Abstract
Background
Most work to understand the controls of alcohol intake by animals involves the two-bottle choice method. Recent experiments involving other nutrients suggest that intakes are profoundly influenced by the number of nutrient choices available. Here, we extended these observations by measuring the alcohol consumption of mice and rats given multiple choices of water and alcohol.
Methods
Four experiments were conducted. In experiments 1 and 2, male C57BL6/J (B6) mice, 129X1/SvJ mice, or Sprague-Dawley rats received a series of six 72- or 48-hr tests in which the number of bottles of 10% alcohol and water was manipulated. One test involved the typical two-bottle choice. In the other five, the rodents always had six bottles with one, two, three, four, or five containing 10% alcohol and the rest containing water. In experiment 3, separate groups of B6 mice received for 16 days (a) the typical two-bottle test, (b) five alcohol bottles and one water bottle, (c) three alcohol bottles and three water bottles, or (d) one alcohol bottle and five water bottles. In experiment 4, groups of B6 mice received either a two-bottle test or five alcohol bottles and one water bottle for 24 days.
Results
In all experiments, the volume of alcohol consumed was strongly and positively related to the number of alcohol bottles available and inversely related to the number of water bottles available. The effect of alcohol availability on alcohol intake persisted for at least 24 days.
Conclusions
Alcohol intake is strongly influenced by availability. The results point to a simple method of manipulating murine alcohol intake over a wide range. They provide an animal model that might be useful for understanding the influence of alcohol availability on human alcohol consumption.
Keywords: Choice, Availability, Preference, Ethanol
It is common practice to assess the alcohol intake of mice and rats by providing them with a choice between a bottle of alcohol and a bottle of water. Because the rodent is not forced to drink the alcohol to assuage hunger or thirst, this method provides a measure of “voluntary” or “spontaneous” consumption, and the ratio of alcohol intake to total intake provides a measure of alcohol preference that is relatively free of nonspecific effects, such as body size, customary fluid intake, or minor motor dysfunctions (Bachmanov et al., 1996a, 2002).
Intakes during two-bottle choice tests are believed to reflect the physiologic status of the animal. This notion, called nutritional wisdom, is based primarily on findings that animals select a balanced diet and grow rapidly when allowed to choose from separate sources of macronutrients (e.g., Richter, 1942–1943–1956). However, we recently showed that macronutrient selection and intake are strongly dependent on the number of sources of each macronutrient available (Tordoff, 2002). In one study, we compared rats given to drink five bottles of 32% sucrose and one bottle of water with those given one bottle of 32% sucrose and five bottles of water. The group with five bottles of sucrose drank approximately twice the volume of sucrose, gained significantly more weight, and accrued significantly more fat than did the group with only one bottle of sucrose. In another study, we found that mice given any of four archetypical taste solutions (saccharin, citric acid, quinine hydrochloride, or NaCl) drank more of the taste solution when given two bottles of it and one of water than when given either a standard two-bottle test or a test with one bottle of taste solution and two of water (Tordoff and Bachmanov, 2003). These experiments indicate that simple manipulations of the availability of nutrients or taste solutions can have profound effects on intake and preference.
Alcohol, of course, is both a nutrient and a taste solution. In the work reported here, we sought to expand our findings with other nutrients by examining the extent to which alcohol intake was influenced by the number of sources available. In experiments 1 and 2, we examined the behavior of C57BL6/J (B6) and 129X1/SvJ (129) mice and Sprague-Dawley rats given access to various combinations of 10% alcohol and water in short-term tests, using a within-subject design. In experiments 3 and 4, we examined the response of separate groups of B6 mice given various numbers of alcohol and water bottles for 16 or 24 days.
METHODS
Subjects, Housing, and Drinking Tubes
All subjects were maintained at 23°C on a 12:12-hr light/dark cycle with lights off at 7:00 PM. All were allowed at least a week to adapt to vivarium conditions before testing began. The animals used in these experiments were maintained in facilities accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care. The research protocols were approved by the Institutional Animal Care and Use Committee of the Monell Chemical Senses Center and were in accordance with Institute of Laboratory Animal Resources (1996) guidelines.
Mice
Male B6 and 129 mice were purchased from The Jackson Laboratory (catalog Nos. 000664 and 000691; Bar Harbor, ME). They were 5 weeks old when they arrived in our facility. The mice were individually housed in plastic tub cages (26.5 × 17 × 12 cm) with a stainless-steel grid lid and wood shavings scattered on the floor. Before tests began, they could drink deionized water from an inverted 300-ml bottle with a stainless-steel spout. They ate Teklad 8604 diet (Harlan, Madison, WI), which was available from a hopper built into the cage lid. During tests, the water bottle was replaced by drinking tubes, and food was scattered on the cage floor (to provide sufficient space to place the drinking tubes). The drinking tubes were arranged along the top of the cage at even intervals, with tips approximately 2 cm apart and extending into the cage 2.5 cm (Fig. 1). They were constructed from graduated pipette tubes with rubber stoppers and stainless-steel spouts. Each spout had a 3.175-mm-diameter hole from which the mice could lick fluids. Specifics of drinking-tube construction and other general test procedures are available elsewhere (Tordoff and Bachmanov, 2001).
Fig. 1.
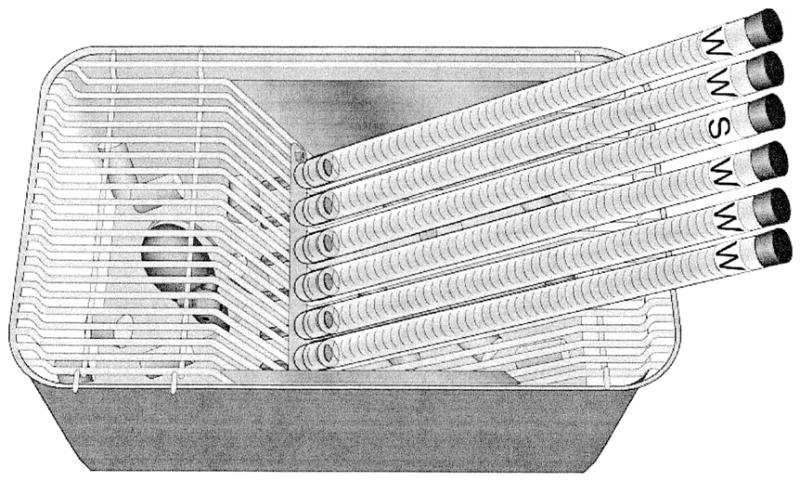
Cage layout for testing mice with six drinking tubes. The labels on the tubes stand for deionized water (W) or solution (S), which was 10% alcohol in all the studies reported here. For two-bottle tests, only the middle two tubes were presented.
Rats
Male Sprague-Dawley rats [Crl:CD (SD)IGS BR] aged 7 weeks were purchased from Charles River Laboratories (Wilmington, MA). The rats were individually housed in stainless-steel “guinea pig” cages (41 × 56 × 23 cm) with pine shavings on the floor. Powdered Purina (St. Louis, MO) Rodent Chow No. 5001 was available from a glass jar held against the middle of the back wall of the cage by a stainless-steel spring. Deionized water was available from an inverted 300-ml bottle with a stainless-steel spout.
During tests, the water bottle was replaced by 50-ml inverted centrifuge tubes with rubber stoppers and stainless-steel drinking spouts. When six tubes were available, the tubes were lined across the front of the cage at approximately 3-cm intervals and held in place by steel springs. Each drinking-spout tip projected into the cage approximately 3 cm to rest approximately 5 cm above the cage floor.
Procedure
Experiments 1 and 2
Experiment 1 involved mice and experiment 2 involved rats, but otherwise they used similar methods. At the start of testing there were 16 B6 mice, 16 129 mice, and 12 Sprague-Dawley rats, weighing (mean ± SE) 27.7 ± 0.3 g, 29.9 ± 0.5 g, and 316 ± 6 g, respectively.
Each animal was tested six times according to a counterbalanced design, with two or three subjects of the same strain receiving each condition during each test. For the mice, the tests lasted 3 days and were separated by 3 days with only a single bottle of deionized water to drink. For the rats, the tests lasted 2 days, and these were separated by 1 to 3 days with a single bottle of water to drink. During one test, the subject received a two-bottle choice between water and 10% alcohol (i.e., 10% ethanol, v/v). The alcohol was available on the subject’s left and the water on the subject’s right. In the other five tests, six drinking tubes were available, with one, two, three, four, or five containing 10% alcohol and the rest containing deionized water. The tubes were arranged across the front of the cage according to the following sequences, with W denoting water and A denoting alcohol: (a) WWWAWW, (b) WAWWAW, (c) WAWAWA, (d) AWAAWA, and (e) AAAWAA (Fig. 1). Intakes were calculated by comparing the volume remaining in the drinking tubes, read from graduated scales (±0.1 ml for mice; ±0.5 ml for rats), at the beginning and end of each test.
Experiment 3
Experiments 3 and 4 were designed to examine the chronic effects of alcohol and water availability on alcohol intake. Experiment 3 involved 48 male B6 mice. After 7 days to adapt to vivarium conditions, the mice were assigned to one of four groups, matched for body weight (average, 21.5 ± 0.2 g). The groups received (a) a standard two-bottle choice between 10% alcohol and water (WA), (b) one bottle of alcohol and five of water (WWWAWW), (c) three bottles of alcohol and three of water (WAWAWA), or (d) five bottles of alcohol and one of water (AAAWAA). Intakes from each bottle were measured every 2 days for 16 days.
Because of concerns about spillage, the water and alcohol solutions were colored by the addition of 100 mg/liter of Derifil (copper chlorophyllin complex; Menley & James, Horsham, PA) or 50 mg/liter of amaranth (Red Dye No. 2; Sigma Chemical Corp, St. Louis, MO). These are two food dyes that turn fluids green or red, respectively, but that are not absorbed, so they are excreted in feces. The dyes made it easy to recognize spillage in bedding and provided color cues that helped ensure that mice received the appropriate fluids in each tube. Pilot work found that these concentrations were either undetected or treated indifferently by mice in two-bottle choice tests.
After the 16-day test, the mice were given one bottle of water for 3 days and then received a two-bottle choice between water and 10% alcohol for 4 days. This test was given to all 48 mice to determine whether the previous procedure had altered the mice’s response to alcohol.
Experiment 4
This experiment involved 24 B6 mice (weighing 25.3 ± 0.3 g). Twelve received a two-bottle choice between water and 10% alcohol. The others received five bottles of alcohol and one of water (AAAWAA). The fluids were not dyed in this experiment. Intakes were measured every 2 days for 24 days. Cage bedding was changed every 8 days, at the same time that blood was collected (see below).
A blood sample was collected from each mouse on days 2, 8, 16, and 24 of access to alcohol, in the middle of the light period. Each mouse was removed from its cage and wrapped in a paper towel, and the tip of its tail was cut with a scalpel blade. Approximately 45 μl of blood was milked into a heparinized microhematocrit tube by gently stroking the tail. The blood was centrifuged at 2000 × g for 3 min to produce plasma, and a 20-μl aliquot was analyzed for alcohol concentration by using a kit based on the conversion of alcohol to acetaldehyde by alcohol dehydrogenase (kit No. 333-UV; Sigma Chemical Corp.). To increase the assay’s sensitivity to detect low concentrations of alcohol, the kit instructions were modified by using 20 μl rather than 10 μl of plasma and a total reaction volume of 1.02 ml rather than 3.01 ml.
To assess the contribution of spillage and evaporation to the results, in parallel with experiment 4, 24 empty cages (6 for each condition used in experiment 3) were interspersed in the rack housing the mice. These extra cages were treated as if they had mice in them: drinking tubes were placed on and removed from the cages at the same times that this was done for the mice. “Intakes” (i.e., changes in fluid levels) were recorded every 2 days.
Data Analysis
Intakes for each measurement period were collated to provide total consumption for each fluid and divided by the test duration to provide intakes per day. Alcohol intakes adjusted for body weight were calculated for each mouse by multiplying the volume of pure alcohol ingested (i.e., 10% of the volume of alcohol solution ingested) by 0.791 (alcohol’s specific gravity) and dividing this by the animal’s average body weight (collected at the beginning and end of each test series). Preferences were calculated according to the formula
For experiments 1 and 2, intakes of each fluid and alcohol preference were analyzed by separate ANOVAs involving a within-subject factor of test and (for the mice) a between-subject factor of strain. For experiments 3 and 4, intakes and alcohol preference were analyzed by mixed-design ANOVAs with group as a between-subject factor and day as a within-subject factor. In experiment 3, colored dye indicating spillage was detected in the bedding on 7 occasions (out of 1920 measurements). To avoid problems associated with missing data in the within-subject factor, in these cases intakes were estimated on the basis of the average of intakes on the previous and subsequent 2-day period.
In all experiments, significant differences between pairs of means were determined with post hoc least significant difference tests. The criterion for significance for all tests was p < 0.05. In preliminary analyses, we examined the distribution of drinking when more than one tube of the same fluid was available. Some subjects drank nearly exclusively from just one or two tubes; others drank from each tube containing the same fluid more or less equally. These patterns of intake seemed to be fairly constant for a given animal but differed so much between animals that they produced no interesting insights and so are not presented here.
RESULTS
Experiment 1: 3-Day Tests of Mice
For both strains, alcohol intakes and preference scores were highly dependent on the number of alcohol-containing drinking tubes available [intakes: F(5,150) = 24.7, p < 0.00001; preference scores: F(5,150) = 41.2, p < 0.00001], and water intake showed the inverse pattern [F(5,150) = 23.8, p < 0.00001; Fig. 2; Tables 1 and 2]. Total fluid intakes of the five conditions involving six bottles were all similar (range of condition means: B6 mice, 6.7–7.5 ml/day; 129 mice, 5.3–6.0 ml/day) but were significantly higher than the total intakes of the two-bottle test (B6 mice, 5.1 ± 0.2 ml/day; 129 mice, 4.2 ± 0.1 ml/day).
Fig. 2.
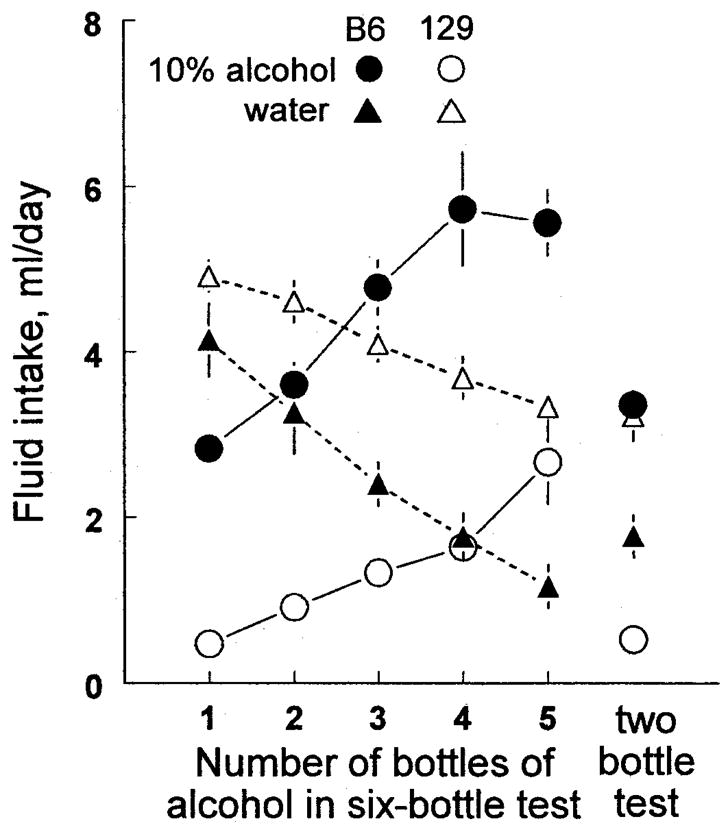
Mean daily intakes of 10% alcohol and water by 16 male C57BL/6J (B6) mice and 16 129X1/SvJ (129) mice given a standard 2-bottle test (right) or 6-bottle tests with 1, 2, 3, 4, or 5 bottles containing alcohol and the rest containing water (left). Each test lasted 72 hr, was given in a counterbalanced order, and was separated from subsequent tests by 3 days, during which the mice had one bottle of water to drink. Vertical bars show SEM.
Table 1.
Daily Alcohol Intakes in Grams of Alcohol per Kilogram Body Weight of Mice and Rats Given One to Five Bottles of 10% Alcohol to Drink (Experiments 1 and 2)
| Bottles of alcohol vs. bottles of water | C57BL/6J mice (n = 16) | 129X1/SvJ mice (n = 16) | Sprague-Dawley rats (n = 12) |
|---|---|---|---|
| 1 vs. 5 | 8.1 ± 0.7a | 1.3 ± 0.2a | 0.3 ± 0.2a |
| 2 vs. 4 | 10.3 ± 0.7a | 2.4 ± 0.4ab | 0.7 ± 0.3ab |
| 3 vs. 3 | 13.7 ± 0.9b | 3.5 ± 0.4bc | 1.7 ± 0.7bc |
| 4 vs. 2 | 16.5 ± 2.0c | 4.4 ± 0.3c | 1.5 ± 0.3ab |
| 5 vs. 1 | 16.0 ± 1.0bc | 7.2 ± 1.4d | 2.8 ± 0.5c |
| 1 vs. 1 | 9.6 ± 0.7a | 1.8 ± 0.2a | 1.6 ± 0.5bc |
Means with the same letter superscript do not differ significantly from each other (p < 0.05; least significant difference post hoc tests). Effect of test type for each group: B6, F(5,75) = 13.1, p < 0.00001; 129, F(5,75) = 13.6, p < 0.00001; rats, F(5,55) = 3.8, p < 0.01.
Table 2.
Alcohol Preference Scores of Mice and Rats Given One to Five Bottles of 10% Alcohol to Drink (Experiments 1 and 2)
| Bottles of alcohol vs. bottles of water | C57BL/6J mice (n = 16) | 129X1/SvJ mice (n = 16) | Sprague-Dawley rats (n = 12) |
|---|---|---|---|
| 1 vs. 5 | 42 ± 4a | 9 ± 2a | 3 ± 2a |
| 2 vs. 4 | 54 ± 4b | 16 ± 2b | 7 ± 2a |
| 3 vs. 3 | 67 ± 3c | 24 ± 3c | 15 ± 5ab |
| 4 vs. 2 | 76 ± 3d | 32 ± 4d | 16 ± 4ab |
| 5 vs. 1 | 83 ± 3d | 42 ± 3e | 31 ± 6c |
| 1 vs. 1 | 66 ± 5c | 22 ± 2c | 22 ± 8bc |
Means with the same letter superscript do not differ significantly from each other (p < 0.05, least significant difference post hoc tests). Effect of test type for each group: B6, F(5,75) = 24.2, p < 0.00001; 129, F(5,75) = 32.3, p < 0.00001; rats, F(5,55) = 4.2, p < 0.01.
As expected on the basis of previous studies with two-bottle tests (Bachmanov et al., 1996b; Tordoff and Bachmanov, 2002; Tordoff et al., 2002), there were very large differences between B6 and 129 mice in alcohol intake, water intake, total fluid intake, and alcohol preference (all p < 0.001). There were no interactions between strain and the type of test (all p > 0.05).
Experiment 2: 2-Day Tests of Rats
The results of the experiment with rats showed a similar pattern of response as observed with the mice. Alcohol intakes and preference scores were highly dependent on the number of alcohol-containing drinking tubes available [intakes: F(5,55) = 3.78, p < 0.01; preference scores: F(5,55) = 4.16, p < 0.005; Fig. 3; Tables 1 and 2]. Water intake showed the inverse relationship [F(5,55) = 6.95; p < 0.0001]. Total fluid intake was similar among the five conditions involving six bottles (means ranged from 43 ± 2 ml/day to 51 ± 4 ml/day) but was significantly lower in the condition involving two bottles [40 ± 2 ml/day; F(5,55) = 3.14; p < 0.05].
Fig. 3.
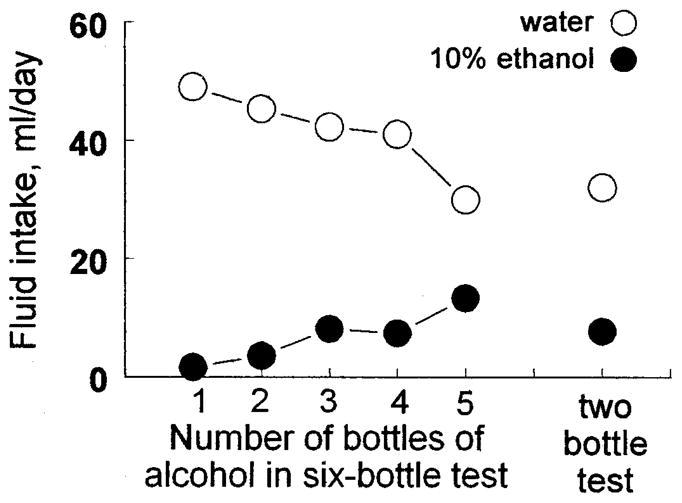
Mean daily intakes of 10% alcohol and water by 12 Sprague-Dawley rats given a standard 2-bottle test (right) or 6-bottle tests with 1, 2, 3, 4, or 5 bottles containing alcohol and the rest containing water (left). Each test lasted 48 hr, was given in a counterbalanced order, and was separated from subsequent tests by 1–3 days, during which the rats had one bottle of water to drink. SEM values were smaller than the symbols.
Experiment 3: 16-Day Test of B6 Mice With One, Three, or Five Bottles of Alcohol
Intakes
There were consistent differences among the four groups in all measures throughout the 16-day test (Fig. 4; Table 3). Mice in the AAAWAA group drank significantly more alcohol, significantly less water, and had significantly higher alcohol preference scores than did mice in any of the other three groups. Conversely, mice in the WWWAWW group drank significantly less alcohol, significantly more water, and had significantly lower preference scores than did mice in any of the other three groups. The WA and WAWAWA groups were intermediate between the AAAWAA and WWWAWW groups on all measures and did not differ on any of them [main effects of group: alcohol intake, F(3,44) = 7.51, p < 0.0001; water intake, F(3,44) = 4.83, p = 0.005; alcohol preference, F(3,44) = 11.0, p < 0.0001]. There were no significant differences among the four groups in total fluid intake.
Fig. 4.
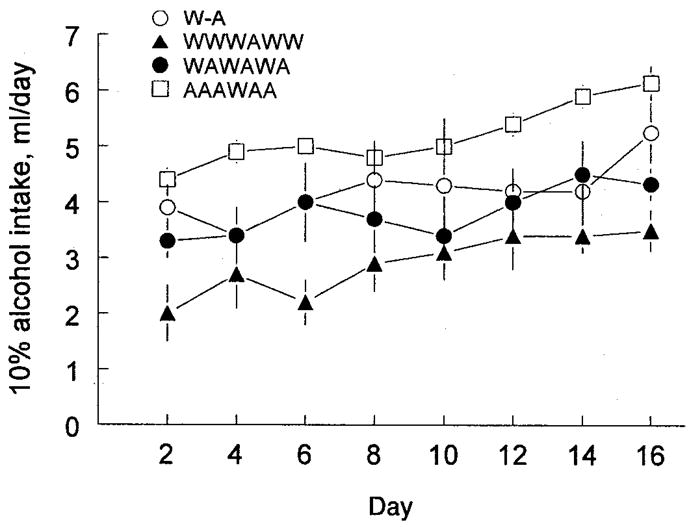
Average daily intakes of 10% alcohol by groups of 12 male C57BL6/J mice given a choice between various combinations of water and 10% alcohol (experiment 3). W-A, one bottle of water and one bottle of 10% alcohol; WWWAWW, five bottles of water and one bottle of 10% alcohol; WAWAWA, three bottles of water and three bottles of 10% alcohol; AAAWAA, one bottle of water and five bottles of 10% alcohol. Values are mean ± SEM. Table 3 lists significant differences among the groups. In this experiment, intakes of 4 ml/day of 10% alcohol were equivalent to approximately 15 g/kg/day.
Table 3.
Average Daily Intakes and Preferences of B6 Mice Given Access to Four Combinations of 10% Alcohol and Water for 16 Days (Experiment 3)
| Bottles of alcohol vs. bottles of water
|
||||
|---|---|---|---|---|
| Measure | 1 vs. 5 | 3 vs. 3 | 5 vs. 1 | 1 vs. 1 |
| 10% Alcohol intake (ml) | 2.7 ± 0.4a | 4.4 ± 0.4bc | 5.2 ± 0.1c | 3.8 ± 0.4ab |
| Alcohol intake (g/kg BW) | 11.0 ± 1.5a | 16.3 ± 1.6bc | 19.2 ± 0.6c | 14.1 ± 1.5ab |
| Water intake (ml) | 2.8 ± 0.4c | 2.3 ± 0.5bc | 0.7 ± 0.2a | 1.5 ± 0.4ab |
| Total fluid intake (ml) | 5.5 ± 0.2a | 6.7 ± 0.7a | 5.9 ± 0.2a | 5.3 ± 0.2a |
| 10% alcohol preference (%) | 45 ± 7a | 66 ± 8b | 89 ± 2c | 72 ± 7b |
Values are means ± SEM (n = 12/group). BW, body weight. Values with the same superscripts did not differ significantly from each other.
There were no interactions affecting intakes of individual groups over time. However, over the 16-day test for all four groups combined, alcohol and total fluid intakes progressively increased [main effect of time: alcohol intake, F(7,308) = 8.40, p < 0.0001; total fluid intake, F(7,308) = 4.20, p = 0.0002]. Water intakes did not change significantly, and the changes in alcohol intake were not sufficient to lead to significant increases in alcohol preference scores over time.
Two-Bottle Test
When all four groups of mice were given a two-bottle test for 2 days, they drank statistically similar volumes of alcohol and had similar preferences (WA group, 4.1 ± 0.4 ml/day, 80 ± 7%; AAAWAA group, 4.5 ± 0.2 ml/day, 89 ± 4%; WAWAWA group, 4.4 ± 0.2 ml/day, 90 ± 1%; WWWAWW group, 4.1 ± 0.2 ml/day, 87 ± 5%).
Experiment 4: 24-Day Test of B6 Mice With One or Five Bottles of Alcohol
Intakes
Mice given five bottles of alcohol and one of water drank significantly and consistently more alcohol than did mice given just one bottle of alcohol and one of water [main effect of group: F(1,22) = 9.46, p = 0.006; Fig. 5]. The mice with six bottles also had significantly greater alcohol preferences and total fluid intakes [Table 4; F(1,22) = 14.3, p = 0.001; F(1,22) = 4.93, p = 0.037, respectively]. There were no differences between the two groups in water intake.
Fig. 5.
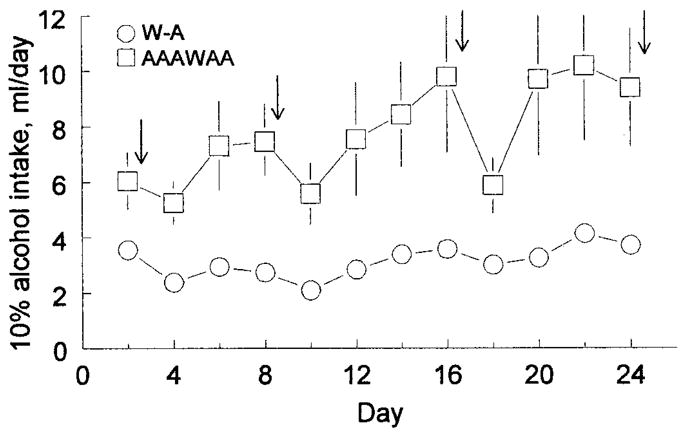
Average daily intakes of 10% alcohol by groups of 12 male C57BL6/J mice given a choice between either one bottle of water and one of 10% alcohol (W-A) or one bottle of water and five of 10% alcohol (AAAWAA). Values are mean ± SEM (SEM values for the W-A group were smaller than the symbols). Arrows show when tail blood samples were collected. In this experiment, intakes of 6 ml/day of 10% alcohol were equivalent to approximately 17.5 g/kg/day.
Table 4.
Average Daily Intakes and Preferences of B6 Mice Given Access to Either One Bottle of 10% Alcohol and One of Water or Five Bottles of 10% Alcohol and One of Water for 24 Days (Experiment 4)
| Bottles of alcohol vs. bottles of water
|
||
|---|---|---|
| Measure | 1 vs. 1 | 5 vs. 1 |
| 10% Alcohol intake (ml) | 3.1 ± 0.3 | 7.7 ± 1.5* |
| Alcohol intake (g/kg BW) | 9.4 ± 0.8 | 23.4 ± 4.4* |
| Water intake (ml) | 2.6 ± 0.3 | 3.5 ± 1.1 |
| Total fluid intake (ml) | 5.7 ± 0.2 | 11.2 ± 2.5* |
| 10% alcohol preference (%) | 55 ± 4 | 76 ± 4* |
Values are means ± SEM (n = 12/group). BW, body weight.
p < 0.001 relative to 1-vs.-1 group.
Alcohol intakes increased progressively over the 24-day test, but there was a marked reduction of alcohol and total fluid intake on days after blood samples were taken [Fig. 5; F(11,242) = 3.04, p < 0.0001; F(11,242) = 2.02, p = 0.027, respectively]. The decrease in total fluid intake was larger in the mice with five bottles rather than one bottle of alcohol [group × day interaction: F(11,242) = 1.88; p = 0.042].
Plasma Alcohol Concentrations
Mice with five bottles of alcohol had significantly higher plasma alcohol concentrations than did mice with one bottle of alcohol [F(1,22) = 8.82; p = 0.007 for the four samples combined]. Average values for the four samples taken were 2.57 ± 0.14 mg/dl for the WA group and 3.69 ± 0.15 mg/dl for the AAAWAA group. Planned comparisons revealed that the two groups differed significantly at three of the four times the mice were sampled (the comparison on day 8 was marginally nonsignificant: day 2, 2.49 ± 0.98 mg/dl versus 4.29 ± 0.98 mg/dl; day 8, 3.00 ± 0.19 mg/dl versus 3.6 ± 0.18 mg/dl; day 16, 2.46 ± 0.27 mg/dl versus 3.74 ± 0.27 mg/dl; day 24, 2.30 ± 0.23 mg/dl versus 3.04 ± 0.24 mg/dl).
Spillage From Empty Cages
The average volume lost from each of the 120 tubes mounted on empty cages was (±SD) 0.14 ± 0.028 ml/day over the 24-day test. Significantly more alcohol than water was lost [alcohol, 0.16 ± 0.017 ml/day per drinking tube; water, 0.11 ± 0.016 ml/day per drinking tube; t(118) = 14.7; p < 0.0001]. Loss of fluid from each spout did not depend on the number of drinking tubes a cage had (average loss per drinking tube: cages with two drinking tubes, 0.15 ± 0.031 ml/day per drinking tube; cages with six drinking tubes, 0.13 ± 0.028 ml/day per drinking tube).
We reanalyzed the data from all four experiments after correcting each intake for spillage by subtracting the values calculated previously (i.e., 0.11 ml/day from each water intake and 0.16 ml/day from each alcohol intake). ANOVAs conducted on these modified data produced F scores that were slightly lower than those for the uncorrected data, although all differences reported as significant with the uncorrected data were also significant with the corrected data.
DISCUSSION
This study shows that alcohol intake and preference of mice and rats is profoundly influenced by the number of sources of alcohol available. The more bottles of alcohol the animals had, the more they drank. The difference in intake when drinking from one rather than five bottles of alcohol was quite dramatic, even obscuring strain differences. For example, in experiment 1, preference scores for B6 and 129 mice drinking 10% alcohol in a standard two-bottle test were 66 ± 5% and 22 ± 2%, respectively, which are similar to results obtained in many other studies and are consistent with the use of these strains as genetic models of high and low alcohol consumption (e.g., Bachmanov et al., 1996b; Belknap et al., 1993). However, with one bottle of alcohol and five of water available, the B6 mice showed only a 42 ± 4% alcohol preference score (i.e., avoidance relative to water), and with five bottles of alcohol and one bottle of water, the 129 mice also showed a 42 ± 3% preference score. Thus, with conditions differing only by the choices available, the high and low alcohol-preferring strains had similar alcohol preferences. We conclude that apparently trivial manipulations of alcohol availability can profoundly influence alcohol intake and preference, even to the extent that they can override the genetic controls of alcohol intake.
Alcohol intake and preference seemed to depend on the number of bottles of water as well as alcohol. Preferences were similar whether mice or rats had three bottles of alcohol and three bottles of water or one bottle of alcohol and one of water (Tables 2 and 3). This implies that alcohol consumption is determined by the availability of alcohol relative to the total number of choices; that is, the choice to ingest alcohol is made within the context of other available options. There are several examples of rodents given a choice between various concentrations of alcohol, or alcohol and a sweet solution (e.g., Fuller, 1964; Gentry and Dole, 1987; Lester and Greenberg, 1952; Mardones et al., 1955; Rodd-Henricks et al., 2001). Although it is often assumed that intake of each fluid is independent from other choices in such tests, our results suggest that this is probably not the case. They also suggest that the interpretation of studies in which multiple concentrations of alcohol are presented requires the inclusion of controls for the number of sources of alcohol provided. As far as we aware, such controls have not been conducted.
Fluid spillage (and evaporation) is a methodological concern with the use of multiple bottles. This is particularly the case for experiments with mice drinking alcohol, because intakes are relatively low and alcohol is particularly liable to spill due to its low surface tension. A contribution of spillage to these studies was suggested by our findings that measured total fluid intakes were significantly higher during six-bottle than during two-bottle tests. We suspect that actual total fluid intakes were unaffected by the number of bottles available, and so the higher recorded intakes were due to spillage. However, we cannot rule out the possibility that greater availability increased actual total fluid intake, as is clearly the case for rats drinking from multiple bottles of sucrose (Tordoff, 2002). Evidence against spillage making a substantial contribution to the results comes from several sources. In one experiment, we added nonabsorbable dyes to the water and alcohol to make spillage easily visible, but it was detected in bedding on only 7 out of 1920 measurements. We also measured the change in volume that occurred in drinking tubes mounted on empty cages. This suggested that loss of alcohol due to spillage and evaporation was 0.16 ml/day per drinking tube. In comparison, each extra alcohol-containing drinking tube increased alcohol intake by approximately 0.7 ml/day. Thus, we suspect that spillage and evaporation are responsible for 20–25% of the effect of alcohol availability on intake in mice [see Tordoff and Bachmanov (2003) for additional discussion].
Our finding that plasma alcohol concentrations were higher in mice given five rather than one bottle of 10% alcohol provides a spillage-independent confirmation that the groups differed in alcohol intake. The higher intakes associated with the four extra bottles of alcohol increased plasma alcohol concentrations by approximately 40%, although in both the one- and five-bottle conditions these were low and fairly variable. This is to be expected given that the blood samples were collected during the middle of the light period, when mice are least active and were thus unlikely to have consumed alcohol recently. Nevertheless, we doubt that concentrations at any time were high enough to induce intoxication or addiction. Also arguing against addiction is our finding that 16 days of experience with several bottles of alcohol did not influence alcohol intake or preference in a subsequent two-bottle choice test.
The influence of availability on intake is not restricted to just alcohol. We have observed this effect with 32% sucrose solutions (Tordoff, 2002), solid macronutrients (Tordoff, 2002), and representative sweet, sour, bitter, and salty solutions (2 mM saccharin, 5 mM citric acid, 30 μM quinine hydrochloride, and 75 mM NaCl, respectively; Tordoff and Bachmanov, 2003). This work and unpublished studies also show that there is no interaction between mouse strain (B6 versus 129 mice) and the number of sources of nutrient or taste solution. These findings argue that the effect of availability on intake is independent of the genetic differences between rodents and is common to all foods and fluids.
Why does an animal drink from several bottles rather than just one if they all contain the same fluid? This makes sense from an ethological standpoint because consuming a little of each source would enhance survival by allowing partially nibbled vegetation to regrow and predated-on animal populations to regenerate. Selecting from distributed sources might also prevent consumption of large amounts of toxins. We have suggested elsewhere (Tordoff, 2002) that the “availability effect” is related to the “variety effect,” in which rats given foods of different flavors or textures overconsume relative to those given foods of only one flavor or texture. In this sense, each drinking tube provides variety. This is not simply novelty, because novelty decays but the high intakes persisted, occurring over several days of testing with alcohol (i.e., 16 days in experiment 3 and 24 days in experiment 4) and for longer than 35 days with sucrose (Tordoff, 2002). The “variety effect” is usually attributed to hedonic factors or the lack of sensory-specific satiety (e.g., Rolls, 1986; Rolls et al., 1983), but these explanations are more descriptive than mechanistic. Until a physiologic substrate is found, we prefer a more operational explanation. We suspect that the rodent simply takes advantage of the additional opportunities to ingest: it drinks more when extra spouts are available because it encounters them more frequently. One implication of this is that rodents often drink because the alcohol is there and not always in response to nutritional or pharmacological needs. This is contrary to the widely held view that nutritional wisdom is the basis of nutrient intake in animals, although it is probably more similar to the generally accepted case for humans.
The limits of the availability effect remain to be tested. A critical study will be to determine whether intakes can be increased even higher, perhaps to levels producing intoxication, by providing more than five bottles of alcohol. There are also a number of potentially interesting parametric investigations involving manipulations of the number of sources and difficulty of obtaining alcohol that might tie the phenomenon into economic models of foraging (e.g., Collier, 1985; Liguori, 1993). It would also be interesting to know whether the availability-induced consumption of alcohol is influenced by pharmacological agents used in the treatment of alcoholism and its comorbid disorders.
It is generally assumed that alcohol consumption by animals is a function of three factors: chemosensory perception, caloric need, and pharmacologic susceptibility. However, this is based almost entirely on studies in which the availability of alcohol is limited to one bottle. The present results highlight that environmental contingencies are just as important as physiologic factors in determining alcohol intake, if not more so. Availability is clearly a factor in the consumption of alcohol by mice and rats, as it most likely is by humans. As such, these results point to a new animal model that could be useful for understanding the influence of alcohol availability on human alcohol consumption.
Acknowledgments
We thank Diane Pilchak and Julie Williams for their excellent technical assistance.
Supported by NIH Grant AA-12715.
References
- Bachmanov AA, Reed DR, Beauchamp GK, Tordoff MG. Body weight, food intake, water intake, and drinking spout side preference of 28 mouse strains. Behav Genet. 2002;32:435–443. doi: 10.1023/a:1020884312053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmanov AA, Reed DR, Tordoff MG, Price RA, Beauchamp GK. Intake of ethanol, sodium chloride, sucrose, citric acid, and quinine hydrochloride solutions by mice: a genetic analysis. Behav Genet. 1996a;26:563–573. doi: 10.1007/BF02361229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmanov AA, Tordoff MG, Beauchamp GK. Ethanol consumption and taste preferences in C57BL/6ByJ and 129/J mice. Alcohol Clin Exp Res. 1996b;20:201–206. doi: 10.1111/j.1530-0277.1996.tb01630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belknap J, Crabbe J, Young E. Voluntary consumption of alcohol in 15 inbred mouse strains. Psychopharmacology. 1993;112:503–510. doi: 10.1007/BF02244901. [DOI] [PubMed] [Google Scholar]
- Collier GH. Satiety: an ecological perspective. Brain Res Bull. 1985;14:693–700. doi: 10.1016/0361-9230(85)90120-0. [DOI] [PubMed] [Google Scholar]
- Fuller JL. Measurement of alcohol preference in genetic experiments. J Comp Physiol Psychol. 1964;57:85–88. doi: 10.1037/h0043100. [DOI] [PubMed] [Google Scholar]
- Gentry RT, Dole VP. Why does a sucrose choice reduce consumption of alcohol in C57BL/6J mice? Life Sci. 1987;40:2191–2194. doi: 10.1016/0024-3205(87)90010-5. [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council. Guide for the Care and Use of Laboratory Animals. 7. National Academy Press; Washington, DC: 1996. [Google Scholar]
- Lester D, Greenberg LA. Nutrition and etiology of alcoholism: the effect of sucrose, saccharin and fat on the self-selection of ethyl alcohol by rats. Q J Stud Alcohol. 1952;13:553–560. [PubMed] [Google Scholar]
- Liguori A. Ethanol-maintained behavior in a foraging context: effects of search and procurement cost. Psychopharmacology. 1993;113:231–236. doi: 10.1007/BF02245703. [DOI] [PubMed] [Google Scholar]
- Mardones J, Segovia-Riquelme N, Hederra A, Alcajno G. Effect of some self-selection conditions on the voluntary alcohol intake of rats. Q J Stud Alcohol. 1955;13:425–437. [PubMed] [Google Scholar]
- Richter CP. Total self-regulatory functions in animals and human beings. Harvey Lect Ser. 1942–1943;38:63–103. [Google Scholar]
- Richter CP. Salt appetite of mammals: its dependence on instinct and metabolism. In: Cie ME, editor. L’Instinct dans le Comportement des Animaux et de l’Homme. Librares de l’Academie de Medécine; Paris: 1956. pp. 577–629. [Google Scholar]
- Rodd-Henricks ZA, Bell RL, Kuc KA, Murphy JM, McBride WJ, Lumeng L, Li T-K. Effects of concurrent access to multiple ethanol concentrations and repeated deprivations on alcohol intake of alcohol-preferring rats. Alcohol Clin Exp Res. 2001;25:1140–1150. [PubMed] [Google Scholar]
- Rolls BJ. Sensory specific satiety. Nutr Rev. 1986;44:93–101. doi: 10.1111/j.1753-4887.1986.tb07593.x. [DOI] [PubMed] [Google Scholar]
- Rolls BJ, van Duijvenvoorde PM, Rowe EA. Variety in the diet contributes to the development of obesity in the rat. Physiol Behav. 1983;30:21–27. doi: 10.1016/0031-9384(83)90091-4. [DOI] [PubMed] [Google Scholar]
- Tordoff MG. Obesity by choice: the powerful effect of nutrient availability on nutrient intake. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1536–R1539. doi: 10.1152/ajpregu.00739.2001. [DOI] [PubMed] [Google Scholar]
- Tordoff MG, Bachmanov AA. [Accessed: February 9, 2003];Monell mouse taste phenotyping project. 2001 Available at: http://www.monell.org/MMTPP.
- Tordoff MG, Bachmanov AA. Influence of test duration on the sensitivity of the two-bottle choice test. Chem Senses. 2002;27:759–768. doi: 10.1093/chemse/27.9.759. [DOI] [PubMed] [Google Scholar]
- Tordoff MG, Bachmanov AA. Mouse taste preference tests: why only two bottles? Chem Senses. 2003 doi: 10.1093/chemse/28.4.315. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tordoff MG, Pilchak DM, Williams JA, McDaniel AH, Bachmanov AA. The maintenance diets of C57BL/6J and 129X1/SvJ mice influence their taste solution preferences: implications for large-scale phenotyping projects. J Nutr. 2002;132:2288–2297. doi: 10.1093/jn/132.8.2288. [DOI] [PMC free article] [PubMed] [Google Scholar]


