Abstract
Peroxisome proliferator-activated receptors (PPARs) are ligand-activated transcription factors that belong to the nuclear hormone receptor superfamily. PPARα is mainly expressed in the liver, where it activates fatty acid catabolism. PPARα activators have been used to treat dyslipidemia, causing a reduction in plasma triglyceride and elevation of high-density lipoprotein cholesterol. PPARδ is expressed ubiquitously and is implicated in fatty acid oxidation and keratinocyte differentiation. PPARδ activators have been proposed for the treatment of metabolic disease. PPARγ2 is expressed exclusively in adipose tissue and plays a pivotal role in adipocyte differentiation. PPARγ is involved in glucose metabolism through the improvement of insulin sensitivity and represents a potential therapeutic target of type 2 diabetes. Thus PPARs are molecular targets for the development of drugs treating metabolic syndrome. However, PPARs also play a role in the regulation of cancer cell growth. Here, we review the function of PPARs in tumor growth.
1. INTRODUCTION
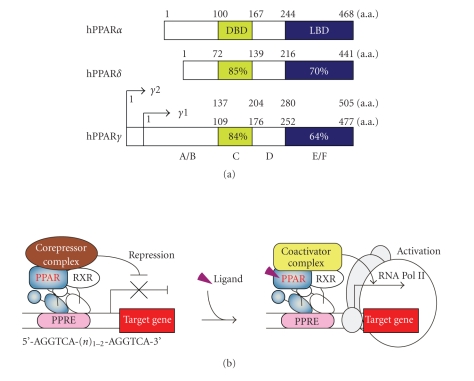
Peroxisome proliferator-activated receptors (PPARs) are ligand-activated transcription factors that belong to the nuclear hormone receptor superfamily [1]. PPARs bind to a direct repeat of two hexanucleotides, spaced by one or two nucleotides (the DR1 or DR2 motif) as heterodimers with the retinoid X receptor (RXR), and activate several target genes [2–4]. These peroxisome proliferator responsive elements (PPREs) are found in various genes that are involved in lipid metabolism and energy homeostasis,including lipid storage or catabolism, and fatty acid transport, uptake, and intracellular binding [5]. Three subtypes, PPARα, PPARδ (also known as PPARβ), and PPARγ, have been identified and these subtypes with a high degree of sequence conservation of each subtype across various species have been characterized. The DNA-binding domains of the three subtypes are 80% identical, while their ligand-binding domains exhibit a lower degree (approximately 65%) of identity (Figure 1). Consistent with this relatively high divergence among the subtype-specific ligand-binding domains, differential activation of PPARs by endogenous and exogenous compounds may account for the specific biological activity of the three PPAR subtypes [6, 7].
Figure 1.
The general features of human PPARs. (a) Structure and functional domain of human PPARs. A/B, C, D, and E/F indicate N-terminal A/B domain containing a ligand-independent activation function 1, DNA-binding domain (DBD), hinge region, and C-terminal ligand-binding domain (LBD), respectively. The number inside each domain corresponds to the percentage of amino acid sequence identity of human PPARδ and PPARγ relative to PPARα. (b) PPAR/RXR heterodimers bind to a PPRE located in the promoter of target genes through the DBD. Unliganded PPAR associates with the corepressor complex. In the presence of ligand, the ligand-bound LBD associates with the coactivator complex.
PPARα is expressed in the liver, kidney, small intestine, heart, and muscle, where it activates fatty acid catabolism and is involved in the control of lipoprotein assembly [8]. PPARα is activated by several molecules, such as long chain unsaturated fatty acids, eicosanoids, and hypolipidemic drugs (e.g., fenofibrate) [9–12]. PPARα activators have been used to treat dyslipidemia, causing a reduction in plasma triglyceride and elevation of high-density lipoprotein (HDL) cholesterol [13, 14]. PPARδ is expressed ubiquitously and is implicated in fatty acid oxidation, keratinocyte differentiation, wound healing, and the response of macrophages for very low-density lipoprotein [15–19]. PPARδ activators have been proposed for the treatment of metabolic disease and are under clinical trial [20, 21]. There are two PPARγ isoforms: PPARγ1 and γ2 [22, 23]. PPARγ2, which is generated by alternative splicing, contains an additional 28 amino acids at the N-terminal compared to PPARγ1. PPARγ3 is a splicing variant of PPARγ1 and gives rise to the same protein [24]. PPARγ2 is expressed exclusively in adipose tissue and plays a pivotal role in adipocyte differentiation, lipid storage in the white adipose tissue, and energy dissipation in the brown adipose tissue [22, 25]. On the other hand, PPARγ1 is expressed in the colon, the immune system (e.g., monocytes and macrophages), and others. Except for the function of PPARγ2 in adipose tissue, PPARγ also participates in inflammation, cell cycle regulation, and other functions [26]. PPARγ is involved in glucose metabolism through the improvement of insulin sensitivity and represents a potential therapeutic target of type 2 diabetes [26]. Indeed, insulin-sensitizing thiazolidinedione (TZD) drugs are PPARγ ligands [27]. Thus PPARs are molecular targets for the development of drugs to treat type 2 diabetes and metabolic syndrome. On the other hand, PPARs also play a role in the regulation of cancer cell growth.
2. PPARα AND CANCER
Fibrates, which are relatively weak PPARα ligands, are useful for the treatment of dyslipidemia [7, 9–11]. Fibrates lower serum triglyceride levels and increase HDL levels through the activation of PPARα [5]. PPARα induces lipoprotein lipase (LPL) expression, reduces the expression levels of apolipoprotein C-III (ApoC-III), a natural LPL inhibitor, and stimulates the uptake of cellular fatty acids and the conversion of fatty acids to acyl-CoA derivatives [5, 28, 29]. These catabolism functions are mediated by upregulating the expression of a series of genes-related carbohydrate and lipid metabolism [5, 30]. In addition, PPARα increases the expressions of ApoA-I and ApoA-II, resulting in raising HDL cholesterol levels in humans [31, 32]. Thus PPARα plays a central role in the control of fatty acid and lipoprotein metabolism, and improves plasma lipid profiles. Although peroxisome proliferators have carcinogenic consequences in the liver of rodents, epidemiological studies suggest that similar effects are unlikely to occur in humans [10, 33–36].
Several mechanisms have been proposed to explain the carcinogenesis of peroxisome proliferators in rodents. Peters et al. reported that wild-type mice treated with the Wy-14,643 showed increase of replicative DNA synthesis in hepatic cells and developing liver tumors with 100% incidence, whereas PPARα-null mice were refractory to this effect [37]. Peroxisome proliferators increase the peroxisome volume and number and result in an increase in hydrogen peroxide (H2O2) levels [38–40]. These effects may be mediated in part by the increased expression of peroxisomal enzymes that produce H2O2, such as acyl CoA oxidase (ACO) [39–41]. PPARα upregulates the expression levels of ACO via PPRE in the promoter region [42, 43]. A stably transfected African green monkey kidney cells (CV-1) overexpressing rat ACO increased H2O2 production, formed transformed foci, and grew efficiently in soft agar when the cells were treated with linoleic acid [44]. Furthermore, when these cells were transplanted into nude mice, these cells formed solid tumors [44]. An increase of intracellular levels of H2O2 could lead to DNA damage via a variety of mechanisms [45]. Any reduced iron present can catalyze the cleavage of H2O2, via the Fenton reaction, to produce hydroxyl radicals (HO•) [46]. The HO• attacks guanine residues, producing residues of 8-oxo-7,8-dihydroguanine (8-oxoguanine). When DNA synthesis occurs before the 8-oxoguanine is repaired, this damaged base will have a chance to pair with adenine nucleotide, resulting in a mutation in the daughter cells [47]. In addition, antioxidants inhibit ciprofibrate-induced hepatic tumorigenesis by scavenging active oxygen [48]. Thus oxidative stress by peroxisome proliferators acts as a driving force to malignancy. The activation of PPARα also leads to increased hepatocellular proliferation and inhibition of apoptosis. Chronic administration of nafenopin, PPARα agonist, to mice causes significant increase in the liver weight, hepatic DNA synthesis, and the development of hepatocellular carcinomas [49]. Nafenopin treatment of primary cultures of adult rat hepatocytes also stimulated DNA synthesis [50]. Indeed, Peters et al. showed that mRNAs encoding cyclin-dependent kinase (CDK) 1, CDK4, cyclin D1, and c-myc and their proteins, which induce cell proliferation, increased in wild-type mice fed by Wy-14,643 but not in PPARα-null mice [51]. Increase of the average liver weight and the levels of mRNAs encoding cell cycle regulation, such as CDK4, proliferating cell nuclear antigen (PCNA) and cyclin B1, were also found in wild-type mice fed by bezafibrate, the less specific PPARα agonist, and these effects were not found in PPARα-null mice [52]. Moreover, the treatment of the primary culture of rat hepatocytes and the rat hepatoma cell line, FaO, with nafenopin suppressed apoptosis [53, 54]. Thus the activation of PPARα leads to the increase of oxidative stress, induction of cell proliferation and inhibition of apoptosis, indicating that PPARα increases hepatocarcinogenesis in mice.
A number of experimental observations suggest that there is a species difference between rodents and humans in the response to PPARα agonists, although the functional differences of PPARα derived from species are not clear (Table 1). One possible explanation for the difference is the expression levels of PPARα in the liver. The expression levels of PPARα in human liver are approximately one order less than that observed in mouse liver [55]. Small expression levels of PPARα could allow PPREs to be occupied by other members of the nuclear receptor superfamily,such as RXR, the chicken ovalbumin upstream promoter transcription factor I (COUP-TFI), COUP-TFII, hepatocyte nuclear factor-4 (HNF4), retinoic acid receptor (RAR), and thyroid hormone receptor (TR), and affect responsiveness to peroxisome proliferators [56–62]. We and others have shown that elevated expression of PPARα in HepG2 cells dramatically increased the expression of several target genes, such as 3-hydroxy-3-methylglutaryl-CoA synthase 2 (mitochondrial) (HMGCS2), carnitine palmitoyltransferase 1A (CPT1A), and long chain fatty acyl-CoA synthetase (ACS) [30, 63, 64]. In this way, the lower expression levels of PPARα in human liver might contribute to holding down peroxisome proliferation and subsequent pathologic effects. Another explanation is that several PPARα variants, which lack the entire exon 6 or contain mutations, are detected in human cells and these variants act as a dominant negative regulator of PPAR-mediated gene transcription [55, 65, 66]. But this has not been found in rodents yet. One PPARα variant containing the mutation prevents the suppression of hepatocyte apoptosis by nafenopin [55, 65, 66]. Thus the expression levels of PPARα variants might affect the response to peroxisome proliferators. Next, there appears to be sequence differences in the PPRE found in the promoter region of ACO. Osumi et al. identified ACO to be a direct PPARα target gene and a functional PPRE located in the proximal promoter of the rat ACO gene [42]. In contrast to the rodent ACO gene promoter, the human ACO gene promoter differs at three bases within the PPRE from the rat ACO promoter and appears refractory to PPARα [42, 67, 68]. This human PPRE was unable to drive peroxisome proliferators-induced gene transcription in cell-based assays [67–69]. Indeed, human liver cell lines and primary hepatocytes did not induce ACO mRNA by treatment with fibrates or other PPARα agonists [63, 64]. A similar pattern, such differences between human and other species, was observed in the expression of ApoA-I gene [31]. Fibrates influence the ApoA-I gene expression, raising it in humans, and lowering it in rodents. These differences are due to a combination of two distinct mechanisms implicating the nuclear receptors PPARα and Rev-erbα, a negative regulator of gene transcription [31]. The species-distinct regulation is due to sequence differences in cis-acting elements in their respective promoters leading to repression by Rev-erbα of rat ApoA-I and activation by PPARα of human ApoA-I. There is a positive PPRE in the human ApoA-I promoter but not in rats. The expression of Rev-erbα is induced by fibrates [3, 31]. In the case of rat, this induction leads to the repression of the ApoA-I gene expression through an Rev-erbα response element. On the other hand, there is no Rev-erbα response element in the human ApoA-I gene [31]. Thus the sequence differences in cis-acting elements cause the species-distinct regulation of target genes expression by peroxisome proliferators. However, the mechanism of the species differences is not known in detail.
Table 1.
Summary of the species differences of PPARα.
| Human | Rodent | |
|---|---|---|
| PPARα expression levels | + | ++ |
| PPARα variants | Yes | ? |
| Peroxisome proliferation | +/− | + |
| Fatty acid metabolism | + | + |
| Expression of cell cycle regulator genes | +/− | + |
| Expression of miRNA (let-7C) | + | − |
| Hepatocellular proliferation | +/− | + |
| Apoptosis | + | − |
| Liver tumor | +/− | ++ |
To determine the mechanism of species difference in response to peroxisome proliferators, Gonzalez et al. generated a liver-specific PPARα humanized mouse line (hPPARα TetOff mice) in which the human PPARα was expressed in the liver in a PPARα-null background under the control of the tetracycline (Tet) responsive regulatory system [70–72]. The expression of several target genes encoding peroxisomal and mitochondrial fatty acid metabolizing enzymes were elevated in hPPARα TetOff mice fed Wy-14,643 or fenofibrate, resulting in the decrease of serum triglycerides [70, 73]. However, the expressions of various genes involved in cell cycle regulation (PCNA, c-myc, CDK1, CDK4, and cyclins) in the liver were unaffected by Wy-14,643. In addition, hPPARα TetOff mice were resistant to Wy-14,643-induced hepatocarcinogenesis [70, 73]. Recently, Shah et al. showed that Wy-14,643 regulated mice hepatic MicroRNA (miRNA) expression via a PPARα-dependent pathway [74]. miRNAs are a class of nonprotein-coding, endogenous,small RNAs, and regulate gene expression by translational repression and mRNA cleavage [75]. Some miRNAs regulate cell proliferation and apoptosis processes that are important in cancer formation [76]. The activation of PPARα with Wy-14,643 inhibits the expression of miRNA let-7C, which functions as a tumor suppressor gene [74]. let-7C degrades c-myc mRNA by binding to 3' untranslated region (UTR) of the c-myc gene. Treatment of mice with Wy-14,643 showed that let-7C expression was decreased and a subsequent increase in c-myc was observed. Following an increase in c-myc, the levels of the oncogenic mir-17 miRNA cluster were increased [74]. In this way, inhibition of the let-7C signaling cascade may lead to increased hepatocellular proliferation and tumorigenesis. In contrast, hPPARα TetOff mice do not exhibit downregulation of let-7C and induced c-myc and mir-17 expression [74]. Furthermore, Yang et al. generated another type of PPARα humanized mice, hPPARα PAC mice, that has the complete human PPARα gene sequence including 5' and 3' flanking sequences on a P1 phage artificial chromosome (PAC) genomic clone, introduced onto the mouse PPARα-null background [71]. Upon treatment with the peroxisome proliferators (Wy-14,643 or fenofibrate), hPPARα PAC mice exhibited peroxisome proliferation, lowering of serum triglycerides, and induction of PPARα target genes encoding enzymes involved in fatty acid metabolism. However, let-7C expression was not decreased and the expression levels of c-myc, cyclin D1 and CDK4 were not increased [71]. Thus these observations suggest that the species differences in response to peroxisome proliferators could be due in part to a differential ability of the mouse and human PPARα to suppress let-7C gene expression [74]. However, the mechanism involved in PPARα-dependent repression of let-7C is unclear. The differences between the wild-type mice and PPARα humanized mice could be caused by the structural differences between human and mouse PPARα and differential coactivator recruitment. However, additional investigation is required to better understand and clarify the mechanism of action of PPARα in causing hepatocarcinogenesis.
3. PPARδ AND CANCER
The role of PPARδ in oncogenesis is controversial, especially in colon cancer. Some reports show that PPARδ promotes tumorigenesis by increasing cell proliferation. Indeed, the levels of PPARδ mRNA are increased in both human and rodent colorectal carcinomas [77, 78]. PPARδ is a potential downstream target gene of the adenomatous polyposis coli (APC)/β-catenin/T cell factor-4 (TCF-4) pathway [77]. APC is a tumor suppressor gene and is mutated in familial adenomatous polyposis (FAP) and most sporadic colorectal tumors [79–83]. β-catenin, which binds to APC and axin in a large protein complex, can be phosphorylated by glycogen synthase kinase-3β (GSK3β) and is followed by ubiquitination and degradation. Mutation of APC results in the accumulation of β-catenin, which in turn translocates to the nucleus and associates with the transcription factor TCF-4 [84]. The β-catenin-TCF-4 transcription complex increases the transcription of growth-promoting genes, such as c-myc and cyclin D1 [85, 86]. The β-catenin-TCF-4 transcription complex also activates the human PPARδ promoter activity via TCF-4 binding sites, namely, APC suppresses the PPARδ expression through the degradation of β-catenin [77]. K-Ras mutation is found in colorectal cancer [80, 87]. Activation mutations in Ras result in the activation of the mitogen-activated protein kinase (MAPK) pathway and induce tumor growth and progression [88]. The expression levels and activity of PPARδ were increased by the induction of mutated K-Ras in conditionally K-Ras-transformed rat intestinal epithelial cells [89]. Thus PPARδ is also a downstream target gene of Ras/Raf/MAPK and extracellular signal-regulated kinase (ERK) kinase (MEK)/ERK pathway [89]. In this way, PPARδ may play a role in colon cancer.
Epidemiological studies have shown that nonsteroidal anti-inflammatory drugs (NSAIDs), such as aspirin, indomethacin, and sulindac, reduce the overall number and size of adenomas in patients with FAP. Healthy individuals using NSAIDs regularly can lead to a 40–50% reduction in the relative risk of developing colon cancer [90]. NSAIDs inhibit cyclooxygenase (COX) activity and thereby reduce prostaglandin synthesis [91]. COX is a key enzyme in arachidonic acid metabolism and prostaglandin production. COX catalyzes a two-step reaction that converts arachidonic acid to prostaglandin H2 (PGH2), which in turn serves as the precursor for the synthesis of all biologically active prostaglandins, including PGD2, PGE2, PGF2 α, prostacyclin (PGI2), and thromboxane A2 (TXA2) [92]. COX exists in two isoforms that are encoded by two separate genes. COX-1 is constitutively expressed in most tissues, on the other hand, the expression of COX-2 is normally low or absent in most tissues but is rapidly upregulated by proinflammatory cytokines [93]. Expression of COX-2 is also elevated in colorectal cancer and in a subset of adenomas [94]. Moreover, since both the introduction of the knockout mutation of the COX-2 gene into A p c Δ716 mice, a model of human FAP, and treating A p c Δ716 mice with NSAIDs reduce the development of intestinal tumors, COX-2 inhibitors have been considered as therapeutic agents for colorectal polyposis and cancer [95]. He et al. reported that NSAIDs inhibited the transcriptional activity of PPARδ by disruption of the DNA binding ability of PPARδ/RXR heterodimers, and ectopic expression of PPARδ in the human colorectal cancer cell line, HCT116, protected the cells from sulindac-induced apoptosis [77]. PPARδ and COX-2 mRNA are expressed in similar regions in human colon cancer, and the stable PGI2 analog, carbaprostacyclin (cPGI), acts as a PPARδ ligand [11, 78]. Indeed, ectopic expression of COX-2 and PGI synthase (PGIS) in the human osteosarcoma cell line, U2OS, produced high levels of endogenous PGI2 and transactivation of PPARδ [78]. PGE2 levels are also elevated in human colorectal cancers and adenomas, and PGE2 increases the growth and motility of colorectal carcinoma cells [96, 97]. D. Wang et al. showed that PGE2 promoted resistance to serum starvation-induced apoptosis of cultured human colon carcinoma cells, LS-174T, through indirectly upregulation PPARδ transcriptional activity via a phosphotidylinositol-3-kinase (PI3K)-Akt pathway [98]. Furthermore, PGE2 accelerates intestinal adenoma growth of A p c min mice, a model of human FAP that harbors a mutation in the apc gene, via PPARδ [98]. Xu et al. showed that PGE2 activated cytosolic phospholipase A2 α (cPLA2 α) through PI3K or MAPK pathway, and subsequently cPLA2 α enhanced PPARδ activity in the human cholangiocarcinoma cells [99]. They also showed that PPARδ enhanced COX2 expression and PGE2 production. This positive feedback loop may play an important role in cholangiocarcinoma cell growth, although it is not known whether this kind of positive feedback loop exists in the colorectal cancer cells [99]. Thus PPARδ induces the cell proliferation through the inhibition of apoptosis. However, sulindac sulfide induces apoptosis not only in wild-type HCT116, but also in HCT116 PPARδ-null cell lines [100]. On the basis of these observations, although NSAIDs may reduce tumorigenesis through the inhibition of PPARδ activity, PPARδ is not a major mediator of sulindac-mediated apoptosis.
Recent evidence supports the hypothesis that PPARδ promotes tumor progression. HCT116 PPARδ-null cell lines grew slightly more slowly than wild-type HCT116 cells, and exhibited a decreased ability to form tumors compared with wild-type mice when inoculated as xenografts in nude mice [100]. Gupta et al. showed that exposure of A p c min mice to 10 mg/kg of GW501516, a high-affinity PPARδ-selective agonist, led to a two-fold increase in polyp number in the small intestine [101]. The most prominent effect was on polyp size, mice treated with the PPARδ activator had a five-fold increase in the number of polyps larger than 2 mm, suggesting that PPARδ activation primarily affected the rate of polyp growth rather than initiating polyp formation. Pretreatment of wild-type HCT116 cells with GW501516 significantly suppressed serum starvation-induced apoptosis in a dose-dependent manner, but not HCT116 PPARδ-null cells [101]. Furthermore, D. Wang et al. showed that PPARd −/−/A p c min mice decreased intestinal adenoma growth and inhibited the tumor-promoting effect of GW501516 [102]. They also showed that PPARδ activation with GW501516 upregulated vascular endothelial growth factor (VEGF) transcription, expression, and peptide release in intestinal epithelial tumor cells, and subsequently activated PI3K-Akt signaling [102]. Similar results were obtained in the human endothelial cells [103, 104]. Piqueras et al. showed that GW501516 induced VEGF mRNA and peptide release, and thus PPARδ induced endothelial cell proliferation and angiogenesis [103]. Stephen et al. showed that the activation of PPARδ resulted in increased expression of VEGF and its receptor fms-related tyrosine kinase 1 (FLT-1), and they suggested that PPARδ might initiate an autocrine loop for cellular proliferation and possibly angiogenesis [104]. These results demonstrate that VEGF mediates the antiapoptotic effects of PPARδ in intestinal epithelial tumor cells by activating the PI3K-Akt cell survival pathway, and the VEGF autocrine loop plays an important role in cell survival. Diminished apoptosis is also linked to downregulated 15-lipoxygenase-1 (15-LOX-1) expression in colorectal cancer cells. 13-S-hydroxyoctadecadienoic acid (13-S-HODE), which is the primary product of 15-LOX-1 metabolism of linoleic acid, inhibits cell proliferation and induces cell cycle arrest and apoptosis in transformed colonic epithelial cells [105]. 15-LOX-1 protein expression and 13-S-HODE intracellular levels are decreased in human colonic tumors [105]. Shureiqi et al. showed that 13-S-HODE bound to PPARδ and then downregulated PPARδ expression and activation in colorectal cancer cells, DLD-1 and RKO, and that the loss of PPARδ expression in HCT116 markedly suppressed 13-S-HODE-mediated apoptosis [106]. 15-LOX-1 expression also downregulated PPARδ expression and transcriptional activity in these colorectal cancer cells [106]. Furthermore, NSAIDs increase 15-LOX-1 protein expression and its product 13-S-HODE levels and downregulate PPARδ expression in association with subsequent growth inhibition and apoptosis [106, 107]. Thus it is considered possible that PPARδ promotes the growth of colon cancers.
On the contrary, other reports suggest that ligand activation of PPARδ promotes the induction of terminal differentiation and inhibition of cell growth. PPARδ was found in intestinal epithelial cells in both the normal intestine and adenomas of A p c min mice [101]. Reed et al. reported that targeted deletion of the APC alleles in mouse intestines decreased the expression levels of PPARδ mRNA and protein, although β-catenin and c-myc were increased [108]. Marin et al. showed that PPARδ expression was reduced in both the A p c min mouse colon polyps and azoxymethane (AOM)-treated wild-type mouse polyps, though the expression levels of PPARδ mRNA in colonic epithelium were not different between A p c min mice and wild-type mice with or without AOM-treatment [109]. Several reports identified that the transcription factor binding sites for AP-1, CCAAT/enhancer-binding proteins, vitamin D receptor, and others were found in human or mouse PPARδ promoter, and these transcription factors regulated PPARδ expression [16, 110, 111]. However, further investigation is required to certify the regulation of PPARδ expression in cancer.
Hollingshead et al. reported that GW501516 and GW0742, highly specific PPARδ ligands, did not increase the growth of human colon cancer cell lines (HT-29, HCT116, and LS-174T) and liver cancer cell lines (HepG2 and HuH7) cultured in the presence or absence of serum [112]. In addition, treatment of these cell lines with either GW501516 or GW0742 did not change the phosphorylation of Akt, and no increase in the expression levels of COX2 or VEGF were detected [112]. Similar results were observed in the colon or liver of A p c min mice treated with GW501516 or GW0742 [109, 112]. Barak et al. showed that the average number of intestinal polyps was not significantly different between PPARd +/+/A p c min, PPARd +/−/A p c min, and PPARd −/−/A p c min mice, although this study was limited to a small number [113]. On the other hand, several studies showed that colon polyp formation was enhanced in the absence of PPARδ expression in both PPARd −/−/A p c min and AOM-treated PPARd −/− mice [108, 109, 114]. Moreover, Marin et al. showed that the administration of GW0742 had no effect on colon or small intestinal tumorigenesis in either PPARd −/−/A p c min or PPARd +/+/A p c min mice as compared with controls [109]. In addition, decrease of colon polyp multiplicity was observed in PPARd +/+ AOM-treated mice administrated with GW0742 compared with control wild-type mice. This effect was likely due in part to PPARδ-dependent induction of colonocyte differentiation and enhancement of apoptosis [109]. Indeed, PPARδ induces keratinocyte terminal differentiation, which normally opposes cell proliferation [115, 116]. Hatae et al. also showed that intracellular PGI2, an endogenous PPARδ ligand, formed by expressing PGIS in human embryonic kidney 293 (HEK293) cells, promoted apoptosis by activating PPARδ [117]. In this way, PPARδ inhibits tumor growth by inducing apoptosis or differentiation.
Thus the conflicting reports in the literature suggest that PPARδ either potentiates or attenuates colon cancer. Similar discrepancies were observed in other tissues. Di-Poï et al. showed that the activation of PPARδ inhibited apoptosis in keratinocyte [118]. The activation of PPARδ by L-165041, one type of PPARδ ligand, upregulates 3-phosphoinositide-dependent kinase-1 (PDK1) and integrin-linked kinase (ILK) gene expression via PPRE and downregulates phosphatase and tensin homolog (PTEN) protein expression, and subsequently leads to the activation of Akt1 in a PI3K- dependent manner in mouse primary keratinocytes and human keratinocyte HaCaT cells [118]. Yin et al. showed that PPARδ ligand GW501516 accelerated progestin- and carcinogen-induced mouse mammary carcinogenesis [119]. Stephen et al. reported that PPARδ selective agonists stimulated the proliferation of human breast and prostate cancer cell lines and primary endothelial cells [104]. On the other hand, Burdick et al. reported that ligand activation of PPARδ with GW0742 inhibited the cell growth of either human keratinocyte cell line N/TERT1 or mouse primary keratinocytes [120]. In these cells, ligand activation of PPARδ by GW0742 did not alter expression and/or modulation of the PTEN/PDK1/ILK1/Akt pathway [120]. Girroir et al. reported that both GW0742 and GW501516 inhibited the growth of the human breast cancer cell line, MCF7, and human melanoma cell line, UACC903 [121].
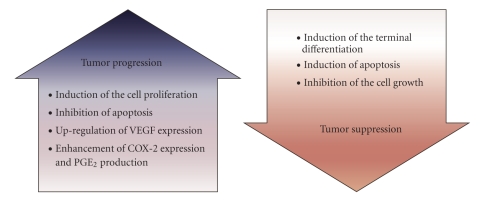
To date, however, the reason for the contradiction in these observations is unclear. One explanation for these conflicting results may be the ability of PPARδ to repress the transcription of target genes. We and others observed that unliganded PPARδ repressed target gene expression, though ligand-activated PPARδ induced these genes [30, 122–124]. It has been reported that unliganded PPARδ bound to PPRE and recruited corepressors, such as B-cell lymphoma 6 (BCL-6), silencing mediator for retinoid and thyroid hormone receptor (SMRT), nuclear receptor corepressor (NCoR), and others. On the other hand, liganded PPARδ is thought to release the corepressor and form a complex with coactivators [122–124]. Furthermore, binding of ligand to the PPARδ or deletion of PPARδ expression may lead to the release of BCL-6. Subsequently, BCL-6 represses the transcription of a number of inflammatory cytokine genes [124]. Thus the PPARδ activity may be influenced by the cellular environment, such as the existence of PPARδ ligands, cofactors, and others. From this viewpoint, the conflicting results may be due to differences in the condition of cell cultures or the genetic background of animal models. Secondly, prostaglandins, some of which act as PPAR ligands, have a variety of biological activities. Prostaglandins, synthesized via the COX pathway from arachidonic acid, are released outside the cells and lead to changes in the cellular levels of cyclic AMP and Ca2+ through binding to G-protein-coupled receptors on the plasma membrane [90]. Indeed, Hatae et al. suggested that cAMP produced by the PGI2-PGI receptor (IP)-cAMP pathway might protect vascular endothelial cells from intracellular PGI2-PPARδ-mediated apoptosis [117]. On the other hand, Fauti et al. showed that the ectopic expression of COX-2 and PGIS in HEK293 cells results in a dramatic induction of PGI2 synthesis, but no increase in PPARδ transcriptional activity is observed [125]. Thus they suggest that PGI2 lacks agonistic activity for PPARδ. Since PGI2 is unstable and rapidly hydrolyzed to 6-keto-PGF1α within minutes and increases the production of intracellular cAMP via stimulation of adenylyl cyclase through the cell surface IP receptor, further investigation is necessary to certify the mechanism of the effect of the PGI2 on PPARδ activity in detail. Therefore, additional analyses are necessary to define the PPARδ functions in cancer (Figure 2).
Figure 2.
Does PPARδ progress or suppress tumor growth?
4. PPARγ AND CANCER
Cancer cells represent dysregulaton of the cell cycle and lead to cell proliferation. In this viewpoint, modulators of the cell cycle and/or apoptosis are useful as chemotherapeutic agents for cancer [126, 127]. A number of investigators have shown that PPARγ was expressed in a variety of tumor cells, and the activation of PPARγ by ligands led to either inhibition of cell proliferation or induction of apoptosis (Table 2) [128, 129]. PPARγ is expressed in colonic tumors, normal colonic mucosa, and colon cancer cell lines [130–135]. Kitamura et al. showed that TZDs, such as troglitazone and rosiglitazone, inhibited the cell growth and induced G1 cell cycle arrest of rat intestinal epithelial cells [136]. The cell growth inhibition by TZDs was caused by the decrease of the expression of cyclin D1, critical for entering the S phase of the cell cycle. TZDs suppressed the cyclin D1 promoter activity through inhibition of the transcriptional activities of AP-1 and Ets [136]. Shao et al. demonstrated that treatment with rosiglitazone inhibited the K-Ras-induced elevation of the expression levels of cyclin D1 by inhibition of the K-Ras-induced phosphorylation of Akt, resulting in the G1 cell cycle arrest [89]. Furthermore, J.-A. Kim et al. showed that treatment of the human colorectal cell line, HCT15, with troglitazone induced the expression of p21Cip1/Waf1, that is, a CDK inhibitor (CKI) and negatively regulates the cell cycle progression, through the ERK pathway, and inhibited HCT15 cell growth [155]. PPARγ ligands also induce apoptosis in human colon cancer cells [156]. Chen et al. showed that PPARγ ligands, 15-Deoxy-Δ12,14-prostaglandin J2 (15dPGJ2), or ciglitazone, induced apoptosis in HT-29 by inhibiting nuclear factor kappa B (NF-κB) activity, which upregulates various antiapoptotic genes, and suppressing the expression of BCL-2, which protects cells against apoptosis [133]. Furthermore, using the in vivo mouse model, the administration of TZD to mice reduced AOM and/or dextran sodium sulfate-induced formation of aberrant crypts foci and colon carcinogenesis [131, 157]. In addition, PPARγ ligands also inhibit the cell growth of several breast cancer cell lines and mammary gland tumor development [137, 158–162]. Elstner et al. showed that PPARγ ligands, troglitazone, 15dPGJ2, and indomethacin, caused inhibition of proliferation in several human breast cancer cell lines, such as MCF7, MDA-MB-231, BT474, and T47D [162]. Troglitazone also inhibited MCF7 tumor growth in triple-immunodeficient BNX nude mice [162]. Clay et al. reported that 15dPGJ2 and troglitzaone attenuated cellular proliferation of MDA-MB-231 by blocking cell cycle progression and inducing apoptosis [160]. Pretreatment of MDA-MB-231 cells with 15dPGJ2 attenuated the capacity of these cells to induce tumors in nude mice [160]. Yin et al. showed that treatment of MCF7 with troglitazone also decreased the expression of several regulators of pRb phosphorylation, such as cyclin D1, CDK4, CDK6, and CDK2 [158]. pRB is a retinoblastoma tumor suppressor gene product, and phosphorylated pRB induces cell cycle progression [163]. Troglitazone induced the G1 cell cycle arrest by attenuation of pRb phosphorylation, resulting in inhibition of cell proliferation [158]. Suh et al. showed that GW7845, synthetic PPARγ ligand, prevented mammary carcinogenesis in the rat model that used nitrosomethylurea as the carcinogen [159]. Mehta et al. also reported that troglitazone prevented the induction of preneoplastic lesions by 7, 12-dimethylbenz[a]anthracene in a mouse mammary gland organ culture model [161]. Moreover, PPARγ ligands inhibit the cell proliferation in other types of cancer. PPARγ ligands inhibited the growth of esophageal squamous carcinoma cell lines by inducing G1 arrest associated with an increased level of several CKIs, such as p27Kip1, p21Cip1/Waf1, and p18Ink4c [138]. PPARγ ligands also induced apoptosis and G1 cell cycle arrest in human gastric cancer cells, and that inhibited cell proliferation [139, 164]. In human pancreatic cancer cells, PPARγ ligands induced apoptosis and growth inhibition associated with G1 cell cycle arrest through increasing p27Kip1 protein expression [140, 165–167]. In human hepatocellular carcinoma cell lines, PPARγ ligands induced cell cycle arrest through increased expression of p21Cip1/Waf1, p27Kip1, and p18Ink4c protein levels [141, 168]. Troglitazone also induced the activation of the cell death protease, caspase 3, and that induced apoptosis of human liver cancer cell lines [169]. PPARγ is abundantly expressed in human adrenal tumors including adrenocortical carcinomas and normal adrenal tissues. PPARγ agonists suppress adrenocortical tumor cell proliferation, increase apoptosis, and induce adrenal differentiation [142, 170]. Moreover, PPARγ ligand showed antitumor effect against human prostate cancer cells and human lung cancer cells [143, 144, 171–173]. Thus PPARγ ligands could suppress the tumorigenesis. Therefore, PPARγ ligands could be used as antineoplastic drugs.
Table 2.
The expression of PPARγ in cancer.
| References | |
|---|---|
| Colonic tumor | [135] |
| Breast tumor | [137] |
| Esophageal tumor | [138] |
| Gastric cancer | [139] |
| Pancreatic cancer | [140] |
| Hepatocellular carcinoma | [141] |
| Adrenocortical carcinoma | [142] |
| Lung tumor | [143] |
| Prostate cancer | [144] |
| Liposarcoma | [145] |
| Thyroid carcinoma | [146] |
| Bladder cancer | [147] |
| Renal cell carcinoma | [148] |
| Melanoma | [149] |
| Squamous cell carcinoma | [150] |
| Cervical carcinoma | [151] |
| Testicular cancer | [152] |
| Neuroblastoma | [153] |
| Pituitary tumor | [154] |
In contrast, both troglitazone and rosiglitazone treatment increased the frequency and size of colon tumors in A p c min mice [174, 175]. Treatment with rosiglitazone also increased the expression levels of β-catenin, a protein involved in Wnt signaling and correlating with enhanced cell proliferation, in the colon of A p c min mice and HT-29 cells [174]. To investigate the basis for this contradiction, Girnun et al. used mice heterozygous for PPARγ with both chemical and genetic models of human colon cancer [176]. Heterozygous loss of PPARγ caused a greater incidence of colon cancer when these mice were treated with AOM. Although there was no difference in β-catenin expression levels in colorectal tumors between AOM-treated PPARg +/− and wild-type mice, β-catenin expression levels in the colonic epithelium of untreated PPARg +/− mice were greater than that of untreated wild-type mice. When crossing to A p c 1638N mice, the mouse model for FAP, there were also no difference in β-catenin levels between PPARg +/−/A p c 1638N and PPARg +/+/A p c 1638N mice before polyp formation. Survival and the number of tumors formed in the colon also showed no difference in both mice. Thus although PPARγ has the potential to suppress β-catenin levels and colon carcinogenesis, PPARγ has no effect on β-catenin levels or tumorigenesis in the presence of APC signaling dysfunction [176]. Furthermore, PPARγ mutations, some of which show the loss of the transactivation ability, are found in colon cancers in humans, and that PPARγ may be considered as a tumor suppressor gene [134]. On the other hand, to evaluate the contribution of PPARγ to breast cancer, Saez et al. generated transgenic mice, MMTV-VpPPARγ mice, that express a constitutively active form of PPARγ in mammary gland [177]. MMTV-VpPPARγ mice showed normal development of mammary gland and no increased tendency to develop tumors. To assess the influence of increased PPARγ signaling on mammary gland neoplasia, MMTV-VpPPARγ mice were crossed to mice that express a polyoma virus middle T antigen (PyV-MT) in mammary tissue, MMTV-PyV mice, which rapidly develop tumors. These mice that expressed both activated PPARγ and PyV-MT showed accelerated development of mammary tumors. Therefore, although increased PPARγ activation does not initiate tumor formation in normal mammary tissue, once a tumor-initiating event occurs, PPARγ signaling serves as a tumor promoter in the mammary gland. Furthermore, there is no difference in tumor development between MMTV-PyV mice and the mice, generated by crossing PPARg +/− mice to MMTV-PyV mice [177]. Thus in this model, PPARγ does not act as a tumor suppressor gene.
Furthermore, PPARγ ligands exert their biological effects through a PPARγ-independent pathway. Palakurthi et al. reported that troglitazone and ciglitazone induced G1 arrest by inhibiting translation initiation in both PPARg −/− and PPARg +/+ mouse embryonic stem cells. Thus TZDs inhibit cell proliferation and tumor growth in a PPARγ-independent manner [178]. Therefore, although PPARγ ligands are used as insulin sensitizers, further investigation is needed to clarify whether PPARγ ligands are effective chemotherapeutic agents for cancer in humans.
5. SUMMARY
PPARs are linked to metabolic disorders and are interesting pharmaceutical targets. Among the synthetic ligands, fibrates are hypolipidemic compounds that activate PPARα, and TZDs, which selectively activate PPARγ, are hypoglycaemic molecules that are used to treat type 2 diabetes. PPARδ agonists might form effective drugs for obesity, diabetes, and cardiovascular disease. Moreover, recent evidence suggests that PPAR modulators may have beneficial effects as chemopreventive agents [179]. However, as mentioned above, it remains unclear whether PPARs act as oncogenes or as tumor suppressors. From this viewpoint, current strategies are aimed at reducing the side effects and improving the efficacy and safety profile of PPAR agonists, termed selective PPAR modulators (SPPARMs) [180, 181]. This model proposes that SPPARMs bind in distinct manners to the ligand binding pocket of PPAR and induce distinct conformational changes of the receptor, resulting in differential interactions with cofactors according to the combination of their expression levels in different organs. Thus each SPPARM leads to differential gene expression and biological response. However, what kinds of cofactors are recruited to PPAR by each SPPARM is still unknown. Thus it is important to identify the cofactor complex for PPAR with each SPPARM and the expression patterns of cofactors in various tissues. Furthermore, recent evidence suggests that the ligand binding protein in the cytosol that transports ligands into the nucleus is important to modulate the action of nuclear receptors. Long-chain fatty acids, endogenous PPAR ligands, are highly hydrophobic and fatty acids are bound to fatty acid binding proteins (FABPs) in the aqueous intracellular compartment [182]. FABPs also bind to PPAR ligands and transport them from the cytosol into the nucleus [183–191]. In the nucleus, FABPs interact directly with PPARs and deliver ligands to PPARs, and the activity of PPARs is modulated [186, 187, 190–192]. Recently, Schug et al. showed that when the cellular retinoic acid binding protein-II (CRABP-II) expression levels were higher than FABP5 in the cells, retinoic acid (RA) bound to CRABP-II. Subsequently, CRABP-II relocated to the nucleus and delivered RA to RAR, resulting in inhibition of cell proliferation and induction of apoptosis [187, 193]. On the contrary, when the FABP5 to CRABP-II ratio is high, RA serves as a physiological ligand for PPARδ, which induces cell survival and proliferation [187, 194]. Therefore, it is important to identify the cytosolic ligand binding proteins and the expression levels of the proteins for defining the physiological effects of ligands. Furthermore, several ligands exert their biological effects through a PPAR-independent pathway [195]. Thus further studies are required to elucidate the role of PPARs for developing new efficiently and safety chemotherapeutic agents for cancer.
References
- 1.Mangelsdorf DJ, Thummel C, Beato M, et al. The nuclear receptor superfamily: the second decade. Cell. 1995;83(6):835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hsu M-H, Palmer CNA, Song W, Griffin KJ, Johnson EF. A carboxyl-terminal extension of the zinc finger domain contributes to the specificity and polarity of peroxisome proliferator-activated receptor DNA binding. Journal of Biological Chemistry. 1998;273(43):27988–27997. doi: 10.1074/jbc.273.43.27988. [DOI] [PubMed] [Google Scholar]
- 3.Gervois P, Chopin-Delannoy S, Fadel A, et al. Fibrates increase human REV-ERBα expression in liver via a novel peroxisome proliferator-activated receptor response element. Molecular Endocrinology. 1999;13(3):400–409. doi: 10.1210/mend.13.3.0248. [DOI] [PubMed] [Google Scholar]
- 4.Kliewer SA, Umesono K, Noonan DJ, Heyman RA, Evans RM. Convergence of 9-cis retinoic acid and peroxisome proliferator signalling pathways through heterodimer formation of their receptors. Nature. 1992;358(6389):771–774. doi: 10.1038/358771a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schoonjans K, Staels B, Auwerx J. Role of the peroxisome proliferator-activated receptor (PPAR) in mediating the effects of fibrates and fatty acids on gene expression. Journal of Lipid Research. 1996;37(5):907–925. [PubMed] [Google Scholar]
- 6.Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocrine Reviews. 1999;20(5):649–688. doi: 10.1210/edrv.20.5.0380. [DOI] [PubMed] [Google Scholar]
- 7.Willson TM, Brown PJ, Sternbach DD, Henke BR. The PPARs: from orphan receptors to drug discovery. Journal of Medicinal Chemistry. 2000;43(4):527–550. doi: 10.1021/jm990554g. [DOI] [PubMed] [Google Scholar]
- 8.Mandard S, Müller M, Kersten S. Peroxisome proliferator-activated receptor α target genes. Cellular and Molecular Life Sciences. 2004;61(4):393–416. doi: 10.1007/s00018-003-3216-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krey G, Braissant O, L'Horset F, et al. Fatty acids, eicosanoids, and hypolipidemic agents identified as ligands of peroxisome proliferator-activated receptors by coactivator-dependent receptor ligand assay. Molecular Endocrinology. 1997;11(6):779–791. doi: 10.1210/mend.11.6.0007. [DOI] [PubMed] [Google Scholar]
- 10.Issemann I, Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature. 1990;347(6294):645–650. doi: 10.1038/347645a0. [DOI] [PubMed] [Google Scholar]
- 11.Forman BM, Chen J, Evans RM. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors α and δ . Proceedings of the National Academy of Sciences of the United States of America. 1997;94(9):4312–4317. doi: 10.1073/pnas.94.9.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kliewer SA, Sundseth SS, Jones SA, et al. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors α and γ . Proceedings of the National Academy of Sciences of the United States of America. 1997;94(9):4318–4323. doi: 10.1073/pnas.94.9.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fruchart J-C. Peroxisome proliferator-activated receptor-α activation and high-density lipoprotein metabolism. The American Journal of Cardiology. 2001;88(12, supplement 1):24–29. doi: 10.1016/s0002-9149(01)02149-x. [DOI] [PubMed] [Google Scholar]
- 14.Staels B, Dallongeville J, Auwerx J, Schoonjans K, Leitersdorf E, Fruchart J-C. Mechanism of action of fibrates on lipid and lipoprotein metabolism. Circulation. 1998;98(19):2088–2093. doi: 10.1161/01.cir.98.19.2088. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y-X, Lee C-H, Tiep S, et al. Peroxisome-proliferator-activated receptor δ activates fat metabolism to prevent obesity. Cell. 2003;113(2):159–170. doi: 10.1016/s0092-8674(03)00269-1. [DOI] [PubMed] [Google Scholar]
- 16.Tan NS, Michalik L, Noy N, et al. Critical roles of PPARβ/δ in keratinocyte response to inflammation. Genes & Development. 2001;15(24):3263–3277. doi: 10.1101/gad.207501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dressel U, Allen TL, Pippal JB, Rohde PR, Lau P, Muscat GEO. The peroxisome proliferator-activated receptor β/δ agonist, GW501516, regulates the expression of genes involved in lipid catabolism and energy uncoupling in skeletal muscle cells. Molecular Endocrinology. 2003;17(12):2477–2493. doi: 10.1210/me.2003-0151. [DOI] [PubMed] [Google Scholar]
- 18.Chawla A, Lee C-H, Barak Y, et al. PPARδ is a very low-density lipoprotein sensor in macrophages. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(3):1268–1273. doi: 10.1073/pnas.0337331100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanaka T, Yamamoto J, Iwasaki S, et al. Activation of peroxisome proliferator-activated receptor δ induces fatty acid β-oxidation in skeletal muscle and attenuates metabolic syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(26):15924–15929. doi: 10.1073/pnas.0306981100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barish GD, Narkar VA, Evans RM. PPARδ: a dagger in the heart of the metabolic syndrome. Journal of Clinical Investigation. 2006;116(3):590–597. doi: 10.1172/JCI27955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takahashi S, Tanaka T, Kodama T, Sakai J. Peroxisome proliferator-activated receptor δ (PPARδ), a novel target site for drug discovery in metabolic syndrome. Pharmacological Research. 2006;53(6):501–507. doi: 10.1016/j.phrs.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 22.Tontonoz P, Hu E, Graves RA, Budavari AI, Spiegelman BM. mPPARγ2: tissue-specific regulator of an adipocyte enhancer. Genes & Development. 1994;8(10):1224–1234. doi: 10.1101/gad.8.10.1224. [DOI] [PubMed] [Google Scholar]
- 23.Fajas L, Auboeuf D, Raspé E, et al. The organization, promoter analysis, and expression of the human PPARγ gene. Journal of Biological Chemistry. 1997;272(30):18779–18789. doi: 10.1074/jbc.272.30.18779. [DOI] [PubMed] [Google Scholar]
- 24.Fajas L, Fruchart J-C, Auwerx J. PPARγ3 mRNA: a distinct PPARγ mRNA subtype transcribed from an independent promoter. FEBS Letters. 1998;438(1-2):55–60. doi: 10.1016/s0014-5793(98)01273-3. [DOI] [PubMed] [Google Scholar]
- 25.Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPARγ2, a lipid-activated transcription factor. Cell. 1994;79(7):1147–1156. doi: 10.1016/0092-8674(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 26.Lehrke M, Lazar MA. The many faces of PPARγ . Cell. 2005;123(6):993–999. doi: 10.1016/j.cell.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 27.Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor γ (PPARγ) Journal of Biological Chemistry. 1995;270(22):12953–12956. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- 28.Schoonjans K, Peinado-Onsurbe J, Lefebvre A-M, et al. PPARα and PPARγ activators direct a distinct tissue-specific transcriptional response via a PPRE in the lipoprotein lipase gene. The EMBO Journal. 1996;15(19):5336–5348. [PMC free article] [PubMed] [Google Scholar]
- 29.Staels B, Vu-Dac N, Kosykh VA, et al. Fibrates downregulate apolipoprotein C-III expression independent of induction of peroxisomal acyl coenzyme A oxidase. A potential mechanism for the hypolipidemic action of fibrates. Journal of Clinical Investigation. 1995;95(2):705–712. doi: 10.1172/JCI117717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tachibana K, Kobayashi Y, Tanaka T, et al. Gene expression profiling of potential peroxisome proliferator-activated receptor (PPAR) target genes in human hepatoblastoma cell lines inducibly expressing PPAR isoforms. Nuclear Receptor. 2005;3, article 3:1–17. doi: 10.1186/1478-1336-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vu-Dac N, Chopin-Delannoy S, Gervois P, et al. The nuclear receptors peroxisome proliferator-activated receptor α and rev-erbα mediate the species-specific regulation of apolipoprotein A-I expression by fibrates. Journal of Biological Chemistry. 1998;273(40):25713–25720. doi: 10.1074/jbc.273.40.25713. [DOI] [PubMed] [Google Scholar]
- 32.Vu-Dac N, Schoonjans K, Kosykh V, et al. Fibrates increase human apolipoprotein A-II expression through activation of the peroxisome proliferator-activated receptor. Journal of Clinical Investigation. 1995;96(2):741–750. doi: 10.1172/JCI118118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saha SA, Kizhakepunnur LG, Bahekar A, Arora RR. The role of fibrates in the prevention of cardiovascular disease—a pooled meta-analysis of long-term randomized placebo-controlled clinical trials. American Heart Journal. 2007;154(5):943–953. doi: 10.1016/j.ahj.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 34.Ashby J, Brady A, Elcombe CR, et al. Mechanistically-based human hazard assessment of peroxisome proliferator-induced hepatocarcinogenesis. Human & Experimental Toxicology. 1994;13(supplement 2):S1–S117. doi: 10.1177/096032719401300201. [DOI] [PubMed] [Google Scholar]
- 35.Hess R, Stäubli W, Riess W. Nature of the hepatomegalic effect produced by ethyl-chlorophenoxy-isobutyrate in the rat. Nature. 1965;208(5013):856–858. doi: 10.1038/208856a0. [DOI] [PubMed] [Google Scholar]
- 36.Reddy JK, Azarnoff DL, Hignite CE. Hypolipidaemic hepatic peroxisome proliferators form a novel class of chemical carcinogens. Nature. 1980;283(5745):397–398. doi: 10.1038/283397a0. [DOI] [PubMed] [Google Scholar]
- 37.Peters JM, Cattley RC, Gonzalez FJ. Role of PPARα in the mechanism of action of the nongenotoxic carcinogen and peroxisome proliferator Wy-14,643. Carcinogenesis. 1997;18(11):2029–2033. doi: 10.1093/carcin/18.11.2029. [DOI] [PubMed] [Google Scholar]
- 38.Goel SK, Lalwani ND, Reddy JK. Peroxisome proliferation and lipid peroxidation in rat liver. Cancer Research. 1986;46(3):1324–1330. [PubMed] [Google Scholar]
- 39.Ozasa H, Miyazawa S, Furuta S, Osumi T, Hashimoto T. Induction of peroxisomal β-oxidation enzymes in primary cultured rat hepatocytes by clofibric acid. Journal of Biochemistry. 1985;97(5):1273–1278. doi: 10.1093/oxfordjournals.jbchem.a135178. [DOI] [PubMed] [Google Scholar]
- 40.Reddy JK, Goel SK, Nemali MR, et al. Transcriptional regulation of peroxisomal fatty acyl-CoA oxidase and enoyl-CoA hydratase/3-hydroxyacyl-CoA dehydrogenase in rat liver by peroxisome proliferators. Proceedings of the National Academy of Sciences of the United States of America. 1986;83(6):1747–1751. doi: 10.1073/pnas.83.6.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Furuta S, Miyazawa S, Hashimoto T. Biosynthesis of enzymes of peroxisomal β-oxidation. Journal of Biochemistry. 1982;92(2):319–326. doi: 10.1093/oxfordjournals.jbchem.a133937. [DOI] [PubMed] [Google Scholar]
- 42.Osumi T, Wen J-K, Hashimoto T. Two cis-acting regulatory sequences in the peroxisome proliferator-responsive enhancer region of rat acyl-CoA oxidase gene. Biochemical and Biophysical Research Communications. 1991;175(3):866–871. doi: 10.1016/0006-291x(91)91645-s. [DOI] [PubMed] [Google Scholar]
- 43.Tugwood JD, Issemann I, Anderson RG, Bundell KR, McPheat WL, Green S. The mouse peroxisome proliferator activated receptor recognizes a response element in the 5′ flanking sequence of the rat acyl CoA oxidase gene. The EMBO Journal. 1992;11(2):433–439. doi: 10.1002/j.1460-2075.1992.tb05072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chu S, Huang Q, Alvares K, Yeldandi AV, Rao MS, Reddy JK. Transformation of mammalian cells by overexpressing H2O2-generating peroxisomal fatty acyl-CoA oxidase. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(15):7080–7084. doi: 10.1073/pnas.92.15.7080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yeldandi AV, Rao MS, Reddy JK. Hydrogen peroxide generation in peroxisome proliferator-induced oncogenesis. Mutation Research. 2000;448(2):159–177. doi: 10.1016/s0027-5107(99)00234-1. [DOI] [PubMed] [Google Scholar]
- 46.Henle ES, Linn S. Formation, prevention, and repair of DNA damage by iron/hydrogen peroxide. Journal of Biological Chemistry. 1997;272(31):19095–19098. doi: 10.1074/jbc.272.31.19095. [DOI] [PubMed] [Google Scholar]
- 47.Sekiguchi M, Tsuzuki T. Oxidative nucleotide damage: consequences and prevention. Oncogene. 2002;21(58):8895–8904. doi: 10.1038/sj.onc.1206023. [DOI] [PubMed] [Google Scholar]
- 48.Rao MS, Lalwani ND, Watanabe TK, Reddy JK. Inhibitory effect of antioxidants ethoxyquin and 2(3)-tert-butyl-4-hydroxyanisole on hepatic tumorigenesis in rats fed ciprofibrate, a peroxisome proliferator. Cancer Research. 1984;44(3):1072–1076. [PubMed] [Google Scholar]
- 49.Reddy JK, Rao MS, Moody DE. Hepatocellular carcinomas in acatalasemic mice treated with nafenopin, a hypolipidemic peroxisome proliferator. Cancer Research. 1976;36(4):1211–1217. [PubMed] [Google Scholar]
- 50.Bieri F, Bentley P, Waechter F, Stäubli W. Use of primary cultures of adult rat hepatocytes to investigate mechanisms of action of nafenopin, a hepatocarcinogenic peroxisome proliferator. Carcinogenesis. 1984;5(8):1033–1039. doi: 10.1093/carcin/5.8.1033. [DOI] [PubMed] [Google Scholar]
- 51.Peters JM, Aoyama T, Cattley RC, Nobumitsu U, Hashimoto T, Gonzalez FJ. Role of peroxisome proliferator-activated receptor α in altered cell cycle regulation in mouse liver. Carcinogenesis. 1998;19(11):1989–1994. doi: 10.1093/carcin/19.11.1989. [DOI] [PubMed] [Google Scholar]
- 52.Hays T, Rusyn I, Burns AM, et al. Role of peroxisome proliferator-activated receptor-α (PPARα) in bezafibrate-induced hepatocarcinogenesis and cholestasis. Carcinogenesis. 2005;26(1):219–227. doi: 10.1093/carcin/bgh285. [DOI] [PubMed] [Google Scholar]
- 53.Bayly AC, Roberts RA, Dive C. Suppression of liver cell apoptosis in vitro by the non-genotoxic hepatocarcinogen and peroxisome proliferator nafenopin. Journal of Cell Biology. 1994;125(1):197–203. doi: 10.1083/jcb.125.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bayly AC, French NJ, Dive C, Roberts RA. Non-genotoxic hepatocarcinogenesis in vitro: the FaO hepatoma line responds to peroxisome proliferators and retains the ability to undergo apoptosis. Journal of Cell Science. 1993;104(2):307–315. doi: 10.1242/jcs.104.2.307. [DOI] [PubMed] [Google Scholar]
- 55.Palmer CNA, Hsu M-H, Griffin KJ, Raucy JL, Johnson EF. Peroxisome proliferator activated receptor-α expression in human liver. Molecular Pharmacology. 1998;53(1):14–22. [PubMed] [Google Scholar]
- 56.IJpenberg A, Tan NS, Gelman L, et al. In vivo activation of PPAR target genes by RXR homodimers. The EMBO Journal. 2004;23(10):2083–2091. doi: 10.1038/sj.emboj.7600209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hertz R, Bishara-Shieban J, Bar-Tana J. Mode of action of peroxisome proliferators as hypolipidemic drugs. Suppression of apolipoprotein C-III. Journal of Biological Chemistry. 1995;270(22):13470–13475. doi: 10.1074/jbc.270.22.13470. [DOI] [PubMed] [Google Scholar]
- 58.Miyamoto T, Kaneko A, Kakizawa T, et al. Inhibition of peroxisome proliferator signaling pathways by thyroid hormone receptor. Competitive binding to the response element. Journal of Biological Chemistry. 1997;272(12):7752–7758. doi: 10.1074/jbc.272.12.7752. [DOI] [PubMed] [Google Scholar]
- 59.Miyata KS, Zhang B, Marcus SL, Capone JP, Rachubinski RA. Chicken ovalbumin upstream promoter transcription factor (COUP-TF) binds to a peroxisome proliferator-responsive element and antagonizes peroxisome proliferator-mediated signaling. Journal of Biological Chemistry. 1993;268(26):19169–19172. [PubMed] [Google Scholar]
- 60.Han K, Song H, Moon I, et al. Utilization of DR1 as true RARE in regulating the Ssm, a novel retinoic acid-target gene in the mouse testis. Journal of Endocrinology. 2007;192(3):539–551. doi: 10.1677/JOE-06-0115. [DOI] [PubMed] [Google Scholar]
- 61.Torra IP, Jamshidi Y, Flavell DM, Fruchart J-C, Staels B. Characterization of the human PPARα promoter: identification of a functional nuclear receptor response element. Molecular Endocrinology. 2002;16(5):1013–1028. doi: 10.1210/mend.16.5.0833. [DOI] [PubMed] [Google Scholar]
- 62.Nakshatri H, Bhat-Nakshatri P. Multiple parameters determine the specificity of transcriptional response by nuclear receptors HNF-4, ARP-1, PPAR, RAR and RXR through common response elements. Nucleic Acids Research. 1998;26(10):2491–2499. doi: 10.1093/nar/26.10.2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hsu M-H, Savas Ü, Griffin KJ, Johnson EF. Identification of peroxisome proliferator-responsive human genes by elevated expression of the peroxisome proliferator-activated receptor α in HepG2 cells. Journal of Biological Chemistry. 2001;276(30):27950–27958. doi: 10.1074/jbc.M100258200. [DOI] [PubMed] [Google Scholar]
- 64.Lawrence JW, Li Y, Chen S, et al. Differential gene regulation in human versus rodent hepatocytes by peroxisome proliferator-activated receptor (PPAR) α. PPARα fails to induce peroxisome proliferation-associated genes in human cells independently of the level of receptor expression. Journal of Biological Chemistry. 2001;276(34):31521–31527. doi: 10.1074/jbc.M103306200. [DOI] [PubMed] [Google Scholar]
- 65.Roberts RA, James NH, Woodyatt NJ, Macdonald N, Tugwood JD. Evidence for the suppression of apoptosis by the peroxisome proliferator activated receptor alpha (PPARα) Carcinogenesis. 1998;19(1):43–48. doi: 10.1093/carcin/19.1.43. [DOI] [PubMed] [Google Scholar]
- 66.Gervois P, Torra IP, Chinetti G, et al. A truncated human peroxisome proliferator-activated receptor α splice variant with dominant negative activity. Molecular Endocrinology. 1999;13(9):1535–1549. doi: 10.1210/mend.13.9.0341. [DOI] [PubMed] [Google Scholar]
- 67.Woodyatt NJ, Lambe KG, Myers KA, Tugwood JD, Roberts RA. The peroxisome proliferator (PP) response element upstream of the human acyl CoA oxidase gene is inactive among a sample human population: significance for species differences in response to PPs. Carcinogenesis. 1999;20(3):369–372. doi: 10.1093/carcin/20.3.369. [DOI] [PubMed] [Google Scholar]
- 68.Lambe KG, Woodyatt NJ, Macdonald N, Chevalier S, Roberts RA. Species differences in sequence and activity of the peroxisome proliferator response element (PPRE) within the acyl CoA oxidase gene promoter. Toxicology Letters. 1999;110(1-2):119–127. doi: 10.1016/s0378-4274(99)00151-4. [DOI] [PubMed] [Google Scholar]
- 69.Hasmall SC, James NH, Macdonald N, Soames AR, Roberts RA. Species differences in response to diethylhexylphthalate: suppression of apoptosis, induction of DNA synthesis and peroxisome proliferator activated receptor alpha-mediated gene expression. Archives of Toxicology. 2000;74(2):85–91. doi: 10.1007/s002040050657. [DOI] [PubMed] [Google Scholar]
- 70.Cheung C, Akiyama TE, Ward JM, et al. Diminished hepatocellular proliferation in mice humanized for the nuclear receptor peroxisome proliferator-activated receptor α . Cancer Research. 2004;64(11):3849–3854. doi: 10.1158/0008-5472.CAN-04-0322. [DOI] [PubMed] [Google Scholar]
- 71.Yang Q, Nagano T, Shah Y, Cheung C, Ito S, Gonzalez FJ. The PPARα-humanized mouse: a model to investigate species differences in liver toxicity mediated by PPARα . Toxicological Sciences. 2008;101(1):132–139. doi: 10.1093/toxsci/kfm206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gonzalez FJ, Shah YM. PPARα: mechanism of species differences and hepatocarcinogenesis of peroxisome proliferators. Toxicology. 2008;246(1):2–8. doi: 10.1016/j.tox.2007.09.030. [DOI] [PubMed] [Google Scholar]
- 73.Morimura K, Cheung C, Ward JM, Reddy JK, Gonzalez FJ. Differential susceptibility of mice humanized for peroxisome proliferator-activated receptor α to Wy-14,643-induced liver tumorigenesis. Carcinogenesis. 2006;27(5):1074–1080. doi: 10.1093/carcin/bgi329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shah YM, Morimura K, Yang Q, Tanabe T, Takagi M, Gonzalez FJ. Peroxisome proliferator-activated receptor α regulates a microRNA-mediated signaling cascade responsible for hepatocellular proliferation. Molecular and Cellular Biology. 2007;27(12):4238–4247. doi: 10.1128/MCB.00317-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Meister G, Tuschl T. Mechanisms of gene silencing by double-stranded RNA. Nature. 2004;431(7006):343–349. doi: 10.1038/nature02873. [DOI] [PubMed] [Google Scholar]
- 76.Zhang B, Pan X, Cobb GP, Anderson TA. microRNAs as oncogenes and tumor suppressors. Developmental Biology. 2007;302(1):1–12. doi: 10.1016/j.ydbio.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 77.He T-C, Chan TA, Vogelstein B, Kinzler KW. PPARδ is an APC-regulated target of nonsteroidal anti-inflammatory drugs. Cell. 1999;99(3):335–345. doi: 10.1016/s0092-8674(00)81664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gupta RA, Tan J, Krause WF, et al. Prostacyclin-mediated activation of peroxisome proliferator-activated receptor δ in colorectal cancer. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(24):13275–13280. doi: 10.1073/pnas.97.24.13275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Groden J, Thliveris A, Samowitz W, et al. Identification and characterization of the familial adenomatous polyposis coli gene. Cell. 1991;66(3):589–600. doi: 10.1016/0092-8674(81)90021-0. [DOI] [PubMed] [Google Scholar]
- 80.Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87(2):159–170. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- 81.Powell SM, Zilz N, Beazer-Barclay Y, et al. APC mutations occur early during colorectal tumorigenesis. Nature. 1992;359(6392):235–237. doi: 10.1038/359235a0. [DOI] [PubMed] [Google Scholar]
- 82.Kinzler KW, Nilbert MC, Su L-K, et al. Identification of FAP locus genes from chromosome 5q21. Science. 1991;253(5020):661–665. doi: 10.1126/science.1651562. [DOI] [PubMed] [Google Scholar]
- 83.Nishisho I, Nakamura Y, Miyoshi Y, et al. Mutations of chromosome 5q21 genes in FAP and colorectal cancer patients. Science. 1991;253(5020):665–669. doi: 10.1126/science.1651563. [DOI] [PubMed] [Google Scholar]
- 84.Polakis P. The many ways of Wnt in cancer. Current Opinion in Genetics & Development. 2007;17(1):45–51. doi: 10.1016/j.gde.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 85.Tetsu O, McCormick F. β-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398(6726):422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- 86.He T-C, Sparks AB, Rago C, et al. Identification of c-MYC as a target of the APC pathway. Science. 1998;281(5382):1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 87.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61(5):759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 88.Sebolt-Leopold JS, Herrera R. Targeting the mitogen-activated protein kinase cascade to treat cancer. Nature Reviews Cancer. 2004;4(12):937–947. doi: 10.1038/nrc1503. [DOI] [PubMed] [Google Scholar]
- 89.Shao J, Sheng H, DuBois RN. Peroxisome proliferator-activated receptors modulate K-Ras-mediated transformation of intestinal epithelial cells. Cancer Research. 2002;62(11):3282–3288. [PubMed] [Google Scholar]
- 90.Gupta RA, DuBois RN. Colorectal cancer prevention and treatment by inhibition of cyclooxygenase-2. Nature Reviews Cancer. 2001;1(1):11–21. doi: 10.1038/35094017. [DOI] [PubMed] [Google Scholar]
- 91.DeWitt DL. Cox-2-selective inhibitors: the new super aspirins. Molecular Pharmacology. 1999;55(4):625–631. [PubMed] [Google Scholar]
- 92.Subbaramaiah K, Dannenberg AJ. Cyclooxygenase 2: a molecular target for cancer prevention and treatment. Trends in Pharmacological Sciences. 2003;24(2):96–102. doi: 10.1016/S0165-6147(02)00043-3. [DOI] [PubMed] [Google Scholar]
- 93.Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Annual Review of Biochemistry. 2000;69:145–182. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- 94.Eberhart CE, Coffey RJ, Radhika A, Giardiello FM, Ferrenbach S, DuBois RN. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994;107(4):1183–1188. doi: 10.1016/0016-5085(94)90246-1. [DOI] [PubMed] [Google Scholar]
- 95.Oshima M, Dinchuk JE, Kargman SL, et al. Suppression of intestinal polyposis in A p c Δ716 knockout mice by inhibition of cyclooxygenase 2 (COX-2) Cell. 1996;87(5):803–809. doi: 10.1016/s0092-8674(00)81988-1. [DOI] [PubMed] [Google Scholar]
- 96.Pugh S, Thomas GAO. Patients with adenomatous polyps and carcinomas have increased colonic mucosal prostaglandin E2 . Gut. 1994;35(5):675–678. doi: 10.1136/gut.35.5.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sheng H, Shao J, Washington MK, DuBois RN. Prostaglandin E2 increases growth and motility of colorectal carcinoma cells. Journal of Biological Chemistry. 2001;276(21):18075–18081. doi: 10.1074/jbc.M009689200. [DOI] [PubMed] [Google Scholar]
- 98.Wang D, Wang H, Shi Q, et al. Prostaglandin E2 promotes colorectal adenoma growth via transactivation of the nuclear peroxisome proliferator-activated receptor δ . Cancer Cell. 2004;6(3):285–295. doi: 10.1016/j.ccr.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 99.Xu L, Han C, Wu T. A novel positive feedback loop between peroxisome proliferator-activated receptor-δ and prostaglandin E2 signaling pathways for human cholangiocarcinoma cell growth. Journal of Biological Chemistry. 2006;281(45):33982–33996. doi: 10.1074/jbc.M600135200. [DOI] [PubMed] [Google Scholar]
- 100.Park BH, Vogelstein B, Kinzler KW. Genetic disruption of PPARδ decreases the tumorigenicity of human colon cancer cells. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(5):2598–2603. doi: 10.1073/pnas.051630998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gupta RA, Wang D, Katkuri S, Wang H, Dey SK, DuBois RN. Activation of nuclear hormone receptor peroxisome proliferator-activated receptor-δ accelerates intestinal adenoma growth. Nature Medicine. 2004;10(3):245–247. doi: 10.1038/nm993. [DOI] [PubMed] [Google Scholar]
- 102.Wang D, Wang H, Guo Y, et al. Crosstalk between peroxisome proliferator-activated receptor δ and VEGF stimulates cancer progression. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(50):19069–19074. doi: 10.1073/pnas.0607948103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Piqueras L, Reynolds AR, Hodivala-Dilke KM, et al. Activation of PPARβ/δ induces endothelial cell proliferation and angiogenesis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2007;27(1):63–69. doi: 10.1161/01.ATV.0000250972.83623.61. [DOI] [PubMed] [Google Scholar]
- 104.Stephen RL, Gustafsson MCU, Jarvis M, et al. Activation of peroxisome proliferator-activated receptor δ stimulates the proliferation of human breast and prostate cancer cell lines. Cancer Research. 2004;64(9):3162–3170. doi: 10.1158/0008-5472.can-03-2760. [DOI] [PubMed] [Google Scholar]
- 105.Shureiqi I, Wojno KJ, Poore JA, et al. Decreased 13-S-hydroxyoctadecadienoic acid levels and 15-lipoxygenase-1 expression in human colon cancers. Carcinogenesis. 1999;20(10):1985–1995. doi: 10.1093/carcin/20.10.1985. [DOI] [PubMed] [Google Scholar]
- 106.Shureiqi I, Jiang W, Zuo X, et al. The 15-lipoxygenase-1 product 13-S-hydroxyoctadecadienoic acid down-regulates PPAR-δ to induce apoptosis in colorectal cancer cells. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(17):9968–9973. doi: 10.1073/pnas.1631086100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shureiqi I, Chen D, Lee JJ, et al. 15-LOX-1: a novel molecular target of nonsteroidal anti-inflammatory drug-induced apoptosis in colorectal cancer cells. Journal of the National Cancer Institute. 2000;92(14):1136–1142. doi: 10.1093/jnci/92.14.1136. [DOI] [PubMed] [Google Scholar]
- 108.Reed KR, Sansom OJ, Hayes AJ, et al. PPARδ status and Apc-mediated tumourigenesis in the mouse intestine. Oncogene. 2004;23(55):8992–8996. doi: 10.1038/sj.onc.1208143. [DOI] [PubMed] [Google Scholar]
- 109.Marin HE, Peraza MA, Billin AN, et al. Ligand activation of peroxisome proliferator-activated receptor β inhibits colon carcinogenesis. Cancer Research. 2006;66(8):4394–4401. doi: 10.1158/0008-5472.CAN-05-4277. [DOI] [PubMed] [Google Scholar]
- 110.Di-Poï N, Desvergne B, Michalik L, Wahli W. Transcriptional repression of peroxisome proliferator-activated receptor β/δ in murine keratinocytes by CCAAT/enhancer-binding proteins. Journal of Biological Chemistry. 2005;280(46):38700–38710. doi: 10.1074/jbc.M507782200. [DOI] [PubMed] [Google Scholar]
- 111.Dunlop TW, Väisänen S, Frank C, Molnár F, Sinkkonen L, Carlberg C. The human peroxisome proliferator-activated receptor δ gene is a primary target of 1α,25-dihydroxyvitamin D3 and its nuclear receptor. Journal of Molecular Biology. 2005;349(2):248–260. doi: 10.1016/j.jmb.2005.03.060. [DOI] [PubMed] [Google Scholar]
- 112.Hollingshead HE, Killins RL, Borland MG, et al. Peroxisome proliferator-activated receptor-β/δ (PPARβ/δ) ligands do not potentiate growth of human cancer cell lines. Carcinogenesis. 2007;28(12):2641–2649. doi: 10.1093/carcin/bgm183. [DOI] [PubMed] [Google Scholar]
- 113.Barak Y, Liao D, He W, et al. Effects of peroxisome proliferator-activated receptor δ on placentation, adiposity, and colorectal cancer. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(1):303–308. doi: 10.1073/pnas.012610299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Harman FS, Nicol CJ, Marin HE, Ward JM, Gonzalez FJ, Peters JM. Peroxisome proliferator-activated receptor-δ attenuates colon carcinogenesis. Nature Medicine. 2004;10(5):481–483. doi: 10.1038/nm1026. [DOI] [PubMed] [Google Scholar]
- 115.Kim DJ, Bility MT, Billin AN, Willson TM, Gonzalez FJ, Peters JM. PPARβ/δ selectively induces differentiation and inhibits cell proliferation. Cell Death & Differentiation. 2006;13(1):53–60. doi: 10.1038/sj.cdd.4401713. [DOI] [PubMed] [Google Scholar]
- 116.Schmuth M, Haqq CM, Cairns WJ, et al. Peroxisome proliferator-activated receptor (PPAR)-β/δ stimulates differentiation and lipid accumulation in keratinocytes. Journal of Investigative Dermatology. 2004;122(4):971–983. doi: 10.1111/j.0022-202X.2004.22412.x. [DOI] [PubMed] [Google Scholar]
- 117.Hatae T, Wada M, Yokoyama C, Shimonishi M, Tanabe T. Prostacyclin-dependent apoptosis mediated by PPARδ . Journal of Biological Chemistry. 2001;276(49):46260–46267. doi: 10.1074/jbc.M107180200. [DOI] [PubMed] [Google Scholar]
- 118.Di-Poï N, Tan NS, Michalik L, Wahli W, Desvergne B. Antiapoptotic role of PPARβ in keratinocytes via transcriptional control of the Akt1 signaling pathway. Molecular Cell. 2002;10(4):721–733. doi: 10.1016/s1097-2765(02)00646-9. [DOI] [PubMed] [Google Scholar]
- 119.Yin Y, Russell RG, Dettin LE, et al. Peroxisome proliferator-activated receptor δ and γ agonists differentially alter tumor differentiation and progression during mammary carcinogenesis. Cancer Research. 2005;65(9):3950–3957. doi: 10.1158/0008-5472.CAN-04-3990. [DOI] [PubMed] [Google Scholar]
- 120.Burdick AD, Bility MT, Girroir EE, et al. Ligand activation of peroxisome proliferator-activated receptor-β/δ (PPARβ/δ) inhibits cell growth of human N/TERT-1 keratinocytes. Cellular Signalling. 2007;19(6):1163–1171. doi: 10.1016/j.cellsig.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Girroir EE, Hollingshead HE, Billin AN, et al. Peroxisome proliferator-activated receptor-β/δ (PPARβ/δ) ligands inhibit growth of UACC903 and MCF7 human cancer cell lines. Toxicology. 2008;243(1-2):236–243. doi: 10.1016/j.tox.2007.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Krogsdam A-M, Nielsen CAF, Neve S, et al. Nuclear receptor corepressor-dependent repression of peroxisome-proliferator-activated receptor δ-mediated transactivation. Biochemical Journal. 2002;363(1):157–165. doi: 10.1042/0264-6021:3630157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shi Y, Hon M, Evans RM. The peroxisome proliferator-activated receptor δ, an integrator of transcriptional repression and nuclear receptor signaling. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(5):2613–2618. doi: 10.1073/pnas.052707099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lee C-H, Chawla A, Urbiztondo N, Liao D, Boisvert WA, Evans RM. Transcriptional repression of atherogenic inflammation: modulation by PPARδ . Science. 2003;302(5644):453–457. doi: 10.1126/science.1087344. [DOI] [PubMed] [Google Scholar]
- 125.Fauti T, Müller-Brüsselbach S, Kreutzer M, et al. Induction of PPARβ and prostacyclin (PGI2) synthesis by Raf signaling: failure of PGI2 to activate PPARβ . FEBS Journal. 2006;273(1):170–179. doi: 10.1111/j.1742-4658.2005.05055.x. [DOI] [PubMed] [Google Scholar]
- 126.Schwartz GK, Shah MA. Targeting the cell cycle: a new approach to cancer therapy. Journal of Clinical Oncology. 2005;23(36):9408–9421. doi: 10.1200/JCO.2005.01.5594. [DOI] [PubMed] [Google Scholar]
- 127.Bremer E, van Dam G, Kroesen BJ, de Leij L, Helfrich W. Targeted induction of apoptosis for cancer therapy: current progress and prospects. Trends in Molecular Medicine. 2006;12(8):382–393. doi: 10.1016/j.molmed.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 128.Grommes C, Landreth GE, Heneka MT. Antineoplastic effects of peroxisome proliferator-activated receptor γ agonists. The Lancet Oncology. 2004;5(7):419–429. doi: 10.1016/S1470-2045(04)01509-8. [DOI] [PubMed] [Google Scholar]
- 129.Theocharis S, Margeli A, Vielh P, Kouraklis G. Peroxisome proliferator-activated receptor-γ ligands as cell-cycle modulators. Cancer Treatment Reviews. 2004;30(6):545–554. doi: 10.1016/j.ctrv.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 130.Brockman JA, Gupta RA, DuBois RN. Activation of PPARγ leads to inhibition of anchorage-independent growth of human colorectal cancer cells. Gastroenterology. 1998;115(5):1049–1055. doi: 10.1016/s0016-5085(98)70072-1. [DOI] [PubMed] [Google Scholar]
- 131.Osawa E, Nakajima A, Wada K, et al. Peroxisome proliferator-activated receptor γ ligands suppress colon carcinogenesis induced by azoxymethane in mice. Gastroenterology. 2003;124(2):361–367. doi: 10.1053/gast.2003.50067. [DOI] [PubMed] [Google Scholar]
- 132.Shimada T, Kojima K, Yoshiura K, Hiraishi H, Terano A. Characteristics of the peroxisome proliferator activated receptor γ (PPARγ) ligand induced apoptosis in colon cancer cells. Gut. 2002;50(5):658–664. doi: 10.1136/gut.50.5.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chen GG, Lee JFY, Wang SH, Chan UPF, Ip PC, Lau WY. Apoptosis induced by activation of peroxisome-proliferator activated receptor-gamma is associated with Bcl-2 and Nf-κB in human colon cancer. Life Sciences. 2002;70(22):2631–2646. doi: 10.1016/s0024-3205(02)01510-2. [DOI] [PubMed] [Google Scholar]
- 134.Sarraf P, Mueller E, Smith WM, et al. Loss-of-function mutations in PPARγ associated with human colon cancer. Molecular Cell. 1999;3(6):799–804. doi: 10.1016/s1097-2765(01)80012-5. [DOI] [PubMed] [Google Scholar]
- 135.Sarraf P, Mueller E, Jones D, et al. Differentiation and reversal of malignant changes in colon cancer through PPARγ . Nature Medicine. 1998;4(9):1046–1052. doi: 10.1038/2030. [DOI] [PubMed] [Google Scholar]
- 136.Kitamura S, Miyazaki Y, Hiraoka S, et al. PPARγ agonists inhibit cell growth and suppress the expression of cyclin D1 and EGF-like growth factors in ras-transformed rat intestinal epithelial cells. International Journal of Cancer. 2001;94(3):335–342. doi: 10.1002/ijc.1470. [DOI] [PubMed] [Google Scholar]
- 137.Mueller E, Sarraf P, Tontonoz P, et al. Terminal differentiation of human breast cancer through PPARγ . Molecular Cell. 1998;1(3):465–470. doi: 10.1016/s1097-2765(00)80047-7. [DOI] [PubMed] [Google Scholar]
- 138.Rumi MAK, Sato H, Ishihara S, Ortega C, Kadowaki Y, Kinoshita Y. Growth inhibition of esophageal squamous carcinoma cells by peroxisome proliferator-activated receptor-γ ligands. Journal of Laboratory and Clinical Medicine. 2002;140(1):17–26. doi: 10.1067/mlc.2002.125055. [DOI] [PubMed] [Google Scholar]
- 139.Sato H, Ishihara S, Kawashima K, et al. Expression of peroxisome proliferator-activated receptor (PPAR)γ in gastric cancer and inhibitory effects of PPARγ agonists. British Journal of Cancer. 2000;83(10):1394–1400. doi: 10.1054/bjoc.2000.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Motomura W, Okumura T, Takahashi N, Obara T, Kohgo Y. Activation of peroxisome proliferator-activated receptor γ by troglitazone inhibits cell growth through the increase of p27Kip1 in human pancreatic carcinoma cells. Cancer Research. 2000;60(19):5558–5564. [PubMed] [Google Scholar]
- 141.Koga H, Sakisaka S, Harada M, et al. Involvement of p21WAF1/Cip1, p27Kip1, and p18INK4c in troglitazone-induced cell-cycle arrest in human hepatoma cell lines. Hepatology. 2001;33(5):1087–1097. doi: 10.1053/jhep.2001.24024. [DOI] [PubMed] [Google Scholar]
- 142.Ferruzzi P, Ceni E, Tarocchi M, et al. Thiazolidinediones inhibit growth and invasiveness of the human adrenocortical cancer cell line H295R. Journal of Clinical Endocrinology & Metabolism. 2005;90(3):1332–1339. doi: 10.1210/jc.2004-0978. [DOI] [PubMed] [Google Scholar]
- 143.Chang T-H, Szabo E. Induction of differentiation and apoptosis by ligands of peroxisome proliferator-activated receptor γ in non-small cell lung cancer. Cancer Research. 2000;60(4):1129–1138. [PubMed] [Google Scholar]
- 144.Mueller E, Smith M, Sarraf P, et al. Effects of ligand activation of peroxisome proliferator-activated receptor γ in human prostate cancer. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(20):10990–10995. doi: 10.1073/pnas.180329197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tontonoz P, Singer S, Forman BM, et al. Terminal differentiation of human liposarcoma cells induced by ligands for peroxisome proliferator-activated receptor γ and the retinoid X receptor. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(1):237–241. doi: 10.1073/pnas.94.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ohta K, Endo T, Haraguchi K, Hershman JM, Onaya T. Ligands for peroxisome proliferator-activated receptor γ inhibit growth and induce apoptosis of human papillary thyroid carcinoma cells. The Journal of Clinical Endocrinology & Metabolism. 2001;86(5):2170–2177. doi: 10.1210/jcem.86.5.7493. [DOI] [PubMed] [Google Scholar]
- 147.Guan Y-F, Zhang Y-H, Breyer RM, Davis L, Breyer MD. Expression of peroxisome proliferator-activated receptor γ (PPARγ) in human transitional bladder cancer and its role in inducing cell death. Neoplasia. 1999;1(4):330–339. doi: 10.1038/sj.neo.7900050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Inoue K, Kawahito Y, Tsubouchi Y, et al. Expression of peroxisome proliferator-activated receptor γ in renal cell carcinoma and growth inhibition by its agonists. Biochemical and Biophysical Research Communications. 2001;287(3):727–732. doi: 10.1006/bbrc.2001.5640. [DOI] [PubMed] [Google Scholar]
- 149.Mössner R, Schulz U, Krüger U, et al. Agonists of peroxisome proliferator-activated receptor γ inhibit cell growth in malignant melanoma. Journal of Investigative Dermatology. 2002;119(3):576–582. doi: 10.1046/j.1523-1747.2002.01861.x. [DOI] [PubMed] [Google Scholar]
- 150.Nijsten T, Geluyckens E, Colpaert C, Lambert J. Peroxisome proliterator-activated receptors in squamous cell carcinoma and its precursors. Journal of Cutaneous Pathology. 2005;32(5):340–347. doi: 10.1111/j.0303-6987.2005.00345.x. [DOI] [PubMed] [Google Scholar]
- 151.Jung T-I, Baek W-K, Suh S-I, et al. Down-regulation of peroxisome proliferator-activated receptor gamma in human cervical carcinoma. Gynecologic Oncology. 2005;97(2):365–373. doi: 10.1016/j.ygyno.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 152.Hase T, Yoshimura R, Mitsuhashi M, et al. Expression of peroxisome proliferator-activated receptors in human testicular cancer and growth inhibition by its agonists. Urology. 2002;60(3):542–547. doi: 10.1016/s0090-4295(02)01747-8. [DOI] [PubMed] [Google Scholar]
- 153.Han SW, Greene ME, Pitts J, Wada RK, Sidell N. Novel expression and function of peroxisome proliferator-activated receptor gamma (PPARγ) in human neuroblastoma cells. Clinical Cancer Research. 2001;7(1):98–104. [PubMed] [Google Scholar]
- 154.Heaney AP, Fernando M, Yong WH, Melmed S. Functional PPAR-γ receptor is a novel therapeutic target for ACTH-secreting pituitary adenomas. Nature Medicine. 2002;8(11):1281–1287. doi: 10.1038/nm784. [DOI] [PubMed] [Google Scholar]
- 155.Kim J-A, Park K-S, Kim H-I, et al. Troglitazone activates p21Cip/WAF1 through the ERK pathway in HCT15 human colorectal cancer cells. Cancer Letters. 2002;179(2):185–195. doi: 10.1016/s0304-3835(01)00869-2. [DOI] [PubMed] [Google Scholar]
- 156.Yang W-L, Frucht H. Activation of the PPAR pathway induces apoptosis and COX-2 inhibition in HT-29 human colon cancer cells. Carcinogenesis. 2001;22(9):1379–1383. doi: 10.1093/carcin/22.9.1379. [DOI] [PubMed] [Google Scholar]
- 157.Tanaka T, Kohno H, Yoshitani S, et al. Ligands for peroxisome proliferator-activated receptors α and γ inhibit chemically induced colitis and formation of aberrant crypt foci in rats. Cancer Research. 2001;61(6):2424–2428. [PubMed] [Google Scholar]
- 158.Yin F, Wakino S, Liu Z, et al. Troglitazone inhibits growth of MCF-7 breast carcinoma cells by targeting G1 cell cycle regulators. Biochemical and Biophysical Research Communications. 2001;286(5):916–922. doi: 10.1006/bbrc.2001.5491. [DOI] [PubMed] [Google Scholar]
- 159.Suh N, Wang Y, Williams CR, et al. A new ligand for the peroxisome proliferator-activated receptor-γ (PPAR-γ), GW7845, inhibits rat mammary carcinogenesis. Cancer Research. 1999;59(22):5671–5673. [PubMed] [Google Scholar]
- 160.Clay CE, Namen AM, Atsumi G, et al. Influence of J series prostaglandins on apoptosis and tumorigenesis of breast cancer cells. Carcinogenesis. 1999;20(10):1905–1911. doi: 10.1093/carcin/20.10.1905. [DOI] [PubMed] [Google Scholar]
- 161.Mehta RG, Williamson E, Patel MK, Koeffler HP. A ligand of peroxisome proliferator-activated receptor γ, retinoids, and prevention of preneoplastic mammary lesions. Journal of the National Cancer Institute. 2000;92(5):418–423. doi: 10.1093/jnci/92.5.418. [DOI] [PubMed] [Google Scholar]
- 162.Elstner E, Müller C, Koshizuka K, et al. Ligands for peroxisome proliferator-activated receptorγ and retinoic acid receptor inhibit growth and induce apoptosis of human breast cancer cells in vitro and in BNX mice. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(15):8806–8811. doi: 10.1073/pnas.95.15.8806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Giacinti C, Giordano A. RB and cell cycle progression. Oncogene. 2006;25(38):5220–5227. doi: 10.1038/sj.onc.1209615. [DOI] [PubMed] [Google Scholar]
- 164.Takahashi N, Okumura T, Motomura W, Fujimoto Y, Kawabata I, Kohgo Y. Activation of PPARγ inhibits cell growth and induces apoptosis in human gastric cancer cells. FEBS Letters. 1999;455(1-2):135–139. doi: 10.1016/s0014-5793(99)00871-6. [DOI] [PubMed] [Google Scholar]
- 165.Eibl G, Wente MN, Reber HA, Hines OJ. Peroxisome proliferator-activated receptor γ induces pancreatic cancer cell apoptosis. Biochemical and Biophysical Research Communications. 2001;287(2):522–529. doi: 10.1006/bbrc.2001.5619. [DOI] [PubMed] [Google Scholar]
- 166.Itami A, Watanabe G, Shimada Y, et al. Ligands for peroxisome proliferator-activated receptor γ inhibit growth of pancreatic cancers both in vitro and in vivo. International Journal of Cancer. 2001;94(3):370–376. doi: 10.1002/ijc.1488. [DOI] [PubMed] [Google Scholar]
- 167.Toyota M, Miyazaki Y, Kitamura S, et al. Peroxisome proliferator-activated receptor γ reduces the growth rate of pancreatic cancer cells through the reduction of cyclin D1. Life Sciences. 2002;70(13):1565–1575. doi: 10.1016/s0024-3205(01)01524-7. [DOI] [PubMed] [Google Scholar]
- 168.Rumi MAK, Sato H, Ishihara S, et al. Peroxisome proliferator-activated receptor γ ligand-induced growth inhibition of human hepatocellular carcinoma. British Journal of Cancer. 2001;84(12):1640–1647. doi: 10.1054/bjoc.2001.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Toyoda M, Takagi H, Horiguchi N, et al. A ligand for peroxisome proliferator activated receptor γ inhibits cell growth and induces apoptosis in human liver cancer cells. Gut. 2002;50(4):563–567. doi: 10.1136/gut.50.4.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Betz MJ, Shapiro I, Fassnacht M, Hahner S, Reincke M, Beuschlein F. Peroxisome proliferator-activated receptor-γ agonists suppress adrenocortical tumor cell proliferation and induce differentiation. Journal of Clinical Endocrinology & Metabolism. 2005;90(7):3886–3896. doi: 10.1210/jc.2004-1267. [DOI] [PubMed] [Google Scholar]
- 171.Tsubouchi Y, Sano H, Kawahito Y, et al. Inhibition of human lung cancer cell growth by the peroxisome proliferator-activated receptor-γ agonists through induction of apoptosis. Biochemical and Biophysical Research Communications. 2000;270(2):400–405. doi: 10.1006/bbrc.2000.2436. [DOI] [PubMed] [Google Scholar]
- 172.Hisatake J, Ikezoe T, Carey M, Holden S, Tomoyasu S, Koeffler HP. Down-regulation of prostate-specific antigen expression by ligands for peroxisome proliferator-activated receptor γ in human prostate cancer. Cancer Research. 2000;60(19):5494–5498. [PubMed] [Google Scholar]
- 173.Kubota T, Koshizuka K, Williamson EA, et al. Ligand for peroxisome proliferator-activated receptor γ (troglitazone) has potent antitumor effect against human prostate cancer both in vitro and in vivo. Cancer Research. 1998;58(15):3344–3352. [PubMed] [Google Scholar]
- 174.Lefebvre A-M, Chen I, Desreumaux P, et al. Activation of the peroxisome proliferator-activated receptor γ promotes the development of colon tumors in C57BL/6J-APCMin/+ mice. Nature Medicine. 1998;4(9):1053–1057. doi: 10.1038/2036. [DOI] [PubMed] [Google Scholar]
- 175.Saez E, Tontonoz P, Nelson MC, et al. Activators of the nuclear receptor PPARγ enhance colon polyp formation. Nature Medicine. 1998;4(9):1058–1061. doi: 10.1038/2042. [DOI] [PubMed] [Google Scholar]
- 176.Girnun GD, Smith WM, Drori S, et al. APC-dependent suppression of colon carcinogenesis by PPARγ . Proceedings of the National Academy of Sciences of the United States of America. 2002;99(21):13771–13776. doi: 10.1073/pnas.162480299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Saez E, Rosenfeld J, Livolsi A, et al. PPARγ signaling exacerbates mammary gland tumor development. Genes & Development. 2004;18(5):528–540. doi: 10.1101/gad.1167804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Palakurthi SS, Aktas H, Grubissich LM, Mortensen RM, Halperin JA. Anticancer effects of thiazolidinediones are independent of peroxisome proliferator-activated receptor γ and mediated by inhibition of translation initiation. Cancer Research. 2001;61(16):6213–6218. [PubMed] [Google Scholar]
- 179.Sertznig P, Seifert M, Tilgen W, Reichrath J. Present concepts and future outlook: function of peroxisome proliferator-activated receptors (PPARs) for pathogenesis, progression, and therapy of cancer. Journal of Cellular Physiology. 2007;212(1):1–12. doi: 10.1002/jcp.20998. [DOI] [PubMed] [Google Scholar]
- 180.Olefsky JM. Treatment of insulin resistance with peroxisome proliferator-activated receptor γ agonists. Journal of Clinical Investigation. 2000;106(4):467–472. doi: 10.1172/JCI10843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sporn MB, Suh N, Mangelsdorf DJ. Prospects for prevention and treatment of cancer with selective PPARγ modulators (SPARMs) Trends in Molecular Medicine. 2001;7(9):395–400. doi: 10.1016/s1471-4914(01)02100-1. [DOI] [PubMed] [Google Scholar]
- 182.Ockner RK, Manning JA, Poppenhausen RB, Ho WKL. A binding protein for fatty acids in cytosol of intestinal mucosa, liver, myocardium, and other tissues. Science. 1972;177(4043):56–58. doi: 10.1126/science.177.4043.56. [DOI] [PubMed] [Google Scholar]
- 183.Cannon JR, Eacho PI. Interaction of LY171883 and other peroxisome proliferators with fatty-acid-binding protein isolated from rat liver. Biochemical Journal. 1991;280(2):387–391. doi: 10.1042/bj2800387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Huang H, Starodub O, McIntosh A, et al. Liver fatty acid-binding protein colocalizes with peroxisome proliferator activated receptor α and enhances ligand distribution to nuclei of living cells. Biochemistry. 2004;43(9):2484–2500. doi: 10.1021/bi0352318. [DOI] [PubMed] [Google Scholar]
- 185.Wolfrum C, Börchers T, Sacchettini JC, Spener F. Binding of fatty acids and peroxisome proliferators to orthologous fatty acid binding proteins from human, murine, and bovine liver. Biochemistry. 2000;39(6):1469–1474. doi: 10.1021/bi991638u. [DOI] [PubMed] [Google Scholar]
- 186.Adida A, Spener F. Adipocyte-type fatty acid-binding protein as inter-compartmental shuttle for peroxisome proliferator activated receptor γ agonists in cultured cell. Biochimica et Biophysica Acta. 2006;1761(2):172–181. doi: 10.1016/j.bbalip.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 187.Schug TT, Berry DC, Shaw NS, Travis SN, Noy N. Opposing effects of retinoic acid on cell growth result from alternate activation of two different nuclear receptors. Cell. 2007;129(4):723–733. doi: 10.1016/j.cell.2007.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Huang H, Starodub O, McIntosh A, Kier AB, Schroeder F. Liver fatty acid-binding protein targets fatty acids to the nucleus. Real time confocal and multiphoton fluorescence imaging in living cells. Journal of Biological Chemistry. 2002;277(32):29139–29151. doi: 10.1074/jbc.M202923200. [DOI] [PubMed] [Google Scholar]
- 189.Lawrence JW, Kroll DJ, Eacho PI. Ligand-dependent interaction of hepatic fatty acid-binding protein with the nucleus. Journal of Lipid Research. 2000;41(9):1390–1401. [PubMed] [Google Scholar]
- 190.Tan N-S, Shaw NS, Vinckenbosch N, et al. Selective cooperation between fatty acid binding proteins and peroxisome proliferator-activated receptors in regulating transcription. Molecular and Cellular Biology. 2002;22(14):5114–5127. doi: 10.1128/MCB.22.14.5114-5127.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wolfrum C, Borrmann CM, Börchers T, Spener F. Fatty acids and hypolipidemic drugs regulate peroxisome proliferator-activated receptors α- and γ-mediated gene expression via liver fatty acid binding protein: a signaling path to the nucleus. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(5):2323–2328. doi: 10.1073/pnas.051619898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Helledie T, Antonius M, Sørensen RV, et al. Lipid-binding proteins modulate ligand-dependent trans-activation by peroxisome proliferator-activated receptors and localize to the nucleus as well as the cytoplasm. Journal of Lipid Research. 2000;41(11):1740–1751. [PubMed] [Google Scholar]
- 193.Dong D, Ruuska SE, Levinthal DJ, Noy N. Distinct roles for cellular retinoic acid-binding proteins I and II in regulating signaling by retinoic acid. Journal of Biological Chemistry. 1999;274(34):23695–23698. doi: 10.1074/jbc.274.34.23695. [DOI] [PubMed] [Google Scholar]
- 194.Shaw N, Elholm M, Noy N. Retinoic acid is a high affinity selective ligand for the peroxisome proliferator-activated receptor β/δ . Journal of Biological Chemistry. 2003;278(43):41589–41592. doi: 10.1074/jbc.C300368200. [DOI] [PubMed] [Google Scholar]
- 195.Peraza MA, Burdick AD, Marin HE, Gonzalez FJ, Peters JM. The toxicology of ligands for peroxisome proliferator-activated receptors (PPAR) Toxicological Sciences. 2006;90(2):269–295. doi: 10.1093/toxsci/kfj062. [DOI] [PubMed] [Google Scholar]