Abstract
Because of the prominent psychoactive effects of cannabis and its preparations, much research has focused on the actions of cannabinoids, the primary psychoactive components of cannabis, on neuronal function. A convergence of research has identified (1) cannabinoid receptors, (2) endogenous compounds that activate these receptors (endocannabinoids), and (3) drugs that interact with these receptors and the proteins that synthesize and degrade the endocannabinoids. This review will first consider how endogenous cannabinoids signal through cannabinoid receptors and the various forms of synaptic plasticity mediated by endocannabinoids. Next the interactions between exogenous cannabinoids such as Δ9-tetrahydrocannabinol and endocannabinoids and endocannabinoid-mediated plasticity will be examined. Finally, a model will be presented that can explain the prominent psychoactivity of these plant-derived cannabinoids.
Keywords: Neuronal plasticity, cannabinoids, long term depression, endocannabinoid
1. Introduction
The primary cannabinoid receptors in the CNS are CB1 receptors. The CB1 receptor is a member of the large family of G protein-coupled receptors (GPCR’s) (Howlett et al., 2002). Thus, they are cell surface proteins that consist of seven transmembrane domains, with an extracellular amino terminus, and an intracellular C terminus. CB1 receptors predominately couple to inhibitory G proteins (Gi and Go), but under certain conditions they can couple to either Gs or Gq/11 (Howlett et al., 2002). Coupling to Gi and Go means that the primary effects of CB1 activation are inhibition of adenylyl cyclase and certain calcium channels together with the activation of inwardly rectifying potassium channels and several different MAP kinases (Howlett et al., 2002). A second cannabinoid receptor is the CB2 cannabinoid receptor. Although this receptor is primarily found in cells of the immune system, credible data supports the expression of CB2 in neurons under certain circumstances (Van Sickle et al., 2005, Wotherspoon et al., 2005). However, while its biology is fascinating (Whiteside et al., 2007) a consideration of this receptor is beyond the scope of the current review. There are additional receptors that can interact with exogenous and endogenous cannabinoids, including GPR55 (Pertwee, 2007). Whether these receptors play a role in modulating neurotransmission remains controversial (Hajos and Freund, 2002, Hoffman et al., 2005, Takahashi and Castillo, 2006) and won’t be considered here.
2. Cannabinoid receptor localization
Key to understanding the function of a receptor is determining its localization. CB1 receptors have been localized by autoradiography, in situ hybridization, and immunocytochemistry reviewed by, (Mackie, 2005). These studies reveal several interesting properties of CB1 receptors and their distribution. The first is that CB1 receptors are among the most abundant GPCR’s in the central nervous system (Herkenham et al., 1990). The second is that the pattern of CB1 receptor expression is consistent with the psychoactive effects of cannabis. That is, high levels are found in the brain regions implicated in the actions of cannabis, including cortex, amygdala, basal ganglia, cerebellum, and brainstem emetic centers (Herkenham et al., 1991). In contrast, relatively low levels are found in other brainstem nuclei, such as those involved in controlling respiration (a feature that distinguishes CB1 from opiate receptors) and thalamus. The third is that CB1 receptors have a striking presynaptic localization. The vast majority of CB1 receptors detected in immunocytochemical studies are found on the plasma membranes of axons and axon terminals (Nyiri et al., 2005). Many of the remaining CB1 receptors appear to be associated with synthetic pathways or are in the process of being trafficked to axons (Nyiri et al., 2005). Together, these findings suggest CB1 receptors play a major role in modulating synaptic transmission in a variety of brain regions associated with higher cognitive function.
3. Endogenous cannabinoids (endocannabinoids)
The presence of cannabinoid receptors suggests an endogenous ligand. Indeed, this is the case. Two endogenous ligands for the CB1 receptor have been well characterized. The first is anandamide, the amide of arachidonic acid and ethanolamine (Devane et al., 1992). The second is 2-arachidonoyl glycerol (2-AG), the ester (at the sn two position) of arachidonic acid and glycerol (Stella et al., 1997, Sugiura et al., 1995). Both share the similarity that they exist as precursors in the cell membrane and are produced in response to specific stimuli. However, they differ in their pharmacological properties (e.g., 2-AG is a much more efficacious agonist than anandamide) and are produced and degraded by very different enzymatic pathways (Alexander and Kendall, 2007). Thus, anandamide and 2-AG are likely produced by different physiological stimuli and their effects on neurons may well differ both in impact and duration. However, both endocannabinoids are made following periods of intense neuronal activity, activity that typically increases intracellular calcium and activates metabotropic receptors, such as group I mGlu receptors or muscarinic receptors (Stella and Piomelli, 2001).
4. Endocannabinoid-mediated synaptic plasticity
As mentioned above, the majority of CB1 receptors are found presynaptically. While the highest levels in forebrain are found on CCK positive interneurons (Katona et al., 1999), they are also present on many forebrain glutamatergic terminals (Katona et al., 2006, Kawamura et al., 2006). Activation of presynaptic CB1 receptors decreases neurotransmitter release, an effect first demonstrated unequivocally in cultured hippocampal neurons (Shen et al., 1996). Since endocannabinoids are synthesized during periods of intense neuronal activity, the localization of CB1 receptors suggests that they might participate in a form of feedback inhibition, where the production of endocannabinoids in the post-synaptic cell inhibits release of transmitter. Indeed, this appears to be the case at a number of synapses throughout the CNS, from the spinal cord to cortex (Hashimotodani et al., 2007), at least in mature animals. This phenomenon is referred to as “endocannabinoid-mediated plasticity.” It is important to appreciate that this is a mechanism that serves to either attenuate or enhance excitability, depending on the release of an excitatory or inhibitory neurotransmitter is being reduced (e.g., glutamate or GABA). While this phenomenon is well established to occur in young animals, its importance over the full lifespan of the animal remains to be determined.
4.1 Depolarization induced suppression of neurotransmission (DSI/DSE)
The first form of synaptic plasticity where endocannabinoids were implicated was depolarization-induced suppression of inhibition (DSI). DSI is a transient suppression of inhibitory neurotransmitter release onto a neuron following depolarization of that neuron (Llano et al., 1991, Pitler and Alger, 1992). While first identified and best studied in hippocampus and cerebellum, this form of plasticity occurs widely. The working model for DSI is that depolarization of the post-synaptic cell increases dendritic calcium levels which stimulates the production of an endocannabinoid (Figure 1A). The identity of this endocannabinoid (e.g., 2-AG vs. anandamide) remains controversial, though the balance of evidence supports a more prominent role for 2-AG (Hashimotodani et al., 2007). This endocannabinoid is believed to travel retrogradely, from the dendrite, across the synaptic cleft to activate CB1 receptors on the presynaptic terminal and preterminal axon segment. The activated CB1 receptors inhibit calcium channels (and may also stimulate potassium channels or have direct effects on the synaptic vesicle release machinery) thus decreasing neurotransmitter release (Wilson et al., 2001). A similar phenomenon also occurs at glutamatergic synapses where CB1 receptors are presynaptically expressed. In this case the phenomenon is referred to as depolarization induced suppression of excitation (DSE) (Kreitzer and Regehr, 2002).
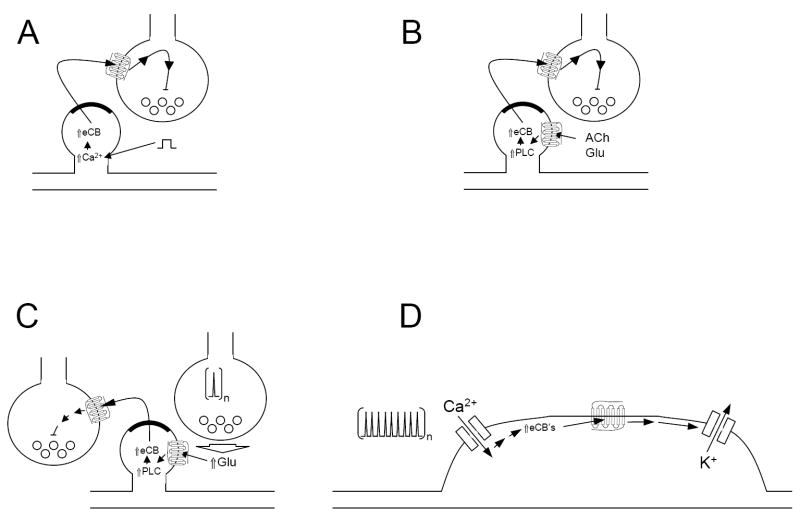
Figure 1. Schematic illustrations of four types of endocannabinoid-mediated plasticity.
A. Depolarization-induced suppression of inhibition or excitation. Depolarization of the post-synaptic cell (depicted by the square wave) increases intracellular calcium, leading to a production of endocannabinoids (eCB). These endocannabinoids then diffuse across the synaptic cleft, activating presynaptic CB1 receptors, leading to the transient inhibition of neurotransmission. B. Metabotropic suppression of inhibition or excitation. Acetylcholine (ACh) or glutamate (Glu) released from neighboring cells activates dendritically localized group I glutamate or m1 or m3 muscarinic receptors, activating phospholipase C (PLC) producing eCB’s. These eCB’s diffuse across the synaptic cleft, activating presynaptic CB1 receptors, leading to the transient inhibition of neurotransmission. C. Endocannabinoid-mediated long-term depression (LTD). Repeated low frequency stimulation of glutamatergic pathways leads to the prolonged activation of group I metabotropic glutamate receptors and high levels of endocannabinoid production. The prolonged stimulation of presynaptic CB1 receptors sets in motion a process that leads to a long-term inhibition of neurotransmitter release that outlasts the production of endocannabinoids. Shown is an example of heterosynaptic LTD. If the CB1 receptors are on the stimulated terminals, then homosynaptic LTD will be produced. D. Endocannabinoid-mediated inhibition of neuronal excitability. Repeated rapid depolarization of a neuron leads to increases in intracellular calcium, activating endocannabinoid production. These endocannabinoids activate CB1 receptors, which in turn activate inwardly rectifying potassium channels, efflux of potassium and hyperpolarization of the neuron. Note that in contrast to the other forms of endocannabinoid-mediated plasticity shown in the other panels, in this form of plasticity the endocannabinoid is produced and acts on the same cell.
4.2 Metabotropic induced suppression of neurotransmission (MSI/MSE)
Endocannabinoids can also be synthesized from membrane phospholipids following activation of post-synaptic Gq/11-linked GPCR’s, most notably, group I metabotropic glutamate receptors and M1 and M3 muscarinic receptors (Ohno-Shosaku et al., 2003, Varma et al., 2001) (Figure 1B). Most likely the endocannabinoid produced by this route is 2-AG. The proposed synthetic pathway is through the sequential activation of phospholipase Cß (PLCß) and diacylglycerol lipase (Sugiura et al., 2002), though additional synthetic routes have been proposed. Like with DSI and DSE, endocannabinoids synthesized by metabotropic receptor activation are thought to travel presynaptically to inhibit neurotransmitter release (Chevaleyre et al., 2006), although alternative processes involving nitric oxide have been suggested (Makara et al., 2007). This mechanism occurs at both inhibitory and excitatory synapses and has been designated metabotropic suppression of inhibition (MSI) or excitation (MSE). Unlike DSI or DSE, post-synaptic calcium need not increase to produce MSI or MSE (Ohno-Shosaku et al., 2005). A permissive level of intracellular calcium is sufficient for PLCß activity (ca. 100 nM). However, increasing intracellular calcium will augment endocannabinoid production during MSI/MSE (Hashimotodani et al., 2005). Thus, MSI and MSE have been proposed to serve as a coincidence detector between metabotropic receptor activation (e.g., by glutamate or acetylcholine) and depolarization-induced calcium influx (Hashimotodani et al., 2005).
4.3 Endocannabinoid-mediated long-term depression (LTD)
Endocannabinoids also participate in other forms of synaptic plasticity. The best described of these is one form of long-term depression (LTD). Endocannabinoid mediated LTD can be evoked by periods of extended low frequency stimulation of glutamatergic fibers (Gerdeman et al., 2002, Robbe et al., 2002) (Figure 1C). Two broad classifications of endocannabinoid mediated LTD have been described: homosynaptic and heterosynaptic. Homosynaptic LTD has been well characterized in glutamatergic projections from cortex to both dorsal striatum and the nucleus accumbens (Gerdeman et al., 2002, Robbe et al., 2002). Here, prolonged (minutes) low frequency stimulation leads to a suppression of glutamate release that persists long after the stimulation ends. The induction of this form of LTD requires CB1 receptors, however its maintenance does not (Robbe et al., 2002).
A second form of endocannabinoid-mediated LTD is heterosynaptic LTD. This form of LTD occurs at synapses adjacent to the glutamatergic fibers being stimulated. This was originally exhaustively described in hippocampus where low frequency stimulation of Schaffer collaterals leads to a long-term depression of inhibitory transmission from adjacent GABAergic terminals (Chevaleyre and Castillo, 2003). It is easy to imagine how weakening of inhibitory input in the face of sustained excitatory input could lead to long lasting strengthening of synaptic connections (Chevaleyre and Castillo, 2004). Similar heterosynaptic LTD also has been found in the amygdala (Marsicano et al., 2002) and is likely a common form of endocannabinoid modulation of synaptic plasticity. Figure 1C is a cartoon showing the highlights of heterosynaptic LTD.
4.4 Endocannabinoid-mediated spike timing-dependent plasticity (STDP)
Another form of persistent synaptic plasticity where endocannabinoids have been implicated is in some types of spike timing-dependent plasticity (STDP) (Dan and Poo, 2006, Kampa et al., 2007). STDP is a form of synaptic plasticity that occurs following repeated pairing of presynaptic release of glutamate from excitatory terminals and postsynaptic depolarization. Interestingly, if postsynaptic depolarization precedes glutamate release by a few to tens of milliseconds long term depression is typically produced. Conversely, if the glutamate release precedes the postsynaptic depolarization than long-term potentiation (LTP) is produced. A substantial body of evidence suggests that some forms of LTD produced by STDP are mediated by endocannabinoids. The working model is that the postsynaptic depolarization increases intracellular calcium, leading to the generation of endocannabinoids, which then act on presynaptic cannabinoid receptors triggering a series of events culminating in LTD. This form of endocannabinoid-mediated LTD has been most extensively characterized in visual cortex (Sjostrom et al., 2003, Sjostrom et al., 2004). But endocannabinoid involvement in STDP also has been found in other cortical regions including auditory and somatosensory (Tzounopoulos et al., 2007) (Bender et al., 2006).
4.5 Endocannabinoid-mediated cerebellar LTD
A mechanistically unique form of endocannabinoid-mediated LTD has been described in cerebellum (Safo and Regehr, 2005). The previous forms of LTD are manifested by decreases in neurotransmitter release (that is, a presynaptic site of action). However, cerebellar LTD produced by concurrent activation of parallel and climbing fibers, while requiring presynaptic cannabinoid receptors, is manifested by a decreased responsiveness of the postsynaptic cell (in this case Purkinje neurons) (Safo and Regehr, 2005). Thus, this form of LTD first requires the retrograde transmission of endocannabinoids from the Purkinje neuron back to presynaptic terminals and then the anterograde travel of a messenger from the presynaptic terminal to the Purkinje neuron from the expression of LTD. It has been speculated the nitric oxide might be the anterograde messenger (Safo et al., 2006). This form of LTD has been suggested to be involved in certain forms of cerebellar learning (Kishimoto and Kano, 2006, Skosnik et al., 2007). It is not known if this type of endocannabinoid-mediated LTD is found outside of the cerebellum.
4.6 Endocannabinoid inhibition of neuronal excitability
Endocannabinoids can also evoke changes in neuronal excitability independent of their effects on synaptic transmission. Here, endocannabinoids produced by depolarization of a neuron act on somatic CB1 receptors to activate potassium channels, hyperpolarizing the neuron and inhibiting firing (Figure 1D). This phenomenon has been described for basket cells in the cerebellum (Kreitzer et al., 2002) and low-threshold-spiking interneurons in cortex (Bacci et al., 2004), but likely occurs in a more widespread fashion. It is interesting to note that the previous forms of endocannabinoid-mediated synaptic plasticity exert their actions over a very restricted area, typically on the order of twenty microns or so (Wilson et al., 2001). In contrast, by attenuating action potentials, cannabinoid activation of somatic potassium channels has a much wider sphere of influence through inhibiting the synaptic output of that neuron.
5. Interactions between Δ9THC and endocannabinoid-mediated synaptic plasticity
The last several years have seen the emergence of an, albeit partial, understanding of the multiple roles endocannabinoids play in modulating synaptic transmission and neuronal excitability. An important question for the field is how Δ9THC (THC), the primary psychoactive component of cannabis interacts with these multiple forms of plasticity. That is, what underlies the psychoactivity of cannabis? Two broad possibilities might be the explanation. The first is that since THC is an agonist at CB1 receptors, perhaps its mode of action is to mimic the actions of endocannabinoids and widely inhibit synaptic transmission through the indiscriminate activation of CB1 receptors. The second possibility is that since THC is a low efficacy agonist, and the endogenous cannabinoid most frequently implicated in the various forms of endocannabinoid-mediated synaptic plasticity is 2-AG, a high efficacy agonist, perhaps the actions of THC are more complex and THC is actually antagonizing the effects of 2-AG.
This question has been difficult to address in brain slice preparations, the model system most frequently used to study endocannabinoid-mediated plasticity. The reason for this is that THC is quite hydrophobic and penetrates brain slices slowly and incompletely. To overcome this technical limitation we developed a simplified neuronal cell culture system that recapitulates DSE/DSI, MSE/MSI, and endocannabinoid-mediated LTD. These experiments were conducted in autaptic cultures (Bekkers and Stevens, 1991). In autaptic cultures a single neuron is grown on an island of glia in a sea of non-permissive substrate. This causes the neuron to form synapses back on itself. Thus, all of the synaptic inputs on the neuron arise from a single neuron, giving a high level of control to the system. Autaptic cultures prepared from early postnatal mice have a predominance of glutamatergic neurons, although some inhibitory neurons are also found. The single neuron nature of the preparation also allows very rapid and complete solution exchanges, allowing the actions of THC to be precisely determined.
Somewhat surprisingly, in examining DSE, MSE, and endocannabinoid-mediated LTD, we found that increasing concentrations of THC antagonized all three forms of plasticity (Straiker and Mackie, 2005, Straiker and Mackie, 2007) (and R. Kellogg, 5/22/2008, and Straiker, unpublished observations). Interestingly, long-term treatment with THC caused desensitization of cannabinoid responses (Straiker and Mackie, 2005). Thus, while occupancy of CB1 receptors by THC antagonizes endocannabinoid inhibition of neurotransmission in autaptic cultures, THC still stimulates CB1 receptors sufficiently to set in motion the cellular machinery necessary for desensitization. This appears to be an example of functional selectivity or biased agonism in CB1 receptor signaling (Urban et al., 2007). Thus, at least from the cell culture results it appears that a major action of THC is to antagonize endogenous cannabinoid signaling. Does this mean that the psychoactivity of THC and cannabis are simply due to the antagonism of endocannabinoid signaling? This is not the case because the indiscriminant antagonism of CB1 receptors by the CB1 antagonist rimonabant generally does not mimic the effects of THC (Le Foll and Goldberg, 2005, Navarro et al., 2001). (However, it’s important to note that CB1 antagonism can produce reward as assayed by conditioned place preference, indicating the complex nature of the interactions of the endocannabinoid and reward systems (Cheer et al., 2000).) More likely the psychoactivity of cannabis is due to complex interactions of THC (and related compounds) as a partial agonist with CB1 receptors, in part through the antagonism of high efficacy endocannabinoids (e.g., 2-AG) and the mimicking of low efficacy endocannabinoids such as anandamide. Additional support for this notion comes from experiments with human volunteers where even sustained high doses of rimonabant only slightly attenuate the subjective measures of cannabis-induced psychoactivity (Gorelick et al., 2006, Huestis et al., 2001).
6. Conclusions and perspectives
The past twenty years have seen the emergence of the endocannabinoid system from the receptors “hijacked” by cannabis to a complex neuromodulatory system involved in processes as diverse as cognition, reinforcement, energy balance and reproduction. Endocannabinoids mediate several forms of synaptic plasticity, an action that may underlie their varied psychoactive and behavioral actions. In addition to the diverse processes influenced by the endocannabinoid system, it is becoming increasingly apparent that the pharmacology of this system is highly complex. In particular, multiple endocannabinoids target the CB1 cannabinoid receptor leading to varied physiological effects. The divergent routes of synthesis and degradation of the different endocannabinoids enriches the diversity of these effects. The interactions between endocannabinoid-mediated plasticity and THC are similarly complex, with substantial evidence supporting an antagonist relationship between THC and the well-studied forms of synaptic plasticity.
Acknowledgments
I would like to thank my collaborators and colleagues for their comments and experiments that have shaped many of the ideas presented here. Funding has been provided by NIH grants DA11322 and DA021696
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander SP, Kendall DA. The complications of promiscuity: endocannabinoid action and metabolism. Br J Pharmacol. 2007;152:602–23. doi: 10.1038/sj.bjp.0707456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacci A, Huguenard JR, Prince DA. Long-lasting self-inhibition of neocortical interneurons mediated by endocannabinoids. Nature. 2004;431:312–6. doi: 10.1038/nature02913. [DOI] [PubMed] [Google Scholar]
- Bekkers JM, Stevens CF. Excitatory and inhibitory autaptic currents in isolated hippocampal neurons maintained in cell culture. Proc Natl Acad Sci U S A. 1991;88:7834–8. doi: 10.1073/pnas.88.17.7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender VA, Bender KJ, Brasier DJ, Feldman DE. Two coincidence detectors for spike timing-dependent plasticity in somatosensory cortex. J Neurosci. 2006;26:4166–77. doi: 10.1523/JNEUROSCI.0176-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheer JF, Kendall DA, Marsden CA. Cannabinoid receptors and reward in the rat: a conditioned place preference study. Psychopharmacology (Berl) 2000;151:25–30. doi: 10.1007/s002130000481. [DOI] [PubMed] [Google Scholar]
- Chevaleyre V, Castillo PE. Heterosynaptic LTD of hippocampal GABAergic synapses: a novel role of endocannabinoids in regulating excitability. Neuron. 2003;38:461–72. doi: 10.1016/s0896-6273(03)00235-6. [DOI] [PubMed] [Google Scholar]
- Chevaleyre V, Castillo PE. Endocannabinoid-mediated metaplasticity in the hippocampus. Neuron. 2004;43:871–81. doi: 10.1016/j.neuron.2004.08.036. [DOI] [PubMed] [Google Scholar]
- Chevaleyre V, Takahashi KA, Castillo PE. Endocannabinoid-mediated synaptic plasticity in the CNS. Annu Rev Neurosci. 2006;29:37–76. doi: 10.1146/annurev.neuro.29.051605.112834. [DOI] [PubMed] [Google Scholar]
- Dan Y, Poo MM. Spike timing-dependent plasticity: from synapse to perception. Physiol Rev. 2006;86:1033–48. doi: 10.1152/physrev.00030.2005. [DOI] [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–9. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Gerdeman GL, Ronesi J, Lovinger DM. Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nat Neurosci. 2002;5:446–51. doi: 10.1038/nn832. [DOI] [PubMed] [Google Scholar]
- Gorelick DA, Heishman SJ, Preston KL, Nelson RA, Moolchan ET, Huestis MA. The cannabinoid CB1 receptor antagonist rimonabant attenuates the hypotensive effect of smoked marijuana in male smokers. Am Heart J. 2006;151:754 e1–754 e5. doi: 10.1016/j.ahj.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Hajos N, Freund TF. Pharmacological separation of cannabinoid sensitive receptors on hippocampal excitatory and inhibitory fibers. Neuropharmacology. 2002;43:503–10. doi: 10.1016/s0028-3908(02)00157-0. [DOI] [PubMed] [Google Scholar]
- Hashimotodani Y, Ohno-Shosaku T, Tsubokawa H, Ogata H, Emoto K, Maejima T, Araishi K, Shin HS, Kano M. Phospholipase Cbeta serves as a coincidence detector through its Ca2+ dependency for triggering retrograde endocannabinoid signal. Neuron. 2005;45:257–68. doi: 10.1016/j.neuron.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Hashimotodani Y, Ohno-Shosaku T, Kano M. Endocannabinoids and synaptic function in the CNS. Neuroscientist. 2007;13:127–37. doi: 10.1177/1073858406296716. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, Rice KC. Cannabinoid receptor localization in brain. Proc Natl Acad Sci U S A. 1990;87:1932–6. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci. 1991;11:563–83. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman AF, Macgill AM, Smith D, Oz M, Lupica CR. Species and strain differences in the expression of a novel glutamate-modulating cannabinoid receptor in the rodent hippocampus. Eur J Neurosci. 2005;22:2387–91. doi: 10.1111/j.1460-9568.2005.04401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, Felder CC, Herkenham M, Mackie K, Martin BR, Mechoulam R, Pertwee RG. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- Huestis MA, Gorelick DA, Heishman SJ, Preston KL, Nelson RA, Moolchan ET, Frank RA. Blockade of effects of smoked marijuana by the CB1-selective cannabinoid receptor antagonist SR141716. Arch Gen Psychiatry. 2001;58:322–8. doi: 10.1001/archpsyc.58.4.322. [DOI] [PubMed] [Google Scholar]
- Kampa BM, Letzkus JJ, Stuart GJ. Dendritic mechanisms controlling spike-timing-dependent synaptic plasticity. Trends Neurosci. 2007;30:456–63. doi: 10.1016/j.tins.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Katona I, Sperlagh B, Sik A, Kafalvi A, Vizi ES, Mackie K, Freund TF. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci. 1999;19:4544–58. doi: 10.1523/JNEUROSCI.19-11-04544.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona I, Urban GM, Wallace M, Ledent C, Jung KM, Piomelli D, Mackie K, Freund TF. Molecular composition of the endocannabinoid system at glutamatergic synapses. J Neurosci. 2006;26:5628–37. doi: 10.1523/JNEUROSCI.0309-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura Y, Fukaya M, Maejima T, Yoshida T, Miura E, Watanabe M, Ohno-Shosaku T, Kano M. The CB1 cannabinoid receptor is the major cannabinoid receptor at excitatory presynaptic sites in the hippocampus and cerebellum. J Neurosci. 2006;26:2991–3001. doi: 10.1523/JNEUROSCI.4872-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto Y, Kano M. Endogenous cannabinoid signaling through the CB1 receptor is essential for cerebellum-dependent discrete motor learning. J Neurosci. 2006;26:8829–37. doi: 10.1523/JNEUROSCI.1236-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Carter AG, Regehr WG. Inhibition of interneuron firing extends the spread of endocannabinoid signaling in the cerebellum. Neuron. 2002;34:787–96. doi: 10.1016/s0896-6273(02)00695-5. [DOI] [PubMed] [Google Scholar]
- Kreitzer AC, Regehr WG. Retrograde signaling by endocannabinoids. Curr Opin Neurobiol. 2002;12:324–30. doi: 10.1016/s0959-4388(02)00328-8. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR. Cannabinoid CB1 receptor antagonists as promising new medications for drug dependence. J Pharmacol Exp Ther. 2005;312:875–83. doi: 10.1124/jpet.104.077974. [DOI] [PubMed] [Google Scholar]
- Llano I, Leresche N, Marty A. Calcium entry increases the sensitivity of cerebellar Purkinje cells to applied GABA and decreases inhibitory synaptic currents. Neuron. 1991;6:565–74. doi: 10.1016/0896-6273(91)90059-9. [DOI] [PubMed] [Google Scholar]
- Mackie K. Distribution of cannabinoid receptors in the central and peripheral nervous system. Handb Exp Pharmacol. 2005:299–325. doi: 10.1007/3-540-26573-2_10. [DOI] [PubMed] [Google Scholar]
- Makara JK, Katona I, Nyiri G, Nemeth B, Ledent C, Watanabe M, de Vente J, Freund TF, Hajos N. Involvement of nitric oxide in depolarization-induced suppression of inhibition in hippocampal pyramidal cells during activation of cholinergic receptors. J Neurosci. 2007;27:10211–22. doi: 10.1523/JNEUROSCI.2104-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgansberger W, Di Marzo V, Lutz B. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–4. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- Navarro M, Carrera MR, Fratta W, Valverde O, Cossu G, Fattore L, Chowen JA, Gomez R, del Arco I, Villanua MA, Maldonado R, Koob GF, Rodriguez de Fonseca F. Functional interaction between opioid and cannabinoid receptors in drug self-administration. J Neurosci. 2001;21:5344–50. doi: 10.1523/JNEUROSCI.21-14-05344.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyiri G, Cserep C, Szabadits E, Mackie K, Freund TF. CB1 cannabinoid receptors are enriched in the perisynaptic annulus and on preterminal segments of hippocampal GABAergic axons. Neuroscience. 2005;136:811–22. doi: 10.1016/j.neuroscience.2005.01.026. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Matsui M, Fukudome Y, Shosaku J, Tsubokawa H, Taketo MM, Manabe T, Kano M. Postsynaptic M1 and M3 receptors are responsible for the muscarinic enhancement of retrograde endocannabinoid signalling in the hippocampus. Eur J Neurosci. 2003;18:109–16. doi: 10.1046/j.1460-9568.2003.02732.x. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Hashimotodani Y, Maejima T, Kano M. Calcium signaling and synaptic modulation: regulation of endocannabinoid-mediated synaptic modulation by calcium. Cell Calcium. 2005;38:369–74. doi: 10.1016/j.ceca.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. GPR55: a new member of the cannabinoid receptor clan? Br J Pharmacol. 2007;152:984–6. doi: 10.1038/sj.bjp.0707464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitler TA, Alger BE. Postsynaptic spike firing reduces synaptic GABAA responses in hippocampal pyramidal cells. J Neurosci. 1992;12:4122–32. doi: 10.1523/JNEUROSCI.12-10-04122.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbe D, Kopf M, Remaury A, Bockaert J, Manzoni OJ. Endogenous cannabinoids mediate long-term synaptic depression in the nucleus accumbens. Proc Natl Acad Sci U S A. 2002;99:8384–8. doi: 10.1073/pnas.122149199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safo PK, Regehr WG. Endocannabinoids control the induction of cerebellar LTD. Neuron. 2005;48:647–59. doi: 10.1016/j.neuron.2005.09.020. [DOI] [PubMed] [Google Scholar]
- Safo PK, Cravatt BF, Regehr WG. Retrograde endocannabinoid signaling in the cerebellar cortex. Cerebellum. 2006;5:134–45. doi: 10.1080/14734220600791477. [DOI] [PubMed] [Google Scholar]
- Shen M, Piser TM, Seybold VS, Thayer SA. Cannabinoid receptor agonists inhibit glutamatergic synaptic transmission in rat hippocampal cultures. J Neurosci. 1996;16:4322–34. doi: 10.1523/JNEUROSCI.16-14-04322.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjostrom PJ, Turrigiano GG, Nelson SB. Neocortical LTD via coincident activation of presynaptic NMDA and cannabinoid receptors. Neuron. 2003;39:641–54. doi: 10.1016/s0896-6273(03)00476-8. [DOI] [PubMed] [Google Scholar]
- Sjostrom PJ, Turrigiano GG, Nelson SB. Endocannabinoid-dependent neocortical layer-5 LTD in the absence of postsynaptic spiking. J Neurophysiol. 2004;92:3338–43. doi: 10.1152/jn.00376.2004. [DOI] [PubMed] [Google Scholar]
- Skosnik PD, Edwards CR, O’Donnell BF, Steffen A, Steinmetz JE, Hetrick WP. Cannabis Use Disrupts Eyeblink Conditioning: Evidence for Cannabinoid Modulation of Cerebellar-Dependent Learning. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stella N, Schweitzer P, Piomelli D. A second endogenous cannabinoid that modulates long-term potentiation. Nature. 1997;388:773–8. doi: 10.1038/42015. [DOI] [PubMed] [Google Scholar]
- Stella N, Piomelli D. Receptor-dependent formation of endogenous cannabinoids in cortical neurons. Eur J Pharmacol. 2001;425:189–96. doi: 10.1016/s0014-2999(01)01182-7. [DOI] [PubMed] [Google Scholar]
- Straiker A, Mackie K. Depolarization-induced suppression of excitation in murine autaptic hippocampal neurones. J Physiol. 2005;569:501–17. doi: 10.1113/jphysiol.2005.091918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straiker A, Mackie K. Metabotropic Suppression of Excitation in Autaptic Hippocampal Neurons. J Physiol. 2007;578:773–85. doi: 10.1113/jphysiol.2006.117499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, Yamashita A, Waku K. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun. 1995;215:89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Kobayashi Y, Oka S, Waku K. Biosynthesis and degradation of anandamide and 2-arachidonoylglycerol and their possible physiological significance. Prostaglandins Leukot Essent Fatty Acids. 2002;66:173–92. doi: 10.1054/plef.2001.0356. [DOI] [PubMed] [Google Scholar]
- Takahashi KA, Castillo PE. The CB1 cannabinoid receptor mediates glutamatergic synaptic suppression in the hippocampus. Neuroscience. 2006;139:795–802. doi: 10.1016/j.neuroscience.2006.01.024. [DOI] [PubMed] [Google Scholar]
- Tzounopoulos T, Rubio ME, Keen JE, Trussell LO. Coactivation of pre- and postsynaptic signaling mechanisms determines cell-specific spike-timing-dependent plasticity. Neuron. 2007;54:291–301. doi: 10.1016/j.neuron.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H, Javitch JA, Roth BL, Christopoulos A, Sexton PM, Miller KJ, Spedding M, Mailman RB. Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther. 2007;320:1–13. doi: 10.1124/jpet.106.104463. [DOI] [PubMed] [Google Scholar]
- Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, Stella N, Makriyannis A, Piomelli D, Davison JS, Marnett LJ, Di Marzo V, Pittman QJ, Patel KD, Sharkey KA. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005;310:329–32. doi: 10.1126/science.1115740. [DOI] [PubMed] [Google Scholar]
- Varma N, Carlson GC, Ledent C, Alger BE. Metabotropic glutamate receptors drive the endocannabinoid system in hippocampus. J Neurosci. 2001;21:RC188. doi: 10.1523/JNEUROSCI.21-24-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside GT, Lee GP, Valenzano KJ. The role of the cannabinoid CB2 receptor in pain transmission and therapeutic potential of small molecule CB2 receptor agonists. Curr Med Chem. 2007;14:917–36. doi: 10.2174/092986707780363023. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Kunos G, Nicoll RA. Presynaptic specificity of endocannabinoid signaling in the hippocampus. Neuron. 2001;31:453–62. doi: 10.1016/s0896-6273(01)00372-5. [DOI] [PubMed] [Google Scholar]
- Wotherspoon G, Fox A, McIntyre P, Colley S, Bevan S, Winter J. Peripheral nerve injury induces cannabinoid receptor 2 protein expression in rat sensory neurons. Neuroscience. 2005;135:235–45. doi: 10.1016/j.neuroscience.2005.06.009. [DOI] [PubMed] [Google Scholar]