Abstract
Microglia and astrocytes express numerous members of the Toll-like receptor (TLR) family that are pivotal for recognizing conserved microbial motifs expressed by a wide array of pathogens. Despite the critical role for TLRs in pathogen recognition, when dysregulated these pathways can also exacerbate CNS tissue destruction. Therefore, a critical balance must be achieved to elicit sufficient immunity to combat CNS infectious insults and downregulate these responses to avoid pathological tissue damage. We performed a comprehensive survey on the efficacy of various PPAR-γ agonists to modulate proinflammatory mediator release from primary microglia and astrocytes in response to numerous TLR ligands relevant to CNS infectious diseases. The results demonstrated differential abilities of select PPAR-γ agonists to modulate glial activation. For example, 15d-PGJ2 and pioglitazone were both effective at reducing IL-12 p40 release by TLR ligand-activated glia, whereas CXCL2 expression was either augmented or inhibited by 15d-PGJ2, effects that were dependent on the TLR ligand examined. Pioglitazone and troglitazone demonstrated opposing actions on microglial CCL2 production that were TLR ligand-dependent. Collectively, this information may be exploited to modulate the host immune response during CNS infections to maximize host immunity while minimizing inappropriate bystander tissue damage that is often characteristic of such diseases.
1. INTRODUCTION
Microglia and astrocytes participate in the genesis of innate immune responses in the CNS parenchyma [1, 2]. Their strategic placement at or near the blood-brain barrier likely makes both glial types sentinel cells for surveying pathogen entry in the CNS parenchyma. Indeed, both microglia and astrocytes are capable of producing a wide range of proinflammatory mediators in response to a diverse array of microbial stimuli [3, 4]. Therefore, it is likely that resident glia are pivotal for eliciting a local CNS inflammatory response through the initial production of inflammatory mediators, which in turn, leads to the recruitment of additional immune effector cells from the peripheral circulation.
Toll-like receptors (TLRs) are a group of pattern recognition receptors (PRRs) responsible for recognizing conserved motifs expressed on broad classes of microbes termed pathogen-associated molecular patterns (PAMPs) [5, 6]. Typically, PAMPs represent structural or nucleic acid motifs that are less likely to undergo mutation, ensuring efficient pathogen recognition with a limited receptor arsenal [7]. A total of 13 TLRs have been identified to date, each conferring recognition of conserved motifs from large subclasses of bacteria, viruses, yeast, and fungi [5–7]. In addition, recent evidence indicates that TLRs are also capable of sensing endogenous ligands produced during stress or injury referred to as danger-associated molecular patterns (DAMPs) that may participate in autoimmune induction [8–10]. Numerous TLRs have been identified on microglia and astrocytes and both glial types are responsive to numerous TLR ligands implicating their role in pathogen recognition (reviewed in [11, 12]). In addition, recent evidence links TLRs with the host response to CNS injury presumably via recognition of endogenous “danger signals” since classical microbial TLR ligands are not present (reviewed in [13–15]). Since TLRs have been implicated in both infectious and noninfectious diseases of the CNS (reviewed in [11, 13]), understanding their potential to influence the course of neuroinflammation is paramount and under certain conditions inappropriate TLR activation may contribute to excessive tissue destruction. Therefore, modulating TLR activity to achieve optimal benefit for the host may be a plausible strategy for minimizing tissue damage during neuroinflammatory disorders.
A group of compounds with reported anti-inflammatory effects in several models of inflammation, including the CNS, are ligands that interact with peroxisome proliferator-activated receptor-gamma (PPAR-γ) [16–18]. PPAR-γ is a member of the nuclear hormone receptor superfamily of ligand-activated transcription factors that regulate the expression of genes involved in reproduction, metabolism, development, and immune responses [17, 19]. A wide array of both natural and synthetic agonists for PPAR-γ have been identified including the naturally occurring prostaglandin metabolite 15d-PGJ2, thiazolidinediones (TZDs) a group of synthetic PPAR-γ agonists used for the treatment of diabetes, polyunsaturated fatty acids, and certain high affinity tyrosine derivatives. With regard to the CNS, several PPAR-γ agonists have been documented for their ability to attenuate both microglial and astrocyte activation in response to a diverse array of stimuli as well as impact the course of several neurodegenerative diseases [16, 18, 20–25]. Although we and others have demonstrated that select PPAR-γ agonists are potent inhibitors of TLR2 and TLR4 activation (PGN and LPS, resp.) [23, 24, 26–29], a comprehensive examination of the effects of PPAR-γ agonists on a wide array of TLR ligands is lacking. In addition, although several studies describing the responses of microglia and astrocytes to TLR ligands exist [30–34], no reports have systematically investigated the ability of PPAR-γ ligands to modulate glial activation in response to TLR signals. Therefore, the purpose of this study was to define the actions of a panel of PPAR-γ agonists on TLR ligand-induced activation of microglia and astrocytes. Although within the same class of compounds, not all PPAR-γ agonists shared similar regulatory properties in response to various TLR ligands. Indeed, in some cases, inflammatory mediator production was enhanced following PPAR-γ agonist treatment. Collectively, these results suggest selective actions of PPAR-γ agonists on glial responses to TLR ligands that could be exploited for specific neuroinflammatory/infectious conditions of the CNS.
2. MATERIALS AND METHODS
2.1. TLR ligands and PPAR-γ agonists
The following TLR agonists were used in this study (see Table 1) with the concentration of each and its TLR target identified in parenthesis: Pam3CSK4 (TLR2, 1 μg/ml), polyinosine-polycytidylic acid (polyI:C; TLR3, 25 μg/ml), lipopolysaccharide from E. coli O111:B4 (LPS; TLR4, 100 ng/ml), flagellin from Salmonella typhimurium (TLR5, 10 μg/ml), single-stranded RNA (ssRNA; TLR7/8, 10 μg/ml), and synthetic unmethylated CpG oligonucleotide (ODN; TLR9, 0.1 μM and 5 μM for microglia and astrocytes, resp.). All TLR ligands were obtained from InvivoGen (San Diego, Calif, USA).
Table 1.
Summary of PPAR-γ effects on PAMP-activated microglia reported in this study.
| TLR ligand | ||||||
|---|---|---|---|---|---|---|
| PPAR-γ agonist | Pam3Cys | polyI:C | LPS | Flagellin | ssRNA | ODN |
| 15d-PGJ2 | ↓ IL-12 | ↓ IL-12 | ↓ IL-12 | ↓ IL-12 | ↓ IL-12 | ↓ IL-12 |
| ↓ TNF-α | ↓ TNF-α | ↓ TNF-α | ↓ TNF-α | TNF-α ND* | ↓ TNF-α | |
| ↓ CXCL2 | ↑ CXCL2 | ↑ CXCL2 | No effect CXCL2 | No effect CXCL2 | ↑ CXCL2 | |
| ↓ CCL2 | ↓ CCL2 | ↓ CCL2 | ↓ CCL2 | No effect CCL2 | ↓ CCL2 | |
|
| ||||||
| Rosiglitazone | ↓ IL-12 | ↓ IL-12 | ↓ IL-12 | ↓ IL-12 | ↓ IL-12 | ↓ IL-12 |
| ↓ TNF-α | ↓ TNF-α | No effect TNF-α | ↓ TNF-α | TNF-α ND | No effect TNF-α | |
|
| ||||||
| Pioglitazone | ↓ IL-12 | ↓ IL-12 | ↓ IL-12 | ↓ IL-12 | ↓ IL-12 | ↓ IL-12 |
| ↑ CXCL2 | ↑ CXCL2 | |||||
*ND; not detectable.
The natural PPAR-γ agonist 15d-PGJ2 and synthetic TZDs ciglitazone, rosiglitazone, pioglitazone, and troglitazone were purchased from Cayman Chemical (see Table 2; PPAR-γ Pak; Ann Arbor , Mich, USA). Dose-response studies were performed for all TZDs (10, 30, and 100 μM) and 15d- PGJ2 (5, 10, and 20 μM) in both TLR-activated microglia and astrocytes.
Table 2.
Summary of PPAR-γ effects on PAMP-activated astrocytes reported in this study.
| TLR ligand | ||||||
|---|---|---|---|---|---|---|
| PPAR-γ agonist | Pam3Cys | polyI:C | LPS | Flagellin | ssRNA | ODN |
| ↓ IL-12 | ↓ IL-12 | ↓ IL-12 | ↓ IL-12 | ↓ IL-12 | ↓ IL-12 | |
| 15d-PGJ2 | ↑ CXCL2 | ↓ NO | ↓ NO | |||
| ↑ CXCL2 | ||||||
|
| ||||||
| Ciglitazone | ↓ CXCL2 | ↓ CXCL2 | ↓ CXCL2 | ↓ CXCL2 | NR* | ↓ CXCL2 |
|
| ||||||
| Pioglitazone | ↓ IL-12 | ↓ IL-12 | ↓ IL-12 | ↓ IL-12 | ↓ IL-12 | ↓ IL-12 |
| ↑ CXCL2 | ↑ CXCL2 | ↑ CXCL2 | ||||
| ↑ CCL2 | ↑ CCL2 | |||||
|
| ||||||
| Troglitazone | ↓ CCL2 | ↓ CCL2 | NR | ↓ CCL2 | NR | ↓ CCL2 |
*NR: not reported.
2.2. Primary microglia and astrocyte cultures
Primary microglia and astrocytes were isolated from C57BL/6 pups (2 to 4 days of age) as previously described [35, 36]. The purity of glial cultures was evaluated by immunohistochemical staining using antibodies against CD11b (BD Pharmingen) and glial fibrillary acidic protein (GFAP, Invitrogen, Carlsbad, Calif, USA) to identify microglia and astrocytes, respectively. The purity of primary microglia and astrocyte cultures was approximately 98% and 95%, respectively.
Throughout this study, microglia and astrocytes were seeded into 96-well plates at 2 × 105 or 1 × 105 cells/well, respectively, and incubated overnight. The following day, glia were pretreated with various PPAR-γ agonists for 1 hour prior to stimulation with the TLR ligand panel for 24 hours. Cell-conditioned supernatants were collected at 24 hours following TLR ligand treatment for quantitation of proinflammatory mediator expression by ELISA.
2.3. Cell viability assays
The effects of PPAR-γ and TLR agonists on glial cell viability were evaluated using a standard MTT assay based upon the mitochondrial conversion of (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) (MTT) into formazan crystals. Results are reported as the raw OD570 values (mean ± SD).
2.4. Enzyme-linked immunosorbent assays (ELISAs)
Protein levels of TNF-α and CXCL2 (MIP-2) (Biosource) and IL-12 p40 and CCL2 (MCP-1, OptEIA, BD Pharmingen, San Jose, Calif, USA) were quantified in conditioned medium from PPAR-γ and TLR ligand-treated glia using ELISA kits according to the manufacturer's instructions (level of sensitivity = 15.6 pg/ml).
2.5. Nitrite assay
Nitrite levels, a stable end product resulting from the reaction of NO with molecular oxygen, were determined in astrocytes by adding 50 μl of cell-conditioned culture medium with 50 μl of Griess reagent (0.1% naphtyletylenediamine dihydrochloride, 1% sulfanilamide, 2.5% phosphoric acid, all from Sigma) in a 96-well plate. The absorbance at 550 nm was measured on a plate reader (Spectra Max 190, Molecular Devices, Sunnyvale , Calif , USA), and nitrite concentrations were calculated using a standard curve with sodium nitrite (NaNO2, Sigma, level of sensitivity, 0.4 μM). Based on our previous findings where S. aureus-derived TLR ligands were potent inducers of NO in astrocytes but not microglia [24, 33, 34], we only quantitated NO levels in the former in the current study.
2.6. Statistics
Significant differences between experimental groups were determined by the Student's t-test at the 95% confidence interval using SigmaStat (SPSS Science, Chicago, Ill, USA).
In this study, we performed a minimum of two independent replicates of each experiment to confirm the results obtained. The reporting of our results as representative of “x” number of independent experiments was required since it is difficult to achieve identical levels of proinflammatory mediator expression with distinct preparations of primary glia. As a result, the absolute concentrations of the various proinflammatory mediators differed between individual experiments; however, the trends were consistent. This required us to report results from a single study where each experimental treatment was represented by 3-4 individual wells (i.e., biological replicates) and statistical analysis conducted.
3. RESULTS
3.1. Ability of PPAR-γ agonists to modulate microglial cytokine production in response to diverse TLR ligands
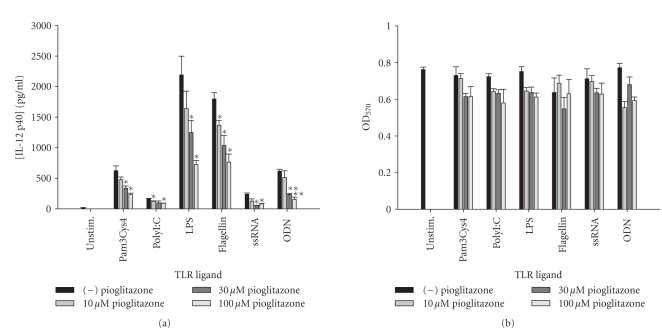
Microglia represent the main innate immune effector in the CNS parenchyma as evident by their expression of numerous TLRs [32, 37, 38]. Although much emphasis has been placed on the neurodestructive properties of activated microglia, recent studies have revealed that in the correct context microglia can also facilitate CNS repair [39–41]. Therefore, striking the correct balance between regulated and inappropriate microglial activation may lead to optimal outcomes for a wide range of CNS neuroinflammatory conditions. To determine whether PPAR-γ agonists could serve to modulate microglial activation in the context of CNS infection, the effects of these compounds on microglial cytokine production in response to diverse TLR ligands were examined. The natural PPAR-γ agonist 15d-PGJ2 was uniformly found to inhibit IL-12 p40 release in response to all TLR ligands tested including Pam3Cys4, polyI:C, LPS, flagellin, ssRNA, and ODN (Figure 1). Fairly comprehensive reductions in IL-12 p40 production were also observed with all TLR ligands tested in response to the synthetic PPAR-γ agonists rosiglitazone (Figure 2) and pioglitazone (Figure 3) although the extent of inhibition was dramatically less compared to 15d-PGJ2. Rosiglitazone exhibited significant toxicity to primary microglia at the highest dose tested (i.e., 100 μM), hence it was not included in the final analysis. In contrast, ciglitazone did not dramatically affect IL-12 p40 production in response to the majority of TLR ligands examined (data not shown). Troglitazone exhibited microglial toxicity at the two highest doses of agonist tested (i.e., 100 and 30 μM); therefore, the results of this PPAR-γ agonist on microglial mediator production in response to TLR ligands are not presented.
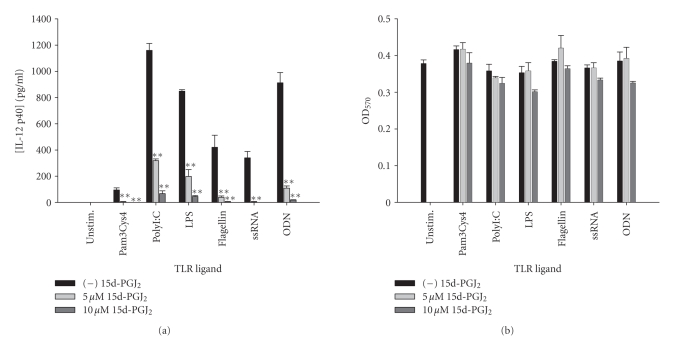
Figure 1.
The natural PPAR-γ agonist 15d-PGJ2 is a potent inhibitor of microglial IL-12 p40 production in response to a vast array of TLR ligands. Primary microglia were plated at 2 × 105 cells/well in 96-well plates and incubated overnight. The following day, cells were pretreated for 1 hour with the indicated concentrations of 15d-PGJ2 prior to the addition of TLR ligands. Cell-conditioned supernatants were collected at 24 hours following TLR ligand exposure, whereupon IL-12 p40 levels were determined by ELISA (a). The effects of 15d-PGJ2 on microglial viability were assessed using an MTT assay (b). Significant differences between microglia treated with TLR ligands alone versus TLR ligands + 15d-PGJ2 are noted with asterisks (**P < .001). Results presented are representative of two independent experiments.
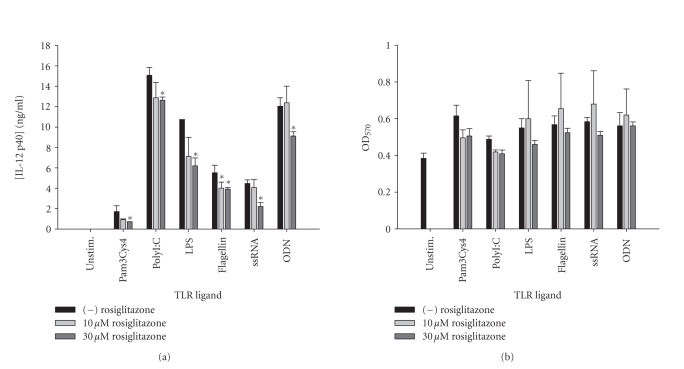
Figure 2.
The synthetic PPAR-γ agonist rosiglitazone attenuates IL-12 p40 production in response to TLR ligands in microglia. Primary microglia were plated at 2 × 105 cells/well in 96-well plates and incubated overnight. The following day, cells were pretreated for 1 hour with the indicated concentrations of rosiglitazone prior to the addition of TLR ligands. Cell-conditioned supernatants were collected at 24 hours following TLR ligand exposure, whereupon IL-12 p40 levels were determined by ELISA (a). The effects of rosiglitazone on microglial viability were assessed using an MTT assay (b). Significant differences between microglia treated with TLR ligands alone versus TLR ligands + rosiglitazone are noted with asterisks (* P < .05). Results presented are representative of two independent experiments.
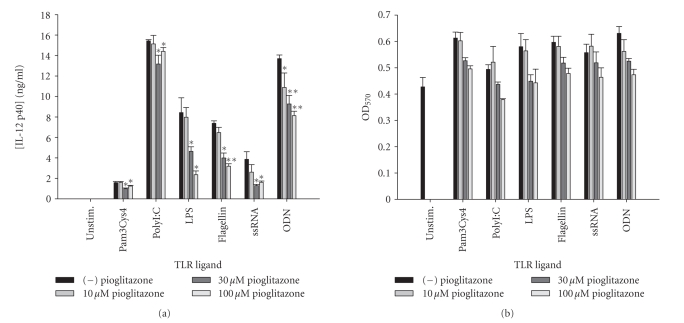
Figure 3.
The TZD pioglitazone inhibits microglial IL-12 p40 expression in response to diverse TLR ligands. Primary microglia were plated at 2 × 105 cells/well in 96-well plates and incubated overnight. The following day, cells were pretreated for 1 hour with the indicated concentrations of pioglitazone prior to the addition of TLR ligands. Cell-conditioned supernatants were collected at 24 hours following TLR ligand exposure, whereupon IL-12 p40 levels were determined by ELISA (a). The effects of pioglitazone on microglial viability were assessed using an MTT assay (b). Significant differences between microglia treated with TLR ligands alone versus TLR ligands + pioglitazone are noted with asterisks (* P < .05;**P < .001). Results presented are representative of two independent experiments.
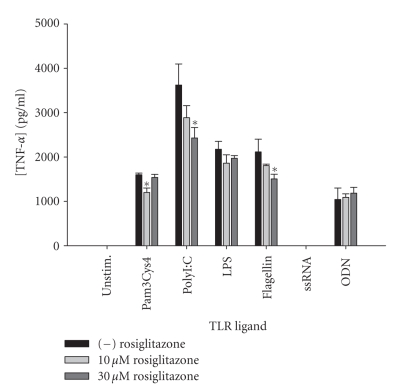
Another proinflammatory cytokine with potent effects on the blood-brain barrier as well as glial activation is TNF-α [42]. This cytokine is expressed at high levels in numerous CNS infectious diseases including bacterial meningitis, brain abscess, as well as viral infections [43–46]. In some cases, excessive TNF-α production during these infectious diseases has been implicated in contributing to bystander damage to surrounding host tissue [45, 46]. Therefore, strategies aimed at achieving optimized cytokine expression may prove beneficial for favorable disease outcomes. Of the PPAR-γ agonists tested, 15d-PGJ2 was found to exert the most global inhibition of TNF-α production in response to the battery of TLR ligands tested (Figure 4). Specifically, TNF-α release by microglia in response to Pam3Cys4, polyI:C, flagellin, and ODN was significantly attenuated by 15d-PGJ2, whereas cytokine production following LPS treatment was not as dramatically affected. Single-stranded RNA (ssRNA) was a poor inducer of TNF-α by primary microglia (Figure 4). Similar to results with IL-12 p40, rosiglitazone was the next more global inhibitor of TNF-α production in response to the TLR ligands tested (Figure 5), whereas the other PPAR-γ agonists (i.e., ciglitazone and pioglitazone) were largely without effect (data not shown). Collectively, these results indicate that not all PPAR-γ agonists are equally effective at modulating proinflammatory cytokine release from primary microglia and suggest that tailored responses to specific pathogen motifs may be achieved through the use of distinct PPAR-γ agonists.
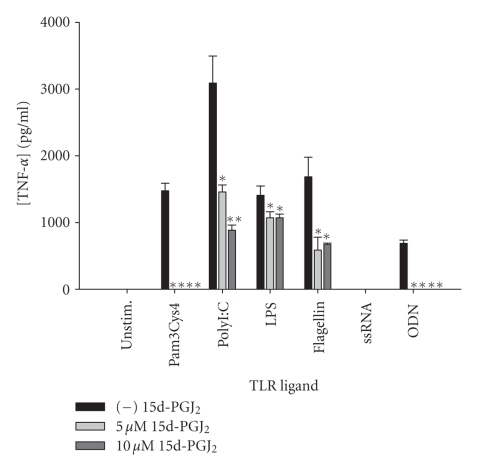
Figure 4.
The potency of 15d-PGJ2 to attenuate TNF-α production varies according to the TLR ligand examined. Primary microglia were plated at 2 × 105 cells/well in 96-well plates and incubated overnight. The following day, cells were pretreated for 1 hour with the indicated concentrations of 15d-PGJ2 prior to the addition of TLR ligands. Cell-conditioned supernatants were collected at 24 hours following TLR ligand exposure, whereupon TNF-α levels were determined by ELISA. Significant differences between microglia treated with TLR ligands alone versus TLR ligands + 15d-PGJ2 are noted with asterisks (*P < .05;**P < .001). Results presented are representative of two independent experiments.
Figure 5.
The synthetic PPAR-γ agonist rosiglitazone selectively inhibits microglial TNF-α expression in response to TLR ligands. Primary microglia were plated at 2 × 105 cells/well in 96-well plates and incubated overnight. The following day, cells were pretreated for 1 hour with the indicated concentrations of rosiglitazone prior to the addition of TLR ligands. Cell-conditioned supernatants were collected at 24 hours following TLR ligand exposure, whereupon TNF-α levels were determined by ELISA. Significant differences between microglia treated with TLR ligands alone versus TLR ligands + rosiglitazone are noted with asterisks (*P < .05). Results presented are representative of two independent experiments.
3.2. PPAR-γ agonists differentially affect chemokine release by microglia following TLR ligand treatment
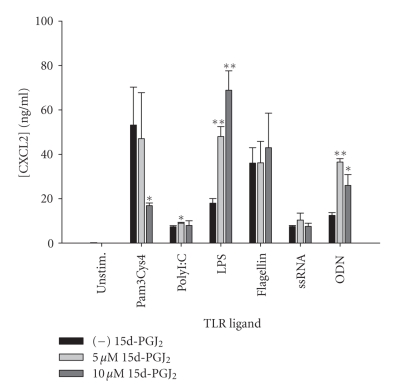
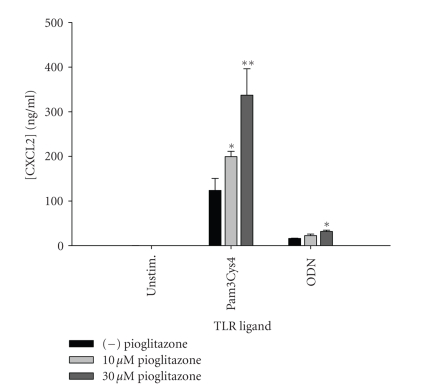
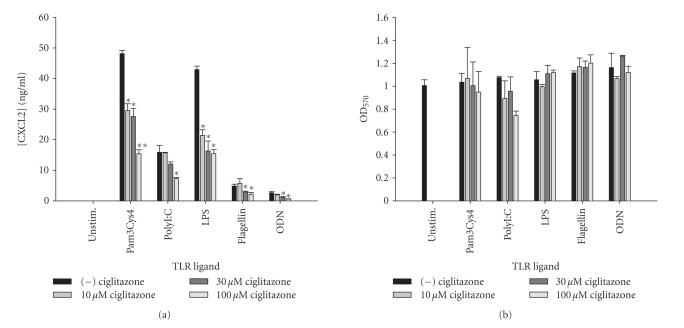
Chemokines are small molecular weight (8–14 kDa) chemotactic cytokines that are produced locally at sites of inflammation and establish a concentration gradient resulting in the active recruitment of target cell populations [47]. Chemokines are a structurally and functionally related family of proteins subdivided into four groups based on the relative position of conserved N-terminal cysteine residues [47–49]. In general, the chemokine subfamilies show similar, often overlapping specificity with regards to the movements of the target cell populations they orchestrate. One key chemokine involved in the recruitment of neutrophils into areas of inflammation, including the CNS, is CXCL2 (MIP-2) [50–52]. The effects of 15d-PGJ2 on microglial CXCL2 expression were complex and varied with each TLR ligand. Specifically, CXCL2 release was either enhanced (polyI:C, LPS, and ODN), reduced (Pam3Cys4), or remained unchanged (flagellin and ssRNA) (Figure 6). Increases in CXCL2 production were also observed following pioglitazone treatment in Pam3Cys- and ODN-stimulated microglia (Figure 7). The overall stimulatory activity of 15d-PGJ2 on CXCL2 production is in agreement with reports from other groups [53, 54].
Figure 6.
15d-PGJ2 demonstrates differential effects on CXCL2 production by microglia, which are TLR ligand-dependent. Primary microglia were plated at 2 × 105 cells/well in 96-well plates and incubated overnight. The following day, cells were pretreated for 1 hour with the indicated concentrations of 15d-PGJ2 prior to the addition of TLR ligands. Cell-conditioned supernatants were collected at 24 hours following TLR ligand exposure, whereupon CXCL2 levels were determined by ELISA. Significant differences between microglia treated with TLR ligands alone versus TLR ligands + 15d-PGJ2 are noted with asterisks (*P < .05;**P < .001). Results presented are representative of two independent experiments.
Figure 7.
Pioglitazone augments microglial CXCL2 production in response to Pam3Cys4 and ODN. Primary microglia were plated at 2 × 105 cells/well in 96-well plates and incubated overnight. The following day, cells were pretreated for 1 hour with the indicated concentrations of pioglitazone prior to the addition of Pam3Cys4 or ODN. Cell-conditioned supernatants were collected at 24 hours following TLR ligand exposure, whereupon CXCL2 levels were determined by ELISA. Significant differences between microglia treated with TLR ligands alone versus TLR ligands + pioglitazone are noted with asterisks (*P < .05;**P < .001). Results presented are representative of two independent experiments.
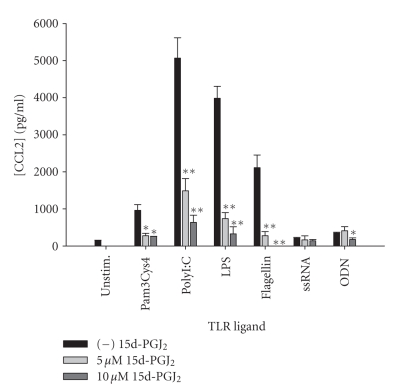
Another chemokine that is associated with mononuclear cell infiltration during various CNS infections is CCL2 (MCP-1), which targets monocyte and lymphocyte entry [52, 55, 56]. Unlike CXCL2, which was differentially regulated by 15d-PGJ2 in response to diverse TLR ligands, CCL2 production was uniformly and potently attenuated by this PPAR-γ agonist in response to the full array of TLR ligands tested (Figure 8). Similar to IL-12 p40 production, the synthetic TZDs demonstrated differential effects on CCL2 release from TLR ligand activated microglia. Specifically, rosiglitazone was fairly comprehensive in its ability to attenuate CCL2 production with significant reductions observed in response to Pam3Cys4, polyI:C, LPS, flagellin, and ODN, whereas the other TZDs tested (ciglitazone and pioglitazone) did not have much effect on CCL2 release in response to the majority of TLR ligands tested (data not shown). In summary, these results reveal that PPAR-γ agonists display a wide range of effects on chemokine production by microglia elicited by TLR ligands.
Figure 8.
15d-PGJ2 is a potent inhibitor of CCL2 release by microglia in response to a wide range of TLR ligands. Primary microglia were plated at 2 × 105 cells/well in 96-well plates and incubated overnight. The following day, cells were pretreated for 1 hour with the indicated concentrations of 15d-PGJ2 prior to the addition of TLR ligands. Cell-conditioned supernatants were collected at 24 hours following TLR ligand exposure, whereupon CCL2 levels were determined by ELISA. Significant differences between microglia treated with TLR ligands alone versus TLR ligands + 15d-PGJ2 are noted with asterisks (*P < .05;**P < .001). Results presented are representative of two independent experiments.
3.3. Effects of PPAR-γ agonists on astrocytic proinflammatory mediator production in response to TLR ligands
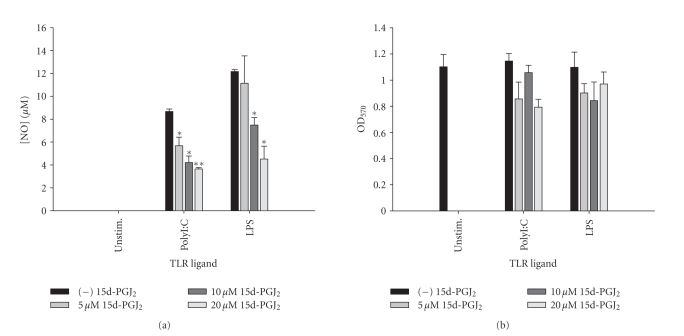
Astrocytes participate in CNS innate immune responses as evident by their ability to produce a wide array of inflammatory mediators in response to diverse stimuli [1, 4]. As already mentioned, these molecules can have dramatic consequences on CNS infection and tissue damage with net effects dictated by factors such as timing and duration of release. To determine the effects of PPAR-γ agonists on astrocyte responses to TLR ligands, we examined the production of two proinflammatory mediators produced by activated astrocytes, namely, NO and IL-12 p40. Both polyI:C and LPS were capable of reproducibly inducing NO expression in astrocytes as previously described [31, 57], which was attenuated by 15d-PGJ2 in a dose-dependent manner (Figure 9). Similar inhibitory effects on astrocytic NO release in response to polyI:C and LPS were observed with troglitazone and ciglitazone, whereas rosiglitazone and pioglitazone did not modulate NO production (data not shown). Of note was the fact that unlike microglia, which exhibited significant cell death in response to the highest dose of 15d-PGJ2 tested (i.e., 20 μM), astrocyte viability was not adversely affected by 15d-PGJ2 at any of the concentrations examined. This finding is in agreement with previous reports demonstrating that, in general, primary astrocytes are more refractory to the toxic effects of PPAR-γ agonists compared to primary microglia [23, 24, 27].
Figure 9.
15d-PGJ2 attenuates NO production by astrocytes in response to polyI:C and LPS stimulation. Primary astrocytes were plated at 1 × 105 cells/well in 96-well plates and incubated overnight. The following day, cells were pretreated for 1 hour with the indicated concentrations of 15d-PGJ2 prior to the addition of polyI:C or LPS. Cell-conditioned supernatants were collected at 24 hours following TLR ligand exposure, whereupon NO levels were determined by the Griess reagent (a). The effects of 15d-PGJ2 on astrocyte viability were assessed using an MTT assay (b). Significant differences between astrocytes treated with TLR ligands alone versus TLR ligands + 15d-PGJ2 are noted with asterisks (*P < .05;**P < .001). Results presented are representative of two independent experiments.
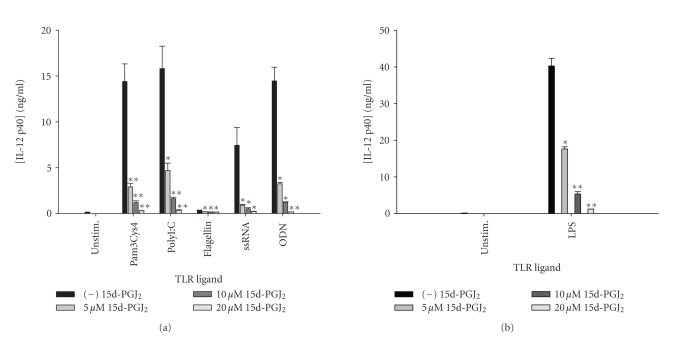
Similar to the findings obtained with microglia, 15d-PGJ2 was a universal and potent inhibitor of IL-12 p40 production by astrocytes in response to all TLR ligands tested (Figure 10). In addition, both pioglitazone and troglitazone were capable of attenuating IL-12 p40 expression in response to the full array of TLR ligands examined (Figure 11 and data not shown), whereas the effects of ciglitazone were variable.
Figure 10.
15d-PGJ2 is a global inhibitor of astrocytic IL-12 p40 release following TLR ligand exposure. Primary astrocytes were plated at 1 × 105 cells/well in 96-well plates and incubated overnight. The following day, cells were pretreated for 1 hour with the indicated concentrations of 15d-PGJ2 prior to the addition of TLR ligands. Cell-conditioned supernatants were collected at 24 hours following TLR ligand exposure, whereupon IL-12 p40 levels were determined by ELISA (a) and (b). Significant differences between astrocytes treated with TLR ligands alone versus TLR ligands + 15d-PGJ2 are noted with asterisks (*P < .05;**P < .001). Results presented are representative of two independent experiments.
Figure 11.
The TZD pioglitazone inhibits astrocytic IL-12 p40 expression in response to diverse TLR ligands. Primary astrocytes were plated at 1 × 105 cells/well in 96-well plates and incubated overnight. The following day, cells were pretreated for 1 hour with the indicated concentrations of pioglitazone prior to the addition of TLR ligands. Cell-conditioned supernatants were collected at 24 hours following TLR ligand exposure, whereupon IL-12 p40 levels were determined by ELISA (a). The effects of pioglitazone on astrocyte viability were assessed using an MTT assay (b). Significant differences between astrocytes treated with TLR ligands alone versus TLR ligands + pioglitazone are noted with asterisks (*P < .05;**P < .001). Results presented are representative of two independent experiments.
3.4. PPAR-γ agonists modulate chemokine production by astrocytes following TLR ligand exposure
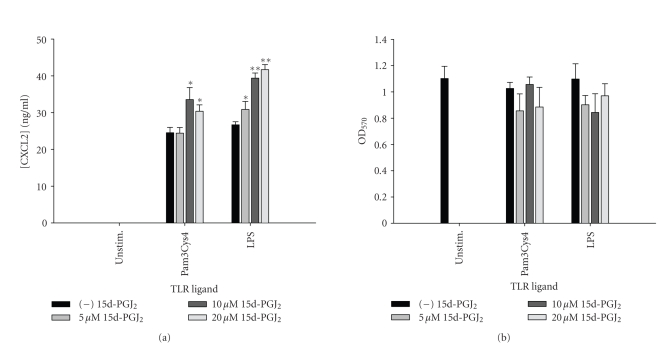
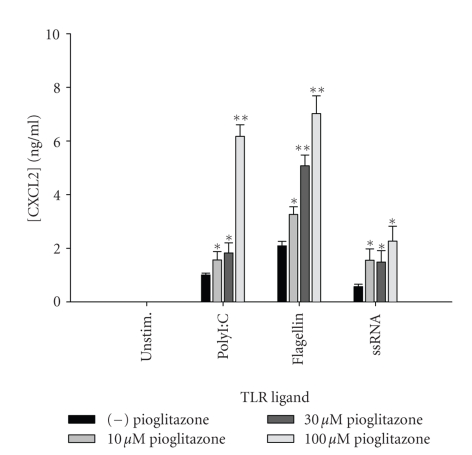
Although astrocytes are capable of releasing cytokines in response to diverse antigens, they are often considered the major source of chemokines during CNS inflammation [1, 4]. Similar to our recent report, 15d-PGJ2 slightly augmented CXCL2 production by astrocytes in response to several TLR ligands examined, namely Pam3Cys4, PGN, and LPS (Figure 12 and data not shown) [24]. Similar increases in CXCL2 release following PPAR-γ agonist exposure have also been reported by others [53, 54]. In contrast, each synthetic TZD appeared to differentially regulate CXCL2 release. For example, ciglitazone inhibited CXCL2 expression in response to the entire battery of TLR ligands examined (Figure 13). Conversely, pioglitazone significantly augmented CXCL2 expression in response to polyI:C, flagellin, and ssRNA particularly at the highest dose examined (i.e., 100 μM; Figure 14), whereas rosiglitazone did not dramatically alter CXCL2 levels response to any of the TLR ligands tested in astrocytes (data not shown).
Figure 12.
CXCL2 release is augmented in astrocytes by 15d-PGJ2 in response to distinct TLR ligands. Primary astrocytes were plated at 1 × 105 cells/well in 96-well plates and incubated overnight. The following day, cells were pretreated for 1 hour with the indicated concentrations of 15d-PGJ2 prior to the addition of Pam3Cys4 or LPS. Cell-conditioned supernatants were collected at 24 hours following TLR ligand exposure, whereupon CXCL2 levels were determined by ELISA (a). The effects of 15d-PGJ2 on astrocyte viability were assessed using an MTT assay (b). Significant differences between astrocytes treated with TLR ligands alone versus TLR ligands + 15d-PGJ2 are noted with asterisks (*P < .05;**P < .001). Results presented are representative of two independent experiments.
Figure 13.
Ciglitazone attenuates astrocytic CXCL2 expression in response to several TLR ligands. Primary astrocytes were plated at 1 × 105 cells/well in 96-well plates and incubated overnight. The following day, cells were pretreated for 1 hour with the indicated concentrations of ciglitazone prior to the addition of TLR ligands. Cell-conditioned supernatants were collected at 24 hours following TLR ligand exposure, whereupon CXCL2 levels were determined by ELISA (a). The effects of ciglitazone on astrocyte viability were assessed using an MTT assay (b). Significant differences between astrocytes treated with TLR ligands alone versus TLR ligands + ciglitazone are noted with asterisks (*P < .05;**P < .001). Results presented are representative of two independent experiments.
Figure 14.
Pioglitazone enhances CXCL2 release by astrocytes following TLR ligand exposure. Primary astrocytes were plated at 1 × 105 cells/well in 96-well plates and incubated overnight. The following day, cells were pretreated for 1 hour with the indicated concentrations of pioglitazone prior to the addition of polyI:C, flagellin, or ssRNA. Cell-conditioned supernatants were collected at 24 hours following TLR ligand exposure, whereupon CXCL2 levels were determined by ELISA. Significant differences between astrocytes treated with TLR ligands alone versus TLR ligands + pioglitazone are noted with asterisks (*P < .05;**P < .001). Results presented are representative of two independent experiments.
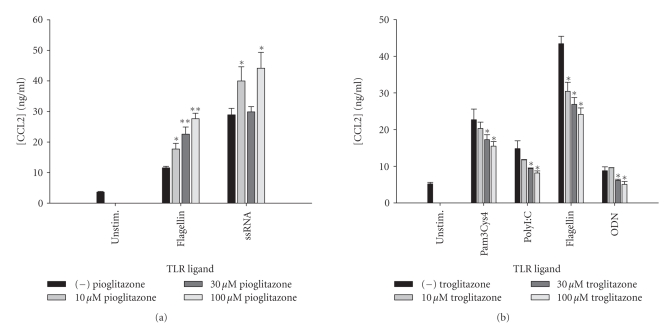
Similar to CXCL2, pioglitazone treatment increased CCL2 production in response to flagellin, and ssRNA primarily at the highest dose tested, whereas troglitazone led to significant reductions in CCL2 release following Pam3Cys4, polyI:C, flagellin, and ODN treatment (Figure 15). In general, ciglitazone and rosiglitazone had little effect on CCL2 production by astrocytes in response to the majority of TLR ligands tested (data not shown). These results indicate that despite their inclusion within the same family, specific PPAR-γ agonists differentially target proinflammatory genes in distinct manners.
Figure 15.
TZDs exert differential effects on astrocytic CCL2 production following TLR ligand treatment. Primary astrocytes were plated at 1 × 105 cells/well in 96-well plates and incubated overnight. The following day, cells were pretreated for 1 hour with the indicated concentrations of pioglitazone (a) or troglitazone, (b) prior to the addition of TLR ligands. Cell-conditioned supernatants were collected at 24 hours following TLR ligand exposure, whereupon CCL2 levels were determined by ELISA (a) and (b). Significant differences between astrocytes treated with TLR ligands alone versus TLR ligands + TZDs are noted with asterisks (*P < .05;**P < .001). Results presented are representative of two independent experiments.
4. DISCUSSION
In order for the CNS to respond to infectious insults, a rapid and efficient host immune response must be initiated and directed to expedite pathogen elimination. One means to achieve this goal is through the triggering of TLRs expressed on resident glia that signal proinflammatory mediator release in an attempt to quell infection [11, 12]. However, recent evidence also suggests that these normally protective immune responses can become dysregulated, culminating in the destruction of surrounding normal CNS parenchyma [13]. Therefore, fine tuning the resultant immune response to achieve maximal pathogen clearance concomitant with minimal tissue damage would represent a best case scenario for the management of a wide array of CNS infectious diseases including bacterial meningitis, HIVE, brain abscess, and other viral infections. The purpose of this study was to perform a comprehensive analysis of the ability of several PPAR-γ agonists to regulate glial activation in response to a panel of TLR ligands that may be encountered during native CNS infections. It is envisioned that this information may be exploited as a first step towards the derivation of specific treatment strategies that could be implemented with existing therapies for CNS infections to maximize benefit to patients.
One obvious distinction between the various PPAR-γ agonists tested to modulate TLR-dependent glial activation was the finding that 15d-PGJ2 consistently led to more dramatic and widespread decreases in inflammatory mediator production compared to the synthetic TZDs. This relationship was observed with both primary microglia and astrocytes and is in agreement with previous reports by us and other laboratories where 15d-PGJ2 exerted potent inhibitory effects at lower effective concentrations compared to TZDs, despite the fact that the former exhibits a lower binding affinity to PPAR-γ [26, 27, 58, 59]. In addition, differences were observed between the immune modulatory effects within the group of TZDs tested. This was somewhat surprising; however, Storer et al. also reported that ciglitazone and pioglitazone had no effect on TNF-α or CCL2 production by microglia in response to LPS [27], similar to our findings in the present study. However, a few discrepancies between these reports also exist, which might be explained by the fact that only a single TLR ligand was examined (i.e., LPS) and the concentrations of several TZDs exceeded the maximal dose tested in the current study. In general, only one TZD, rosiglitazone, exerted rather global effects on the inflammatory mediators examined here, whereas the other compounds (i.e., ciglitazone, troglitazone, and pioglitazone) demonstrated differential effects that were dependent on the TLR ligand as well as the proinflammatory mediator measured. The reason responsible for these differences is not clear but distinct chemical structures of the various TZDs have been suggested to contribute to their unique properties [60]. The widespread effects of rosiglitazone on glial inflammatory mediator production are in agreement with the fact that this TZD has been reported to impact numerous PPAR-γ isoforms including PPAR-γ1 and PPAR-γ2, whereas other TZDs have been reported to selectively target a single PPAR-γ subtype [61, 62]. Other studies have revealed distinct differences in the effectiveness of TZD members in regulating changes in glucose metabolism in astrocytes [63] and mitochondrial function [64]. In addition, the EC50 of individual TZD compounds for PPAR-γ does vary within this group of molecules depending on the experimental readout examined, which may contribute to their differential effects [61, 62]. Alternatively, studies have shown that the coactivator proteins interacting with PPAR-γ differ in a ligand-dependent manner [65]. Unfortunately, there are few reports where comprehensive side-by-side comparisons have been made with regard to the effects of various TZD compounds on neuroinflammation either in vitro or in vivo. Making direct comparisons between various TZDs in disparate models of neuroinflammation should be viewed cautiously since differences in disease models, inflammatory stimuli, and species examined all have the potential to influence the results obtained.
Our findings demonstrating the inhibitory effects of 15d-PGJ2 on TLR ligand-induced glial activation are similar to those observed following LPS stimulation. Specifically, 15d-PGJ2 has been shown to inhibit LPS-induced NO [27–29, 66], TNF-α [27, 28, 66], and IL-12 family member expression [26] in microglia and/or astrocytes. Importantly, this report has provided a more comprehensive analysis of the effects of PPAR-γ agonists on glial activation with the inclusion of a battery of TLR ligands that would be encountered during various CNS infectious diseases. Earlier studies have primarily focused on the immune modulatory effects of PPAR-γ agonists in response to the TLR4 ligand LPS, and therefore, this study is also novel from the perspective that numerous TLR ligands were evaluated.
Interestingly, both 15d-PGJ2 and pioglitazone were found to augment CXCL2 release by microglia and astrocytes in response to specific TLR ligands. In particular, 15d-PGJ2 enhanced CXCL2 production in response to LPS stimulation in both microglia and astrocytes, in agreement with previous reports [53, 54]. In addition, recent results from our laboratory have revealed that CXCL2 release was slightly increased by 15d-PGJ2 and downregulated by ciglitazone in response to the gram-positive pathogen S. aureus in primary astrocytes [24], in corroboration with the current report.
Elevated levels of endogenous 15d-PGJ2 have been associated with the resolution of inflammation in vivo, suggesting that it functions as negative feedback regulator of inflammatory responses [67, 68]. This study demonstrates that 15d-PGJ2 is an effective and selective inhibitor of glial activation in response to TLR ligands, suggesting that it may be capable of modulating chronic microglial and astrocyte responses during the course of CNS infectious diseases. Evidence to support this concept is provided by our findings that 15d-PGJ2 effectively inhibited IL-12 p40 and CCL2 expression in microglia and astrocytes, two molecules with important functions in the differentiation of CD4+ Th1 cells and monocyte and T cell influx into the infected CNS [52, 55, 56, 69, 70]. It is possible that the downregulation of these mediators in microglia and astrocytes following 15d-PGJ2 treatment results in the failure to recruit and/or stimulate Ag-specific T cells in the CNS parenchyma. Therefore, preventing chronic microglial activation by 15d-PGJ2 or synthetic PPAR-γ agonists may help to resolve inflammation earlier, resulting in less damage to surrounding normal brain parenchyma.
In summary, these studies demonstrate that not all PPAR-γ agonists are created equal in terms of their ability to modulate proinflammatory mediator release by activated microglia and astrocytes. Specifically, differences were specific to the type of TLR ligand examined. When considering the potential utility of PPAR-γ agonists for modulating pathological inflammation typical of several CNS infectious diseases, critical issues such as the timing and length of PPAR-γ administration and doses of compound must be considered. For example, it appears likely that compounds should be delivered at periods at or nearing pathogen clearance since a significant attenuation of the host immune response would be counterproductive to infection resolution. Upon pathogen elimination, PPAR-γ agonists may minimize damage to surrounding tissue by downregulating exaggerated CNS immune responses that could be perpetuated by microbial debris (i.e., cell wall fragments such as LPS and PGN or pathogen nucleic acids) via continued engagement of TLRs. Indeed, recent studies by our laboratory have revealed that the PPAR-γ agonist ciglitazone demonstrates beneficial effects with delayed administration in an experimental brain abscess model as revealed by accelerated abscess encapsulation and reduction in bacterial burdens [71]. In addition, recent studies by other groups have revealed beneficial effects of PPAR-γ agonists in other infectious disease paradigms [72–76]. Therefore, the current study can be used as a guide to facilitate the selection of PPAR-γ agonists as candidates for intervention during CNS infectious diseases.
ACKNOWLEDGMENTS
The authors would like to thank Paul Malbrough and Gail Wagoner for excellent technical assistance. This work was supported by the NIH National Institute of Neurological Disorders and Stroke (NINDS; 2RO1 NS40730) to T. Kielian and the NINDS-supported Core facility at UAMS (P30 NS047546).
References
- 1.Farina C, Aloisi F, Meinl E. Astrocytes are active players in cerebral innate immunity. Trends in Immunology. 2007;28(3):138–145. doi: 10.1016/j.it.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 2.Aloisi F. Immune function of microglia. Glia. 2001;36(2):165–179. doi: 10.1002/glia.1106. [DOI] [PubMed] [Google Scholar]
- 3.Hanisch U-K. Microglia as a source and target of cytokines. Glia. 2002;40(2):140–155. doi: 10.1002/glia.10161. [DOI] [PubMed] [Google Scholar]
- 4.Dong Y, Benveniste EN. Immune function of astrocytes. Glia. 2001;36(2):180–190. doi: 10.1002/glia.1107. [DOI] [PubMed] [Google Scholar]
- 5.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124(4):783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 6.Kaisho T, Akira S. Pleiotropic function of Toll-like receptors. Microbes and Infection. 2004;6(15):1388–1394. doi: 10.1016/j.micinf.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 7.Carpenter S, O'Neill LAJ. How important are Toll-like receptors for antimicrobial responses? Cellular Microbiology. 2007;9(8):1891–1901. doi: 10.1111/j.1462-5822.2007.00965.x. [DOI] [PubMed] [Google Scholar]
- 8.Sitia G, Iannacone M, Müller S, Bianchi ME, Guidotti LG. Treatment with HMGB1 inhibitors diminishes CTL-induced liver disease in HBV transgenic mice. Journal of Leukocyte Biology. 2007;81(1):100–107. doi: 10.1189/jlb.0306173. [DOI] [PubMed] [Google Scholar]
- 9.Krieg AM, Vollmer J. Toll-like receptors 7, 8, and 9: linking innate immunity to autoimmunity. Immunological Reviews. 2007;220(1):251–269. doi: 10.1111/j.1600-065X.2007.00572.x. [DOI] [PubMed] [Google Scholar]
- 10.Marsland BJ, Kopf M. Toll-like receptors: paving the path to T cell-driven autoimmunity? Current Opinion in Immunology. 2007;19(6):611–614. doi: 10.1016/j.coi.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 11.Kielian T. Toll-like receptors in central nervous system glial inflammation and homeostasis. Journal of Neuroscience Research. 2006;83(5):711–730. doi: 10.1002/jnr.20767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carpentier PA, Duncan DS, Miller SD. Glial toll-like receptor signaling in central nervous system infection and autoimmunity. Brain, Behavior, and Immunity. 2008;22(2):140–147. doi: 10.1016/j.bbi.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larsen PH, Holm TH, Owens T. Toll-like receptors in brain development and homeostasis. Science's STKE. 2007;2007(402) doi: 10.1126/stke.4022007pe47. [DOI] [PubMed] [Google Scholar]
- 14.Babcock AA, Wirenfeldt M, Holm T, et al. Toll-like receptor 2 signaling in response to brain injury: an innate bridge to neuroinflammation. Journal of Neuroscience. 2006;26(49):12826–12837. doi: 10.1523/JNEUROSCI.4937-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kigerl KA, Lai W, Rivest S, Hart RP, Satoskar AR, Popovich PG. Toll-like receptor (TLR)-2 and TLR-4 regulate inflammation, gliosis, and myelin sparing after spinal cord injury. Journal of Neurochemistry. 2007;102(1):37–50. doi: 10.1111/j.1471-4159.2007.04524.x. [DOI] [PubMed] [Google Scholar]
- 16.Kielian T, Drew PD. Effects of peroxisome proliferator-activated receptor-γ agonists on central nervous system inflammation. Journal of Neuroscience Research. 2003;71(3):315–325. doi: 10.1002/jnr.10501. [DOI] [PubMed] [Google Scholar]
- 17.Feige JN, Gelman L, Michalik L, Desvergne B, Wahli W. From molecular action to physiological outputs: peroxisome proliferator-activated receptors are nuclear receptors at the crossroads of key cellular functions. Progress in Lipid Research. 2006;45(2):120–159. doi: 10.1016/j.plipres.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 18.Kapadia R, Yi J-H, Vemuganti R. Mechanisms of anti-inflammatory and neuroprotective actions of PPAR-gamma agonists. Frontiers in Bioscience. 2008;13(5):1813–1826. doi: 10.2741/2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kostadinova R, Wahli W, Michalik L. PPARs in diseases: control mechanisms of inflammation. Current Medicinal Chemistry. 2005;12(25):2995–3009. doi: 10.2174/092986705774462905. [DOI] [PubMed] [Google Scholar]
- 20.Heneka MT, Landreth GE. PPARs in the brain. Biochimica et Biophysica Acta. 2007;1771(8):1031–1045. doi: 10.1016/j.bbalip.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 21.Drew PD, Xu J, Storer PD, Chavis JA, Racke MK. Peroxisome proliferator-activated receptor agonist regulation of glial activation: relevance to CNS inflammatory disorders. Neurochemistry International. 2006;49(2):183–189. doi: 10.1016/j.neuint.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 22.Bernando A, Minghetti L. PPAR-γ agonists as regulators of microglial activation and brain inflammation. Current Pharmaceutical Design. 2006;12(1):93–109. doi: 10.2174/138161206780574579. [DOI] [PubMed] [Google Scholar]
- 23.Kielian T, McMahon M, Bearden ED, Baldwin AC, Drew PD, Esen N. S. aureus-dependent microglial activation is selectively attenuated by the cyclopentenone prostaglandin 15-deoxy-Δ12,14-prostaglandin J2 (15d-PGJ2) Journal of Neurochemistry. 2004;90(5):1163–1172. doi: 10.1111/j.1471-4159.2004.02579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phulwani NK, Feinstein DL, Gavrilyuk V, Akar C, Kielian T. 15-deoxy-Δ12,14-prostaglandin J2 (15d-PGJ2) and ciglitazone modulate Staphylococcus aureus-dependent astrocyte activation primarily through a PPAR-γ-independent pathway. Journal of Neurochemistry. 2006;99(5):1389–1402. doi: 10.1111/j.1471-4159.2006.04183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang Q, Heneka M, Landreth GE. The role of peroxisome proliferator-activated receptor-γ (PPARγ) in Alzheimer's disease: therapeutic implications. CNS Drugs. 2008;22(1):1–14. doi: 10.2165/00023210-200822010-00001. [DOI] [PubMed] [Google Scholar]
- 26.Xu J, Drew PD. Peroxisome proliferator-activated receptor-γ agonists suppress the production of IL-12 family cytokines by activated glia. Journal of Immunology. 2007;178(3):1904–1913. doi: 10.4049/jimmunol.178.3.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Storer PD, Xu J, Chavis J, Drew PD. Peroxisome proliferator-activated receptor-gamma agonists inhibit the activation of microglia and astrocytes: implications for multiple sclerosis. Journal of Neuroimmunology. 2005;161(1-2):113–122. doi: 10.1016/j.jneuroim.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 28.Bernardo A, Levi G, Minghetti L. Role of the peroxisome proliferator-activated receptor-γ (PPAR-γ) and its natural ligand 15-deoxy-Δ12,14-prostaglandin J2 in the regulation of microglial functions. European Journal of Neuroscience. 2000;12(7):2215–2223. doi: 10.1046/j.1460-9568.2000.00110.x. [DOI] [PubMed] [Google Scholar]
- 29.Petrova TV, Akama KT, Van Eldik LJ. Cyclopentenone prostaglandins suppress activation of microglia: down-regulation of inducible nitric-oxide synthase by 15-deoxy-Δ12,14-prostaglandin J2 . Proceedings of the National Academy of Sciences of the United States of America. 1999;96(8):4668–4673. doi: 10.1073/pnas.96.8.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jack CS, Arbour N, Manusow J, et al. TLR signaling tailors innate immune responses in human microglia and astrocytes. Journal of Immunology. 2005;175(7):4320–4330. doi: 10.4049/jimmunol.175.7.4320. [DOI] [PubMed] [Google Scholar]
- 31.Carpentier PA, Begolka WS, Olson JK, Elhofy A, Karpus WJ, Miller SD. Differential activation of astrocytes by innate and adaptive immune stimuli. Glia. 2005;49(3):360–374. doi: 10.1002/glia.20117. [DOI] [PubMed] [Google Scholar]
- 32.Olson JK, Miller SD. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. Journal of Immunology. 2004;173(6):3916–3924. doi: 10.4049/jimmunol.173.6.3916. [DOI] [PubMed] [Google Scholar]
- 33.Esen N, Tanga FY, DeLeo JA, Kielian T. Toll-like receptor 2 (TLR2) mediates astrocyte activation in response to the Gram-positive bacterium Staphylococcus aureus . Journal of Neurochemistry. 2004;88(3):746–758. doi: 10.1046/j.1471-4159.2003.02202.x. [DOI] [PubMed] [Google Scholar]
- 34.Esen N, Kielian T. Central role for MyD88 in the responses of microglia to pathogen-associated molecular patterns. Journal of Immunology. 2006;176(11):6802–6811. doi: 10.4049/jimmunol.176.11.6802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Esen N, Kielian T. Effects of low dose GM-CSF on microglial inflammatory profiles to diverse pathogen-associated molecular patterns (PAMPs) Journal of Neuroinflammation. 2007;4, article 10 doi: 10.1186/1742-2094-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Esen N, Shuffield D, Syed MM, Kielian T. Modulation of connexin expression and gap junction communication in astrocytes by the gram-positive bacterium S. aureus . Glia. 2007;55(1):104–117. doi: 10.1002/glia.20438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bsibsi M, Ravid R, Gveric D, van Noort JM. Broad expression of Toll-like receptors in the human central nervous system. Journal of Neuropathology & Experimental Neurology. 2002;61(11):1013–1021. doi: 10.1093/jnen/61.11.1013. [DOI] [PubMed] [Google Scholar]
- 38.Kielian T, Esen N, Bearden ED. Toll-like receptor 2 (TLR2) is pivotal for recognition of S. aureus peptidoglycan but not intact bacteria by microglia. Glia. 2005;49(4):567–576. doi: 10.1002/glia.20144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Byram SC, Carson MJ, DeBoy CA, Serpe CJ, Sanders VM, Jones KJ. CD4-positive T cell-mediated neuroprotection requires dual compartment antigen presentation. Journal of Neuroscience. 2004;24(18):4333–4339. doi: 10.1523/JNEUROSCI.5276-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carson MJ, Bilousova TV, Puntambekar SS, Melchior B, Doose JM, Ethell IM. A rose by any other name? The potential consequences of microglial heterogeneity during CNS health and disease. Neurotherapeutics. 2007;4(4):571–579. doi: 10.1016/j.nurt.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Turrin NP, Rivest S. Molecular and cellular immune mediators of neuroprotection. Molecular Neurobiology. 2006;34(3):221–242. doi: 10.1385/MN:34:3:221. [DOI] [PubMed] [Google Scholar]
- 42.Gosselin D, Rivest S. Role of IL-1 and TNF in the brain: twenty years of progress on a Dr. Jekyll/Mr. Hyde duality of the innate immune system. Brain, Behavior, and Immunity. 2007;21(3):281–289. doi: 10.1016/j.bbi.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 43.Sriram K, O'Callaghan JP. Divergent roles for tumor necrosis factor-α in the brain. Journal of NeuroImmune Pharmacology. 2007;2(2):140–153. doi: 10.1007/s11481-007-9070-6. [DOI] [PubMed] [Google Scholar]
- 44.Kielian T. Immunopathogenesis of brain abscess. Journal of Neuroinflammation. 2004;1, article 16 doi: 10.1186/1742-2094-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nau R, Brück W. Neuronal injury in bacterial meningitis: mechanisms and implications for therapy. Trends in Neurosciences. 2002;25(1):38–45. doi: 10.1016/s0166-2236(00)02024-5. [DOI] [PubMed] [Google Scholar]
- 46.Saha RN, Pahan K. Tumor necrosis factor-α at the crossroads of neuronal life and death during HIV-associated dementia. Journal of Neurochemistry. 2003;86(5):1057–1071. doi: 10.1046/j.1471-4159.2003.01942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ransohoff RM, Liu L, Cardona AE. Chemokines and chemokine receptors: multipurpose players in neuroinflammation. International Review of Neurobiology. 2007;82:187–204. doi: 10.1016/S0074-7742(07)82010-1. [DOI] [PubMed] [Google Scholar]
- 48.Luster AD. Chemokines—chemotactic cytokines that mediate inflammation. The New England Journal of Medicine. 1998;338(7):436–445. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- 49.Rebenko-Moll NM, Liu L, Cardona A, Ransohoff RM. Chemokines, mononuclear cells and the nervous system: heaven (or hell) is in the details. Current Opinion in Immunology. 2006;18(6):683–689. doi: 10.1016/j.coi.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 50.Kielian T, Barry B, Hickey WF. CXC chemokine receptor-2 ligands are required for neutrophil-mediated host defense in experimental brain abscesses. Journal of Immunology. 2001;166(7):4634–4643. doi: 10.4049/jimmunol.166.7.4634. [DOI] [PubMed] [Google Scholar]
- 51.Bell MD, Taub DD, Kunkel SJ, et al. Recombinant human adenovirus with rat MIP-2 gene insertion causes prolonged PMN recruitment to the murine brain. European Journal of Neuroscience. 1996;8(9):1803–1811. doi: 10.1111/j.1460-9568.1996.tb01324.x. [DOI] [PubMed] [Google Scholar]
- 52.Ransohoff RM, Kivisäkk P, Kidd G. Three or more routes for leukocyte migration into the central nervous system. Nature Reviews Immunology. 2003;3(7):569–581. doi: 10.1038/nri1130. [DOI] [PubMed] [Google Scholar]
- 53.Zhang X, Wang JM, Gong WH, Mukaida N, Young HA. Differential regulation of chemokine gene expression by 15-deoxy-Δ12,14 prostaglandin J2 . Journal of Immunology. 2001;166(12):7104–7111. doi: 10.4049/jimmunol.166.12.7104. [DOI] [PubMed] [Google Scholar]
- 54.Si Q, Zhao M-L, Morgan AC, Brosnan CF, Lee SC. 15-deoxy-Δ12,14,-prostaglandin J2 inhibits IFN-inducible protein 10/CXC chemokine ligand 10 expression in human microglia: mechanisms and implications. Journal of Immunology. 2004;173(5):3504–3513. doi: 10.4049/jimmunol.173.5.3504. [DOI] [PubMed] [Google Scholar]
- 55.Kielian T. Microglia and chemokines in infectious diseases of the nervous system: views and reviews. Frontiers in Bioscience. 2004;9:732–750. doi: 10.2741/1266. [DOI] [PubMed] [Google Scholar]
- 56.Mahad DJ, Ransohoff RM. The role of MCP-1 (CCL2) and CCR2 in multiple sclerosis and experimental autoimmune encephalomyelitis (EAE) Seminars in Immunology. 2003;15(1):23–32. doi: 10.1016/s1044-5323(02)00125-2. [DOI] [PubMed] [Google Scholar]
- 57.Auch CJ, Saha RN, Sheikh FG, Liu X, Jacobs BL, Pahan K. Role of protein kinase R in double-stranded RNA-induced expression of nitric oxide synthase in human astroglia. FEBS Letters. 2004;563(1–3):223–228. doi: 10.1016/S0014-5793(04)00302-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-γ is a negative regulator of macrophage activation. Nature. 1998;391(6662):79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 59.Straus DS, Glass CK. Cyclopentenone prostaglandins: new insights on biological activities and cellular targets. Medicinal Research Reviews. 2001;21(3):185–210. doi: 10.1002/med.1006. [DOI] [PubMed] [Google Scholar]
- 60.Van Gaal L, Scheen AJ. Are all glitazones the same? Diabetes/Metabolism Research and Reviews. 2002;18(supplement 2):S1–S4. [PubMed] [Google Scholar]
- 61.Willson TM, Cobb JE, Cowan DJ, et al. The structure-activity relationship between peroxisome proliferator-activated receptor γ agonism and the antihyperglycemic activity of thiazolidinediones. Journal of Medicinal Chemistry. 1996;39(3):665–668. doi: 10.1021/jm950395a. [DOI] [PubMed] [Google Scholar]
- 62.Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor γ (PPARγ) Journal of Biological Chemistry. 1995;270(22):12953–12956. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- 63.Dello Russo C, Gavrilyuk V, Weinberg G, et al. Peroxisome proliferator-activated receptor γ thiazolidinedione agonists increase glucose metabolism in astrocytes. Journal of Biological Chemistry. 2003;278(8):5828–5836. doi: 10.1074/jbc.M208132200. [DOI] [PubMed] [Google Scholar]
- 64.Feinstein DL, Spagnolo A, Akar C, et al. Receptor-independent actions of PPAR thiazolidinedione agonists: is mitochondrial function the key? Biochemical Pharmacology. 2005;70(2):177–188. doi: 10.1016/j.bcp.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 65.Kodera Y, Takeyama K, Murayama A, Suzawa M, Masuhiro Y, Kato S. Ligand type-specific interactions of peroxisome proliferator-activated receptor γ with transcriptional coactivators. Journal of Biological Chemistry. 2000;275(43):33201–33204. doi: 10.1074/jbc.C000517200. [DOI] [PubMed] [Google Scholar]
- 66.Drew PD, Chavis JA. The cyclopentone prostaglandin 15-deoxy-Δ12,14 prostaglandin J2 represses nitric oxide, TNF-α, and IL-12 production by microglial cells. Journal of Neuroimmunology. 2001;115(1-2):28–35. doi: 10.1016/s0165-5728(01)00267-3. [DOI] [PubMed] [Google Scholar]
- 67.Gilroy DW, Colville-Nash PR, Willis D, Chivers J, Paul-Clark MJ, Willoughby DA. Inducible cyclooxygenase may have anti-inflammatory properties. Nature Medicine. 1999;5(6):698–701. doi: 10.1038/9550. [DOI] [PubMed] [Google Scholar]
- 68.Lawrence T, Willoughby DA, Gilroy DW. Anti-inflammatory lipid mediators and insights into the resolution of inflammation. Nature Reviews Immunology. 2002;2(10):787–795. doi: 10.1038/nri915. [DOI] [PubMed] [Google Scholar]
- 69.Langrish CL, McKenzie BS, Wilson NJ, de Waal Malefyt R, Kastelein RA, Cua DJ. IL-12 and IL-23: master regulators of innate and adaptive immunity. Immunological Reviews. 2004;202(1):96–105. doi: 10.1111/j.0105-2896.2004.00214.x. [DOI] [PubMed] [Google Scholar]
- 70.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nature Reviews Immunology. 2003;3(2):133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 71.Kielian T, Syed MM, Liu S, Phillips N, Wagoner G, Drew PD, Esen N. The synthetic PPAR-γ agonist ciglitazone attenuates neuroinflammation and accelerates encapsulation in bacterial brain abscesses. Journal of immunology. 2008;180(3):5004–5016. doi: 10.4049/jimmunol.180.7.5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.von Knethen A, Soller M, Brüne B. Peroxisome proliferator-activated receptor γ (PPARγ) and sepsis. Archivum Immunologiae et Therapiae Experimentalis. 2007;55(1):19–25. doi: 10.1007/s00005-007-0005-y. [DOI] [PubMed] [Google Scholar]
- 73.Arnold R, König W. Peroxisome proliferator-activated receptor-γ agonists inhibit the replication of respiratory syncytial virus (RSV) in human lung epithelial cells. Virology. 2006;350(2):335–346. doi: 10.1016/j.virol.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 74.Liu D, Zeng BX, Zhang SH, Yao SL. Rosiglitazone, an agonist of peroxisome proliferator-activated receptor γ, reduces pulmonary inflammatory response in a rat model of endotoxemia. Inflammation Research. 2005;54(11):464–470. doi: 10.1007/s00011-005-1379-0. [DOI] [PubMed] [Google Scholar]
- 75.Kaplan JM, Cook JA, Hake PW, O'Connor M, Burroughs TJ, Zingarelli B. 15-deoxy-Δ12,14-prostaglandin J2 (15D-PGJ2), a peroxisome proliferator activated receptor γ ligand, reduces tissue leukosequestration and mortality in endotoxic shock. Shock. 2005;24(1):59–65. doi: 10.1097/01.shk.0000167108.88376.f2. [DOI] [PubMed] [Google Scholar]
- 76.Ramirez SH, Heilman D, Morsey B, Potula R, Haorah J, Persidsky Y. Activation of peroxisome proliferator-activated receptor γ (PPARγ) suppresses Rho GTPases in human brain microvascular endothelial cells and inhibits adhesion and transendothelial migration of HIV-1 infected monocytes. Journal of immunology. 2008;180(3):1854–1865. doi: 10.4049/jimmunol.180.3.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]