Abstract
Background and Purpose
One hundred twelve patients undergoing elective carotid endarterectomy for symptomatic and asymptomatic carotid artery stenosis were enrolled in a prospective study to evaluate the incidence of change in postoperative cerebral function.
Methods
Patients were evaluated preoperatively and postoperatively before hospital discharge and at follow-up 1 and 5 months later with a battery of neuropsychometric tests. The results were analyzed by both event-rate and group-rate analyses. For event-rate analysis, change was defined as either a decline or improvement in postoperative neuropsychometric performance by 25% or more compared with a preoperative baseline.
Results
Approximately 80% of patients showed decline in one or more test scores, and 60% had one or more improved test scores at the first follow-up examination. The percentage of declined test scores decreased and the percentage of improved test scores increased with subsequent follow-up examinations. Group-rate analysis was similar for group performance on individual tests. However, a decline in performance was seen most commonly on verbal memory tests, and improved performance was seen most commonly on executive and motor tests.
Conclusions
Neuropsychometric evaluation of patients undergoing carotid endarterectomy for significant carotid artery stenosis demonstrates both declines and improvements in neuropsychometric performance. The test changes that showed decreased performance may be associated with ischemia from global hypoperfusion or embolic phenomena, and the improvement seen may be related to increased cerebral blood flow from removal of stenosis.
Keywords: carotid endarterectomy, cerebral ischemia, neuropsychological tests, vascular surgery
Stroke is the leading cause of disability and the third leading cause of death in the United States. It has devastating physical, psychological, and socioeconomic consequences. At present, therapy for completed strokes is limited. Extracranial carotid atherosclerotic disease is thought to be a principal cause of strokes and is the major risk factor in 15% to 20% of patients. One method of prophylactic therapy to prevent strokes from occurring is surgery. More than one million operations for carotid artery stenosis have been performed in the United States since the early 1950s. Within the past decade, a number of prospective multicenter studies have compared medical and surgical therapy in either symptomatic1,2 or asymptomatic3,4 patients with carotid artery stenosis. The conclusions of these studies are that CEA is the most effective means of preventing stroke when the stenosis is greater than 70% in symptomatic and 60% in asymptomatic patients at centers where surgical morbidity and mortality are less than 3%. Because of these studies, we have seen a dramatic increase in the number of patients undergoing this type of surgery.
Although the incidence of major morbidity associated with CEA is low, the incidence of subtle neurological injury is unknown but undoubtedly depends on the measures of assessing injury. For example, it is reasonably well accepted that a significant percentage of patients having cardiac surgery with cardiopulmonary bypass develop neurological injury.5,6 However, while only approximately 5% develop “major” neurological changes determined by a neurological examination, almost 67% have more subtle changes in cerebral function as determined by a battery of neuropsychometric tests.5 Although the etiology of neurological injury has not been definitively established, emboli probably play a significant role.
Stroke is a rare event after CEA; however, subtle changes in neuropsychometric performance may be seen after carotid artery surgery.7 The aim of this study was to determine the incidence of subtle neurological changes in patients undergoing CEA.
Subjects and Methods
One hundred twelve patients undergoing elective CEA were recruited to participate in this institutional review board–approved
Selected Abbreviations and Acronyms.
CBF = cerebral blood flow
CEA = carotid endarterectomy
CLTR = consistency of long-term retrieval (Buschke Selective Reminding test)
EEG = electroencephalogram, electroencephalographic
LTR = long-term retrieval (Buschke Selective Reminding test)
study. All patients had at least 70% stenosis of the operative carotid artery. After written informed consent was obtained, patients were assessed at four times with a battery of neuropsychometric tests: (1) before surgery (112 patients) to establish a performance baseline, (2) between 1 and 6 days after surgery (follow-up 1: 102 patients), (3) 1 month after surgery (follow-up 2: 76 patients), and (4) 5 months after surgery (follow-up 3: 33 patients). Two patients who had baseline evaluations withdrew from the study, 6 patients missed the follow-up 1 examination, and 4 patients missed the follow-up 2 examination but had one or more subsequent follow-up examinations. All examinations were performed 3 hours or more after any analgesic or sedative medication was administered.
Anesthesia
No patients were premedicated. General anesthesia was induced primarily with fentanyl, midazolam (mean total doses [SD] were 2.3 [0.9]) μg/kg and 0.05 [0.14]) mg/kg, respectively), and vecuronium 6.5 [4.9] mg or rocuronium 82.5 [27.5] mg and maintained with isoflurane ([0.2 to 0.5]exp) as tolerated. Standard monitors were applied including an arterial catheter for measuring blood pressure continuously. All hemodynamic data plus temperature were monitored continuously and recorded every minute by a PC-based data acquisition system (Lifelog from MI2) or manually in a small number of cases. In addition, continuous eight-channel EEG monitoring was performed on all patients (Neurotrac II). After the internal carotid artery was cross-clamped, a significant EEG change was defined as 50% or greater decrease in amplitude in the alpha or beta frequencies and a similar increase in the delta or theta frequencies, or complete loss of all cerebral electrical activity.
Surgery
All operations were performed by one of three surgeons (G.J.T., D.O.Q., R.A.S.). The surgery for CEA consisted of positioning the patient supine with the head in an extended midline position. An incision was made along a skin crease from just below the angle of the mandible to near the midline through skin, subcutaneous tissue, and platysma. The common, internal, and external carotid arteries were exposed and controlled. A shunt was prepared and used only if changes consistent with cerebral ischemia were noted on the EEG. After heparin (5000 or 6000 U) was administered intravenously, the common, internal, and external carotid arteries were occluded. A longitudinal incision was made in the common carotid artery proximal to the bifurcation and extended into the internal carotid artery distal to the plaque. The atheroma was removed with the use of a dissector. Firmly attached intact intima was left above and below the area of atheroma resection. When a vein patch was inserted, it was taken from the proximal saphenous vein (G.J.T.). Before the final sutures were placed, back-bleeding from the common, internal, and external carotid arteries was performed, and the lumen was washed with heparinized saline. Debris and air were expelled by releasing the clip on the superior thyroid artery, which provided inflow as the final sutures were secured. Clamps were sequentially removed from the external, common, and internal carotid arteries. Heparin was reversed selectively with protamine by one of the surgeons (G.J.T.).
Neuropsychometric Evaluation
A battery of neuropsychometric tests was administered with essentially the same battery recommended by a consensus group.8 All examinations were administered by a trained neuropsychologist (S.D.S.). Eight scores were generated from this battery of four neuropsychometric tests, each of which was chosen to evaluate a different cognitive domain.9,10 Halstead-Reitan Trails Parts A and B evaluated “visual conceptual and visuomotor tracking” by timing how long it took a subject to connect consecutively numbered circles with a single line on one work sheet (Part A) and then connect the same number of consecutively numbered and lettered circles on another worksheet by alternating between the two sequences (Part B).9 The repetitive Fine Finger Tapping test measured manual dexterity by having the subject tap for 10 seconds with the index finger a device that recorded the number of taps.9 This trial was repeated for five trials if the numbers of taps were within 10% of each other. If they varied by more than 10%, then five additional trials were performed. The score was the average of all the trials. Dominant and nondominant hands were tested separately. The Grooved Pegboard test measured complex motor coordination with dominant and nondominant hands separately by timing how long it took a subject to place 25 ridged pegs into an equal number of slotted holes angled in different directions.9 The Buschke Selective Reminding test was used to determine verbal memory.11 Subjects were read a list of 12 words by the examiner and then asked to repeat them back. The subject was reminded of any word that was not repeated and then asked to repeat the entire list of 12 words. This procedure was repeated for a total of 12 trials. Performance was scored as long-term retrieval (LTR) and consistency of long-term retrieval (CLTR).9 To minimize the practice effect on the Buschke Selective Reminding test, alternate word lists were used postoperatively. These tests primarily evaluate fine motor control, executive function, and verbal memory.
Any new neurological finding was determined by the surgeon, according to usual criteria. With the use of “event-rate” analysis, each patient's overall neuropsychometric performance was assessed by comparing preoperative and postoperative scores for each test (difference scores). For an individual test to be considered changed, there had to be at least a 25% change in the postoperative difference score, ie, deterioration was a 25% decrease and improvement was a 25% increase in postoperative compared with preoperative performance. These changes will hereafter be referred to as a declined or improved test score, respectively.
Statistical Measures
The number of neuropsychometric tests changed by 25% or more was calculated for each patient. Then the number of patients with 0, only 1, only 2, only 3, or 4 or more changed tests (either declined or improved) was determined. We compared this distribution at the three follow-up times using the Wilcoxon signed-rank test. The data were analyzed in two ways: (1) pairwise, ie, comparing the patient's performance for follow-up 1 to 2, 1 to 3, and 2 to 3; and (2) including only patients having all four tests. In addition, the Mann-Whitney rank sum test was used when two different samples were compared. Deterioration on more than three tests is generally considered indicative of significantly impaired neuropsychometric performance.5 In addition, repeated-measures ANOVA on the eight neuropsychometric tests (baseline minus first, baseline minus second, and baseline minus third follow-up examinations) was used to evaluate the effect of time. To determine whether patients lost to follow-up started from different baselines, between-groups analyses were performed. These analyses were performed with SAS Proc GLM (SAS Institute). All statistical tests were evaluated with a two-tailed type 1 error rate of 5%.
Results
The demographic and anesthetic variables are shown in Table 1 for all patients. Patients with a venous patch sewn into the carotid artery had statistically longer durations of cross-clamp times (Table 1). There were equal numbers of male and female patients and left and right CEAs (Table 1). Most patients had a history of hypertension (55.5%), and many had diabetes mellitus (16.4%), previous stroke or transient ischemic attack (36.7%), and previous myocardial infarction (21.1%). A small number of patients had previous CEA (9.4%) on the opposite side (Table 1). The demographic profiles for the patients having follow-up 2 and follow-up 3 examinations, as well as those who missed these follow-up times points, were identical to those of the baseline group, demonstrating that those who were lost to follow-up were no different in their demographic profiles from those actually tested.
TABLE 1.
Demographic and Intraoperative Parameters
| Age, y | 70.4 (9.2) |
| Operative side (right/left) | 49%/51% |
| Sex (male/female) | 49%/51% |
| Handedness (right/left) | 89.1%/10.9% |
| Height, cm | 167.2 (27.8) |
| Weight, kg | 69.9 (16.3) |
| Education, y | 14.4 (3.2) |
| Hypertension | 55.5% |
| Diabetes mellitus | 16.4% |
| Previous stroke or TIA | 36.7% |
| Previous MI | 21.1% |
| Previous CEA | 9.4% |
| Duration of surgery, min | 161.6 (47.2) |
| Without patch | 154.4 (47.0) |
| With patch | 167.9 (62.5) |
| Cross-clamp time, min | 45.1 (16.1) |
| Without patch | 37.4 (10.2) |
| With patch | 62.5 (12.6)* |
| Fentanyl, μg/kg | 2.3 (0.9) |
| Midazolam, mg/kg | 0.05 (0.14) |
TIA indicates transient ischemic attack; MI, myocardial infarction. Values are mean (SD) or percentage (n = 112).
P<0.001 comparing cross-clamp time without and with insertion of a venous patch.
Follow-up examinations were performed at three times: between 1 and 6 days after surgery (mean, 2.6±3.4 days; mode, 1 day; median, 1 day [±SD]), 1 month (1.2±0.7 months), and 5 months (5.6±2.1 months). The number of patients seen at follow-up 3 (n=33) was significantly smaller than those seen at follow-up 1 (n=102), because about a third of our patients were seen off-site at this time, and one surgeon did not follow his cases beyond 1 month.
Neuropsychometric performance was evaluated in two ways: either by event-rate analysis or by group-rate analysis. With the former, difference scores were calculated for each patient and each test. The number of tests that declined or improved by 25% or more was determined for each patient. Then the number of patients with 0, only 1, only 2, only 3, or 4 or more tests changed was counted, and the percentage of the total number of patients was tabulated for declined or improved performance (Table 2). For example, at follow-up 1, 13.7% of all patients had no neuropsychometric tests declined, whereas 18.6% had only 1, 33.3% had only 2, 20.6% had only 3, and 13.3% had 4 or more declined tests (Table 2).
TABLE 2.
Decline or Improvement in Neuropsychometric Performance on Follow-up Examinations
| No. of Abnormal Tests | FU 1 (n = 102) | FU 2 (n = 76) | FU 3 (n = 33) |
|---|---|---|---|
| Declined | |||
| 0 | 13.7% | 31.6% | 45.4% |
| Only 1 | 18.6% | 27.6% | 24.2% |
| Only 2 | 33.3% | 26.3% | 18.2% |
| Only 3 | 20.6% | 7.9% | 12.1% |
| ≥4 | 13.3% | 6.6% | 0% |
| Improved | |||
| 0 | 43.1% | 23.7% | 6.1% |
| Only 1 | 38.2% | 32.9% | 48.5% |
| Only 2 | 11.8% | 29.0% | 18.2% |
| Only 3 | 5.9% | 11.8% | 18.2% |
| ≥4 | 1.0% | 2.6% | 9.1% |
The percentages reported represent the percentage of patients with a certain number of changed (declined or improved) neuropsychometric tests. The numbers of patients used in the Wilcoxon signed-rank test calculation were 66 and 26, respectively, when we compared follow-up (FU) 1 with FU2 and FU 1 with FU 3 (P<0.02, FU 1 vs FU 2 and FU 1 vs FU 3, declined; P=0.002, FU 1 vs FU 2, improved; P=0.011, FU 1 vs FU 3, improved). Twenty-three patients were used in the Wilcoxon signed-rank test calculation when we required that all four tests be completed. The comparison of FU 1 with FU 2, FU 1 with FU 3, and FU 2 with FU 3 yielded P=0.30, P=0.05, and P=0.35 under these circumstances for declined tests, and P=0.08, P=0.005, and P=0.34 for improved tests.
Patients improved significantly between follow-up examinations 1, 2, and 3. This is shown in Table 2, in which with each follow-up examination the percentage of patients with no (zero) declined tests increased, and the percentage of patients with more than two declined tests decreased. Subtle changes were detected by neuropsychometric tests at all follow-up periods. When we compared follow-up 1 with follow-up 2 (n=66, where n is the number of patients) and follow-up 1 with follow-up 3 (n=26), the Wilcoxon signed-rank test yielded P<0.02 for decreased performance and P=0.002 and P=0.011 for improved performance, respectively; there was no significant difference for follow-up 2 compared with follow-up 3 for decreased or improved performance (Table 2). However, when we required that all four tests be completed, there were 23 patients in the Wilcoxon signed-rank test calculation. Under these conditions, the comparison of follow-up 1 to 2, 1 to 3, and 2 to 3 yielded P=0.30, P=0.05, and P=0.35 for declined tests and P=0.08, P=0.005, and P=0.34 for improved tests.
There were fewer patients with more than two declined tests and more patients with more than two improved tests at each follow-up examination (follow-ups 1, 2, and 3). At follow-up 1, no differences in neuropsychometric performance were found depending on the side of the CEA (P=0.62) or whether a venous graft was used (based on the Mann-Whitney rank sum tests [P=0.62 or P=0.47] for declined or improved tests; results not shown).
The data were also analyzed to determine whether age or the presence of hypertension significantly determined the percentage of patients with declined test performance. Patients were divided into two age groups: 72 years or younger (58 patients) and greater than 72 years (45 patients). Seventy-two years was chosen because it was the median age of our patient population as a whole. The mean (SD) in each group was 65.1 (5.9) and 77.1 (3.6) years. The distribution of declined tests was different when we used the Mann-Whitney rank sum test (P=0.033), with older patients having more declined tests. However, while 63 patients had a history of hypertension and 40 did not, the distribution of declined tests was not different between these patient groups when we used the Mann-Whitney rank sum test (P=0.901).
Group means and SDs are shown in Table 3 for four examinations as part of the group-rate analysis. By repeated-measures ANOVA, all tests showed significant changes (P<0.05) as a function of time except for Fine Finger Tapping, dominant and nondominant hands. When compared pairwise with baseline and with significance defined as P<0.05, Halstead-Reitan Trails Parts A and B were unchanged at the first follow-up period and improved significantly at the second and third follow-up periods. The Buschke Selective Reminding tests (LTR and CLTR) were both impaired at the first follow-up period, and only LTR was impaired at the second follow-up period. The Grooved Pegboard test for the dominant hand was impaired only at the first follow-up period, and both were improved at the third follow-up period. To test whether there was any bias due to the fact that only some patients returned for all of the follow-up examinations, we determined whether baseline performance for each follow-up group differed in the initial performance for each neuropsychometric test. We found that patients who had only the first follow-up examination had more impairment at baseline in the Grooved Pegboard test for the dominant hand. All other tests did not show any difference in their baseline group scores for all of the follow-up periods. Therefore, we do not consider this finding for the Grooved Pegboard test to be important.
TABLE 3.
Neuropsychological Test Scores
| Baseline | FU 1 | FU 2 | FU 3 | |
|---|---|---|---|---|
| Trails A | 48.8 (23.7) | 44.7 (19.8) | 39.6 (17.5)* | 36.2 (13.2)* |
| Trails B | 99.1 (41.5) | 94.5 (53.0) | 82.0 (37.9)* | 81.4 (32.3)* |
| Fine Finger | ||||
| Tapping | ||||
| Dominant | 46.0 (12.5) | 48.3 (12.8) | 51.5 (12.4) | 51.0 (10.0) |
| Nondominant | 41.7 (10.7) | 40.9 (9.4) | 44.1 (13.2) | 43.8 (11.3) |
| Buschke | ||||
| LTR | 89.2 (25.2) | 74.6 (24.6)* | 72.6 (31.1)* | 85.0 (38.6) |
| CLTR | 56.8 (27.5) | 39.0 (14.5)* | 44.4 (30.0) | 48.8 (26.6) |
| Grooved | ||||
| Pegboard | ||||
| Dominant | 93.6 (18.6) | 112.4 (37.4)* | 91.2 (24.1) | 84.6 (18.0)* |
| Nondominant | 105.8 (20.0) | 124.2 (56.5) | 99.2 (32.0) | 97.2 (24.6)* |
Values are mean (SD) for patients who had all four evaluations. For Trails A, Trails B, and Grooved Pegboard tests, higher scores reflect poorer performance. For all other neuropsychometric tests, lower scores reflect poorer performance.
P<0.05, follow-up (FU) mean values compared with baseline performance values.
Significant EEG changes were found in seven patients (6.2%), and all had insertion of a shunt. All patients with significant EEG changes had two or more neuropsychometric tests changed. Only one patient had a postoperative stroke (0.9% stroke rate). That patient did not have an intraoperative EEG change and had only one declined neuropsychometric test at the follow-up 1 examination; however, he had three declined neuropsychometric tests at the follow-up 2 examination.
Different neuropsychometric tests showed decreased or improved function (Figure). The percentage of patients with declined performance on the four neuropsychometric tests is plotted on this bar graph for each score at the three follow-up times. For example, at follow-up 1 approximately 18% of patients had a 25% or greater decrease in performance on the Trails A test (Figure, top panel); however, at follow-up 2 and follow-up 3, a smaller percentage of patients had declined performance. The Buschke Selective Reminding test was most likely to be decreased and remain so, while the Grooved Pegboard test was likely to be decreased at the first follow-up but to improve at subsequent follow-up examinations. Trails A and B and Fine Finger Tapping were most likely to have improved performance at the first follow-up examination and to improve further with subsequent examinations.
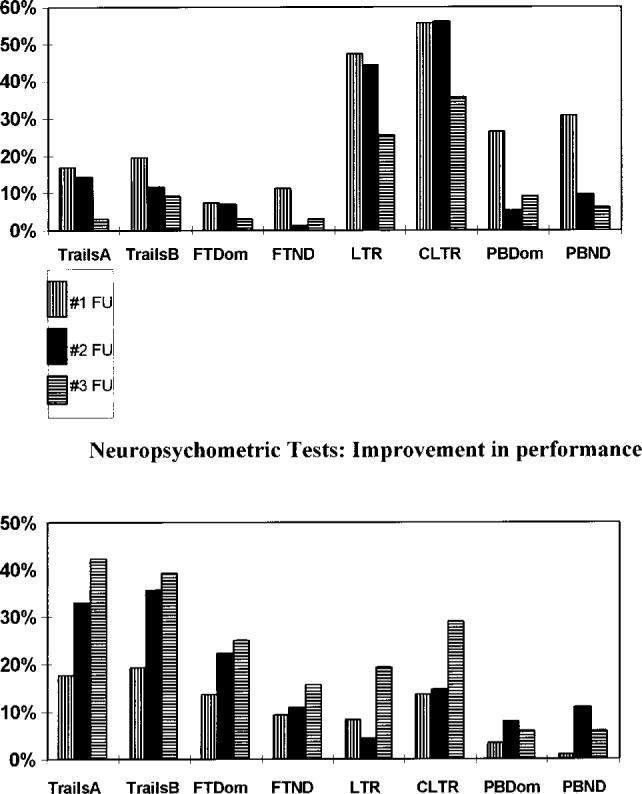
Percentage of patients showing a decline (top) or improvement (bottom) in neuropsychological test performance as a function of the specific tests. The eight tests are noted on the bottom for both graphs. TrailsA indicates Halstead-Reiten Trails A test; TrailsB, Halstead-Reiten Trails B test; FT, Fine Finger Tapping test; Dom, dominant hand; ND, nondominant hand; PB, Grooved Pegboard test; #1 FU, first follow-up examination; #2 FU, second follow-up examination; and #3 FU, third follow-up examination.
Discussion
Although the neurological examination is the traditional measure of injury to the central nervous system, subtle changes in neurological function may be overlooked. A battery of neuropsychometric tests offers a more detailed assessment of higher cortical functioning. Because the results from different clinical studies on patients having cardiac surgery have varied, a consensus group was convened, which suggested that at least four specific neuropsychometric tests be administered so that different studies become more comparable.8 We followed these guidelines with one exception. The Buschke Selective Reminding test was substituted for an equivalent test, the Rey Auditory Verbal Learning test, because we have used it extensively in previous studies and these two tests appear to evaluate similar verbal memory tasks.9
The criterion for defining a significant change in neuropsychometric test performance can affect the reported incidence of cerebral dysfunction.12 For declined and improved performance, we considered a 25% change from baseline to be significant. Using percent change in neuropsychometric performance minimizes the problem posed by patients who score significantly below the mean on their baseline examination and therefore cannot decline on follow-up examinations if a fixed numerical decrease, such as 1 SD or more, is required. There are no clear guidelines for judging what is a significant improvement in performance because such improvement may in part reflect “practice effect.” Even in an elderly surgical population, patients significantly improved their performance with repeated neuropsychometric testing.13
To provide a measure of confidence that significant improvement and deterioration of performance occurred, we also looked at the percentage of patients who showed improved or decreased performance for each test individually. Our logic is as follows. Since some tests like Trails A and B tend to show improvement in part because of practice effect, then this test is an excellent measure to determine decreased performance. Indeed, approximately 15% decreased performance at follow-ups 1 and 2. Since other tests like the Buschke Selective Reminding test are more likely to show deterioration in performance, then this test is an excellent measure of improved performance. In fact, 8% and 14% of patients improved at follow-up 1 and 19% and 29% of patients improved at follow-up 3 on LTR and CLTR, respectively. These percentages are approximately what one would find if significant changes were defined as more than two neuropsychometric tests changed for improved or decreased performance.
Regardless of how neuropsychometric performance is measured, those patients with decreased test performance at the first follow-up examination improved with subsequent examinations, and patients with improved performance continued to have more tests improved. However, all of the tests were not equally likely to show decline or improvement. The verbal memory– based test showed most of the decreased performance, and tests that required executive and motor functions, such as Trails A and B, Finger Tapping, and Grooved Pegboard, showed most improvement.
Our results are similar to other studies that have been performed on similar populations.14,15 However, unlike previous studies in which either improvement16,17 or deterioration7,18 of function was determined, we have evaluated our data by both event- and group-rate analyses to find individuals and tests that show the most improvement or deterioration. Not all tests showed deterioration or improvement to the same degree. Tasks requiring motor functions, ie, mechanical, were more likely to demonstrate improved postoperative function than language-based tasks (Figure). During and after the course of surgery, certain hemodynamic and vascular events might account for some of these changed neuropsychometric performances.
What accounts for decreased function? To perform CEA, the internal and common carotid arteries are cross-clamped. There is a transient decrease in CBF in the ipsilateral middle cerebral artery in many patients reflected in decreased cerebral blood velocity (measured by transcranial Doppler19,20), and CBF (measured by xenon CBF21). If the change in hemispheric CBF is significant enough to change neuronal functioning, there may be a change in the EEG.22 Indeed, patients with EEG changes have a higher probability of having a stroke.23,24 However, that is not the only mechanism producing a postoperative stroke. A large percentage of patients have evidence of gaseous and particulate emboli in the middle cerebral artery associated with CEA.14,25 Particulate embolization during the dissection period appears to be correlated with neuropsychometric deterioration.14 Many clinicians consider thromboembolic phenomena the most common etiology for producing cerebral injury.26–28 One can hypothesize that subtle deficits may result from these emboli.
What explanations account for improved function? This problem has been addressed in a previous study. Bornstein et al17 compared the performance of patients having CEA to two control groups: one a surgical population in which surgery did not involve the brain or cerebral vasculature, and the other a nonoperative population with cerebrovascular symptoms. Performance on their neuropsychometric battery was significantly improved in patients having CEA on the right carotid artery who had had a stroke compared with all other groups.17 In addition to these effects, patients had increased CBF in the ipsilateral middle cerebral artery postoperatively29,30 and increased cerebral area perfused.30 These increases may lead to improved neuropsychometric performance.15 In general, improvement occurred in those patients who had low flow–endangered brains.15,31,32 This may explain why patients having CEA with low preoperative CBF had greater improvement in a battery of neuropsychometric tests than a control group also having CEA for carotid artery stenosis but with hemodynamically insignificant lesions.31 However, rarely, this increased flow may be deleterious.21 Owens et al15 demonstrated that if patients with greater than 50% stenosis had normal CT scans and computerized radionuclide angiograms before and after CEA, they showed cognitive improvement as measured by a battery of neuropsychometric tests in the immediate postoperative period (3 to 10 days) but deteriorated in their cognitive performance if these tests demonstrated evidence of small infarcts or if there was clinical evidence of a stroke. Only those with small infarcts improved at later follow-up testing (3 to 6 months).15 Our patients all had greater than 70% stenosis; some were symptomatic, but most were asymptomatic. This population falls into the group that others have shown to have the most improvement in postoperative neuropsychometric performance.15,31 Whether these explanations are responsible for our observations remains to be demonstrated. Clearly performance on some tests improves with repetition, ie, the practice effect. The contribution of this effect awaits further investigation with a large control population.
In future investigations our hope is to correlate these changes with other independent parenchymal markers of cerebral injury and intraoperative events that occur during surgery, such as changes in CBF or the number of emboli, that may be predictive of those patients who will have deterioration of function so that either technical or pharmaceutical intervention can be instituted to decrease the incidence of these problems.
Acknowledgments
This study was supported in part by a grant from National Institutes of Health, Division of Research Resources, General Clinical Research Centers Program (5 MO1 RR00645) (to D.J.M.) and by an award from Herbert and Florence Irving (to E.J.H). Dr Heyer is a Herbert Irving Assistant Professor of Anesthesiology. We would like to thank Drs S. Mayer and P. Martin for reviewing the manuscript.
References
- 1.NASCET Collaborators Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991;325:445–453. doi: 10.1056/NEJM199108153250701. [DOI] [PubMed] [Google Scholar]
- 2.ECSTC Group MRC European Carotid Surgery Trial: interim results for symptomatic patients with severe (70−99%) or with mild (0−29%) carotid stenosis. Lancet. 1991;337:221–227. [PubMed] [Google Scholar]
- 3.ACA Study Endarterectomy for asymptomatic carotid artery stenosis. JAMA. 1995;273:1421–1428. [PubMed] [Google Scholar]
- 4.Hobson RW, II, Weiss DG, Fields WS, Goldstone J, Moore WS, Towne JB, Wright CB, the TVACS Group Efficacy of carotid endarterectomy for asymptomatic carotid stenosis. N Engl J Med. 1993;328:221–227. doi: 10.1056/NEJM199301283280401. [DOI] [PubMed] [Google Scholar]
- 5.Shaw PJ, Bates D, Cartlidge NEF, French JM, David H, Julian DG, Shaw DA. Neurologic and neuropsychological morbidity following major surgery: comparison of coronary artery bypass and peripheral vascular surgery. Stroke. 1987;18:700–707. doi: 10.1161/01.str.18.4.700. [DOI] [PubMed] [Google Scholar]
- 6.Furlan AJ, Sila CA, Chimowitz MI, Jones SC. Neurologic complications related to cardiac surgery. Neurol Clin. 1992;10:145–166. [PubMed] [Google Scholar]
- 7.Vanninen E, Vanninen R, Aikia M, Tulla H, Kononen M, Koivisto K, Partanen J, Partanen K, Hippelainen M, Kuikka JT. Frequency of carotid endarterectomy-related subclinical cerebral complications. Cerebrovasc Dis. 1996;6:272–280. [Google Scholar]
- 8.Murkin JM, Newman SP, Stump DA, Blumenthal JA. Statement of consensus on assessment of neurobehavioral outcomes after cardiac surgery. Ann Thorac Surg. 1995;59:1289–1295. doi: 10.1016/0003-4975(95)00106-u. [DOI] [PubMed] [Google Scholar]
- 9.Lezak MD. Neuropsychological Assessment. 3rd ed. Oxford University Press; New York, NY: 1995. [Google Scholar]
- 10.Adams RD, Victor M. Principles of Neurology. 3rd ed. McGraw-Hill Book Co; New York, NY: 1985. [Google Scholar]
- 11.Buschke H, Fuld PA. Evaluating storage, retention, and retrieval in disordered memory and learning. Neurology. 1974;24:1019–1025. doi: 10.1212/wnl.24.11.1019. [DOI] [PubMed] [Google Scholar]
- 12.Mahanna EP, Blumenthal JA, White WD, Croughwell ND, Clancy CP, Smith R, Newman MF. Defining neuropsychological dysfunction after coronary artery bypass grafting. Ann Thorac Surg. 1996;61:1342–1347. doi: 10.1016/0003-4975(95)01095-5. [DOI] [PubMed] [Google Scholar]
- 13.Rollason WN, Robertson GS, Cordiner CM, Hall DJ. A comparison of mental function in relation to hypotensive and normotensive anaesthesia in the elderly. Br J Anaesth. 1971;43:561–566. doi: 10.1093/bja/43.6.561. [DOI] [PubMed] [Google Scholar]
- 14.Gaunt ME, Martin PJ, Smith JL, Rimmer T, Cherryman G, Ratliff DA, Bell PRF, Naylor AR. Clinical relevance of intraoperative embolization detected by transcranial Doppler ultrasonography during carotid endarterectomy: a prospective study of 100 patients. Br J Surg. 1994;81:1435–1439. doi: 10.1002/bjs.1800811009. [DOI] [PubMed] [Google Scholar]
- 15.Owens M, Pressman M, Edwards AE, Tourtellotte W, Rose JG, Stern D, Peters G, Stabile BE, Wilson SE. The effect of small infarcts and carotid endarterectomy on postoperative psychologic test performance. J Surg Res. 1980;28:209–216. doi: 10.1016/0022-4804(80)90117-1. [DOI] [PubMed] [Google Scholar]
- 16.Kelly MP, Garron DC, Javid H. Carotid artery disease, carotid endarterectomy, and behavior. Arch Neurol. 1980;37:743–748. doi: 10.1001/archneur.1980.00500610023002. [DOI] [PubMed] [Google Scholar]
- 17.Bornstein RA, Benoit BG, Trites RL. Neuropsychological changes following carotid endarterectomy. Can J Neurol Sci. 1981;8:127–132. doi: 10.1017/s031716710004302x. [DOI] [PubMed] [Google Scholar]
- 18.Williams M, McGee TF. Psychological study of carotid occlusion and endarterectomy. Arch Neurol. 1964;10:293–297. doi: 10.1001/archneur.1964.00460150063006. [DOI] [PubMed] [Google Scholar]
- 19.Halsey JH., Jr Risks and benefits of shunting in carotid endarterectomy: the International Transcranial Doppler Collaborators. Stroke. 1992;23:1583–1587. doi: 10.1161/01.str.23.11.1583. [DOI] [PubMed] [Google Scholar]
- 20.Halsey JH., Jr . Monitoring blood flow velocity in the middle cerebral artery during carotid endarterectomy. In: Babikian V, Wechsler LR, editors. Transcranial Doppler: Clinical and Research Applications. Mosby-Year Book Inc; New York, NY: 1992. [Google Scholar]
- 21.Sundt TM, Jr, Sharbrough FW, Piepgras DG, Kearns TP, Messick J, Jr, O'Fallon WM. Correlation of cerebral blood flow and electroencephalographic changes during carotid endarterectomy: with results of surgery and hemodynamics of cerebral ischemia. Mayo Clin Proc. 1981;56:533–543. [PubMed] [Google Scholar]
- 22.Sharbrough FW, Messick JM, Sundt TMJ. Correlation of continuous electroencephalograms with cerebral blood flow measurements during carotid endarterectomy. Stroke. 1973;4:674–683. doi: 10.1161/01.str.4.4.674. [DOI] [PubMed] [Google Scholar]
- 23.Krul JM, Ackerstaff RG, Eikelboom BC, Vermeulen FE. Stroke-related EEG changes during carotid surgery. Eur J Vasc Surg. 1989;3:423–428. doi: 10.1016/s0950-821x(89)80050-7. [DOI] [PubMed] [Google Scholar]
- 24.Chiappa KH, Burke SR, Young RR. Results of electroencephalographic monitoring during 367 carotid endarterectomies: use of a dedicated minicomputer. Stroke. 1979;10:381–388. doi: 10.1161/01.str.10.4.381. [DOI] [PubMed] [Google Scholar]
- 25.Smith JL, Evans DH, Fan L, Gaunt ME, London NJM, Bell PRF, Naylor AR. Interpretation of embolic phenomena during carotid endarterectomy. Stroke. 1995;26:2281–2284. doi: 10.1161/01.str.26.12.2281. [DOI] [PubMed] [Google Scholar]
- 26.Krul JM, van Gijn J, Ackerstaff RG, Eikelboom BC, Theodorides T, Vermeulen FE. Site and pathogenesis of infarcts associated with carotid endarterectomy. Stroke. 1989;20:324–328. doi: 10.1161/01.str.20.3.324. [DOI] [PubMed] [Google Scholar]
- 27.Sieber FE, Toung TJ, Diringer MN, Wang H, Long DM. Preoperative risks predict neurological outcome of carotid endarterectomy related stroke. Neurosurgery. 1992;30:847–854. doi: 10.1227/00006123-199206000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Steed DL, Peitzman AB, Grundy BL, Webster MW. Causes of stroke in carotid endarterectomy. Surgery. 1982;92:634–641. [PubMed] [Google Scholar]
- 29.Vanninen R, Koivisto K, Tulla H, Manninen H, Partanen K. Hemodynamic effects of carotid endarterectomy by magnetic resonance flow quantification. Stroke. 1995;26:84–89. doi: 10.1161/01.str.26.1.84. [DOI] [PubMed] [Google Scholar]
- 30.Boysen G, Ladegaard-Pedersen HJ, Valentin N, Engell HC. Cerebral blood flow and internal carotid artery flow during carotid surgery. Stroke. 1970;1:253–260. doi: 10.1161/01.str.1.4.253. [DOI] [PubMed] [Google Scholar]
- 31.Jacobs LA, Ganji S, Shirley JG, Morrell RM, Brinkman SD. Cognitive improvement after extracranial reconstruction for the low flow-endangered brain. Surgery. 1983;93:683–687. [PubMed] [Google Scholar]
- 32.Bennion RS, Owens ML, Wilson SE. The effect of unilateral carotid endarterectomy on neuropsychological test performance in 53 patients. J Thorac Cardiovasc Surg. 1985;26:21–26. [PubMed] [Google Scholar]


