Abstract
Human African trypanosomiasis (HAT) is caused by the protozoan parasite Trypanosoma brucei. The cysteine proteases of T.brucei have been shown to be crucial for parasite replication and represent an attractive point for therapeutic intervention. Herein we describe the synthesis of a series of thiosemicarbazones and their activity against the trypanosomal cathepsins TbcatB and rhodesain, as well as human cathepsins L and B. The activity of these compounds was determined against cultured T.brucei, and specificity was assessed with a panel of four mammalian cell lines.
The protozoan parasite Trypanosoma brucei causes Human African trypanosomiasis (HAT), a major health concern in sub-Saharan Africa with an estimated 50,000 cases and 60 million at risk of infection.1 The toxicity and impractical dosing regimens of the currently available drugs requires the development of new therapies. The urgency of the situation is further underscored by emerging clinical resistance to Melarsoprol, the front-line therapy for late stage parasitemia.2–4
One potential strategy for discovering new chemotherapies is to target T.brucei’s cysteine proteases. Irreversible peptidyl cysteine protease inhibitors are potent trypanocides5,6 and can arrest T.brucei infections in a mouse model.7 The parasite’s major papain-like protease,8 known interchangeably as brucipain, trypanopain, and rhodesain, was presumed until recently to be the target of these inhibitors. However, recent studies indicate that a second cysteine protease, TbcatB, may also be a target for these inhibitors.9,10
The biological functions of TbcatB and rhodesain are poorly understood. It has been suggested that they may be involved in nutrient aquisition, degradation of host proteins, evasion of the host immune response, or crossing of the blood brain barrier.8,11 Because T.brucei expresses only two proteases of the papain-like protease family, the biology of these two enzymes should be amenable to study by specific small molecule inhibitors.
Our prior work has shown that thiosemicarbazones have potent activity against rhodesain.12,13 However, the relationships between these compounds’ in vitro activity against rhodesain and TbcatB and their in vivo activity in cultured T.brucei has not been assessed. Here we report the synthesis of a third generation thiosemicarbazone series and its activity against cultured T.brucei proliferation and the parasite’s two cathepsins. In addition, activity against human cathepsins B and L was determined, and cytotoxicity evaluated in a panel of four mammalian cell lines to determine a cellular therapeutic index.
Thiosemicarbazones were synthesized by the general route (Scheme 1) previously described.13 Briefly, acid chloride 1 was reacted with the appropriate boronic acid to yield the ketone intermediate 2. The crude reaction was filtered and concentrated in vacuo, and the resulting solid was partially purified by silica chromatography. Acid catalyzed reaction with thiosemicarbazide afforded the target thiosemicarbazones 3a–m. Purification was achieved by silica chromatography, and the overall yield was 15% to 40%. Purity of target compounds was confirmed by LCMS using both C4 and C18 columns.
Each inhibitor was tested for activity against the trypanosomal cathepsins TbcatB and rhodesain as well as against T.brucei proliferation. In order to assess potential therapeutic utility, activity against human cathepsins B and L was determined, and general human cytotoxicity was evaluated in cultures of Raji (a lymphoblastoid cell line derived from a Burkitt’s lymphoma), HEK 293 (a human embryonic kidney cell line), BJ (a human fibroblast line), and HEP G2 (a human liver cell line derived from a hepatoblastoma). Membrane permeability was assessed in a parallel artificial membrane permeability assay (PAMPA).
Previous research demonstrated that aryl substituents are tolerated at the R and R1 positions by rhodesain.13 We further explored this observation and found that a variety of aryl moieties were well tolerated at these positions (Table 1). Nearly all compounds displayed submicromolar potency against rhodesain. Although several compounds displayed submicromolar potency against TbcatB, the protease was less sensitive to inhibition by this compound series. Unlike rhodesain, TbcatB did not tolerate phenylethyl substituted compounds.
Table 1.
Activity of thiosemicarbazones
| Compound
|
R
|
R1 |
IC50 vs. rhodesain, uM
|
IC50 vs. TbcatB, uM
|
EC50 vs. T.brucei, uM
|
IC50 vs. cat L, uM
|
IC 50 vs. cat B, uM
|
Cellular therapeutic index
|
|---|---|---|---|---|---|---|---|---|
| 3a | 3,5-(CF3)2 Ph- | 2-thiophene | 0.007 ± 0.001 | 0.15 ± 0.01 | 7 ± 1 | 0.0101 ± 0.0009 | 0.30 ± 0.05 | > 4 |
| 3b | 4-Me Ph- | 3-CF3 Ph- | 0.008 ± 0.001 | 0.21 ± 0.03 | 1.5 ± 0.2 | 0.0094 ± 0.0006 | 0.21 ± 0.01 | > 17 |
| 3c | 3,5-Cl2 Ph- | 2-thiophene | 0.011 ± 0.001 | 0.45 ± 0.06 | 7 ± 2 | 0.0036 ± 0.0003 | 0.30 ± 0.02 | > 4 |
| 3d | 3,5-Cl2 Ph- | 4-Me Ph- | 0.046 ± 0.003 | 6 ± 1 | 1.1 ± 0.2 | 0.0110 ± 0.0007 | 1.8 0.2 | > 9 |
| 3e | Ph-CH=CH- | 3-CF3 Ph- | 0.029 ± 0.004 | 7 ± 1 | 2.5 ± 0.3 | 0.081 ± 0.004 | 1.7 ± 0.1 | > 10 |
| 3f | 3,5-(CF3)2 Ph- | 4-Me Ph- | 0.044 ± 0.008 | 15 ± 4 | 1.3 ± 0.8 | 0.0074 ± 0.0006 | 3.3 ± 0.9 | > 19 |
| 3g | 3,5-(CF3)2 Ph- | (CH2)2- | 0.053 ± 0.006 | >25 | 2 ± 1 | 0.017 ± 0.001 | 3.0 ± 0.4 | > 10 |
| 3h | 4-Me Ph- | 3-F Ph- | 0.089 ± 0.009 | >25 | >10 | 0.044 ± 0.003 | 4.5 ± 0.5 | na |
| 3i | 3,5-Cl2 Ph- | Ph-(CH2)2- | 0.057 ± 0.008 | >25 | 1.3 ± 0.1 | 0.039 ± 0.004 | 4.9 ± 0.6 | > 19 |
| 3j | 4-Me Ph- | Ph- | 0.50 ± 0.06 | >25 | >10 | 1.02 ± 0.08 | 13 ± 1 | na |
| 3k | Ph-(CH2)2- | Ph- | 1.5 ± 0.2 | >25 | >10 | 0.91 ± 0.06 | >25 | na |
| 3l | Ph-(CH2)2- | 3-Cl Ph- | 0.042 ± 0.006 | >25 | 3.7 ± 0.7 | 0.021 ± 0.001 | 6 ± 1 | > 6.7 |
| 3m | Ph-(CH2)2- | 3-F Ph- | 0.39 ± 0.03 | >25 | 6 ± 1 | 0.118 ± 0.007 | 22 ± 4 | > 4 |
We hypothesized that thiosemicarbazones might act through TbcatB, or through both rhodesain and TbcatB, to kill the parasite. Regression analysis conducted on the compound series detected only a weak positive association between rhodesain inhibition and trypanocidal activity (R2=0.3). For TbcatB, no statistically significant relationship between inhibition and trypanocidal activity was observed. Membrane permeability of the compound series was tested by PAMPA, and it was found the inhibitors generally exhibited similar permeabilities (Supporting Information). This suggests that differences in intracellular accumulation are unlikely to explain the lack of correlation between protease inhibition and trypanocidal activity.
It is clear that activity against the parasite cannot be explained by either rhodesain or TbcatB inhibition alone, or simply by their acting in synergy. Although it is difficult to interpret the mechanism of action of these inhibitors, it is interesting to note that all reasonably active compounds against TbcatB were also active against T.brucei (3a–c). In contrast, at least one compound highly active against rhodesain and inactive against TbcatB was completely inactive against the parasite (3h). The lack of correlation observed between trypanocidal activity with activity against either protease target suggests that at least some of the compounds in this series exert their effects at unknown targets. This is not unexpected, as the thiosemicarbazone scaffold has reported activity against a wide range of cell types and molecular targets.12,14–18
Significant specificity for the parasitic proteases was not achieved in this compound series relative to the human cathepsins. However, a high degree of specificity was observed for the two cathepsin L-like enzymes (cathepsin L and rhodesain) relative to the two cathepsin B-like enzymes (cathepsin B and TbcatB). These results are similar to previous TbcatB studies with purine nitrile inhibitors, in which TbcatB was generally less sensitive to inhibition than cathepsin L.10 A number of inhibitors displayed absolute specificity for rhodesain over TbcatB. These compounds are potent inhibitors of rhodesain and show little or no toxicity against the parasite. They are also membrane permeable, making these compounds attractive tools for studying rhodesain function in T.brucei.
General cytotoxicity of each inhibitor was evaluated by EC50 determination in cultures of BJ, Raji, HEK 293, and HEP G2. Of the four cell lines, Raji was the most sensitive. A cellular therapeutic index for each cell line was determined and defined as: (EC50 Raji)/(EC50 T.brucei). Compounds in this series generally killed the parasites with significant selectivity relative to mammalian cells, with several exhibiting index values upwards of 20 fold in various cell lines (Supporting Information). These data indicate the trypanocidal effects of these inhibitors are not due to general cytotoxicity, and it is notable that 3f displayed an index value of over 20 fold in all four cell lines.
We have reported the synthesis and evaluation of a series of thiosemicarbazones against two human and two trypanosomal cathepsins. Each compound was assayed for activity against T.brucei and for cytotoxicity in a panel of four mammalian cell lines. This inhibitor series was determined to have low overall cytotoxicity, with several compounds killing the parasite selectively relative to the mammalian cell lines tested. All inhibitors from this series exhibited potent activity against rhodesain, and a subset of these compounds was active against TbcatB. Several compounds were potent trypanocides. However, no strong correlation was observed between trypanocidal activity and potency against either protease. Although the mechanism of action of these compounds remains unclear, the low cytotoxicity and good membrane permeability of this series suggests that thiosemicarbazones warrant further examination as leads for the therapy of Human African trypanosomiasis.
Supplementary Material
Supporting Information
Spectroscopic data, LCMS data, PAMPA data, and complete cytotoxicity data for all listed compounds. Details for regression analysis and cell based assays.
Figure 1.
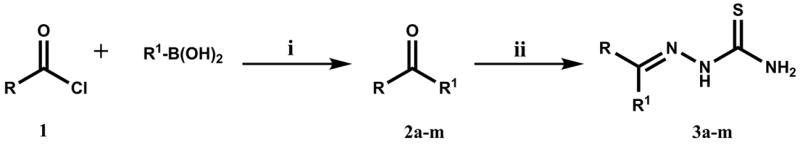
Conditions: (i) PdCl2(PPh3)2, K3PO4, toluene, 70°C, 2 to 5 h; (ii) thiosemicarbazide, HOAc, H2O/EtOH, 80°C, 72 to 96 h.
Acknowledgments
This work was supported by the American Lebanese Syrian Associated Charities (ALSAC) and St. Jude Children’s Research Hospital, NIH grant AI35707, and the Sandler Family Supporting Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.WHO. Sleeping sickness. 2007 http://www.who.int/mediacentre/factsheets/fs259/en/
- 2.WHO. Sleeping sickness treatment schedule. 2007. [Google Scholar]
- 3.Brun R, Schumacher R, Schmid C, Kunz C, Burri C. Trop Med Int Health. 2001;6:906. doi: 10.1046/j.1365-3156.2001.00775.x. [DOI] [PubMed] [Google Scholar]
- 4.Legros D, Evans S, Maiso F, Enyaru JC, Mbulamberi D. Trans R Soc Trop Med Hyg. 1999;93:439. doi: 10.1016/s0035-9203(99)90151-7. [DOI] [PubMed] [Google Scholar]
- 5.Ashall F, Angliker H, Shaw E. Biochem Biophys Res Commun. 1990;170:923. doi: 10.1016/0006-291x(90)92179-4. [DOI] [PubMed] [Google Scholar]
- 6.Troeberg L, Morty RE, Pike RN, Lonsdale-Eccles JD, Palmer JT, McKerrow JH, Coetzer TH. Exp Parasitol. 1999;91:349. doi: 10.1006/expr.1998.4386. [DOI] [PubMed] [Google Scholar]
- 7.Scory S, Caffrey CR, Stierhof YD, Ruppel A, Steverding D. Exp Parasitol. 1999;91:327. doi: 10.1006/expr.1998.4381. [DOI] [PubMed] [Google Scholar]
- 8.Caffrey CR, Hansell E, Lucas KD, Brinen LS, Alvarez Hernandez A, Cheng J, Gwaltney SL, 2nd, Roush WR, Stierhof YD, Bogyo M, Steverding D, McKerrow JH. Mol Biochem Parasitol. 2001;118:61. doi: 10.1016/s0166-6851(01)00368-1. [DOI] [PubMed] [Google Scholar]
- 9.Mackey ZB, O’Brien TC, Greenbaum DC, Blank RB, McKerrow JH. J Biol Chem. 2004;279:48426. doi: 10.1074/jbc.M402470200. [DOI] [PubMed] [Google Scholar]
- 10.Mallari JP, Shelat A, Obrien T, Caffrey C, Kosinski A, Connely M, Harbut M, Greenbaum D, McKerrow JH, Guy RK. Journal of Medicinal Chemistry. 2007 doi: 10.1021/jm070760l. In Press. [DOI] [PubMed] [Google Scholar]
- 11.Nikolskaia OV, de ALAP, Kim YV, Lonsdale-Eccles JD, Fukuma T, Scharfstein J, Grab DJ. J Clin Invest. 2006;116:2739. doi: 10.1172/JCI27798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenbaum DC, Mackey Z, Hansell E, Doyle P, Gut J, Caffrey CR, Lehrman J, Rosenthal PJ, McKerrow JH, Chibale K. J Med Chem. 2004;47:3212. doi: 10.1021/jm030549j. [DOI] [PubMed] [Google Scholar]
- 13.Fujii N, Mallari JP, Hansell EJ, Mackey Z, Doyle P, Zhou YM, Gut J, Rosenthal PJ, McKerrow JH, Guy RK. Bioorg Med Chem Lett. 2005;15:121. doi: 10.1016/j.bmcl.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 14.Du X, Guo C, Hansell E, Doyle PS, Caffrey CR, Holler TP, McKerrow JH, Cohen FE. J Med Chem. 2002;45:2695. doi: 10.1021/jm010459j. [DOI] [PubMed] [Google Scholar]
- 15.Klayman DL, Bartosevich JF, Griffin TS, Mason CJ, Scovill JP. J Med Chem. 1979;22:855–62. doi: 10.1021/jm00193a020. [DOI] [PubMed] [Google Scholar]
- 16.Klayman DL, Lin AJ, McCall JW, Wang SY, Townson S, Grogl M, Kinnamon KE. J Med Chem. 1991;34:1422. doi: 10.1021/jm00108a027. [DOI] [PubMed] [Google Scholar]
- 17.Klayman DL, Scovill JP, Mason CJ, Bartosevich JF, Bruce J, Lin AJ. Arzneimittelforschung. 1983;33:909. doi: 10.1002/chin.198344210. [DOI] [PubMed] [Google Scholar]
- 18.Creasey WA, Agrawal KC, Capizzi RL, Stinson KK, Sartorelli AC. Cancer Res. 1972;32:565. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Spectroscopic data, LCMS data, PAMPA data, and complete cytotoxicity data for all listed compounds. Details for regression analysis and cell based assays.


