Abstract
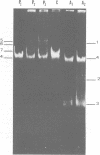
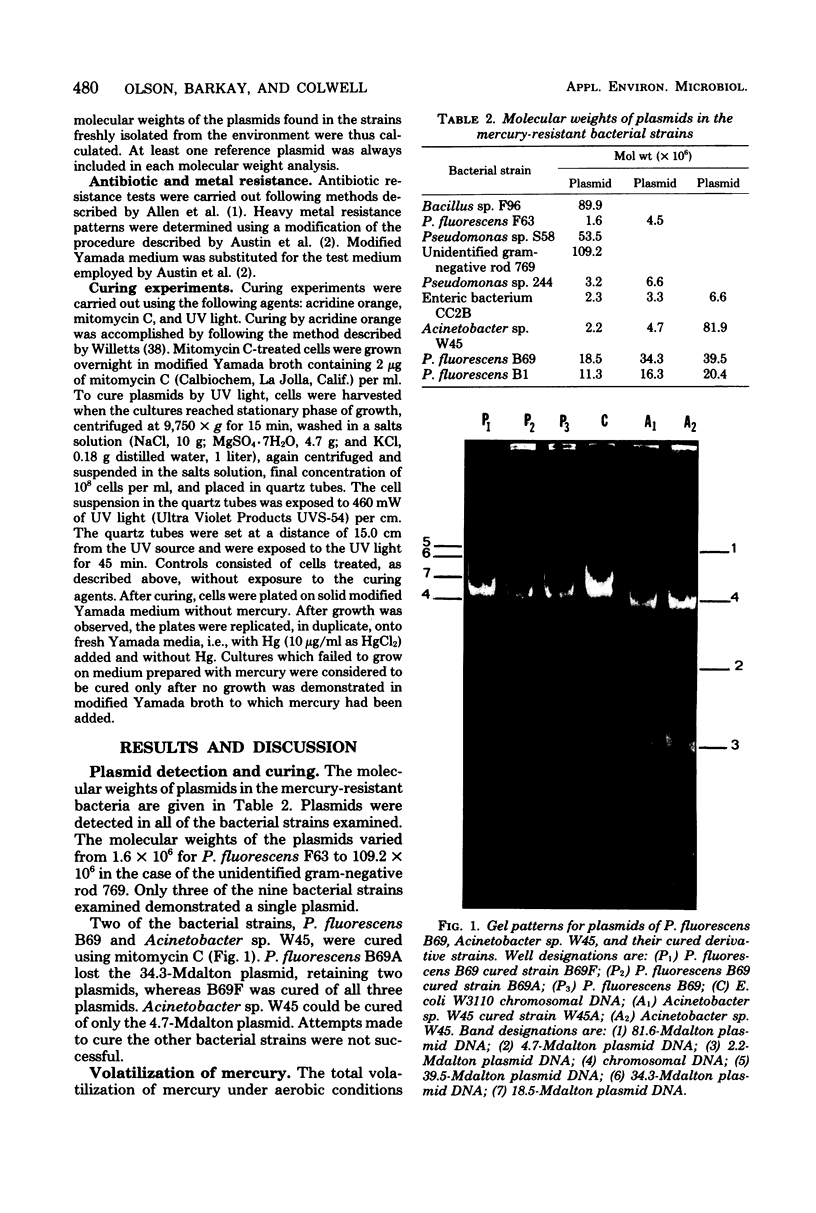
Eight mercury-resistant bacterial strains isolated from the Chesapeake Bay and one strain isolated from the Cayman Trench were examined for ability to volatilize mercury. Mercury volatilization was found to be variable in the strains tested. In addition, plasmids were detected in all strains. After curing, two of the bacterial strains lost mercury resistance, indicating that volatilization is plasmid mediated in these strains. Only two cultures demonstrated ability to methylate mercuric chloride under either aerobic or anaerobic conditions. Methylation of mercury, compared with volatilization, appears to be mediated by a separate genetic system in these bacteria. It is concluded that mercury volatilization in the estuarine environment can be mediated by genes carried on plasmids.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. A., Austin B., Colwell R. R. Antibiotic resistance patterns of metal-tolerant bacteria isolated from an estuary. Antimicrob Agents Chemother. 1977 Oct;12(4):545–547. doi: 10.1128/aac.12.4.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin B., Allen D. A., Mills A. L., Colwell R. R. Numerical taxonomy of heavy metal-tolerant bacteria isolated from an estuary. Can J Microbiol. 1977 Oct;23(10):1433–1447. doi: 10.1139/m77-212. [DOI] [PubMed] [Google Scholar]
- Chakrabarty A. M. Plasmids in Pseudomonas. Annu Rev Genet. 1976;10:7–30. doi: 10.1146/annurev.ge.10.120176.000255. [DOI] [PubMed] [Google Scholar]
- Clark D. L., Weiss A. A., Silver S. Mercury and organomercurial resistances determined by plasmids in Pseudomonas. J Bacteriol. 1977 Oct;132(1):186–196. doi: 10.1128/jb.132.1.186-196.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerry P., LeBlanc D. J., Falkow S. General method for the isolation of plasmid deoxyribonucleic acid. J Bacteriol. 1973 Nov;116(2):1064–1066. doi: 10.1128/jb.116.2.1064-1066.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadeishi T., McLaughlin R. D. Hyperfine zeeman effect atomic absorption spectrometer for mercury. Science. 1971 Oct 22;174(4007):404–407. doi: 10.1126/science.174.4007.404. [DOI] [PubMed] [Google Scholar]
- Hamdy M. K., Noyes O. R. Formation of methyl mercury by bacteria. Appl Microbiol. 1975 Sep;30(3):424–432. doi: 10.1128/am.30.3.424-432.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izaki K. Enzymatic reduction of mercurous ions in Escherichia coli bearing R factor. J Bacteriol. 1977 Aug;131(2):696–698. doi: 10.1128/jb.131.2.696-698.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komura I., Izaki K. Mechanism of mercuric chloride resistance in microorganisms. I. Vaporization of a mercury compound from mercuric chloride by multiple drug resistant strains of Escherichia coli. J Biochem. 1971 Dec;70(6):885–893. doi: 10.1093/oxfordjournals.jbchem.a129718. [DOI] [PubMed] [Google Scholar]
- Kondo I., Ishikawa T., Nakahara H. Mercury and cadmium resistances mediated by the penicillinase plasmid in Staphylococcus aureus. J Bacteriol. 1974 Jan;117(1):1–7. doi: 10.1128/jb.117.1.1-7.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loutit J. S. Investigation of the mating system of Pseudomonas aeruginosa strain I. VI. Mercury resistance associated with the sex factor (FP). Genet Res. 1970 Oct 2;16(2):179–184. doi: 10.1017/s0016672300002408. [DOI] [PubMed] [Google Scholar]
- Meyers J. A., Sanchez D., Elwell L. P., Falkow S. Simple agarose gel electrophoretic method for the identification and characterization of plasmid deoxyribonucleic acid. J Bacteriol. 1976 Sep;127(3):1529–1537. doi: 10.1128/jb.127.3.1529-1537.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahara H., Ishikawa T., Sarai Y., Kondo I. Frequency of heavy-metal resistance in bacteria from inpatients in Japan. Nature. 1977 Mar 10;266(5598):165–167. doi: 10.1038/266165a0. [DOI] [PubMed] [Google Scholar]
- Nelson J. D., Blair W., Brinckman F. E., Colwell R. R., Iverson W. P. Biodegradation of phenylmercuric acetate by mercury-resistant bacteria. Appl Microbiol. 1973 Sep;26(3):321–326. doi: 10.1128/am.26.3.321-326.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P., Roth C. Plasmid-linked resistance to inorganic salts in Staphylococcus aureus. J Bacteriol. 1968 Apr;95(4):1335–1342. doi: 10.1128/jb.95.4.1335-1342.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHMOND M. H., JOHN M. CO-TRANSDUCTION BY A STAPHYLOCOCCAL PHAGE OF THE GENES RESPONSIBLE FOR PENICILLINASE SYNTHESIS AND RESISTANCE TO MERCURY SALTS. Nature. 1964 Jun 27;202:1360–1361. doi: 10.1038/2021360a0. [DOI] [PubMed] [Google Scholar]
- Schottel J., Mandal A., Clark D., Silver S., Hedges R. W. Volatilisation of mercury and organomercurials determined by inducible R-factor systems in enteric bacteria. Nature. 1974 Sep 27;251(5473):335–337. doi: 10.1038/251335a0. [DOI] [PubMed] [Google Scholar]
- Smith D. H. R factors mediate resistance to mercury, nickel, and cobalt. Science. 1967 May 26;156(3778):1114–1116. doi: 10.1126/science.156.3778.1114. [DOI] [PubMed] [Google Scholar]
- Stanisich V. A., Bennett P. M., Richmond M. H. Characterization of a translocation unit encoding resistance to mercuric ions that occurs on a nonconjugative plasmid in Pseudomonas aeruginosa. J Bacteriol. 1977 Mar;129(3):1227–1233. doi: 10.1128/jb.129.3.1227-1233.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanisich V. A. Interaction between an R factor and an element conferring resistance to mercuric ions in Pseudomonas aeruginosa. Mol Gen Genet. 1974 Feb 6;128(3):201–212. doi: 10.1007/BF00267109. [DOI] [PubMed] [Google Scholar]
- Summers A. O., Jacoby G. A. Plasmid-determined resistance to boron and chromium compounds in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1978 Apr;13(4):637–640. doi: 10.1128/aac.13.4.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers A. O., Silver S. Mercury resistance in a plasmid-bearing strain of Escherichia coli. J Bacteriol. 1972 Dec;112(3):1228–1236. doi: 10.1128/jb.112.3.1228-1236.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers A. O., Silver S. Microbial transformations of metals. Annu Rev Microbiol. 1978;32:637–672. doi: 10.1146/annurev.mi.32.100178.003225. [DOI] [PubMed] [Google Scholar]
- Summers A. O., Sugarman L. I. Cell-free mercury(II)-reducing activity in a plasmid-bearing strain of Escherichia coli. J Bacteriol. 1974 Jul;119(1):242–249. doi: 10.1128/jb.119.1.242-249.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonk J. W., Sijpesteijn A. K. Studies on the methylation of mercuric chloride by pure cultures of bacteria and fungi. Antonie Van Leeuwenhoek. 1973;39(3):505–513. doi: 10.1007/BF02578894. [DOI] [PubMed] [Google Scholar]
- Wang P. Y., Relf J., Palchaudhuri S., Iyer V. N. Plasmid conferring increased sensitivity to mercuric chloride and cobalt chloride found in some laboratory strains of Escherichia coli K-12. J Bacteriol. 1978 Feb;133(2):1042–1043. doi: 10.1128/jb.133.2.1042-1043.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A. A., Murphy S. D., Silver S. Mercury and organomercurial resistances determined by plasmids in Staphylococcus aureus. J Bacteriol. 1977 Oct;132(1):197–208. doi: 10.1128/jb.132.1.197-208.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts N. S. The elimination of Flac+ from Escherichia coli by mutagenic agents. Biochem Biophys Res Commun. 1967 Apr 7;27(1):112–117. doi: 10.1016/s0006-291x(67)80048-2. [DOI] [PubMed] [Google Scholar]