tyrosine hydroxylase immunoreactive neurons. J Neurosci Methods. 2007;166:13–9. doi: 10.1016/j.jneumeth.2007.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson K, et al. Conditionally immortalized cell lines, engineered to produce and release GABA, modulate the development of behavioral seizures. Exp Neurol. 2000;161:481–9. doi: 10.1006/exnr.1999.7305. [DOI] [PubMed] [Google Scholar]
- Thompson KW. Genetically engineered cells with regulatable GABA production can affect afterdischarges and behavioral seizures after transplantation into the dentate gyrus. Neuroscience. 2005;133:1029–37. doi: 10.1016/j.neuroscience.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Thompson KW, Suchomelova LM. Transplants of cells engineered to produce GABA suppress spontaneous seizures. Epilepsia. 2004;45:4–12. doi: 10.1111/j.0013-9580.2004.29503.x. [DOI] [PubMed] [Google Scholar]
- Turner DA, Shetty AK. Clinical prospects for neural grafting therapy for hippocampal lesions and epilepsy. Neurosurgery. 2003;52:632–44. doi: 10.1227/01.neu.0000047825.91205.e6. discussion 641–4. [DOI] [PubMed] [Google Scholar]
- Velísková J, Moshé SL. Update on the role of substantia nigra pars reticulata in the regulation of seizures. Epilepsy Curr. 2006;6:83–7. doi: 10.1111/j.1535-7511.2006.00106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahnschaffe U, Löscher W. Selective bilateral destruction of substantia nigra has no effect on kindled seizures induced from stimulation of amygdala or piriform cortex in rats. Neurosci Lett. 1990;113:205–10. doi: 10.1016/0304-3940(90)90304-r. [DOI] [PubMed] [Google Scholar]