Abstract
In mammals, light entrainment of the circadian clock, located in the suprachiasmatic nuclei (SCN), requires retinal input. Traditional rod and cone photoreceptors, however, are not required. Instead, the SCN-projecting retinal ganglion cells (RGCs) function as autonomous photoreceptors and exhibit light responses independent of rod- and cone-driven input. Using whole-cell patch-clamp recording techniques, we have investigated the morphological and electrophysiological properties of this unique class of RGCs. Although SCN-projecting RGCs resemble Type III cells in form, they display strikingly different physiological properties from these neurons. First, in response to the injection of a sustained depolarizing current, SCN-projecting cells fired in a transient fashion, in contrast to most RGCs which fired robust trains of action potentials. Second, in response to light, SCN-projecting RGCs exhibited an intensity-dependent transient depolarization in the absence of rod and cone input. This depolarization reached a peak within 5 s and generated increased spiking activity before decaying to a plateau. Voltage-clamp recordings were used to characterize the light-activated conductance which generated this depolarization. In response to varying light intensities, SCN-projecting RGCs exhibited a graded transient inward current which peaked within 5 s and decayed to a plateau. The voltage dependence of the light-activated current was obtained by subtracting currents elicited by a voltage ramp before and during illumination. The light-activated current displayed both inward and outward rectification and was largely unaffected by substitution of extracellular Na+ with choline. In both respects, the intrinsic light-activated current observed in SCN-projecting RGCs resembles currents carried by ion channels of the transient receptor potential (trp) family, which are known to mediate the light response of invertebrate photoreceptors.
Keywords: circadian rhythms, light response, patch-clamp electrophysiology, photoentrainment, retinal ganglion cells, trp channels
Introduction
In mammals, light entrainment of the circadian clock in the suprachiasmatic nuclei (SCN) requires input from the retina (Johnson et al., 1988; Moore et al., 1995). However, traditional rod and cone photoreceptors are not required (Foster et al., 1991; Freedman et al., 1999). Instead, the retinal ganglion cells (RGCs) that project to the SCN seem to function as autonomous circadian photoreceptors. In striking contrast to their counterparts projecting to the primary visual targets (the dorsal lateral geniculate nucleus and the superior colliculus), SCN-projecting RGCs exhibit light responses independent of rod- and cone-driven synaptic input (Berson et al., 2002). Furthermore, the majority of these neurons express the novel photopigment melanopsin (Provencio et al., 2000; Gooley et al., 2001; Hattar et al., 2002; Hannibal et al., 2002). As an essential part of the neural pathway from the retina to the SCN, these RGCs play a critical role in generating and shaping retinal output to the circadian system.
The primary neural pathway from the retina to the SCN is the retinohypothalamic tract (RHT), which arises from a small subset (1–2%) of RGCs (Moore et al., 1995; Pickard, 1985). In rats and cats, these SCN-projecting ganglion cells morphologically resemble the Type III or gamma RGCs (Boycott & Wassle, 1974; Fukuda, 1977; Perry, 1979; Pu, 1999; Berson et al., 2002). Type III neurons are characterized by relatively small cell bodies and expansive dendritic fields (Perry, 1979; Wassle & Boycott, 1991). This morphology would seem ideal for the circadian system because it could survey the light intensity over a large sector of the retina. The most striking and novel property of SCN-projecting RGCs is an intrinsic sensitivity to light (Berson et al., 2002; Hattar et al., 2002). Using whole-cell patch-clamp techniques, Berson et al., (2002) recorded intrinsic light responses from RGCs that were labelled with retrograde tracers following stereotaxic injection of the SCN. In response to light, SCN-projecting RGCs depolarized slowly and generated increased spiking activity. The light response persisted in the presence of a formidable cocktail of synaptic blockers and in isolated cells, suggesting that it does not require synaptic input derived from excitation of classical rod and cone photoreceptors. Furthermore, the long response latency suggested that the depolarization was mediated by an intracellular signalling pathway. Under the same conditions, RGCs that were not labelled by the retrograde tracer from the SCN did not respond to light.
Due to their distinct functional role, RGCs mediating entrainment might be expected to have physiological properties that are markedly different from those mediating vision. While little is known about the intrinsic membrane properties of the RGCs that project to the SCN, the spiking properties and ionic conductances of enzymatically dispersed rat RGCs projecting to the visual system have been examined using patch-clamp techniques (Lipton & Tauck, 1987; Karschin & Lipton, 1989). Typically, these neurons have a relatively high input resistance and a resting membrane potential ≈−60 mV. In the adult rat, all three types of RGCs produce trains of tetrodotoxin (TTX)-sensitive spikes in response to maintained depolarizing currents (Wang et al., 1997; Schmid & Guenther, 1999).
In the current study, we have examined unique aspects of the morphology and physiology of SCN-projecting RGCs that enable them to generate and propagate intrinsic light responses. While these neurons morphologically resemble Type III neurons of the visual system, we show that they have markedly different physiological properties. Specifically, in addition to their previously reported intrinsic light sensitivity, SCN-projecting neurons exhibit transient spiking patterns to maintained depolarizing currents and little synaptic input at their resting membrane potential. Most significantly, we show that the depolarization observed in response to light is mediated by the activation of an inward current with biophysical characteristics similar to those reported for members of the transient receptor potential (trp) family of ion channels.
Materials and Methods
Stereotaxic injection of fluorescent retrograde tracers
Surgical procedures were carried out in compliance with guidelines from the National Institutes of Health and in accordance with protocols approved by the Institutional Animal Care and Use Committee (IACUC) of Oregon Health & Science University. Because retinas from younger rats were more amenable to patch-clamp recording, we used 6-week-old rats, weighing between 120 and 170g. Fluorescent retrograde tracers (0.04 μm red Fluospheres; Molecular Probes, Eugene, OR, USA) were injected into the SCN using a stereotaxic apparatus under ketamine (0.05 mL/kg) anaesthesia (Cartesian Designs, Inc., Sandy, OR, USA) to enable unequivocal identification of RGCs projecting to the circadian system. Between two and 10 days after injection, animals were killed by cervical dislocation following deep anaesthesia with ether. After the eyes had been removed for electrophysiological recordings, the brain was removed and fixed by immersion in 4% paraformaldehyde for 24 h. The brain was subsequently sectioned on a vibratome, and each section (200 μm) was carefully inspected for signs of possible injection of the optic chiasm, which lies directly below the SCN.
Electrophysiology and light stimulation of retinal wholemounts
The anterior chamber of each eye was removed, and the retinas were gently peeled from the eyecup and stored in oxygenated Eagle's minimal essential medium (Hepes modification) at room temperature. To prepare a wholemount, a small piece of retina was cut from the whole and mounted on a piece of nitrocellulose filter paper with a 2-mm hole to provide access for the recording electrode. This preparation was placed in the recording chamber with the ganglion cell layer facing up. SCN-projecting RGCs were identified by the presence of fluorescent beads using epifluorescent illumination, and the membrane potential and light-activated currents were recorded using whole-cell patch-clamp techniques. Recordings were made at 22 °C with an Axopatch 1D amplifier controlled by pClamp8 software via a Digidata 1320 interface (Axon Instruments, Union City, CA). Data were low-pass filtered at rates between 1 (voltage-clamp) and 5 (current-clamp) kHz and digitized at rates between 2.5 and 10 kHz. Patch pipettes with tip resistances between 3 and 8 MΩ were pulled from borosilicate glass (Sutter Instruments Novato, CA). For current-clamp recordings, electrodes were filled with a solution containing (in mM): NaCl, 5; KCl, 110; CaCl2, 1; MgCl2, 1; Hepes, 10; EGTA, 11; and ATP, 3; with Lucifer Yellow (0.1%) and biocytin (2%), pH7.4. The bath solution, continuously bubbled with oxygen, consisted of (in mM): NaCl, 120; KCl, 5; CaCl2, 3; MgCl2, 2; Hepes, 10; and D-glucose, 5, pH7.4 (290–300 mOsm).
For voltage-clamp recordings, electrodes were filled with a caesium-based intracellular solution containing (in mM): CsCl, 117; Mg-ATP, 4; Na3GTP, 0.3; creatine phosphate (Na+ salt), 2; Hepes, 10; K4BAPTA, 5, pH 7.4. The typical external solution contained (in mM): NaCl, 120; KCl, 6; CaCl2, 0.5; and Hepes, 10, pH7.4, with 500nM TTX. The series resistance and cell capacitance were electronically compensated prior to each recording. During light stimulation, cells were held at −60 mV unless otherwise noted. To determine the voltage-dependence of the light-activated current, a voltage ramp was applied from −100 to + 100mVover 2 s prior to light stimulation and during the peak response. All voltage-clamp data have been corrected for the junction potential.
Light stimulation
Illumination was provided by a 100-W tungsten–halogen light source and the intensity was reduced using a variety of neutral-density filters. The intensity was determined using a radiometer (International Light, Newburyport, MA) with a detector which had a flat response between 400 and 1000nm. All light intensities are given in W/cm2. For a point of reference, detector readings are given for a number of lighting environments: a well-lit room in a laboratory, 1.7 × 10−6 W/cm2; an overcast December day in Oregon, 1.1 × 10−6 W/cm2; a sunny December day in Oregon, 1.8 × 10−4W/cm2. All experimental recordings were conducted in a dimly lit room with an average total irradiance of 2.3 × 10−9W/cm2. By using a calibrated thermocouple (Fluke, Everett, WA) we determined that the broad-spectrum light used in the present study had no thermal effects on the recording chamber or the bath solution.
Morphological analysis
At the end of each recording, a −200 mV hyperpolarizing potential was applied to facilitate diffusion of Lucifer Yellow and biocytin into the cell. Once adequate filling was achieved, the retina was fixed in 4% paraformaldehyde for 6–8 h at 4 °C. Following fixation, retinas were processed using a standard diaminobenzidine reaction (Vectastain ABC kit). SCN-projecting RGCs judged to be fully stained by the diaminobenzidine (DAB) reaction were analysed using the Neurolucida neuronal reconstruction system (MicroBrightField). The dendritic field areas (areas of influence) were calculated using the area of a complex polygon that encompasses the ends of the dendrites. The software application ImageJ (http://rsb.info.nih.gov/ij/) was used to calculate the area of this polygon as well as its circularity [4π × (area of influence/perimeter2)].
In-house software routines were used to convert the 2-D structure of the neuron into polar coordinates to assess dendritic extent, dendritic distribution index and dendritic polarity index. These routines analysed a black-and-white 2-D image of a neuron (1600 × 1200 pixels) and calculated the polar coordinates for each black pixel that made up the soma and dendrites using the centre of the soma as the origin (0,0). Therefore, thicker dendrites had a larger number of polar coordinates per unit length of dendrite than did finer ones. The dendritic extent was determined by summing the lengths of the longest dendrites in symmetrically opposite 30° (π/6 radians) regions of the dendritic field.
The dendritic distribution index was calculated by dividing the polar plot into 16 equal regions (π/8 radians), determining the number of black pixels contained in each region, and normalizing these values by the total number of pixels in the whole neuronal structure. The distribution index is the minimum number of regions required to account for 80% of the total dendritic structure. A value close to 1 indicates that the dendritic mass of a cell is evenly distributed around the soma, while a value close to 0 indicates that the majority of dendrites are restricted to a single region. This value does not account for the distance of a particular part of the dendritic field from the soma, or the relationship between the dendritic masses of neighbouring regions, but merely gives an indication of how tightly clumped dendrites are in regions of space around the soma.
The dendritic polarity index provides a quantitative analysis of how symmetrically distributed the dendritic tree is around the soma. In contrast to the distribution index, the polarity index takes into account both the location and the distance of each dendrite with respect to the soma. However, the polarity index does not give any information regarding the degree of dendritic clumping that may exist within the whole arbor. To calculate the polarity index, the polar coordinate of each pixel that comprises the dendritic tree was converted into a vector, with a distance and angle relative to the centre of the soma. The vector angle was defined as the angle formed by a line connecting the point in the dendritic tree and the centre of the soma with an arbitrarily orientated axis. A vector sum was then calculated to determine the resultant vector length and angle. This method cancels symmetrically opposed parts of the dendritic tree and the resultant vector length indicates the degree of polarity within the dendritic arbor. The resultant angle indicates the direction of polarization. By normalizing the resultant vector length by the sum of the lengths of all the vectors, it was possible to compare the degree of polarity across cells with different sizes of dendritic arbor. A neuron with a symmetrical dendritic tree, such as a starburst amacrine cell, would have a polarity index close to 0, whereas a very polarized cell, such as a cortical pyramidal neuron, would have a polarity index closer to 1. While the resultant angle provides information regarding the direction of polarity, the orientation of each neuron analysed, and its location within the retina, was unknown. Therefore, in the present study a comparison of resultant vector angles across neurons was not meaningful.
Statistical analysis
All values are given as the mean±SD. Significance was determined using the permutation test for paired differences.
Results
Morphology of SCN-projecting RGCs
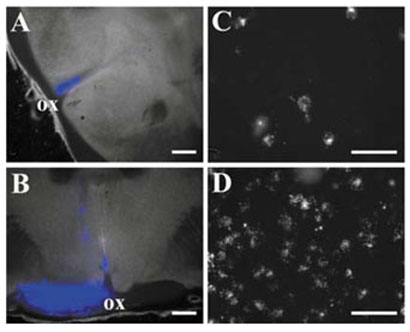
Figure 1A illustrates a successful injection of the SCN with fluorescent polystyrene beads. The site of injection showed a focal area of fluorescence that was entirely contained within 1–2 sections, consistent with a diameter of ≈400 μm. Furthermore, there was no visible labelling in the optic chiasm or nerves. Figure 1C displays a retinal wholemount showing clear labelling of RGCs following injection of the SCN. Following a typical injection, we observed 20–50 labelled cells per retina. This number represents only a fraction of the neurons reported to project to the SCN and probably reflects poor bead uptake. In addition, the injection site never encompassed the entire SCN, which also reduced the total number of labelled neurons one would expect to observe in the retina.
FIG. 1.
Retrograde labelling of RGCs following stereotaxic injection of the SCN. (A) 200-μm brain slice containing the central SCN was imaged using DIC optics and overlaid with a fluorescence image (blue) showing the location of the fluorescent beads; ox, optic chiasm. (B) Similar overlay showing an injection into the optic nerve. (C and D) Photomicrographs of retinal wholemounts showing the distribution of labelled neurons resulting from injection of (C) the SCN and (D) the optic nerve, respectively. Scale bars, 500 μm (A and B), 25 μm (C and D).
In contrast, control experiments were performed in which beads were injected directly into the optic chiasm (Fig. 1B). In these experiments, fluorescent beads were clearly visible in transit along the optic nerve, and the density of labelled cells found in the retina was substantially higher (Fig. 1D). In all experiments the injection site was closely examined to confirm that the optic chiasm had not been injected with fluorescent beads.
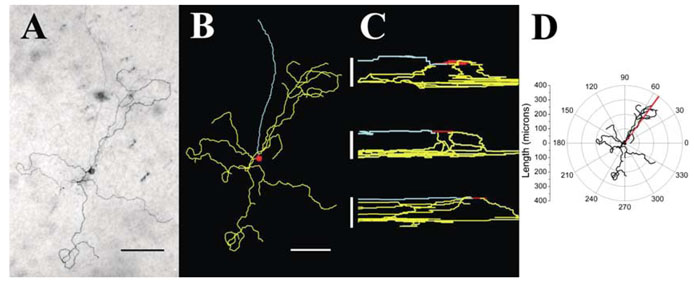
Following recording, SCN-projecting RGCs were filled with biocytin, and the retina was processed using a standard DAB reaction. Figure 2A shows a photomicrograph of an SCN-projecting RGC from which a recording was made. A 2-D representation of a detailed reconstruction of this neuron by Neurolucida is shown in Fig. 2B. The morphology exhibits many characteristics similar to those reported for Type III rat RGCs (Perry, 1979). The somal area of the cell was 204 μm2, and it had two sparsely branched primary dendrites with 17 branch points. The dendritic field covered a relatively large area of retina (extent 587 μm, area 132057 μm2). Similar detailed reconstructions were conducted on five fully filled SCN-projecting RGCs. On average, SCN-projecting RGCs had somal areas of 195±5 μm2 and 2.6±0.6 primary dendrites with 14.0±8.5 branch points. The area of influence ranged from 45758–457 640 μm2. In addition, the dendrites of SCN-projecting RGCs were found to stratify in several different regions of the inner plexiform layer. Of the five reconstructed neurons one stratified in the inner lamina and two in the outer lamina of the inner plexiform layer (IPL) exclusively, while two others were found to be bi-stratified in both inner and outer lamina (Fig. 2C). The average dendritic depths in the inner and outer laminas were 20.8±3.3 and 9.8±1.3 μm, respectively.
FIG. 2.
Morphology of SCN-projecting neurons. (A) Photomicrograph showing the typical morphology of SCN-projecting RGCs. Filled cell was visualized with a DAB reaction and photographed. (B) A 2-D representation of the same neuron using the Neurolucida software. (C) Cross-sectional representations of three Neurolucida reconstructed neurons to show the variety of stratification observed in SCN-projecting RGCs. (D) A polar plot of the neuron from A and B, used to determine dendritic polarity index and dendritic distribution index. Scale bars, 100 μm (A and B), 12.5, 20 and 25 μm (C, from top).
By plotting the 2-D representation of each reconstructed neuron on polar coordinates relative to the centre of the cell soma (Fig. 2D), it was possible to determine the degree of symmetry by calculating the polarity index (cf. Materials and Methods). In this neuron, the resultant vector had a nonzero resultant angle (red line) and a polarity index of 0.21, indicating that its dendritic arbor was biased towards an angle of 54° relative to an arbitrarily chosen 0° axis orientation (Fig. 2D). The average polarity index was 0.49±0.25. The distribution of dendritic mass within the arbor was also assessed by determining the distribution index for each neuron (cf. Materials and Methods). The average distribution index for SCN-projecting RGCs was 0.5±0.08, indicating that the dendrites were not evenly distributed around the soma but tended to be clumped together.
Intrinsic membrane properties of SCN-projecting RGCs
Successful current-clamp recordings were made from 18 SCN-projecting RGCs. The average resting potential was −44.5±5.6mV. At the resting membrane potential, these neurons exhibited no spontaneous spiking activity (cf. Fig. 5), although small and infrequent spontaneous postsynaptic potentials were observed. Application of 400-ms maintained hyperpolarizing current pulses was used to assess the input resistance of SCN-projecting RGCs. The average input resistance was 856±296 MΩ, determined by fitting a plot of the maximum change in membrane potential against the magnitude of injected current with a straight line. The membrane time constant for each neuron was calculated by fitting a single exponential function to the change in membrane potential resulting from a step hyperpolarizing current injection. The average membrane time constant recorded in response to a 45-pA hyperpolarizing maintained current injection was 40.5±20.7 ms.
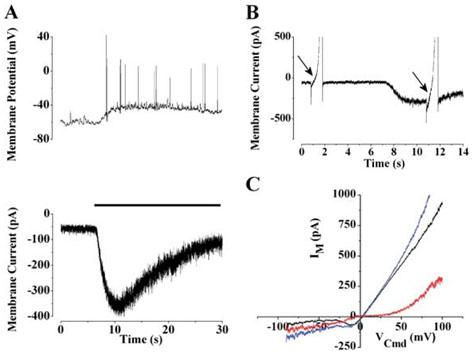
FIG. 5.
Current–voltage relationship of the light-activated current. (A) The lower panel shows the light response (black bar; irradiance 5.8×10−5W/cm2) of an SCN-projecting RGC recorded in voltage-clamp mode. To illustrate the similar time courses, a light response recorded in current-clamp mode is shown in the upper panel. (B)To determine the voltage dependence of the light-activated current, voltage ramps (−100 to +100 mVover 2 s) were applied before and during illumination. (C) The light-activated current (red) was determined by subtracting currents elicited by the voltage ramp before (black) and during (blue) illumination.
In response to injections of maintained depolarizing currents, SCN-projecting RGCs responded with either a single spike (29%) at stimulus onset or with sporadic firing (71%) throughout the stimulus irrespective of its magnitude (Fig. 3A and B, respectively). The number of spikes elicited by a 900-ms maintained depolarization ranged from 1 to 6. While subsequently larger current injections initially increased the number of spikes elicited, above 35 pA of injected depolarizing current no further increases in spike number were observed. Analysis of individual spikes showed that action potentials generated by SCN-projecting neurons had a half-width of 1.12±0.22 ms and maximum rates of rise and decay 97.86±38.6 and −78.12±42.4mV/ms, respectively.
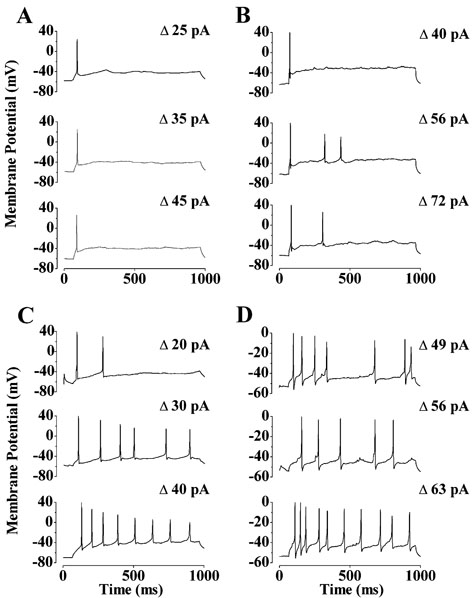
FIG. 3.
Spiking patterns of SCN-projecting RGCs. (A and B) Current-clamp recordings from two SCN-projecting RGCs. The response of each cell to increasing depolarization is shown from bottom to top. The magnitude of the depolarization is shown on the right. (C and D) For comparison, the spiking patterns elicited from a similar stimulus protocol are shown for (C) a Type I and (D) a Type II neuron.
In contrast to RGCs that project to the SCN, unlabelled RGCs exhibited markedly different spiking patterns in response to injection of a maintained depolarizing current. As shown by the typical recordings from Type I (Fig. 3C) and Type II (Fig. 3D) rat RGCs, neurons not projecting to the SCN responded in a sustained manner, with up to 10 spikes being elicited in response to a 900-ms maintained depolarization. In contrast to SCN-projecting RGCs, no response saturation was observed over the range of depolarizing current magnitudes used. Also, unlike SCN-projecting RGCs, Type I and II RGCs often exhibited robust spiking activity at their resting membrane potential as well as higher rates of postsynaptic potentials (cf. Fig. 5B)
Intrinsic light responses of SCN-projecting RGCs
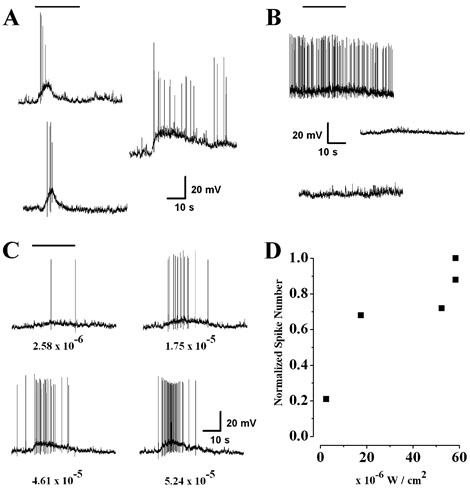
Current-clamp recording in combination with full-field illumination of the retina with a tungsten–halogen lamp was used to examine the intrinsic light sensitivity of SCN-projecting RGCs. The light responses exhibited by these neurons were characterized by a transient depolarization that reached a peak within 5 s and decayed steadily towards a plateau during illumination (n = 9 cells; Fig. 4A). In response to the highest intensity light stimulus (5.8 × 10−5W/cm2), the amplitude of the light-induced depolarization was 14.44±5.5 mV (n = 9). The level of the plateau depolarization, measured 24 s into the light stimulus, varied between 20 and 80% of the peak depolarization. RGCs selected at random did not respond to light under the same conditions (n = 13; Fig. 4B), presumably due to bleaching of the rod and cone photoreceptors resulting from the extensive exposure to bright light during the dissection and epifluorescent scanning of the retina. This insensitivity to light was observed in all randomly selected RGCs whether they exhibited high levels of spontaneous activity or were quiescent at their resting membrane potential.
FIG. 4.
Intrinsic light responses of SCN-projecting RGCs. During recordings, cells were exposed to a 20-s step of white light from a tungsten-halogen lamp (bar). (A) In current-clamp, the cells responded to light (5.8 × 10−5W/cm2) with a slow depolarization which generated increased spiking activity. (B) In contrast the resting membrane activity of unlabelled ganglion cells, chosen at random, did not show any response to the same light stimulus (top two traces). No light responses were observed from RGCs labelled by an optic nerve hit (lower trace). (C) The responses of an SCN-projecting RGC to increasingly brighter illumination. The numbers below each trace represent the light intensity of the stimulus in W/cm2. (D) The relationship between stimulus intensity and spike frequency was approximately linear over the range of light intensities used.
The magnitude of the depolarization and the number of resulting spikes were dependent on the intensity of the light stimulus (Fig. 4C). In response to the lowest intensity used (2.6 × 10−6W/cm2), the cell exhibited a peak depolarization of 5.9 mV, which elicited two spikes. Increasing the light intensity 18-fold caused a peak depolarization of 10.2 mV, which elicited 38 spikes during the 25-s light exposure. We were unable to accurately determine threshold for the light response or demonstrate response saturation because the current experimental setup had a limited range of attainable light intensities (Fig. 4D).
Characteristics of the light-activated current in SCN-projecting RGCs
To characterize the light-activated current that underlies the membrane depolarization, we made voltage-clamp recordings from SCN-projecting RGCs. In response to light, these neurons exhibited a transient inward current (Fig. 5A, lower panel). Using maximal illumination (5.8 × 10−5 W/cm2), the light-activated current developed slowly, with a time-to-peak of 5.35±2.7 s and an average peak amplitude of −135.3±105.3 pA (n = 8). After the peak, the current declined with a time constant of 10.9±4.2 s (n = 6) to a plateau that was typically 20–80% of the maximum. After the step of illumination was terminated, the current returned to its original resting level. The time course of the light-activated current was similar to the membrane depolarization elicited in response to the same light intensity (Fig. 5A, upper panel).
The voltage-dependence of the light-activated current was obtained by subtracting the currents elicited by a voltage ramp before and during light stimulation in the presence of 500nM TTX (Fig. 5B). The resulting light-activated current (Fig. 5C) was inward and linear between −100 and −30 mV with a slope conductance of 1.85±1.2nS (n = 8). As shown by the typical cell in Fig. 5C, there was little net current flow between −10 and +40 mV; however, outward current was observed above +40 mV.
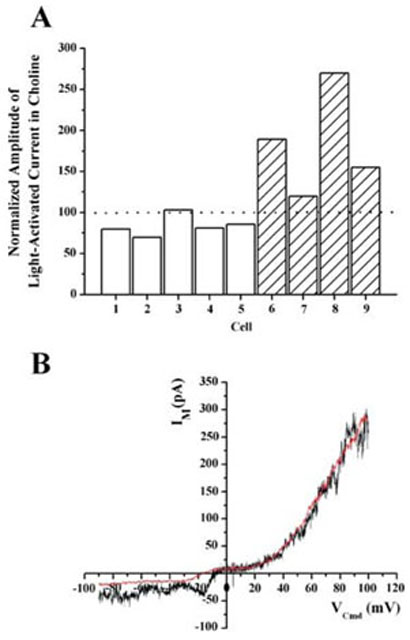
The contribution of Na+ to the light-activated current was examined by substituting the extracellular Na+ with choline to obtain solutions containing either 0 or 20mM Na+. In Fig. 6A, the amplitude of the light-activated current recorded at −60 mV in reduced Na+ is plotted as a percentage of the current amplitude recorded in 120mM Na+. The grey bars show data gathered where the amplitude of the light-activated current was first determined in 120mM Na+ before the extracellular Na+ was lowered to 20mM. The hashed bars represent data in which the amplitude of the light-activated current was first measured in 0mM Na+ and then in 120mM Na+. These data indicate that substitution of extracellular Na+ did not dramatically reduce the amplitude of the light-activated current (184±64%). The differences in current amplitude shown in Fig. 6A can be attributed to run-down of the light-activated current during the course of the experiment. In eight of nine cells recorded, the amplitude of the light-activated current recorded first were greater than that recorded subsequently, regardless of the solution. Moreover, the permutation test for paired differences indicated that the amplitude of the light-sensitive current in low Na+ was not significantly different (P = 0.125) from its amplitude in a normal extracellular solution (120mM Na+).
FIG. 6.
The light-activated current was insensitive to replacement of extracellular Na+. (A) The bars represent the amplitude of the light-activated current recorded in an external solution containing 20mM (grey) or 0mM (hatched) Na+. The amplitudes in low Na+ were normalized to the amplitude of the current recorded in 120mM Na+ extracellular solution. The permutation test for paired differences indicates that the amplitude of the light sensitive current in low Na+ was not significantly different (P = 0.125) from that measured in normal Na+ extracellular solution. (B) I–V characteristics of the light-activated current in 120mM Na+ (black) and 0 Na+ (red). The I–V characteristic in 0 Na+ was obtained first and, to permit comparison, has been scaled to match the amplitude of the current obtained at +100mV in 120mM Na+.
Figure 6B shows the I–V characteristics of the light-activated current obtained first in 0mM Na+ (red trace) and then in 120mM Na+ (black trace). As discussed above, there was an appreciable rundown in the amplitude of the light-activated current over time. Therefore, to facilitate comparison, the I–V characteristic obtained in 0mM Na+ has been scaled so that its amplitude at +100mV matches that obtained at +100 mV in 120 mM Na+. Despite this dramatic reduction in extracellular Na+, the I–V characteristics of the light-activated current was relatively unchanged in this and all other cells examined (n = 4).
Discussion
The photic information required for vision and circadian entrainment are quite different, and they may require different mechanisms for generation and propagation of signals to these systems. These differences are manifest in the distinct physiological responses of RGCs that serve these functions. In this study, we present the first characterization of the light-activated current that underlies the intrinsic light sensitivity of rat SCN-projecting RGCs. Furthermore, we describe the intrinsic membrane properties and morphology of these neurons.
Morphological characteristics, as revealed by Golgi staining, have been used to classify rat RGCs into three groups (I–III; Fukuda, 1977; Perry, 1979). A number of studies have provided strong evidence to suggest that the neurons projecting to the SCN exhibit morphological characteristics similar to those ascribed to Type III RGCs: somal diameters of ≈12 μm, three to five primary dendrites with relatively few branches, and a dendritic extent of ≈340 μm (Moore et al., 1995; Pu, 1999; Berson et al., 2002). All of our reconstructed neurons fit these parameters very closely. In addition, examination of cells not included in the detailed morphometric analysis revealed similar morphologies. It should be noted that we observed a large range in the areas of influence of SCN-projecting RGCs. A possible explanation for this result could be that the neurons with larger dendritic fields were located in more peripheral retina than those with smaller ones. In the present study retinal location was not noted. Another explanation could be that there are different subgroups of SCN-projecting RGCs. The recent observation that between 20 and 30% of SCN-projecting RGCs do not contain melanopsin (Gooley & Saper, 2002; Sollars et al., 2002) supports this idea, although we have yet to observe any distinct groups based on functional criteria.
The similarity between the morphology of SCN-projecting RGCs and Type III RGCs is in agreement with the results obtained by Berson et al. (2002). However, our observation that their dendritic arbors are either mono-stratified in the inner or outer laminas of the IPL, or bi-stratified is in contrast to this previous study, which reported that SCN-projecting neurons were observed predominantly extending to the outer lamina alone. Our results, however, do support the immunocytochemical analysis of Provencio and colleagues, who demonstrated that the dendritic arbors of melanopsin-containing RGCs were bi-stratified in both the inner and outer laminae of the inner plexiform layer (Provencio et al., 2002a,b). Our results are also consistent with physiological studies in cats where SCN-projecting RGCs exhibit ON and ON–OFF receptive field properties (Pu, 1999).
The functional significance of the large dendritic tree and the different levels of stratification have yet to be addressed in an detail. At least some SCN-projecting RGCs appear to receive stimulatory input from rod and/or cone photoreceptors (Dunn & Berson, 2002). It has been proposed that this input arises from synaptic contacts onto the proximal dendrites in the ON lamina of the IPL. In addition, our preliminary data show that these neurons express receptors for both inhibitory and excitatory neurotransmitters, including GABA and glutamate. Together, these findings strongly suggest that conventional retinal circuitry, in addition to the intrinsic light sensitivity, may modulate the activity of SCN-projecting RGCs. Indeed, this is in agreement with the reported sensitivity and action spectrum for the excitation of SCN neurons (Aggelopoulos & Meissl, 2000).
Although the SCN-projecting RGCs are similar in form to Type III cells, they display a markedly different and unique physiology from RGCs that project to the visual system (Wang et al., 1997). In response to injection of depolarizing current, SCN-projecting RGCs generated only transient or sporadic patterns of spiking activity. Such patterns are in contrast to those previously reported for Type I, II and III RGCs, which respond with sustained patterns of activity (Wang et al., 1997). Instead, the responses we observed from SCN-projecting RGCs resembled those reported for immature rat ganglion cells (Skaliora et al., 1993; Wang et al., 1997). During development, the primary determinant of spike pattern is reported to be the rate of recovery from sodium channel inactivation (Wang et al., 1997). The possibility that slow recovery from sodium channel inactivation is responsible for the sporadic firing patterns we observed in SCN-projecting RGCs remains to be determined.
Perhaps the most striking property of SCN-projecting RGCs is their intrinsic sensitivity to light. In agreement with previously published work (Berson et al., 2002), we found that these cells respond to light with a slow depolarization that triggers an intensity-dependent sporadic firing of action potentials. In contrast, RGCs selected at random did not respond to light, presumably because the photopigment in rods and cones was totally bleached during dissection and epifluorescent identification of labelled neurons. Under similar conditions, Berson et al., (2002) also reported that control RGCs did not exhibit intrinsic light responses. The close similarity of our results from photobleached retina with those obtained by Berson et al., (2002) from retina bathed in a cocktail of blockers designed to preclude any residual photoreceptor-driven synaptic input indicates that these blockers are not necessary to isolate the intrinsic light response of SCN-projecting RGCs. We therefore chose not to use them in our experiments due to the uncharacterized pharmacology of the light-activated channel. The ability of SCN-projecting RGCs to respond to light when the photopigment in both rods and cones has presumably been photoisomerized into the all-trans isoform suggests that the photopigment in these neurons may be similar to those found in invertebrates, which are resistant to bleaching.
The light intensities used in the present study (2.6 × 10−6−5.8 × 10−5W/cm2) are within the range observed in the environment during the day (cf. Materials and Methods) but are somewhat brighter than the intensities observed at dusk (2 × 10−8W/cm2) or a dimly lit location (2.3 × 10−9W/cm2). In mice lacking rod and cone photoreceptors, circadian entrainment was observed using light intensities (1 × 10−9−1 × 10−5W/cm2) lower than those used in the present study (Freedman et al., 1999). This inconsistency suggests that the sensitivity of the light-activated pathway may have been reduced, possibly by adaptation, during the dissection and epifluorescent scanning of the retina. Another explanation could be that the sensitivity is regulated in a circadian fashion. In this study retinas were isolated during the animals' day and it would be interesting in future experiments to examine whether SCN-projecting RGCs in retinas isolated during the animals' night exhibit a higher sensitivity to light.
Compared to the light responses reported previously, the majority of those in the present study appear more transient in nature, with the underlying depolarization returning to almost baseline levels within 15 s, even in the continued presence of light. The time course of this transient depolarization was also closely matched by the time course of the light-activated current recorded in voltage-clamp mode with the membrane being held at −60 mV. The differences between our results and those published previously (Berson et al., 2002) cannot be easily explained. However, both the internal and external solutions are quite different. Until much more is known about the ion channel and intracellular signalling pathway that mediate the intrinsic light response, the reasons why our responses are more transient than those reported by Berson et al., (2002) remain unknown.
By recording from SCN-projecting RGCs in the whole-cell voltage-clamp mode, we were able to directly observe the light-activated current that underlies the depolarizing response. The light-activated current was inward and relatively linear between −100mV and −30 mV and was outward at potentials >+40mV. However, there was little to no net current flow between −10 and +40 mV. In this respect, the light-activated current in SCN-projecting RGCs resembles currents carried by certain ion channels of the trp family (cf. Clapham et al., 2001; Minke & Cook, 2002). Specifically, the I–V characteristics closely resemble those reported for the TRPC6 channel in A7r5 smooth muscle cells (Jung et al., 2002), the mTRP6 channel in vascular smooth muscle (Inoue et al., 2001), and the TRPC4 and TRPC5 channels expressed in embryonic kidney cells (Shaefer et al., 2000). Furthermore, the I–V characteristics and amplitude of the light-activated current at −60 mV were largely unaffected by total replacement of extracellular Na+ with choline. While these results do not unequivocally identify the light-activated channel as being a member of the trp family, it does seem to rule out other candidates. For example, inward current carried by the cyclic nucleotide-activated channels found in rod photoreceptors would be nearly abolished under these conditions (Hodgkin et al., 1985).
While further genetic and pharmacological experiments are needed to confirm the identity of the light-activated channel, it is interesting to note that trp channels mediate the light response of invertebrate photoreceptors (Montell & Rubin, 1989; Hardie & Minke, 1992; Hardie & Minke, 1992). Moreover, octopus rhodopsin bears an evolutionary similarity to melanopsin (Oshima, 2001), which is expressed in SCN-projecting RGCs in the rat (Gooley et al., 2001; Hattar et al., 2002; Hannibal et al., 2002). Melanopsin is thought to form a retinal-based photopigment, and its sequence displays several hallmarks of invertebrate opsins, including a tyrosine counter-ion for the retinal Schiff's base, instead of the glutamate that is characteristic of vertebrate opsins (Provencio et al., 1998). A tenable hypothesis is that photoexcited melanopsin activates an intracellular signalling pathway similar to that found in invertebrate photoreceptors, leading to the opening of trp channels and membrane depolarization.
The transient light-induced depolarization seen at stimulus onset, in addition to the apparent transient nature of the spike generating machinery, raises a myriad of questions regarding how such a system could signal the SCN about general luminance information over long periods of time. If one were to design such a system, one might select a nonadapting mechanism in which the retinal input to the circadian clock faithfully follows the light intensity of the environment. Clearly much work is needed to elucidate how a system that appears to respond best to the onset of illumination rather than its duration can result in circadian entrainment.
Acknowledgements
The work was supported by grants from the NIMH (MH 63364) and the OHS Foundation. E.J.W. was supported by an NRSA Training Grant (NS07466). We would like to thank Dr. William Cameron for generously allowing us to use his Neurolucida reconstruction system.
Abbreviations
- DAB
diaminobenzidine
- IPL
inner plexiform layer
- RGC
retinal ganglion cell
- RHT
retinohypothalamic tract
- SCN
suprachiasmatic nucleus
- trp
transient receptor potential
- TTX
tetrodotoxin
References
- Aggelopoulos NC, Meissl H. Responses of neurones of the rat suprachiasmatic nucleus to retinal illumination under photopic and scotopic conditions. J. Physiol. (Lond.) 2000;523:211–222. doi: 10.1111/j.1469-7793.2000.t01-1-00211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- Boycott BB, Wassle H. The morphological types of ganglion cells of the domestic cat's retina. J. Physiol. (Lond.) 1974;240:397–419. doi: 10.1113/jphysiol.1974.sp010616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham DE, Runnels LW, Struübing C. The TRP ion channel family. Nature Rev. Neurosci. 2001;2:387–396. doi: 10.1038/35077544. [DOI] [PubMed] [Google Scholar]
- Dunn FA, Berson DM. 2002 Annual Meeting. Fort Lauderdale FL: Association for Research in Vision and Ophthamology; 2002. Are intrinisically photosensitive retinal ganglion cells influenced by rods and cones? Abstract and Program Planner Accessed at Www.Arvo.Org Abstract number 2982. [Google Scholar]
- Foster RG, Provencio I, Hudson D, Fiske S, De Grip W, Menaker M. Circadian photoreception in the retinally degenerate mouse (rd/rd) J. Comp. Physiol. [a] 1991;169:39–50. doi: 10.1007/BF00198171. [DOI] [PubMed] [Google Scholar]
- Freedman MS, Lucas RJ, Soni B, von Schantz M, Munoz M, David-Gray Z, Foster R. Regulation of mammalian circadian behavior by non-rod, non-cone, ocular photoreceptors. Science. 1999;284:502–504. doi: 10.1126/science.284.5413.502. [DOI] [PubMed] [Google Scholar]
- Fukuda Y. A three-group classification of rat retinal ganglion cells: histological and physiological studies. Brain Res. 1977;119:327–334. doi: 10.1016/0006-8993(77)90314-6. [DOI] [PubMed] [Google Scholar]
- Gooley JJ, Lu J, Chou TC, Scammell TE, Saper CB. Melanopsin in cells of origin of the retinohypothalamic tract. Nature Neurosci. 2001;4:1165. doi: 10.1038/nn768. [DOI] [PubMed] [Google Scholar]
- Gooley JJ, Saper CB. A broad role for melanopsin in non-visual photoreception based on neuroanatomical evidence in rats. Soc. Neurosci. Abstr. 2002;371:16. [Google Scholar]
- Hannibal J, Hindersson P, Knudsen SM, Georg B, Fahrenkrug J. The photopigment melanopsin is exclusively present in pituitary adenylate cyclase-activating polypeptide-containing retinal ganglion cells of the retinohypothalamic tract. J. Neurosci. 2002;22:RC191. doi: 10.1523/JNEUROSCI.22-01-j0002.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie RC, Minke B. The trp gene is essential for a light-activated Ca2+ channel in Drosophila photoreceptors. Neuron. 1992;8:643–651. doi: 10.1016/0896-6273(92)90086-s. [DOI] [PubMed] [Google Scholar]
- Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002;295:1065–1070. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin AL, McNaughton PA, Nunn BJ. The ionic selectivity and calcium dependence of the light-sensitive pathway in toad rods. J. Physiol. (Lond.) 1985;358:447–468. doi: 10.1113/jphysiol.1985.sp015561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue R, Okada T, Onoue H, Hara Y, Shimizu S, Nairoh S, Ito Y, Mori Y. The transient receptor potential protein homologue TRP6 is the essential component of vascular alpha(1)-adrenoceptor-activated Ca(2+)-permeable cation channel. Circ. Res. 2001;88:325–332. doi: 10.1161/01.res.88.3.325. [DOI] [PubMed] [Google Scholar]
- Johnson RF, Moore RY, Morin LP. Loss of entrainment and anatomical plasticity after lesions of the hamster retinohypothalamic tract. Brain Res. 1988;460:297–313. doi: 10.1016/0006-8993(88)90374-5. [DOI] [PubMed] [Google Scholar]
- Jung S, Strotmann R, Schultz G, Plant TD. TRPC6 is a candidate channel involved in receptor-stimulated cation currents in A7r5 smooth muscle cells. Am. J. Physiol. Cell Physiol. 2002;282:C347–C359. doi: 10.1152/ajpcell.00283.2001. [DOI] [PubMed] [Google Scholar]
- Karschin A, Lipton SA. Calcium channels in solitary retinal ganglion cells from post-natal rat. J. Physiol. (Lond.) 1989;418:379–396. doi: 10.1113/jphysiol.1989.sp017847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton SA, Tauck DL. Voltage-dependent conductances of solitary ganglion cells dissociated from the rat retina. J. Physiol. (Lond.) 1987;385:361–391. doi: 10.1113/jphysiol.1987.sp016497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minke M, Cook B. TRP channels and signal transduction. Physiol. Rev. 2002;82:429–472. doi: 10.1152/physrev.00001.2002. [DOI] [PubMed] [Google Scholar]
- Montell C, Rubin GM. Molecular characterization of the Drosophila trp locus: a putative integral membrane protein required for phototransduction. Neuron. 1989;2:1313–1323. doi: 10.1016/0896-6273(89)90069-x. [DOI] [PubMed] [Google Scholar]
- Moore RY, Speh JC, Card JP. The retinohypothalamic tract originates from a distinct subset of retinal ganglion cells. J. Comp. Neurol. 1995;352:351–366. doi: 10.1002/cne.903520304. [DOI] [PubMed] [Google Scholar]
- Oshima N. Direct reception of light by chromatophores of lower vertebrates. Pigment Cell Res. 2001;14:312–319. doi: 10.1034/j.1600-0749.2001.140502.x. [DOI] [PubMed] [Google Scholar]
- Perry VH. The ganglion cell layer of the retina of the rat: a Golgi study. Proc. R. Soc. Lond. B Biol. Sci. 1979;204:363–375. doi: 10.1098/rspb.1979.0033. [DOI] [PubMed] [Google Scholar]
- Pickard GE. Bifurcating axons of retinal ganglion cells terminate in the hypothalamic suprachiasmatic nucleus and the intergeniculate leaflet of the thalamus. Neurosci. Lett. 1985;55:211–217. doi: 10.1016/0304-3940(85)90022-9. [DOI] [PubMed] [Google Scholar]
- Provencio I, Berson DM, Richardson RC, Rollag MD, Castrucci AM. 2002 Annual Meeting. Fort Lauderdale FL: Association for Research in Vision and Ophthamology; 2002a. Melanopsin immunoreactivity in retinal ganglion cells. Abstract number: 1363. [Google Scholar]
- Provencio I, Jiang G, De Grip WJ, Hayes WP, Rollag MD. Melanopsin: An opsin in melanophores, brain, and eye. Proc. Natl Acad. Sci. USA. 1998;95:340–345. doi: 10.1073/pnas.95.1.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencio I, Rodriguez IR, Jiang G, Hayes WP, Moreira EF, Rollag MD. A novel human opsin in the inner retina. J. Neurosci. 2000;20:600–605. doi: 10.1523/JNEUROSCI.20-02-00600.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencio I, Rollag MD, Castrucci AM. Photoreceptive net in the mammalian retina. This mesh of cells may explain how some blind mice can still tell day from night. Nature. 2002b;415:493. doi: 10.1038/415493a. [DOI] [PubMed] [Google Scholar]
- Pu M. Dendritic morphology of cat retinal ganglion cells projecting to suprachiasmatic nucleus. J. Comp. Neurol. 1999;414:267–274. [PubMed] [Google Scholar]
- Schmid S, Guenther E. Voltage-activated calcium currents in rat retinal ganglion cells in situ: changes during prenatal and postnatal development. J. Neurosci. 1999;19:3486–3494. doi: 10.1523/JNEUROSCI.19-09-03486.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaefer M, Plant TD, Obukhov AG, Hofmann T, Gudermann T, Shultz G. Receptor-mediated regulation of the nonselective cation channels TRPC4 and TRPC5. J. Biol. Chem. 2000;275:23965–23972. doi: 10.1074/jbc.275.23.17517. [DOI] [PubMed] [Google Scholar]
- Skaliora I, Scobey RP, Chalupa LM. Prenatal development of excitability in cat retinal ganglion cells: action potentials and sodium currents. J. Neurosci. 1993;13:313–323. doi: 10.1523/JNEUROSCI.13-01-00313.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollars PJ, Smeraski CA, Kaufman JD, Ogilvie MD, Provencio I, Morin LP, Pickard GE. Melanopsin and non-melanopsin expressing retinal ganglion cells innervate the suprachiasmatic nucleus. Soc Neurosci. Abstr. 2002;371:21. doi: 10.1017/s0952523803206027. [DOI] [PubMed] [Google Scholar]
- Wang GY, Ratto G, Bisti S, Chalupa LM. Functional development of intrinsic properties in ganglion cells of the mammalian retina. J. Neurophysiol. 1997;78:2895–2903. doi: 10.1152/jn.1997.78.6.2895. [DOI] [PubMed] [Google Scholar]
- Wassle H, Boycott BB. Functional architecture of the mammalian retina. Physiol. Rev. 1991;71:447–480. doi: 10.1152/physrev.1991.71.2.447. [DOI] [PubMed] [Google Scholar]