Abstract
To elucidate the mechanisms controlling peripheral tolerance, we established two transgenic (Tg) mouse strains expressing different levels of membrane-bound ovalbumin (mOVA) as a skin-associated self-antigen. When we transferred autoreactive TCR-Tg CD8 T cells (OT-I cells), keratin14 (K14)-mOVAhigh Tg mice developed autoreactive skin disease (graft-vs-host disease (GvHD)-like skin lesions) while K14-mOVAlow Tg mice did not. OT-I cells in K14-mOVAhigh Tg mice were fully activated with full development of effector function. In contrast, OT-I cells in K14-mOVAlow Tg mice proliferated but did not gain effector function. Exogenous IL-15 altered the functional status of OT-I cells and concomitantly induced disease in K14-mOVAlow Tg mice. Conversely, neutralization of endogenous IL-15 activity in K14-mOVAhigh Tg mice attenuated GvHD-like skin lesions induced by OT-I cell transfer. Futhermore, K14-mOVAhigh Tg mice on IL-15KO or IL-15RαKO backgrounds did not develop skin lesions after adoptive transfer of OT-I cells. These results identify IL-15 as an indispensable co-stimulator that can determine the functional fate of autoreactive CD8 T cells; whether immunity or tolerance ensues, and suggest that inhibition of IL-15 function may be efficacious in blocking expression of autoimmunity where a breach in peripheral tolerance is suspected.
Keywords: rodent, T cells, cytotoxic, Autoimmunity, Cytokines, Skin
Introduction
Multiple mechanisms contribute to the prevention of autoimmunity to self-antigens. Although self-reactive T cells are efficiently eliminated in the thymus (central tolerance, 1-3), those that recognize tissue-specific self-antigens escape central tolerance (4,5) and undergo surveillance by peripheral tolerance mechanisms (6,7) acting either directly on the self-reactive T cells (ignorance, anergy, phenotype skewing, apoptosis), or indirectly via additional cells (tolerogenic dendritic cells, regulatory T cells) (5).
Two parameters, antigen presenting cell (APC) maturation and self-antigen levels, critically control peripheral tolerance (8,9). APCs, including dendritic cells (DC), capture self-antigens from other cells and present them to self-reactive T cells (cross-presentation) to induce tolerance (9). In the absence of pro-inflammatory signals DC remain relatively immature, and cross-presentation of antigens by such immature DC leads to tolerance induction rather than activation of self-reactive cells (10-13). Peripheral T cell tolerance may also reflect low concentrations of self-antigen (14,15). If efficient cross presentation is coupled with APC maturation, then autoimmunity may ensue. In addition to these two parameters, recent studies suggest the requirement for cytokine signals such as IL-12 in determining the development of tolerance or autoimmunity (16).
To study mechanisms underlying peripheral tolerance, we established two transgenic mouse strains expressing different levels of membrane-associated ovalbumin (mOVA) controlled by a skin-associated K14 promoter. Adoptive transfer of OT-I CD8 T cells that recognize OVA as nominal antigen into OVA-high-expressing (K14-mOVAhigh) mice led to rapid development of GvHD-like autoreactive skin disease (17). In contrast, transfer of OT-I cells failed to cause disease in OVA-low-expressing (K14-mOVAlow) mice despite their in vivo expansion. OT-I cells in K14-mOVAlow Tg mice are not fully activated and do not exhibit effector function as reported in some models of peripheral CD8 T cell tolerance (14, 16, 18-20). We therefore used the K14-mOVAlow Tg strain to elucidate the mechanisms controlling the maintenance or reversal of peripheral tolerance.
We administered several γc-using cytokines (e.g. IL-2, -7, -15 and -21) into these mice since they contribute to the homeostasis of CD8 T cells (21-27). IL-15 converted peripheral tolerance to immunity in K14-OVAlow Tg mice that were adoptively transferred with OT-I cells. The IL-15 acted directly on the OT-I cells not by facilitating CD8 T cell expansion, but by inducing functional changes of the CD8 T cells. Furthermore, neutralization of endogenous IL-15 activity using an anti-IL-2/IL-15Rβ mAb or anti-IL-15 mAb effectively, albeit not completely, inhibited the development of skin lesions in K14-mOVAhigh Tg mice following OT-I transfer. Crossing K14-mOVAhigh Tg mice with IL-15KO or IL-15RαKO mice also effectively abrogated the development of skin lesions after adaptive transfer of OT-I cells.
Collectively, these results indicate that levels of selected cytokines can determine the outcome of immune responses towards self by affecting the checkpoint determining whether peripheral tolerance or autoimmunity ensues through modulating the functional status of self-reactive CD8 T cells in vivo. Our findings provide new evidence that cytokines can be a critical co-stimulator to affect this checkpoint. From a clinical perspective, attempts at blocking IL-15 function may be a promising interventional strategy for some types of graft vs host like reactions or autoimmunity in humans where a breach in peripheral tolerance is suspected.
Materials and Methods
Mice
All mice were obtained from the National Cancer Institute Animal Production Program (Frederick, MD), housed in a clean conventional facility, and bred and used in accordance with institutional guidelines. IL-15KO mice and Rag-1 deficient OT-I mice (17) were purchased from Taconic (Germantown, NY). IL-15RαKO mice were purchased from Jackson Laboratory (Bar Harbor, ME) and were backcrossed to C57BL/6 background for at least 15 generations in house. K14-mOVAhigh Tg mice have been described previously as K14-mOVA Tg mice (17) and are of C57BL/6 background as are the K14-mOVAlow Tg mice. OT-I mice were crossed onto Thy1.1 mice (Jackson Laboratory) to generate Thy1.1+OT-I mice. All animal studies were conducted with prior approval by the Animal Care and Use Committee of the National Institutes of Health.
Real-time PCR
Total RNA was extracted from various tissues and reverse transcribed with StrataScript™ First-Strand Synthesis System (Stratagene, La Jolla, CA). Resulting cDNAs were used for real-time PCR using SYBR-Green PCR Master Mix (Applied Biosystems, Foster city, CA) in triplicate. 5’ (GGCATCAATGGCTTCTGAGAA) and 3’ (CCAACATGCTCATTGTCCCA) primers were used to amplify the mOVA fragment. PCR and data collection were performed on ABIS PRISM™ 7700 Sequence Detector (Perkin Elmer). The data were normalized using actin.
Transfection of 293T cells
Five μg of pK14-mOVA were transfected into 5x105 293T cells using a Lipofectamine 2000 Reagent (Invitrogen, Carsbad, CA). Two days later, cells were harvested and used for western blot. The pK14-mOVA plasmid, which has an HA tag, was previously described (17). To detect mOVA expression level, a monoclonal Ab against HA was used.
Preparation of ear specimen and Western Blot analysis
Ears were harvested, split and subcutaneous tissue was scraped off. Ears were placed dermis-side-down into petri dishes containing 5000U of dispase (BD Biosciences, Bedford, MA) and incubated at 37 °C for 20 min. Epidermis was separated from the dermis, placed in a Dounce homogenizer and disrupted in a buffer containing 50 mM Tris-HCl (pH 7.4), 1% NP-40, 0.25% NaDOC, 0.15 M NaCl, 1 mM EGTA, 1 mM PMSF, 1 μg/ml each of aprotinin and leupeptin. Cell lysates were blotted to a PVDF membrane and probed with an anti-HA mAb followed by HRP-conjugated anti-mouse Ig (Amersham, Piscata way, NJ). Blots were visualized by incubation with chemiluminescence substrate (Pierce, Rockford, IL). To verify equal protein loading, blots were stripped of antibody by washing at 55 °C for 30 min in 62.5 mM Tris-HCl (pH 7.5), 2% SDS, and 0.1 M 2-ME and reprobed with anti-vinculin antibody.
Antibodies and flow cytometry
FITC-conjugated anti-Vα2, Thy1.1, PE-conjugated anti-CD62L, CD25, CD44, APC-conjugated anti-CD8, and biotin-conjugated anti-Vβ5, Vα2 (PharMingen, San Diego, CA) were used for cell surface staining. APC-conjugated streptavidin was used for the visualization of biotin-conjugated mAb. Stained cells were analyzed on a FACSCalibur flow cytometer (Becton Dickinson, Franklin Lakes, NJ).
Preparation of OT-I cells and CD8 T cells
Single cell suspensions were prepared from pooled LNs of Rag-1-/- OT-I mice and used without additional processing. Polyclonal CD8 T cells or Thy1.1+OT-I cells were prepared from single cell suspensions using CD8 columns (R&D system, Minneapolis, Minnesota) from pooled LNs and spleen from C57BL/6 mice or Thy1.1+OT-I mice, respectively.
Adoptive Transfer
Various doses of OT-I cells in 200 μl of PBS were injected intravenously (i.v.) into K14-mOVA Tg mice or C57BL/6 mice. Weight and health status was monitored daily for 14 days after injection. In some experiments, OT-I cells (1×107/ml) were labeled with 2μM CFSE (Molecular Probes, Eugene, OR) in PBS for 10 min at 37°C. Cells were then washed and resuspended in PBS.
Cytokine administration
Recombinant murine IL-15, IL-2, and IL-7 were purchased from PeproTech (Rocky Hill, NJ). Two μg/dose of each cytokine was administered by i.p. injection twice a day for 5-7 days except for the first and the last two days when they were administered once per day.
In vivo antibody treatment
TMβ1, an anti-CD122 (IL-2/IL-15Rβsubunit) mAb was kindly provided by UCB Pharma Ltd. TMβ1 (250 μg/day) or isotype control (rat IgG2a; 250 μg/day) was injected intraperitoneally on days -5, -3, -1 before and 1 and 4 after transfer of OT-I cells. K14-mOVAhigh Tg mice were injected with 500 μg/day (i.p.) of rat anti-mouse IL-15 Ab (AIO3) (28) or isotype control antibody for 7 days starting two days before transfer of OT-I cells.
Culture of OT-I cells with IL-15
Naïve OT-I cells were stimulated with plate-bound anti-CD3 mAb (10μg/ml) with or without mIL-15 (50 ng/ml) at 2x106 cells/well in a 24-well plates in complete RPMI medium. For culture of OT-I cells with IL-15 alone, 500 ng/ml of mIL-15 was used. Five days later, these pretreated OT-I cells were harvested and transferred into K14-mOVAlow Tg mice.
Intracellular cytokine staining
Five million OT-I cells in 200 μl PBS were injected intravenously into C57BL/6 and K14-mOVA Tg mice. On day 5 after injection, single cell suspensions from LN and spleens were prepared. Cells were then cultured at a density of 1×107 cells in a 24-well plate at 37°C in the presence of 2 μg/ml of OVA peptide (SIINFEKL) and 5 μg/ml of Golgiplug (PharMingen). Five hours later, cells were harvested and stained for Vα2, Vβ5, Thy1.1 and CD8. Cells were then fixed and permeabilized by incubation with cytofix/cytoperm solution (PharMingen). The permeabilized cells were incubated with PE-conjugated anti-IFN-γ mAb and the fluorescence intensities were determined by flow cytometry.
Purification of OT-I cells from K14-mOVAlow Tg mice
Five million OT-I cells or Thy1.1+OT-I cells were injected into K14-mOVAlow Tg mice. On day 5 following injection, OT-I cells or Thy1.1+OT-I cells were purified from the pooled LN and spleens of injected K14-mOVAlow Tg mice. The lymphocytes were passed through CD8 columns and the enriched CD8 T cell population was stained with FITC-conjugated anti-Vα2 mAb or anti-Thy1.1mAb. The cells were then incubated with anti-FITC microbeads (Miltenyi Biotec, Auburn, CA) and purified by positive selection. The purity of Vα2/CD8+ cells or Vα2/Thy1.1 cells was 85-99%.
Proliferation assay
Naïve or tolerant OT-I cells (1×105) were cultured with various concentrations of cytokines and APC (1×105). Splenocytes from C57BL/6 were irradiated (3000 rad) and used as APCs. For positive controls, APCs were pulsed with 10 μg/ml of OVA peptide (SIINFEKL) for 1 hr, washed twice and used as peptide-pulsed APCs. In some experiments, naïve or tolerant OT-I cells (2×105) were cultured with 50 ng/ml of mIL-15. 1μCi of [3H] thymidine was added during the last 16 hr of a 3 day culture.
ELISA
The cultures established for the proliferation assay described above were used. Supernatants were removed at 48 h and assayed by ELISA (R&D systems).
Preparation of epidermal sheets and immunofluorescence microscopy
C57BL/6 mice were injected intraperitoneally with PBS or 2 μg/dose of IL-15 twice a day for 3 days. As a positive control for class II MHC and CD86 epidermal cell surface staining, ears were painted with TNCB (29). TNCB was dissolved in acetone : olive oil (4:1) and C57BL/6 ears were treated with 1% TNCB on both sides 24 hr before preparation of epidermal sheets. To obtain epidermal sheets, the ears were split into dorsal and ventral halves and both halves were incubated in 0.5 M ammonium thiocyanate (Sigma, St. Louis, MO) for 20 min at 37°C to allow separation of epidermal sheets from the dermis. The sheets were incubated in cold acetone for 10 min, washed with PBS, and then stained with FITC-MHC class II and PE-CD86 antibodies. Images were viewed with a fluorescence microscope (Carl Zeiss MicroImaging. Inc., Thornwood, NY).
Histological analysis
Tissue samples were fixed in 10% neutral-buffered formalin. Paraffin embedded tissues were then sectioned and stained with hematoxylin and eosin using standard techniques (American Histolabs, Rockville, MD).
Clinical score
Clinical severity scores were determined on day 14 after injection of OT-I cells. 0-2 points was given for each of the following criterion.
skin lesion (erythematous rash, crust formation) : no-0, mild-1, severe-2
alopecia (hair loss) : no-0, mild-1, severe-2
mucosal involvemant (eye, mouth, nose: scale and crust formation) : no-0, mild-1, severe-2
hunched appearance : no-0, yes-1
weight loss: <5%-0, 5-15%-1, >15%-2
Maximal possible clinical severity score for an individual mouse was 9.
Utilization of this clinical severity score system was validated by several fellows from various laboratories in the Dermatology Branch, NCI. Their assessment of clinical severity of GvHD-like skin lesions differed very little from each other.
Statistical analysis
The obtained data were compared using a two-tailed Student’s t-test (Fig1A) or a Kruskal-Wallis test. Values of p < 0.05 were referred to as a significant difference.
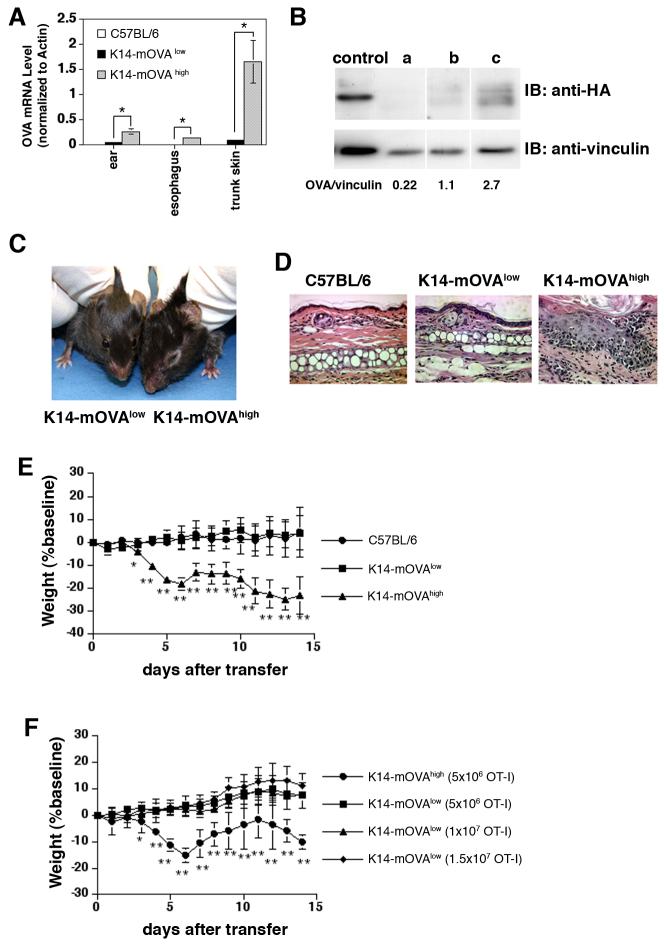
FIGURE 1.
K14-mOVA Tg mice which express low levels of OVA do not develop autoreactive skin disease following injection of OT-I cells
(A) Expression of OVA transgenes was quantified by real-time PCR using total RNA isolated from various tissues of C57BL/6, K14-mOVAlow , and K14-mOVAhigh Tg mice. Error bars indicate standard deviation (SD) of triplicate measurements. *P<0.05.
(B) Expression level of OVA protein in the ear skin was quantified by Western blot. Whole cell or tissue lysates (40 μg/lane for ears) were separated by 10% SDS-PAGE. Western analysis was carried out with anti-HA mAb and blots were subsequently stripped and reprobed with anti-vinculin Ab to verify that similar total amounts of vinculin were loaded in each lane. Signal intensities of HA and vinculin bands were determined by densitometry.
control: pK14-mOVA transfected 293T cells, a: C57BL/6 ear, b: K14-mOVAlow Tg mouse ear, c: K14-mOVAhigh Tg mouse ear
(C) Clinical photo of K14-mOVAhigh and K14-mOVAlow Tg mice taken on day 14 after injection of 5×106 OT-I cells.
(D) Histology of the ears of C57BL/6 mice and K14-mOVA Tg mice 14 days after injection of 5 ×106 OT-I cells. Magnification: ×20
(E) Weight course graph after injection of 5×106 OT-I cells. OT-I cells were injected into C57BL/6 mice and K14-mOVA Tg mice. The mice were weighed and skin lesions were monitored every day for 14 days (n= 7-8 per group). Error bars represent SD. **P<0.001 and *P<0.05 versus C57BL/6 and K14-mOVAlow Tg mice.
(F) Weight course graph after injection of various doses of OT-I cells. K14-mOVAlow Tg mice were injected with 5×10 6, 1×10 7,and 1.5×10 7 OT-I cells. K14-mOVAhigh Tg mice were injected with 5×10 6 OT-I cells (n=6-8 per group). Error bars represent SD. **P<0.001 and *P<0.05 versus K14-mOVAlow Tg mice.
All data are representative of at least three independent experiments.
Results
Levels of OVA dictate the development of autoreactive skin disease following injection of OT-I cells into K14-mOVA Tg mice
To determine mOVA expression levels in various founders of K14-mOVA Tg mice, genomic DNA, mOVA-encoding mRNA, and protein were quantified by PCR (data not shown), by real-time PCR (Fig. 1A) and Western blotting (Fig 1B), respectively. A high expresser (K14-mOVAhigh Fig1B-c) and a low expresser (K14-mOVAlow Fig1B-b) were identified.
Following adoptive transfer of 5×106 naïve OT-I cells into these two mice, the K14-mOVAhigh Tg mice developed acute inflammatory skin lesions and weight loss starting on day 4 (Fig 1C, E) (17), whereas K14-mOVAlow Tg mice did not develop disease (Fig 1C, E). Histology of the ears showed a thickened epidermis, exocytosis, apoptotic epidermal cells, and dermal inflammation in K14-mOVAhigh Tg mice, consistent with GvHD (Fig 1D) (17). K14-mOVAlow Tg mice and C57BL/6 mice did not exhibit skin lesions after transfer of OT-I cells (Fig 1D). To rule out the possibility that the number of injected OT-I cells determined the occurrence of immune reactions, various numbers of OT-I cells were transferred into K14-mOVAlow Tg mice. As previously reported, as few as 1×106 OT-I cells induced disease in K14-mOVAhigh Tg mice (17), whereas K14-mOVAlow Tg mice failed to develop skin lesions even after transfer of 1.5×107 OT-I cells (Fig.1F). These data suggest that low OVA levels rendered transferred OT-I cells unresponsive and that an increase in the number of injected cells does not overcome the effect of the tolerogenic environment.
OT-I cells injected into K14-mOVAlow Tg mice proliferate and exhibit a CD25lowCD44highCD62Lhigh phenotype without gain of effector function
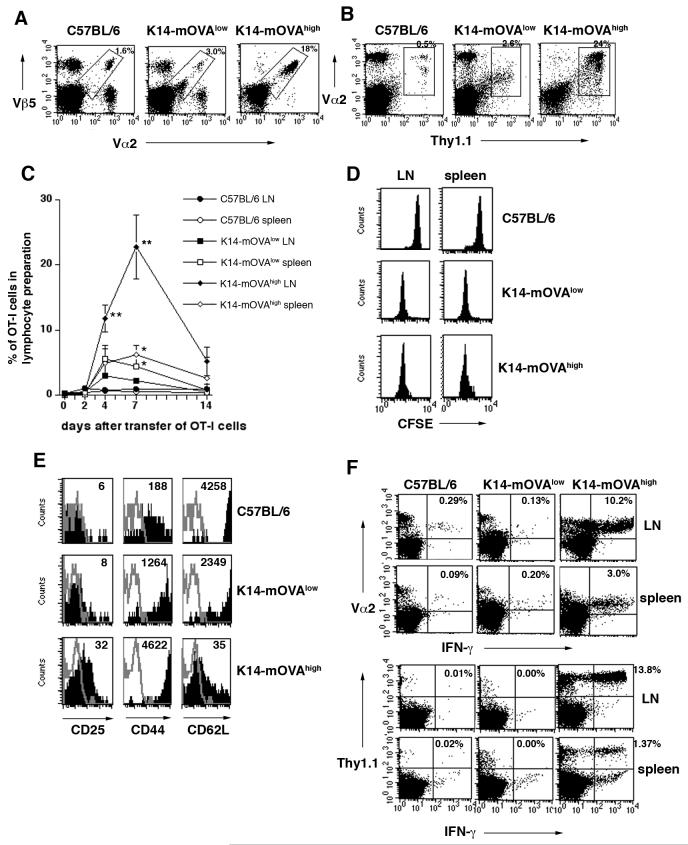
On day 5 after adoptive transfer of OT-I cells into mice, skin-draining (superficial) LN cells were harvested and analyzed for the presence of OT-I cells and for activation markers on these cells. We observed an increase in the percentage of Vα2Vβ5 positive OT-I cells in both K14-mOVA Tg mice compared to C57BL/6 mice (Fig. 2A). To completely exclude the recipient derived Vα2Vβ5 cells, we also transferred Thy1.1+OT-I cells (Fig. 2B). We found a similar increase in the percentage of Thy1.1+OT-I cells in both K14-mOVA Tg mice to Figure 2A. The percentage of OT-I cells in K14-mOVA Tg mice increased in superficial LN on day 4, further expanded by day 7, and decreased by day 14 after transfer (Fig 2C). Thus, transferred OVA-specific OT-I cells recognized OVA antigen in both K14-mOVA Tg mice, but more OT-I cells accummulated in K14-mOVAhigh Tg mice than in K14-mOVAlow Tg mice. CFSE dilution analysis showed that transferred OT-I cells did not proliferate in control mice but proliferated to a similar extent in both K14-mOVA Tg mice (Fig. 2D). Thus, the rate of proliferation did not correlate with the severity of disease. To determine whether disease development correlated with the extent of activation of the OT-I cells in vivo, we recovered OT-I cells from superficial LN on day 4. In control mice, the cells remained phenotypically naive, (CD25low, CD44low and CD62Lhigh expression) (Fig. 2E). In contrast, the expression pattern of these molecules by OT-I cells was characteristic of fully activated effector CD8 T cells (CD25high CD44highCD62Llow) in K14-mOVAhigh Tg mice (Fig. 2E). In K14-mOVAlow Tg mice, OT-I cells were not fully activated (CD25lowCD44highCD62Lhigh, Fig 2E). Thus, proliferation and activation of OT-I cells are separately regulated in these two different environments.
FIGURE 2.
In K14-mOVAlow Tg mice, OT-I cells did not show a fully activated phenotype and nor did they gain effector function
(A) Five million naïve OT-I cells were adoptively transferred into C57BL/6 and K14-mOVA Tg mice. Five days later, superficial LNs were harvested and stained with Vα2-FITC, Vβ5-APC for FACS analysis. The percentage of Vα2Vβ5 cells in the lymphocyte preparation is indicated.
(B) Five million naïve Thy1.1+OT-I cells were adoptively transferred. Five days later, superficial LNs were stained with Thy1.1-FITC, and Vα2-APC. The percentage of Thy1.1Vα2 cells in the lymphocyte preparation is indicated. It was confirmed that Thy1.1Vα2 cells were all Vβ5 positive.
(C) Five million OT-I cells were transferred into C57BL/6 and K14-mOVATg mice. After 2, 4, 7, or 14 days, lymphocytes from superficial LN and spleen were stained with Vα2-FITC and Vβ5-APC. The average percentage of Vα2Vβ5 cells in the lymphocyte preparations is indicated (n=3 per group). Error bars indicate SD of triplicate measurements. **P<0.001 versus the other groups and *P<0.05 versus C57BL/6. Comparison was made among LN samples or spleen samples.
(D) Proliferation of OT-I cells in vivo.
Naïve OT-I cells were labeled with CFSE and adoptively transferred into C57BL/6 and K14-mOVA Tg mice. Superficial LN and spleens were harvested 2 days later for FACS analysis. Cells were gated on CFSE+CD8+ cells.
(E) Five million OT-I cells were transferred into C57BL/6 and K14-mOVA Tg mice. Peripheral LN were harvested on day 5 following iv injection and stained with anti-Vα2, -Vβ5, -CD25, -CD44, and -CD62L for FACS analysis. Cells were gated on Vα2+Vβ5+ cells. Numbers in histograms indicate the mean fluorescence intensity (MFI).
(F) IFN-γ intracellular staining
Five million OT-I cells (upper panel) or Thy1.1+OT-I cells (lower panel) were transferred into C57BL/6 and K14-mOVA Tg mice. Five days later, peripheral LN and spleen were harvested. After in vitro stimulation, cells were stained with Vα2, Thy1.1 and IFN-γ. The percentage of IFN-γ producing Vα2+cells or Thy1.1+cells is indicated. All data are representative of at least three independent experiments with 3-5 mice in each (A, B, D-F).
We next assessed IFN-γ production by transferred OT-I cells as a marker of effector function. In control and K14-mOVAlow Tg mice, IFN-γ production by OT-I cells recovered from LN and spleen at day 5 after transfer was negligible. In contrast, a large percentage of OT-I cells exhibited IFN-γ production in K14-mOVAhigh Tg mice (Fig. 2F). These findings indicate that OT-I cells adoptively transferred into K14-mOVAlow Tg mice proliferated, but were not fully activated and did not gain effector function. We therefore refer to these cells as “tolerant” OT-I cells.
Adoptive transfer of OT-I cells into K14-mOVAlow Tg mice treated with exogenous IL-15 induces GvHD-like skin disease
The importance of γc cytokines in CD8 T cell differentiation, maturation and homeostasis has been demonstrated (27). IL-15 especially acts on CD8 T cells, thus, we administered IL-15 to determine if it could modulate the effect of the OT-I cells in the K14-mOVAlow Tg mice. Five million OT-I cells were adoptively transferred into K14-mOVAlow Tg mice, and IL-15 (2μg per dose) was administered once or twice daily for 5-7 days (Fig. 3A). In this setting, these K14-mOVAlow Tg mice exhibited weight loss and developed skin lesions beginning on day 4 (Fig. 3B), consistent with what occurs when OT-I cells are adoptively transferred into K14-mOVAhigh Tg mice. Histology of the ears of the IL-15 treated K14-mOVAlow Tg mice showed basal vacuolar changes, exocytosis, apoptosis of keratinocytes, and intense dermal infiltrates (Fig. 3C), hallmark changes of GvHD. These results suggest that exogenous IL-15 reverses tolerance of the OT-I cells in K14-mOVAlow Tg mice.
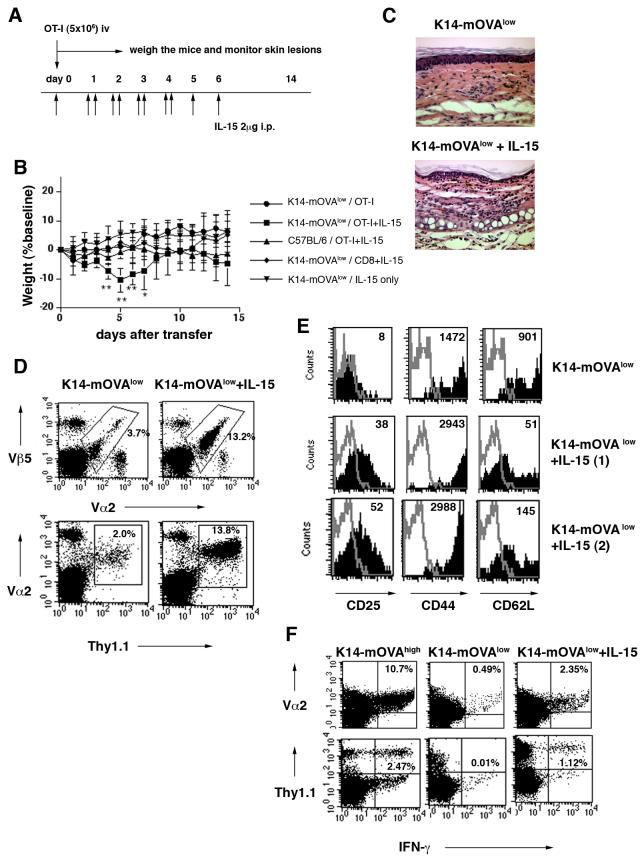
FIGURE 3.
IL-15 administration induces full activation of autoreactive T cells, and leads to the onset of GvHD-like skin disease in K14-mOVAlow Tg mice
(A) The protocol used for IL-15 treatments and cell transfer is illustrated.
(B) OT-I cells or CD8 T cells from C57BL/6 mice were injected into various mice with or without IL-15 treatment (n= 3-6 per group). Error bars represent SD. **P<0.001 and *P<0.05 versus the other groups. Graph is representative of three independent experiments.
(C) On day 7 after injection, tissue sections of ears were stained with hematoxylin-eosin (H&E). Magnification: ×20 One representative result of five is shown.
(D-F) Naïve OT-I cells were adoptively transferred into K14-mOVAlow Tg mice with or without IL-15 treatment. Results shown represent one of five experiments with 3-5 mice in each.
(D) Five days after transfer, peripheral LNs were harvested and cells were stained with anti-Vα2-FITC and anti-Vβ5-APC and subjected to FACS analysis. The percentages of Vα2Vβ5 cells within the lymphocyte gate are indicated (upper panel). Thy1.1+OT-I cells were used instead of OT-I cells and the percentages of Thy1.1Vα2 cells within the lymphocyte gate are indicated (lower panel). It was confirmed that Thy1.1Vα2 cells were all Vβ5 positive.
(E) On day 5 after injection of OT-I cells, LNs were analyzed for expression of various activation markers. Cells were gated on Vα2Vβ5 cells. The thin line represents isotype control staining. Numbers in histograms indicate MFI.
(F) Intracellular IFN-γ staining was performed on splenocytes on day 5 after injection of OT-I cells (upper panel) or Thy1.1+OT-I cells (lower panel). The percentage of IFN-γ producing Vα2+cells and Thy1.1+cells is indicated.
The controls used in these studies helped determine that the effect of the IL-15 on inducing autoreactive skin disease in K14-mOVAlow Tg mice was specific for the OVA antigen. Five different groups of mice were tested; 1) K14-mOVAlow Tg mice injected with OT-I cells, 2) K14-mOVAlow Tg mice injected with OT-I cells and IL-15, 3) C57BL/6 mice injected with OT-I cells and IL-15, 4) K14-mOVAlow Tg mice injected with CD8 T cells from C57BL/6 mice and IL-15, and 5) K14-mOVAlow Tg mice with IL-15 alone. Although IL-15 was able to enhance the proliferation of CD8 T cells, only K14-mOVAlow Tg mice receiving OT-I cells and IL-15 developed skin lesions (Fig. 3B). These results demonstrated that the GvHD-like reaction is specific for OT-I cells and OVA antigen.
Exogenous IL-15 enhances injected OT-I cell function in vivo
Earlier we observed that the activation status of OT-I cells in vivo correlated well with the development of autoreactive skin disease in K14-mOVA Tg mice. Thus, we characterized transferred OT-I cells in the IL-15 treated K14-mOVAlow Tg mice. We observed a significant increase in the percentage of OT-I cells in IL-15 treated K14-mOVAlow Tg mice as compared to non-treated K14-mOVAlow Tg mice (Fig. 3D). As shown above, in K14-mOVAlow Tg mice, OT-I cells had a CD25lowCD44highCD62Lhigh phenotype (Fig. 2E and 3E). Analysis of surface marker expression on OT-I cells in IL-15 treated K14-mOVAlow Tg mice showed a CD25highCD44highCD62Llow phenotype suggestive of full activation (Fig. 3E).
We next determined whether injected OT-I cells gained effector function in IL-15-treated K14-mOVAlow Tg mice. A significant percentage of OT-I cells in IL-15 treated K14-mOVAlow Tg mice produced IFN-γ (Fig. 3F), suggesting that administration of IL-15 can covert tolerant OT-I cells into fully autoreactive OT-I cells in vivo.
IL-15 converts tolerant OT-I cells into fully functional OT-I cells
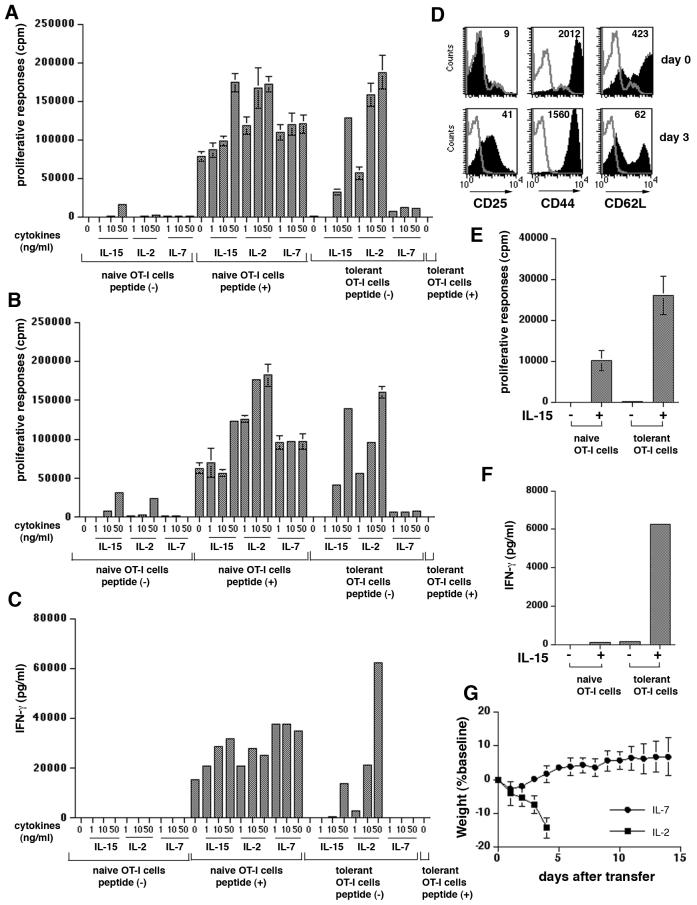
In vitro studies were next conducted to determine the precise mechanism of the effect of IL-15 on OT-I cells in K14-mOVAlow Tg mice. We asked whether IL-15 could alter the functional status of OT-I cells obtained from K14-mOVAlow Tg mice in vitro. We purified OT-I cells from K14-mOVAlow Tg mice on day 5 after adoptive transfer. These tolerant OT-I cells were then cultured with various concentrations of IL-15 and with non-pulsed APC (Fig. 4A). After 3 days of culture, tolerant OT-I cells proliferated in response to 10 ng/ml and 50 ng/ml of IL-15 in the absence of peptide (Fig. 4A). This effect was almost as strong as that seen in the positive controls containing peptide. To rule out the possibility that using anti-Vα2 antibodies for purification affect the function of OT-I cells, we also purified Thy1.1+OT-I cells using anti-Thy1.1 antibodies. The result was almost identical to Figure 4A (Fig. 4B), suggesting that it did not have functional effects. To determine whether they gained effector function, production of IFN-γ was also assessed by ELISA (Fig. 4C). Tolerant OT-I cells without peptide produced IFN-γ in the presence of 50 ng/ml of IL-15. To rule out the possibility that IL-15 might have acted through modulation of APC function, we also performed assays using tolerant OT-I cells in the absence of APC (Fig. 4D-F). Tolerant OT-I cells were cultured with 50 ng/ml of IL-15 alone for 3 days and examined for expression of activation markers. At the start of the culture, tolerant OT-I cells were not fully activated (Fig. 4D). However, after incubation with IL-15, they expressed high levels of CD25 and low levels of CD62L, indicating that IL-15 directly induced full activation of tolerant OT-I cells (Fig. 4D). Cultured OT-I cells proliferated (Fig. 4E) and produced high levels of IFN-γ (Fig. 4F) when stimulated with IL-15 in the absence of peptide or APCs. These results indicate that IL-15 per se altered the nature of tolerant OT-I cells.
FIGURE 4.
IL-15 is able to qualitatively alter the nature of OT-I cells.
Five million OT-I cells or Thy1.1+OT-I cells (B) were injected into K14-mOVAlow Tg mice. Five days later, LNs and spleens from K14-mOVAlow Tg mice were harvested, and OT-I cells were purified by negative and positive selection.
(A) Naïve or tolerant (OT-I cells purified from K14-mOVAlow Tg mice adoptively-transferred with OT-I cells) OT-I cells were cultured with various concentrations of cytokines in the presence of peptide-pulsed or non-pulsed APCs. Naïve OT-I cells cultured with peptide-pulsed APC and with non-pulsed APC were used as positive and negative controls, respectively. 1μCi of [3H] thymidine was added during the last 16 hr of a 3 day culture. Assays were performed in triplicate and the error bars represent SD.
(B) The same as (A) except that naïve and tolerant Thy1.1+OT-I cells were used.
(C) Naïve or tolerant OT-I cells were cultured with various concentrations of cytokines in the presence of peptide-pulsed or non-pulsed APCs. Two days later, supernatants were removed and production of IFN-γ was determined by ELISA.
(D) Tolerant OT-I cells were stimulated with 50 ng/ml of IL-15 in a 24-well plate. Three days later (day 8 after iv), expanded OT-I cells were harvested and analyzed by flow cytometry. The histograms illustrate expression of various markers on Vα2Vβ5 cells (shaded histograms). The thin line represents isotype control staining and numbers in histograms indicate MFI.
(E) Naïve or tolerant OT-I cells were stimulated with 50 ng/ml of IL-15 in a 96-well plate. [3H] thymidine was added during the last 16 hr of a 3 day culture. Assays were performed in triplicate and the error bars represent SD.
(F) Naïve or tolerant OT-I cells were cultured without antigen in the presence or absence of IL-15 (50 ng/ml) for 48 hr, and the amount of IFN-γ production in the supernatant was measured by ELISA.
(G) Five million OT-I cells were injected into K14-mOVAlow Tg mice with IL-2 or IL-7. The same dose of IL-2 and IL-7 as IL-15 was used (n= 3-5 per group). The mice were weighed and skin lesions were monitored for 14 days. Error bars represent SD
All results are representative of al least three independent experiments.
Because a critical role for IL-7 in homeostatic proliferation and survival of naive T cells has been identified (30-32), IL-7 was also assessed. When the same doses of IL-7 were injected, K14-mOVAlow Tg mice failed to develop disease (Fig. 4G), although OT-I cells express IL-7Rα (data not shown). In contrast, K14-mOVAlow Tg mice treated with IL-2 had severe toxic effects and died on day 4 after OT-I transfer (Fig. 4G). Lower doses of IL-2 (0.5-1 μg/dose) did not affect the ability of OT-I cells to cause skin lesions (data not shown).
We also examined the in vitro effects of IL-7 and IL-2 on tolerant OT-I cells. As shown in Fig. 4A-C, IL-15 and IL-7 had differential effects on proliferation and production of IFN-γ by tolerant OT-I cells. In contrast to IL-15, IL-7 failed to stimulate proliferation of tolerant OT-I cells and IL-7 did not induce production of IFN-γ. FACS analysis showed that IL-7Rα was expressed by tolerant OT-I cells but that it was down-regulated compared to the naïve OT-I cells (data not shown). This in vitro effect is consistent with the in vivo effects of IL-7 (Fig. 4G). As in the case with IL-15, IL-2 stimulated tolerant OT-I cells to proliferate (Fig.4A, B) and to produce IFN-γ (Fig. 4C) in the absence of antigen peptide. IL-2 was more potent than IL-15, an effect consistent with the in vivo data (Fig. 4G). However, as mentioned above lower doses of IL-2 in vivo did not result in GvHD when OT-I cells were adoptively transferred. There may be a very limited dose range in which IL-2 could simulate the results obtained with IL-15, which was effective across a broad dose range. Taken together, these results indicate that IL-15, but not IL-7 acts as a specific convertor of proliferating OT-I cells into autoreactive CD8 T cells. Thus IL-15 does not simply expand the cell pool, but it alters the nature of the CD8 T cells.
Injected IL-15 may act directly on OT-I cells
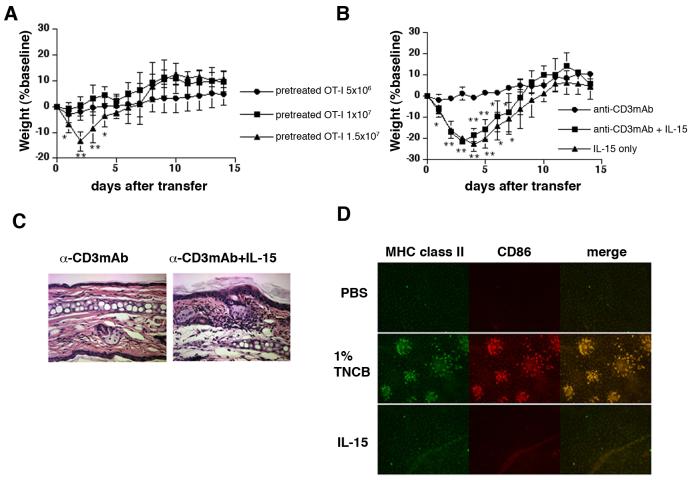
We next determined whether IL-15 directly acts on injected OT-I cells. We adoptively transferred OT-I cells that were pretreated with IL-15 into K14-mOVAlow Tg mice to examine whether they could mimic the in vivo transfer of naïve OT-I cells into IL-15 treated K14-mOVAlow Tg mice. Naïve OT-I cells were cultured with anti-CD3 mAb in the presence of IL-15 and IL-15-primed (pretreated) cells were then transferred. When 1.5×107 pretreated OT-I cells were transferred, K14-mOVAlow Tg mice developed GvHD-like disease (Fig. 5A-C). Furthermore, OT-I cells stimulated with anti-CD3mAb alone failed to induce disease (Fig. 5B, C), while OT-I cells pretreated with high doses (500 ng/ml) of IL-15 induced disease in K14-mOVAlow Tg mice (Fig. 5B), suggesting the importance of IL-15 stimulation. Consistent with observations described above, OT-I cells pretreated with high doses of IL-7 did not induce disease (data not shown). Since K14-mOVAlow Tg mice did not develop disease even when 1.5×107 naïve OT-I cells were injected (Fig. 1F), these results indicate that IL-15-pretreatment directly rendered OT-I cells pathogenic in K14-mOVAlow Tg mice and that this cytokine modulates the functional status of OT-I cells to determine the disease outcome.
FIGURE 5.
IL-15 may directly act on OT-I cells (A) Naïve OT-I cells were pretreated by plate-bound anti-CD3 mAb and by IL-15 in 24-well plate. Five days later, cells were harvested and various number of pretreated OT-I cells were adoptively transferred into K14-mOVAlow Tg mice. The mice were weighed and skin lesions were monitored for 14 days (n=6-7 per group). Error bars represent SD. **P<0.001 and *P<0.05 versus the other groups.
(B) Naïve OT-I cells were pretreated by plate-bound anti-CD3 mAb with or without 50 ng/ml of IL-15. For culture of OT-I cells with IL-15 alone, 500 ng/ml of mIL-15 was used. Five days later, cells were harvested and 1.5 ×107 pretreated OT-I cells were adoptively transferred into K14-mOVAlow Tg mice (n=4-5 per group). Error bars represent SD. **P<0.001 and *P<0.05 versus anti-CD3mAb treated OT-I cells.
(C) On day 7 after injection, tissue sections of ears were stained with hematoxilin-eosin (H&E). Magnification: ×20
(D) Immunofluorescence of epidermal sheets from C57BL/6 mice after either PBS injection, 1% TNCB painting or IL-15 injection. Sheets were stained with FITC-anti-MHC class II and PE-anti-CD86 antibody.
All results are representative of at least three independent experiments.
IL-15 has been implicated in inducing functional maturity of accessory cells upon antigen-presentation (33-35). We therefore determined whether IL-15 induced maturation of DC in our model. We focused on Langerhans cells (LC) because LC are the APC in closest proximity to the keratinocytes which produce OVA. Epidermal sheets were stained for LC after PBS injection, TNCB painting or IL-15 injection. LC in TNCB- treated ears up-regulated CD86 and formed clusters (Fig. 5D), however, when 2 μg/dose of IL-15 were injected (total of 6 doses), LC maintained their immature phenotype (Fig. 5D), indicating that IL-15 did not activate LC.
Endogenous IL-15 plays a role in autoreactive skin disease in K14-mOVAhigh Tg mice
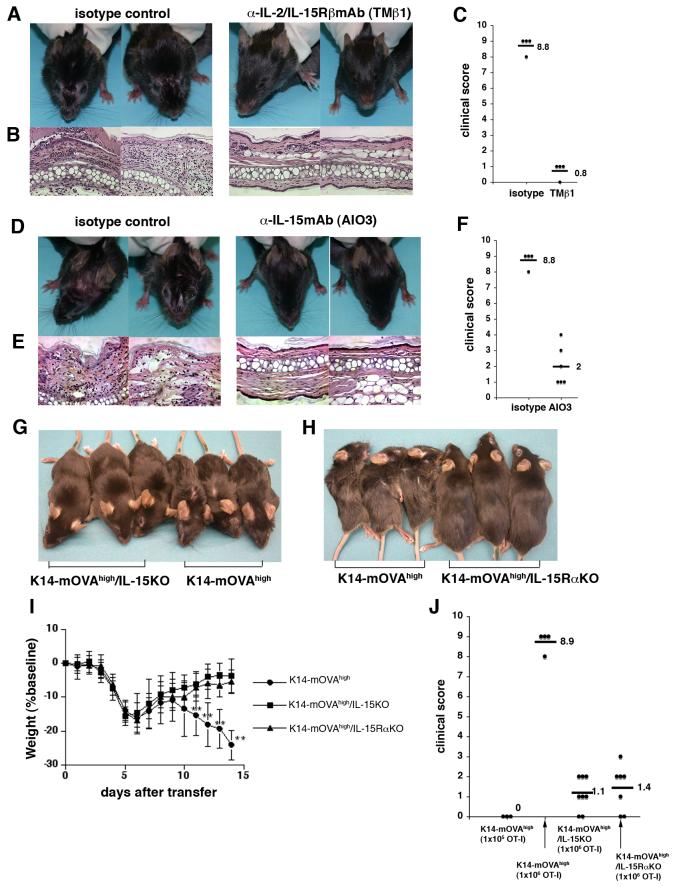
The action of exogenously administered IL-15 in K14-mOVAlow Tg mice does not necessarily mean that this cytokine has a similar role in controlling the development of autoreactive skin disease under physiological conditions. To this end, blocking experiments were performed with anti-IL-2/IL-15Rβ mAb (TMβ1) and with anti-IL-15 mAb in K14-mOVAhigh Tg mice after transfer of OT-I cells. When OT-I cells were administered into K14-mOVAhigh Tg mice that had been injected with the TMβ1 Ab, recipient mice exhibited slight weight loss, but developed no skin lesions (Fig. 6A) and ear histology remained normal (Fig. 6B), confirming that TMβ1 blocked the development of disease (Fig. 6C). However, to ascertain that this blockade by TMβ1 was not due to antibody-dependent cell-mediated cytotoxicity (ADCC) or by blocking IL-2 action in vivo, we tested anti-IL-15 mAb in K14-mOVAhigh Tg mice. Multiple injections of anti-IL-15 mAbs blocked the development of disease in OT-I injected K14-mOVAhigh Tg mice (Fig. 6D). Histology of the ears in anti-IL-15-treated mice revealed minimal to no GvHD (Fig. 6E). It is, however, of note that the effect of this antibody was less complete than that of TMβ1: some K14-mOVAhigh Tg mice treated with anti-IL-15 mAb developed very mild skin lesions, while other mice had no skin lesions at all (Fig. 6C, F).
FIGURE 6.
An anti-IL-2/15R β Ab (TM β 1) and an anti-IL-15 mAb attenuate GvHD-like skin disease in K14-mOVAhigh Tg mice. K14-mOVAhigh Tg mice on either IL-15KO, IL-15RαKO backgrounds do not manifest skin lesions.
(A-C) K14-mOVAhigh Tg mice were treated with TMβ1 on days -5, -3, -1, 1, and 4 and (D-F) with anti-IL-15mAb every day for 7 days starting on day -2. On day 0, 1×106 OT-I cells were intravenously injected into these mice. The health status and skin lesions were monitored every day for 14 days. (A,D) The clinical photos were taken on day 14. (B,E) On day 14 after injection of OT-I cells, the ears were stained with hematoxylin and eosin. Magnification: ×20 (C,F) Mice were scored for clinical symptoms on day 14 (isotype for TMβ1; n=5, TMβ1 treatment; n=5, isotype for AIO3; n=5, AIO3 treatment; n=6). (G,H) Clinical photos taken on day 14 after injection of 1×106 OT-I cells.
(I) Weight course graph after injection of 1×106 OT-I cells. OT-I cells were injected into K14-mOVAhigh, K14-mOVAhigh Tg/IL-15KO, and K14-mOVAhigh Tg/IL-15RαKO mice. The mice were weighed and skin lesions were monitored every day for 14 days (n= 10-15 per group). Error bars represent SD. Data are pooled from two independent experiments. **P<0.001 versus K14-mOVAhigh Tg/IL-15KO, and K14-mOVAhigh Tg/IL-15RαKO mice.
(J) Mice were injected with OT-I cells and scored for clinical symptoms on day 14. (n=5 for K14-mOVAhigh Tg mice injected with 1×105 OT-I cells, n=15 for K14-mOVAhigh Tg mice injected with 1×106 OT-I cells, n=10 for K14-mOVAhigh /IL-15KO, and n=7 for K14-mOVAhigh /IL-15RαKO) Data are pooled from two independent experiments.
All experiments were repeated at least three times.
Nonetheless, these results with blocking antibodies prompted us to generate K14-mOVAhigh Tg mice on an IL-15KO background to further determine the physiological role of IL-15 in developing autoreactive skin disease in K14-mOVAhigh Tg mice. K14-mOVAhigh Tg /IL-15KO mice did not manifest skin lesions clinically (Fig. 6G) or histologically (data not shown) although they transiently lost weight (Fig.6I). Elimination of IL-15 by genetic means did not completely abrogate the disease since some mice developed mild mucocutaneous lesions around the eyes or lost more than 5% of their weight on day 14, giving a clinical severity score 1-3 (fig. 6J). Interestingly, K14-mOVAhigh Tg /IL-15RαKO mice had a phenotype similar to K14-mOVAhigh Tg /IL-15KO mice and developed minimal to no skin lesions (Fig. 6H-J), suggesting that trans-presentation of IL-15 plays a role in K14-mOVAhigh Tg mice.
Discussion
In this study, we have shown that IL-15 regulates the final responses of potentially autoreactive CD8 T cells, i.e., whether peripheral tolerance or autoimmunity ensues. The role of IL-15 in this context seems physiological because the abrogation of endogenous IL-15 activity using anti-IL-2/IL-15RβAb or anti-IL-15 Ab prevented the development of GvHD-like skin lesions. This study provides the first evidence of reliable blocking of murine inflammatory disease model using anti-IL-15 neutralizing Ab. Our observations were further strengthened by the results using gene-targeting because K14-mOVAhigh Tg mice on an IL-15KO background did not develop skin disease after transfer of OT-I cells. Interestingly, K14-mOVAhigh Tg mice on an IL-15RαKO background did not develop skin lesions either suggesting that IL-15 acted on the injected OT-I cells by way of a trans-presentation mechanism (36, 37) rather than as a soluble factor. Despite the critical demonstration that trans-, rather than cis-, presentation is the mode of action of this cytokine upon development of NK and CD8 T cells in vivo, it remains unclear whether trans-presentation operates during more dynamic immune responses, including graft vs host reaction. Thus our model may be a useful in further elucidating the detailed mechanisms underlying the action of IL-15 trans-presentation upon the activation of CD8 T cells.
Outcomes of lymphocyte-mediated immune responses are regulated at many levels. Since T cell activation requires antigen and co-stimulation, antigen dose and DC maturation primarily determine the outcome of the autoreactive CD8 T cell function and control tolerance or autoimmunity (8,9). After antigen stimulation by self antigens, naïve CD8 T cells undergo clonal expansion and may develop effector functions to induce immune responses. However, the presence of autoreactive T cells with effector functions is not sufficient for an autoimmune disease to occur (38). Recent studies have demonstrated that cell proliferation can be separated from development of effector functions (16,19,20). Thus, signals in addition to antigen and co-stimulation may be required for the development of CD8 T cell-induced autoimmune disease.
In our model, OT-I cells adoptively transferred into K14-mOVAlow Tg mice proliferated but they were not fully activated and did not gain effector function (14,16,18,19). Thus, OT-I cells failed to cause disease in K14-mOVAlow Tg mice. However, in vivo administration of IL-15 into OT-I injected K14-mOVAlow Tg mice broke tolerance and caused GvHD-like skin lesions by altering the functional status of the adoptively-transferred OT-I cells. Similarly, injection of OT-I cells that had been pretreated with IL-15 ex vivo into K14-mOVAlow Tg mice caused disease. More importantly, neutralizing IL-15 function by in vivo administration of an anti-IL-2/IL-15Rβ or anti-mouse IL-15 antibody effectively blocked the development of GvHD-like skin lesions in these mice, suggesting that, indeed, the levels of IL-15 physiologically control the onset of tolerance or disease in our experimental model. Our observations collectively demonstrate that in addition to antigen and conventional co-stimulation, a cytokine, in particular IL-15, can be a critical co-factor in the determination of tolerance or autoimmunity,.
We also demonstrated the rather non-redundant relevance of IL-15 in our system because, other members of the γc-using cytokine, such as IL-7 and IL-21, failed to cause disease when injected into K14-mOVAlow Tg mice with OT-I cells (Fig. 4G, and FM, YT, and SIK unpublished observation). In contrast, IL-2 exhibited a similar activity to IL-15 with regard to in vitro activation of OT-I cells. High-doses of IL-2 can cause severe, dose-limiting toxicities in human beings (39). Similarly, IL-2 induced severe toxic side-effects (death) at doses equivalent to those used for IL-15, whereas lower doses of IL-2 did not enable adoptively-transferred OT-I cells to induce GvHD-like skin lesions.
A mechanism for the role of IL-15 on TCR-mediated activation of CD8 T cells has been proposed by Oh et al (40). They demonstrated that IL-15 induced CD8 T cell maturation by promoting greater survival of high-avidity CTLs and induced higher levels of the surface co-receptor CD8αβ (40). While this proposition may help explain our data, CD8αβ induction can be a consequence of, rather than the cause of the acquisition of full CTL activity in our model. In their proposal, the role of IL-15 is defined as a simple enhancer of antigen presentation. However, simultaneous IL-15 and TCR signaling may allow cross-talk between these two distinct pathways, enabling CD8 T cells to undergo diverse events such as maturation, and acquisition of effector functions, as well as the induction of CD8 αβ. In a similar context, Curtsinger et al. (16) have demonstrated that IL-12 provides a critical signal (which they defined as signal 3) for the acquisition of effector functions in CD8 T cells. Interestingly, IL-15 did not replace IL-12 in their system. This same group demonstrated that IFN- αβ may act as signal 3 (41). One crucial question is whether the cytokine signal simply enhances the TCR signal or cytokines represent a distinct T cell-activating entity by inducing intracellular events that are different from those activated by the TCR signal. The detailed mechanism by which cytokines participate in full CD8 T cell maturation requires further biochemical investigation.
Our experimental system provides a unique and convenient model to study the diverse elements involved in the decision of whether peripheral tolerance or autoimmunity ensues after expansion of autoreactive CD8 T cells. IL-15 is an important factor influencing the development of self-reactive immune effector responses in our system. It appears to directly act on the injected OT-I cells via trans-presentation. In addition, the interplay between autoreactive CD8 T cells and resident antigen-expressing skin cells may also have a critical influence on the final clinical outcome. Analysis of the behavior of OT-I cells in K14-mOVA Tg mice crossed with various genetically-engineered mice should help us uncover the various parameters involved in this process. From a clinical perspective, our model may be appropriate for the study of skin-targeted disease such as erythema multiforme, lichen planus, psoriasis and systemic lupus erythematosus (SLE). Also, we expect that our study would further support ongoing attempts to utilize IL-15 and anti-IL-15 or anti-IL-15 receptor antibodies in reversing tolerance in cancer or in preventing autoimmunity in human beings.
Acknowledgments
We thank Jay Linton for technical assistance, and Mark Udey for helpful discussions. The TMβ 1 Ab was supplied by UCB Pharma Ltd, UK.
Footnotes
This work was supported by intramural research funds of the CCR, NCI/ NIH.
- DC
- dendritic cell
- γc
- common-γ chain
- GvHD
- graft versus host disease
- K14
- keratin 14
- LC
- Langerhans cell
- LN
- lymph node
- MFI
- mean fluorescence intensity
- mOVA
- membrane-bound ovalbumin
- Tg
- transgenic
- TNCB
- trinitrochlorobenzene
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Kappler JW, Roehm N, Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987;49:273–280. doi: 10.1016/0092-8674(87)90568-x. [DOI] [PubMed] [Google Scholar]
- 2.Kisielow P, Bluthmann H, Staerz UD, Steinmetz M, von Boehmer H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature. 1988;333:742–746. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- 3.Pircher H, Burki K, Lang R, Hengartner H, Zinkernagel RM. Tolerance induction in double specific T-cell receptor transgenic mice varies with antigen. Nature. 1989;342:559–561. doi: 10.1038/342559a0. [DOI] [PubMed] [Google Scholar]
- 4.Bouneaud C, Kourilsky P, Bousso P. Impact of negative selection on the T cell repertoire reactive to a self-peptide: a large fraction of T cell clones escapes clonal deletion. Immunity. 2000;13:829–840. doi: 10.1016/s1074-7613(00)00080-7. [DOI] [PubMed] [Google Scholar]
- 5.Walker LS, Abbas AK. The enemy within: keeping self-reactive T cells at bay in the periphery. Nat. Rev. Immunol. 2002;2:11–19. doi: 10.1038/nri701. [DOI] [PubMed] [Google Scholar]
- 6.Rocha B, von Boehmer H. Peripheral selection of the T cell repertoire. Science. 1991;251:1225–1228. doi: 10.1126/science.1900951. [DOI] [PubMed] [Google Scholar]
- 7.Jones LA, Chin LT, Longo DL, Kruisbeek AM. Peripheral clonal elimination of functional T cells. Science. 1990;250:1726–1729. doi: 10.1126/science.2125368. [DOI] [PubMed] [Google Scholar]
- 8.Heath WR, Carbone FR. Cross-presentation, dendritic cells, tolerance and immunity. Annu. Rev. Immunol. 2001;19:47–64. doi: 10.1146/annurev.immunol.19.1.47. [DOI] [PubMed] [Google Scholar]
- 9.Ohashi PS, DeFranco AL. Making and breaking tolerance. Curr. Opin. Immunol. 2002;14:744–759. doi: 10.1016/s0952-7915(02)00406-5. [DOI] [PubMed] [Google Scholar]
- 10.Liu K, Iyoda T, Saternus M, Kimura Y, Inaba K, Steinman RM. Immune tolerance after delivery of dying cells to dendritic cells in situ. J. Exp. Med. 2002;196:1091–1097. doi: 10.1084/jem.20021215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J. Exp. Med. 2002;196:1627–1638. doi: 10.1084/jem.20021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hawiger D, Inaba K, Dorsett Y, Guo M, Mahnke K, Rivera M, Ravetch JV, Steinman RM, Nussenzweig MC. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J. Exp. Med. 2001;194:769–779. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scheinecker C, McHugh R, Shevach EM, Germain RN. Constitutive presentation of a natural tissue autoantigen exclusively by dendritic cells in the draining lymph node. J. Exp. Med. 2002;196:1079–1090. doi: 10.1084/jem.20020991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurts C, Sutherland RM, Davey G, Li M, Lew AM, Blanas E, Carbone FR, Miller JF, Heath WR. CD8 T cell ignorance or tolerance to islet antigens depends on antigen dose. Proc. Natl. Acad. Sci. U S A. 1999;96:12703–12707. doi: 10.1073/pnas.96.22.12703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morgan DJ, Kreuwel HT, Sherman LA. Antigen concentration and precursor frequency determine the rate of CD8+ T cell tolerance to peripherally expressed antigens. J. Immunol. 1999;163:723–727. [PubMed] [Google Scholar]
- 16.Curtsinger JM, Lins DC, Mescher MF. Signal 3 determines tolerance versus full activation of naive CD8 T cells: dissociating proliferation and development of effector function. J. Exp. Med. 2003;197:1141–1151. doi: 10.1084/jem.20021910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shibaki A, Sato A, Vogel JC, Miyagawa F, Katz SI. Induction of GVHD-like skin disease by passively transferred CD8(+) T-cell receptor transgenic T cells into keratin 14-ovalbumin transgenic mice. J. Invest. Dermatol. 2004;123:109–115. doi: 10.1111/j.0022-202X.2004.22701.x. [DOI] [PubMed] [Google Scholar]
- 18.Hernandez J, Aung S, Redmond WL, Sherman LA. Phenotypic and functional analysis of CD8(+) T cells undergoing peripheral deletion in response to cross-presentation of self-antigen. J. Exp. Med. 2001;194:707–717. doi: 10.1084/jem.194.6.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hernandez J, Aung S, Marquardt K, Sherman LA. Uncoupling of proliferative potential and gain of effector function by CD8(+) T cells responding to self-antigens. J. Exp. Med. 2002;196:323–333. doi: 10.1084/jem.20011612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang X, Yang Y. Transient gain of effector function by CD8+ T cells undergoing peripheral tolerance to high-dose self-antigen. Eur. J. Immunol. 2004;34:1351–1360. doi: 10.1002/eji.200324734. [DOI] [PubMed] [Google Scholar]
- 21.Zhang X, Sun S, Hwang I, Tough DF, Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8:591–599. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- 22.Ku CC, Murakami M, Sakamoto A, Kappler J, Marrack P. Control of homeostasis of CD8+ memory T cells by opposing cytokines. Science. 2000;288:675–678. doi: 10.1126/science.288.5466.675. [DOI] [PubMed] [Google Scholar]
- 23.Waldmann TA, Dubois S, Tagaya Y. Contrasting roles of IL-2 and IL-15 in the life and death of lymphocytes: implications for immunotherapy. Immunity. 2001;14:105–110. [PubMed] [Google Scholar]
- 24.Schluns KS, Williams K, Ma A, Zheng XX, Lefrancois L. Cutting edge: requirement for IL-15 in the generation of primary and memory antigen-specific CD8 T cells. J. Immunol. 2002;168:4827–4831. doi: 10.4049/jimmunol.168.10.4827. [DOI] [PubMed] [Google Scholar]
- 25.Becker TC, Wherry EJ, Boone D, Murali-Krishna K, Antia R, Ma A, Ahmed R. Interleukin 15 is required for proliferative renewal of virus-specific memory CD8 T cells. J. Exp. Med. 2002;195:1541–1548. doi: 10.1084/jem.20020369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Judge AD, Zhang X, Fujii H, Surh CD, Sprent J. Interleukin 15 controls both proliferation and survival of a subset of memory-phenotype CD8(+) T cells. J. Exp. Med. 2002;196:935–946. doi: 10.1084/jem.20020772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kovanen PE, Leonard WJ. Cytokines and immunodeficiency diseases: critical roles of the gamma(c)-dependent cytokines interleukins 2, 4, 7, 9, 15, and 21, and their signaling pathways. Immunol. Rev. 2004;202:67–83. doi: 10.1111/j.0105-2896.2004.00203.x. [DOI] [PubMed] [Google Scholar]
- 28.Kuwajima S, Sato T, Ishida K, Tada H, Tezuka H, Ohteki T. Interleukin 15-dependent crosstalk between conventional and plasmacytoid dendritic cells is essential for CpG-induced immune activation. Nat. Immunol. 2006;7:740–746. doi: 10.1038/ni1348. [DOI] [PubMed] [Google Scholar]
- 29.Aiba S, Katz SI. Phenotypic and functional characteristics of in vivo-activated Langerhans cells. J. Immunol. 1990;145:2791–2796. [PubMed] [Google Scholar]
- 30.Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat. Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 31.Tan JT, Dudl E, LeRoy E, Murray R, Sprent J, Weinberg KI, Surh CD. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc. Natl. Acad. Sci. U S A. 2001;98:8732–8737. doi: 10.1073/pnas.161126098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fry TJ, Mackall CL. The many faces of IL-7: from lymphopoiesis to peripheral T cell maintenance. J. Immunol. 2005;174:6571–6576. doi: 10.4049/jimmunol.174.11.6571. [DOI] [PubMed] [Google Scholar]
- 33.Ohteki T, Suzue K, Maki C, Ota T, Koyasu S. Critical role of IL-15-IL-15R for antigen-presenting cell functions in the innate immune response. Nat. Immunol. 2001;2:1138–1143. doi: 10.1038/ni729. [DOI] [PubMed] [Google Scholar]
- 34.Loser K, Mehling A, Apelt J, Stander S, Andres PG, Reinecker HC, Eing BR, Skryabin BV, Varga G, Schwarz T, Beissert S. Enhanced contact hypersensitivity and antiviral immune responses in vi vo by keratinocyte-targeted overexpression of IL-15. Eur. J. Immunol. 2004;34:2022–2031. doi: 10.1002/eji.200324785. [DOI] [PubMed] [Google Scholar]
- 35.Dubois SP, Waldmann TA, Muller JR. Survival adjustment of mature dendritic cells by IL-15. Proc. Natl. Acad. Sci. U S A. 2005;102:8662–8667. doi: 10.1073/pnas.0503360102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dubois S, Mariner J, Waldmann TA, Tagaya Y. IL-15Ralpha recycles and presents IL-15 In trans to neighboring cells. Immunity. 2002;17:537–547. doi: 10.1016/s1074-7613(02)00429-6. [DOI] [PubMed] [Google Scholar]
- 37.Lodolce JP, Burkett PR, Boone DL, Chien M, Ma A. T cell-independent interleukin 15Ralpha signals are required for bystander proliferation. J. Exp. Med. 2001;194:1187–1194. doi: 10.1084/jem.194.8.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lang KS, Recher M, Junt T, Navarini AA, Harris NL, Freigang S, Odermatt B, Conrad C, Ittner LM, Bauer S, Luther SA, Uematsu S, Akira S, Hengartner H, Zinkernagel RM. Toll-like receptor engagement converts T-cell autoreactivity into overt autoimmune disease. Nat. Med. 2005;11:138–145. doi: 10.1038/nm1176. [DOI] [PubMed] [Google Scholar]
- 39.Rosenberg SA, Yang JC, Topalian SL, Schwartzentruber DJ, Weber JS, Parkinson DR, Seipp CA, Einhorn JH, White DE. Treatment of 283 consecutive patients with metastatic melanoma or renal cell cancer using high-dose bolus interleukin 2. J.a.m.a. 1994;271:907–913. [PubMed] [Google Scholar]
- 40.Oh S, Perera LP, Burke DS, Waldmann TA, Berzofsky JA. IL-15/IL-15Ralpha-mediated avidity maturation of memory CD8+ T cells. Proc. Natl. Acad. Sci. U S A. 2004;101:15154–15159. doi: 10.1073/pnas.0406649101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Curtsinger JM, Valenzuela JO, Agarwal P, Lins D, Mescher MF. Type I IFNs provide a third signal to CD8 T cells to stimulate clonal expansion and differentiation. J. Immunol. 2005;174:4465–4469. doi: 10.4049/jimmunol.174.8.4465. [DOI] [PubMed] [Google Scholar]