Abstract
Adrenal hormones regulate glucose levels, responses to unpredictable stressors and modulate cognition. Glucocorticoids can have an inverted-U shape relationship with cognition, as very low or high levels impair, whereas moderate elevations facilitate, acquisition and retention of memories. To date these relationships have been tested with humans and rodents in laboratory settings rather than with wild animals in biologically relevant contexts. This study examined whether the elevated cortisol observed in juvenile Belding's ground squirrels (Spermophilus beldingi) at natal emergence might promote both acquisition of adaptive responses to this species' two alarm calls warning of predators and memory of the spatial configuration of mothers' territories. Both experimentally increased and decreased basal cortisol levels interfere with acquisition and retention of an association between a warning call and the appropriate response compared with naturally occurring moderately elevated cortisol. Further, decreased cortisol impairs learning of a novel, complex spatial maze. Thus in the field the brief elevation of cortisol at emergence might facilitate acquisition of spatial memory of a three-dimensional environment and responses to alarm calls during a sensitive period of learning. This novel demonstration of the inverted-U shape function in a wild animal suggests that natural selection has favored a hormonal profile facilitating rapid acquisition of important survival behaviors.
Keywords: cortisol, spatial learning, associative learning, ground squirrel, development
1. Introduction
Stress hormones can have an inverted-U shape effect on cognition which is due to differential activation of mineralocorticoid and glucocorticoid receptors (MRs and GRs) in the hippocampus and amygdala, among other areas. At basal glucocorticoid levels, 80-90% of MRs but only 10-15% of GRs are occupied, and learning and memory are facilitated. However, when MRs and GRs are equally occupied, due to low basal corticoid levels or to high levels as a result of acute stress, acquisition and consolidation of memories are impaired (de Kloet, Oitzl, & Joels, 1999; Ferguson & Sapolsky, 2007; Herbert, Goodyer, Grossman, Hastings, de Kloet, Lightman, Lupien, Roozendaal, & Seckl, 2006; Lupien & McEwen, 1997; Roozendaal & McGaugh, 1996).
This relationship between glucocorticoids and cognition is complex, and can vary by developmental stage, sex, reproductive status, and with the nature of the learning paradigm and the neural structures involved. For example, acute increases in corticosterone facilitate learning by rats in a Morris water maze, but the effect depends on their prior experience (Sandi, Loscertales, & Guaza, 1997). Three months, but not one month, of chronic elevation of corticosterone impair Morris maze learning in middle-age rats, but young rats are not affected by either duration (Bodnoff, Humphrey, Lehmann, Diamone, Rose, & Meaney, 1995; see also Conrad, Lupien, & McEwen, 1999; see also Dachir, Kadar, Robinzon, & Levy, 1993; Roskoden, Linke, & Schwegler, 2005 for studies with y-mazes and radial-arm mazes). Acute stressors do not influence trace eye-blink conditioning (TEBC) in pre- or pubertal rats, but in adults they impair female and enhance male performance (Hodes & Shors, 2005; see also Duncko, Cornwell, Cui, Merikangas, & Grillon, 2007). In contrast, chronic corticosterone implants impair TEBC performance by prepubertal males (Claflin, Hennessy, & Jensen, 2005). Research on the inverted-U shape function has largely focused on humans performing standardized cognitive tests or on laboratory animals learning traditional conditioning tasks (reviewed in de Kloet et al., 1999; Lupien & McEwen, 1997; Roozendaal, Cahill, & McGaugh, 1996). This approach has been very successful in unraveling the mechanisms of glucocorticoid's actions, particularly at the molecular and cellular levels, and we are now at a point where we can apply these findings to freely behaving outbred animals learning species-typical behaviors in their natural environments. Different species experience divergent evolutionary pressures, which in turn result in differing cognitive abilities. Belding's ground squirrels are ideal for this investigation because the development of their cortisol profiles and anti-predator behaviors are both well documented.
Aerial and terrestrial predators hunt S. beldingi and elicit whistle and trill alarm calls, respectively. Listeners respond to whistles by running to or entering a burrow, whereas they typically adopt a bipedal stance (‘post’) in response to trills (Mateo, 1996). Optimal responses (initial reaction, duration of alert behavior) vary by habitat and even location within a meadow (e.g. center versus edge), and this plasticity is likely favored because predator environments change temporally and spatially (Mateo, 2007; Mateo & Holmes, 1999b). Juveniles emerge from natal burrows nearly weaned at about one month of age, and quickly learn appropriate alarm-call responses within five days. During this period young are also learning the spatial configuration of their mother's burrows and aboveground territory, including the locations of escape routes and holes and prime foraging areas. Rapid learning is important because natal emergence attracts predators and up to 30% of juveniles disappear during their first two weeks aboveground, presumably due to predation. In addition, at this time young are vulnerable to infanticide and are becoming nutritionally independent (Mateo, 1996, 2007; Mateo & Holmes, 1997, 1999b).
Acquisition of these survival behaviors thus coincides with considerable potential stressors, and juvenile basal cortisol is increased at natal emergence compared with pre-emergence levels, and at levels 1.3-2.1 times higher than those observed two weeks following emergence (Mateo, 2006; unpubl. data). This brief elevation has been observed in least three ecologically distinct populations with differences in predation risk. Furthermore, cortisol is similarly elevated at the age of emergence in captive juveniles experiencing a constant environment, indicating it is not due to exposure to novelty or stressors associated with emergence. Juvenile cortisol lowers within two weeks to population-specific levels similar to those of adults (Mateo, 2006, 2007).
This study examined whether the moderately elevated glucocorticoids at natal emergence facilitate S. beldingi learning, as they can in humans and inbred strains of rats and mice (Bodnoff et al., 1995; Catalani, Marinelli, Scaccianoce, Nicolai, Muscolo, Porcu, Koranyi, Piazza, & Angelucci, 1993; Conrad et al., 1999; de Kloet, de Kock, Schild, & Veldhuis, 1988; Ferguson & Sapolsky, 2007; Lupien & McEwen, 1997; Lupien, Wilkinson, Briere, Menard, Kin, & Nair, 2002; Shors, Weiss, & Thompson, 1992; Takahashi, 1994). Using two techniques to manipulate cortisol non-invasively, The effects of experimentally lowered and normally elevated basal cortisol on spatial and associative learning were studied in juvenile S. beldingi (Group 1). The associative-learning experiment was also conducted with control and very high basal cortisol levels (Group 2).
2. Material and Methods
2.1. Animals, study site, and cortisol manipulations
Belding's ground squirrels are group-living, burrowing rodents found in alpine and subalpine regions of the western United States (Jenkins & Eshelman, 1984). They are socially active above ground between April and August and hibernate the remainder of the year. Each adult female produces one litter annually of 5-8 pups, which is reared for about a month in an underground burrow (the natal burrow). Young first come above ground (emerge) as nearly weaned, 4-week old juveniles. About one month after natal emergence, juvenile females establish their own burrow system within 25 m of their natal burrow, whereas juvenile males begin to disperse permanently from their birthplace (Holekamp, 1984). Females live an average of 3.4 ± 0.3 years (up to 12 years); males live 2.5 ± 0.4 years (up to 9 years; Sherman & Morton, 1984; pers. obs.).
Research was conducted at the Sierra Nevada Aquatic Research Laboratory (SNARL; administered by University of California at Santa Barbara) near Mammoth Lakes, California, and was approved by the IACUCs of the University of Chicago and UCSB. Group 1 juveniles were born in captivity to field-mated females housed in a laboratory at SNARL. Juvenile basal cortisol was lowered non-invasively by increasing maternal cortisol during lactation. Hydrocortisone (Sigma; WI USA) was suspended in a drop of sesame oil and added to equal parts peanut butter and wheat germ to form a ball ∼1.5 cm diameter. Mothers received one ball/day for 25 d starting the day after parturition (CONTROL1: n = 8 mothers, 0 mg hydrocortisone/g bodyweight; MEDIUM Cortisol: n = 5, 0.045 mg/g; HIGH Cortisol: n = 5, 0.09 mg/g). Doses were adjusted every five days according to maternal weight. In rats, a similar manipulation mimics chronic, mild stress in corticosterone-fed mothers and can produce offspring with lower basal corticosterone (Catalani et al., 1993). Because cortisol of juveniles reared by MEDIUM and HIGH mothers was lower than that of CONTROL1 juveniles, they will hereafter be referred to as LOW1 and LOW2 CORT groups, respectively. Spatial memory of juveniles was tested in the lab with a subset of offspring (CONTROL1: n = 10 males, 15 females from 8 litters; LOW1 CORT: n = 3 males, 11 females from 5 litters; LOW2 CORT: n = 7 males, 10 females from 5 litters; 1-4 juveniles from each litter). After the spatial-maze study was concluded, a larger subset of juveniles was transferred with their mothers to one of three large outdoor enclosures at SNARL for an associative-learning study (CONTROL1: 4 mothers and their 9 sons and 19 daughters; LOW1 CORT: 4 mothers and their 9 sons and 18 daughters; LOW2 CORT: 4 mothers and their 8 sons and 17 daughters; each treatment group was housed together in one enclosure). Each 10 × 10 × 2 m open-air enclosure included natural vegetation, laboratory food and water, and four buried nestboxes connected to the surface by plastic tunnels (see Mateo & Holmes, 1997 for details).
For Group 2, juveniles were field collected with their mothers 0-4 days after natal emergence (average 1.28 days) and placed in two separate enclosures at SNARL. Juveniles were no longer nursing from their mothers when the cortisol manipulation began. Cortisol was raised non-invasively by giving the HIGH CORT group 0.5g hydrocortisone/liter of drinking water for one week prior to and for the duration of behavioral testing (n = 4 mothers and their 10 sons and 7 daughters); The CONTROL2 group received untreated water (n = 4 mothers and their 7 sons and 14 daughters). Hydrocortisone doses for both Studies 1 and 2 were after Catalani et al. (1993). After each study feces were collected from all animals and corticoid metabolites were measured following the methods in Mateo and Cavigelli (2005). Fecal-cortisol values were log-transformed for normality, which was confirmed with Kolmogorov-Smirnov tests. Details of trapping, marking and housing of animals are in Mateo and Holmes (1997). Animals were released at the site of their or their mother's capture at the end of the studies.
2.2. Spatial-maze learning
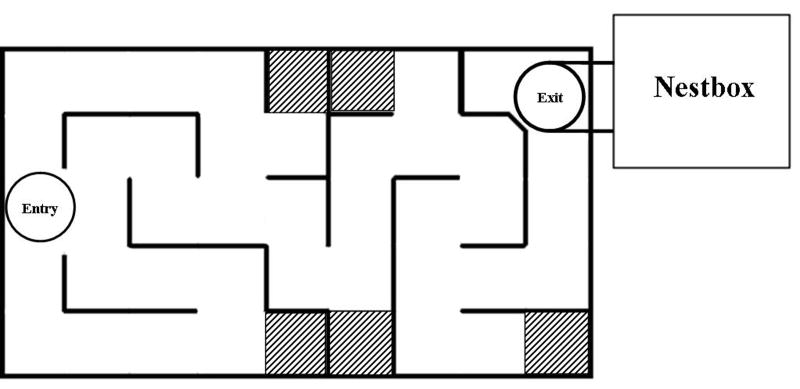
To simulate the exploration and learning of their mother's burrow system and aboveground territory after natal emergence, a subset of Group 1 juveniles was tested in a modified Habitrail Mini Maze for Mice® (Fig. 1; 48 × 25 × 5 cm with two 2.5 cm holes on either side of the lid for entry and exit). This novel maze with its branching and blind alleys was chosen over a Morris water maze or a radial-arm maze to simulate the three-dimensional micro-topographic features surrounding natal burrows in the field, such as vegetation highways, rocks and logs. With its family in a holding box, an animal's home nestbox was connected to the maze's exit hole via a PVC elbow pipe. In a pilot study some young juveniles would travel halfway through the maze and stop, so the nestbox was attached to provide some odor cues to motivate them to continue moving through the maze and return to a familiar location. Juveniles did not immediately orient toward and move to the end of the maze with the nestbox, suggesting that odors did not drift far into the maze. For each trial an animal was placed in the entry hole and removed when either it entered the nest box or after 300 seconds. To familiarize animals with the apparatus, after the first trial (during which no animal exited) each juvenile was placed at the end of the maze and allowed to climb into the nestbox. Testing started at 26 days of age (n = 10), or, because of another ongoing study, at 30 days (n = 25). Trials were recorded with a Sony HandyCam® camcorder for quantification of latencies to exit and numbers of entries to one of five blind alleys (‘errors’), coded by someone blind to the cortisol condition of the animals but aware of the study's hypothesis. Animals ran once daily until they reached criterion of two trials with ≤ two errors per trial to complete the maze. A more stringent criterion of two consecutive trials was not adopted because of high variability in animals' behaviors. Mazes were cleaned with isopropyl alcohol after each trial. Numbers of trials required was not affected by age at the start of testing (26 or 30 days; F1,40 = 0.07, P > 0.80). After reaching criterion animals were tested without the nestbox to confirm that they learned the maze's spatial configuration rather than relied on nestbox odor cues to navigate.
Fig. 1.
Overhead schematic of the modified Habitrail Mini-maze for Mice® (48 × 25 × 5 cm) with 5 cm-wide alleys and two 2.5 cm holes on either side of the transparent lid for entry and exit. Dark lines denote solid walls; hatched areas denote blind alleys, entry into which would be considered ‘errors’. A PVC elbow pipe connected the exit hole to the juvenile's home nest box. Juveniles ranged from 5-11 cm in length (nose to rump) during the course of the study.
2.3. Associative learning
To assess whether glucocorticoids modulate learning of alarm-call responses (Mateo, 1996, 2006), in particular the acquisition of an association between calls and their appropriate behavioral reactions, a classical-conditioning playback study was conducted with Group 1 and 2 juveniles living in outdoor enclosures. During training sessions, a recording of an S. beldingi squeal (juvenile vocalization sometimes made during play) was played 1-3 seconds prior to release of a fast-moving overhead visual stimulus (a frisbee) across the enclosure, as if the squeal warned of an aerial predator. After one daily training session each day for four days, the squeal was played without the frisbee on Day 5 to test for learning of the association (SQ Probe 1) and again on Day 10 to test for retention (SQ Probe 2). Predation risk is high after natal emergence (Mateo, 1996), and juveniles may not have multiple opportunities to hear alarm calls and observe appropriate responses, so next the effects of cortisol on one-trial associative learning were tested. A frisbee was paired with a Brewer's blackbird (Euphagus cyanocephalus) alarm call that warns other blackbirds of a raptor on Day 10, after SQ Probe 2, and the alarm alone was presented on Day 11 (Bird Probe). Squeals and blackbird alarms were used as conditioned stimuli because they are commonly heard after juvenile emergence but S. beldingi do not react behaviorally to either vocalization without training (Mateo, 1996; Mateo & Holmes, 1999b).
During training sessions all juveniles responded, typically by running to the nearest burrow or entering it, characteristic of responses to whistles warning of aerial predators. Some responses were socially facilitated, prompted by another animal's response rather than the frisbee itself (see also Mateo, 1996), but still allowed animals to hear the call and associate it with a rapid escape. Responses to probe trials were videotaped for determination of the number of animals responding, initial responses (look or freeze, post - a bipedal stance, run to a burrow, or enter a burrow) and duration of responses (Mateo, 1996; Mateo & Holmes, 1999b). Reactions of animals out of camera view were included in analyses, although their response durations were not recorded. Significant group differences in frequency data (likelihood of responding and types of initial responses to playbacks) were analyzed with Chi-square tests with partitioned tables used for post-hoc comparisons for significant differences in initial responses (Siegel & Castellan, 1988). Previous playback studies involving over 100 S. beldingi litters have failed to detect litter effects (Mateo, 1996; Mateo & Holmes, 1997, 1999a; 1999b; J. M. Mateo, unpubl. data), and thus individual responses were used as units of analyses (see Mateo, 1996 for details). If juveniles learn to associate the conditioned stimuli with fast-moving, overhead objects similar to aerial predators, then responsivity should be high, with initial responses of running to a burrow and long response durations (Mateo, 1996; Mateo & Holmes, 1999a; 1999b).
3. Results
3.1. Cortisol manipulations
For Group 1, treatment of mothers during lactation with hydrocortisone-laced peanut-butter balls increased maternal cortisol in all five sampling periods compared with pre-treatment levels (‘round 0’), with Medium and High Cortisol levels significantly higher than CONTROL1 levels in rounds 1-5, and High Cortisol significantly higher than Medium Cortisol in round 2 (overall F8,60 = 3.86, P < 0.0001; all Bonferroni-adjusted pairwise t-test post-hoc comparisons, Ps < 0.0001; Fig. 2a). Treatment of mothers with exogenous cortisol resulted in significantly lower juvenile corticoids (ANCOVA using age as a covariate: F2,65 = 5.70, P = 0.005; Fig. 2b). Between litter variation did not exceed within litter variation (CONTROL1: F7,22 = 1.01, P = 0.45; LOW1 CORT: F3,14 = 1.12, P = 0.37; LOW2 CORT: F4,13 = 1.42, P = 0.28). CONTROL1 juveniles weighed significantly more than juveniles in both treatment groups at the beginning of the spatial-maze study (F2,67 = 21.18, P < 0.001; all Bonferroni-adjusted pairwise t-test post-hoc comparisons, Ps < 0.0001), but at the conclusion of the subsequent associative-learning study, juveniles in both treatment groups weighed more than the CONTROL1 juveniles (ANCOVA using age as a covariate: F2,76 = 3.76, P = 0.03), with LOW1 CORT juveniles weighing significantly more based on Bonferroni post-hoc comparisons (P = 0.025). Although cortisol of the LOW1 and LOW2 CORT juveniles did not differ, their learning performance was analyzed separately because differential exposure to maternal cortisol could affect cognitive development.
Fig. 2.
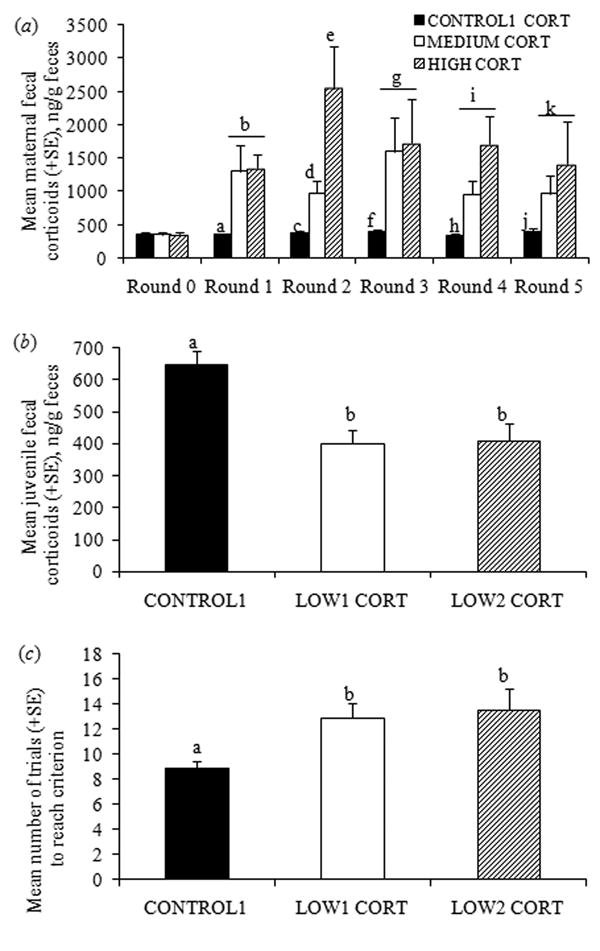
The relationship between maternal cortisol, juvenile cortisol and juvenile spatial learning. (a) Maternal fecal corticoids across six sampling periods, starting at round 0 prior to the onset of cortisol manipulation and repeated every five days (repeated-measures ANOVA comparing three treatments across six sampling periods). Mothers received no (CONTROL1), MEDIUM or HIGH levels of exogenous cortisol. (b) Juvenile fecal corticoids, measured at the conclusion of the spatial-learning study. Because cortisol of juveniles reared by MEDIUM and HIGH mothers was lower than that of CONTROL1 juveniles, they are referred to as LOW1 and LOW2 CORT groups, respectively. (c) Numbers of trials required to reach criterion in a complex spatial maze. Different letters over columns indicate significant differences based on Bonferroni-adjusted post-hoc pairwise t-test comparisons for significant ANOVAs.
In Group 2, the HIGH CORT juveniles received exogenous cortisol in their drinking water, resulting in higher fecal corticoid levels than the CONTROL2 group (X̅ ± SEM: 768.68 ng/g dried feces ± 72.59 and 191.90 ± 29.40 ng/g, respectively; independent t-test on log-transformed data: t36 = 10.50, P < 0.0001; variation between litters did not exceed variation within litters: CONTROL2: F3,17 = 2.76, P = 0.74; HIGH CORT: F3,13 = 0.256, P = 0.86). Body weights did not differ between the groups at emergence in the field (t36 = 1.52, P = 0.14), but the HIGH CORT juveniles weighed less at the conclusion of the associative-learning study (t36 = 4.23, P = 0.001). However, a subset of juveniles was re-captured one week after being returned to the field, and their corticoids did not differ significantly (t8 = 1.03, P = 0.33). Corticoids of Group 2 HIGH CORT juveniles were higher than Group 1 CONTROL1 juveniles (ANCOVA using age as a covariate; F1,59 = 16.76, P = 0.0001).
3.2. Spatial-maze learning
Variation between litters in the number of trials to reach criterion did not exceed that within litters (F13,21 = 1.54, P = 0.18), so individual juveniles were the units of analysis. CONTROL1 juveniles reached criterion (completing the maze with ≤ 2 errors in each of two trials) in significantly fewer trials than both LOW1 and LOW2 CORT juveniles (log-transformed data, normality confirmed with a Kolmogorov-Smirnov test; ANOVA F2,32 = 6.96, P = 0.003; Bonferroni-adjusted pairwise t-test post-hoc comparisons, P = 0.046 and P = 0.006, respectively; Fig. 2c). The groups did not differ statistically in the number of juveniles to reach criterion (CONTROL1: 19/25, LOW1 CORT: 7/14, LOW2 CORT: 9/17; χ2 = 3.54, df= 2, P = 0.17), although CONTROL1 juveniles were more likely to do so. There was no significant difference in the number of juveniles which completed the maze with ≤ 2 errors twice in a row (CONTROL1: 14/19, LOW1 CORT: 6/7, LOW2 CORT: 4/9; χ2= 3.62, df= 2, P = 0.16). Across the first ten trials, for which there were sufficient sample sizes, there were no significant differences between groups in the number of errors made (F2,39 = 0.45, P > 0.64). There were no significant sex, beginning bodyweight or litter-size effects on rates of learning (ANOVAs and ANCOVAs, all Ps > 0.05). Groups did not differ in the likelihood of exiting the maze during this final test without the nest box (CONTROL: 17/19 animals, LOW1 CORT: 6/7, LOW2 CORT: 8/9; χ2 = 0.073, df = 2, P = 0.96). Although this maze could involve both spatial (place) and associative (response or motoric) learning (e.g. Gibson & Shettleworth, 2005; Kesner, Bolland, & Dakis, 1993), within-individual variation in latencies to exit the maze (a proxy for response or motor learning) was larger than between-individual variation (F55,156 = 0.92, P > 0.64). This result is consistent with spatial learning reliant on extra-maze visual cues rather than associative or place learning via motoric responses. In sum, although juveniles in all groups learned the configuration of the maze, which simulates learning their mother's three-dimensional territory in the field, decreased cortisol interfered with acquisition of spatial learning in young S. beldingi.
3.3. Associative learning
The three cortisol groups in Group 1 did not differ in their likelihood of responding to SQ Probe 1 but only the CONTROL1 juveniles reliably responded to SQ Probe 2 (Table 1). The CONTROL1 group remained alert longer than both of the LOW CORT groups following SQ Probe 1 (F2,50 = 18.21, P < 0.0001; Bonferroni pairwise t-test post-hoc comparisons, both Ps < 0.0001) and longer than the LOW2 CORT group following SQ Probe 2 (F2,49 = 5.09, P = 0.01; post-hoc P = 0.008; Fig. 4a). Initial responses to playbacks differed as well, as CONTROL1 juveniles were more likely to run to a burrow whereas LOW2 CORT juveniles were likely to simply freeze to SQ Probe 2 (χ2 = 14.11, df= 6, P = 0.028; Fig. 4b; SQ Probe 1: χ2 = 12.44, df= 6, P = 0.053). The CONTROL1 and LOW1 CORT groups were more likely to respond to the Bird Probe than the LOW2 CORT group (Table 1). CONTROL1 juveniles remained alert more than twice as long as the two LOW CORT groups to the Bird Probe (F2,51 = 17.87, P < 0.0001; Bonferroni pairwise t-test post-hoc comparisons, both Ps < 0.0001; Fig. 4a) and were more likely to run to a burrow whereas LOW1 and LOW2 CORT juveniles looked or posted (overall χ2 = 33.58, df = 6, P < 0.0001; Fig. 4c). Thus juveniles with species-typical elevated glucocorticoid levels are more likely to learn and retain associations between an auditory signal warning of an ‘aerial predator’ and the appropriate behavioral response, compared with juveniles with low cortisol which are less likely to learn the associations and if they did, showed reactions to squeals or bird calls typical of untrained S. beldingi (Mateo, 1996).
Table 1. Numbers of juveniles responding to conditioned acoustic stimulus played alone.
| Squeal probe 1 | Squeal probe 2 | Bird probe | |
|---|---|---|---|
| Group 1 | |||
|
| |||
| CONTROL1 | 18/20 (90.0%) | 26/30 (86.67%) | 22/25 (88.0%) |
| LOW1 CORT | 20/21 (95.24%) | 10/19 (52.63%) | 25/33 (75.76%) |
| LOW2 CORT | 18/24 (75.0%) | 18/39 (46.0%) | 16/28 (57.14%) |
|
χ2 = 4.20, df = 2,
P = 0.12 |
χ2 = 12.58, df = 2,
P = 0.002 |
χ2 = 7.71, df = 2,
P < 0.021 |
|
|
| |||
| Group 2 | |||
|
| |||
| CONTROL2 | 12/12 (100.0%) | 18/19 (94.74%) | 16/16 (100%) |
| HIGH CORT | 14/14 (100.0%) | 2/12 (16.67%) | 5/8 (62.5%) |
|
χ2 = 19.58, df = 1,
P < 0.0001* |
χ2 = 4.10, df = 1,
P = 0.04* |
||
(Animals received one pairing of an S. beldingi squeal with a fast-moving overhead stimulus per day for four days, followed by Probe 1 (squeal played back alone) on Day five, and Probe 2 on Day ten. Animals then received one pairing of a blackbird alarm call with a visual stimulus on Day 11 with the call played back alone on Day 12.
Because of low expected frequencies, these analyses are presented for illustrative purposes only
Fig. 4.
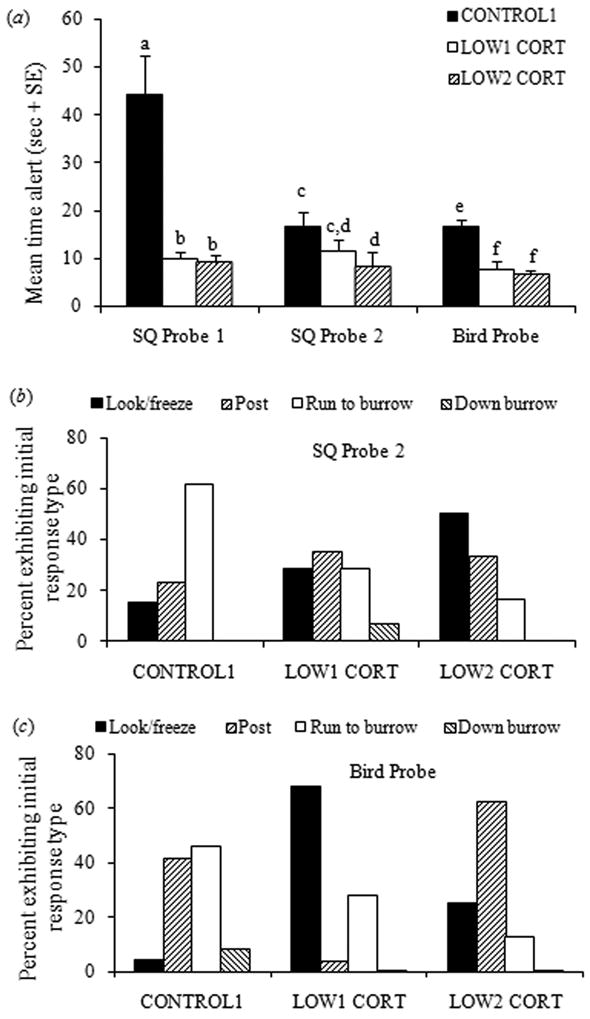
Behavioral responses to probe playbacks in Group 1. Experimental treatment of groups is described in Methods and in Figure 2. (a) Duration of alert behavior following onset of playback. Different letters over columns indicate significant differences based on Bonferroni-adjusted post-hoc pairwise t-test comparisons for significant ANOVAs on log-transformed data. (b) Type of initial responses by juveniles following playback of Squeal Probe 2. (c) Type of initial responses by juveniles following playback of Bird Probe. Data for Squeal Probe 1 (no significant differences) not shown.
All juveniles in Group 2 responded to SQ Probe 1 (Table 1) and there were no significant group differences in their time spent alert (t14 = 1.07, P = 0.30; Fig. 5a) or initial responses (χ2 = 2.41, df= 2, P = 0.30). However more CONTROL2 than HIGH CORT juveniles responded to SQ Probe 2 (Table 1). Of the 18 CONTROL2 juveniles that responded, all but one ran to a burrow, whereas the two responding HIGH CORT juveniles simply looked or posted (expected frequencies too low for χ2 analysis; Fig. 5b). CONTROL2 juveniles also remained alert longer than HIGH CORT juveniles (X̅ ± SEM: 26.19 sec ± 4.54 and 2.0 ± 1.0, respectively; Fig. 5a). CONTROL2 juveniles were more likely than HIGH CORT juveniles to respond to the Bird Probe (Table 1), remained alert longer (t12= 2.38, P = 0.035; X̅ ± SEM: 18.56 sec ± 2.30 and 11.2 ± 1.56, respectively), and were more likely to run to a burrow than the HIGH CORT juveniles (χ2 = 5.83, df = 2, P = 0.054; Fig. 5c). In summary, highly elevated glucocorticoids interfere with acquisition and retention of both multiple- and one-trial conditioning tasks in juveniles 10-20 days past natal emergence. Given the age-dependent effects of glucocorticoids on cognition, it is unknown if similar negative effects would be observed in newly emerged animals as well.
Fig. 5.
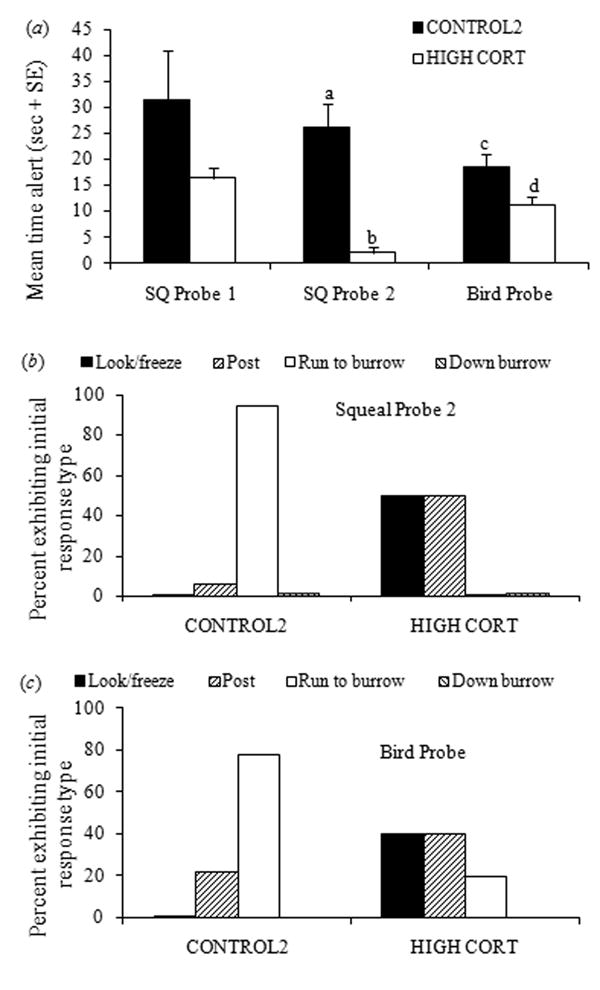
Behavioral responses to probe playbacks in Group 2. HIGH CORT juveniles and adults received exogenous cortisol in their drinking water for one week prior to and for the duration of behavioral testing, whereas the CONTROL2 group received untreated water. (a) Duration of alert behavior following onset of playback. Different letters over columns indicate significant differences based on Bonferroni-adjusted post-hoc pairwise t-test comparisons for significant ANOVAs on log-transformed data. (b) Type of initial responses by juveniles following playback of Squeal Probe 2. (c) Type of initial responses by juveniles following playback of Bird Probe. Data for Squeal Probe 1 (no significant differences) not shown.
Control playbacks were conducted to determine whether group differences in associative learning were due to generalized differences in behavioral reactivity or were specific to the training context. Controls included eight exemplars of each of four call types: S. beldingi whistles, trills (conspecific alarm calls) and squeals (conspecific non-alarm calls; 7 exemplars different from the training exemplar) and house-wren songs (heterospecific non-alarm calls; see Mateo, 1996), and each group was exposed to at least four playbacks of each call type. These playbacks were conducted between Days 2 and 10. Within Group 1 or Group 2, the cortisol groups did not differ in their responses to the control playbacks (data not shown), with the sole exception that LOW1 CORT juveniles in Group 1 remained alert longer than LOW2 CORT animals following wren playbacks (overall F2,43 = 5.97, P = 0.005; Bonferroni post-hoc paired comparison P = 0.004). Therefore the group differences were restricted to responses to Probes, and were the result of learning rather than some generalized change in reactivity.
4. Discussion
In free-living juveniles, cortisol is elevated at natal emergence, and declines to population-specific levels within 1-2 weeks (Mateo, 2006). Similar age-related differences in cortisol were observed here, with the younger CONTROL1 juveniles (measured at ∼ 30-40 days old) having higher natural levels of corticoids than the older CONTROL2 juveniles (at ∼ 50-54 days). The CONTROL2 juveniles acquired and retained the associative-learning tasks, indicating that unlike after emergence, this reduced level of cortisol, which was even lower than the LOW CORT groups in Group 1, did not interfere with cognition, as discussed below next, and illustrates how the modulating effects of cortisol depend on an animal's stage of development.
Together, these studies demonstrate that juvenile S. beldingi with very low or high levels of cortisol perform poorly on association tasks compared with juveniles with moderately elevated cortisol (contrast CONTROL groups with LOW1, LOW2 and HIGH CORT in Figures 4, 5). Specifically, more CONTROL juveniles learned and retained an association between a vocalization and the appropriate behavioral response, placing them in the middle of the inverted-U shape curve. This suggests that the natural elevation of cortisol observed in free-living juveniles at the age of emergence promotes learning of adaptive reactions to S. beldingi's two alarm calls, which require two very different behavioral responses (Mateo, 1996). Cortisol values affected performance on both multiple- and one-trial training tasks, the latter of which is unexpected since other prey species require multiple exposures to heterospecific calls and their eliciting stimuli for acquisition of responses to the calls themselves (Hauser, 1988; Ramakrishnan & Coss, 2000; Shriner, 1999). However, it is unclear how long S. beldingi would retain memories of the one-trial pairings used here.
Cortisol also influences spatial learning in a complex maze, as juveniles with lower levels required significantly more trials to learn how to exit the maze than juveniles with moderately elevated levels (Fig. 2c). In the field, the elevated cortisol at emergence from their natal burrows (compared with before and after; Mateo, 2006) therefore likely assists juveniles in learning to navigate their mother's above ground territory and the safe locations nearby in which to forage and hide from predators. Escape burrows and the routes to them are three-dimensional due to vegetation, rocks and dirt piles. Thus the alleys of the maze simulate the micro-topographical features which juveniles must learn quickly to avoid the predators which increase in numbers after natal emergence. (In contrast, navigating their mother's underground burrows most likely involves either following odor cues or response learning, given the lack of visual cues beyond the first few inches near the entrance.) Group 2 animals were collected from the field after natal emergence and were too large to run in the maze, so as yet it is unclear what affect very high levels of basal cortisol have on spatial learning. In other species high corticoids can improve (Pravosudov, 2003) or impair (Bodnoff et al., 1995; Conrad et al., 1999) spatial learning.
Cortisol was manipulated non-invasively directly through juveniles' drinking water or indirectly through mothers' milk, rather than through adrenalectomy or pharmacological inhibition of cortisol synthesis. This was done to minimize the effects of handling and injections on young animals, and to maximize the numbers of animals which could be studied during a short active season. (S. beldingi hibernate up to nine months and females produce one litter each year. In addition, they are social, group-living animals, and the associative-learning studies conducted in the outdoor enclosures are most successful when multiple litters are housed in each enclosure.) However, it remains unclear the mechanisms by which this exogenous cortisol affected learning in juvenile S. beldingi. Male offspring of rat mothers given corticosterone in their drinking water during lactation have reduced corticosterone, more hippocampal MRs and GRs and improved learning (Casolini, Cigliana, Alema, Ruggieri, Angelucci, & Catalani, 1997; Catalani, Casolini, Scaccianoce, Patacchioli, Spinozzi, & Angelucci, 2000), so it is possible that the LOW CORT juveniles studied here also had reduced corticoid receptor numbers. Likewise, MR numbers decline following chronic elevations in corticoids (de Kloet et al., 1999), so the HIGH CORT juveniles may also have had reduced corticoid receptors, accounting for their poorer cognitive performance.
It is unlikely that the learning differences among Group 1 were due to differences in maternal behavior such as licking or huddling (sensu Liu, Diorio, Tannenbaum, Caldji, Francis, Freedman, Sharma, Pearson, Plotsky, & Meaney, 1997), mediated by the exogenous cortisol, as we noted no differences in the time mothers spent outside of nestboxes or huddling over pups during cage checks at multiple points during the day (see also Catalani et al., 1993). In addition, for both Groups 1 and 2, alarm-call response differences were limited to the training stimuli, demonstrating learning-specific effects of cortisol rather than general differences in behavioral reactivity.
Corticosterone in the drinking water of rat mothers (Catalani et al., 1993) results in lowered glucocorticoids in offspring but improved spatial learning; the manipulation with S. beldingi results in lowered glucocorticoids but impaired learning. The disparate outcomes could be due to species differences in maternal metabolism of the glucocorticoids, juvenile ages, glucocorticoid-receptor distribution, the learning tasks, or to evolutionary histories shaping the relationships between adrenal functioning and cognition during early development. For instance, the rat treatment occurred during the pups' stress hyporesponsive period (postnatal days 4-14; Sapolsky & Meaney, 1986), but no such period is known for S. beldingi. Cortisol and corticosterone are structurally similar, but it is unknown if they have functionally similar effects on neural structures underlying spatial and associative learning. Unlike most laboratory rodents, S. beldingi produce both glucocorticoids above detectable levels, and present a unique opportunity for future studies on the potentially disparate hormonal effects within the same species.
A few studies have examined the acquisition of species-typical behaviors in freely behaving animals (Pfeffer, Fritz, & Kotrschal, 2002; Pravosudov, 2003; Saldanha, Schlinger, & Clayton, 2000), but the results reported here are the first demonstration of an inverted-U shape relationship between glucocorticoids and species-typical learning in a wild animal. Because of multiple sources of mortality after emergence, including predation, infanticide and starvation, natural selection likely favors rapid learning of survival strategies. Cortisol is spontaneously elevated at this time (Mateo, 2006), perhaps to mobilize energy to promote emergence from burrows (similar to the glucocorticoid changes associated with fledging-, dispersal- and migration-related activities in birds; e.g. Belthoff & Dufty, 1998; Heath, 1997; Piersma, Reneerkens, & Ramenofsky, 2000). Yet the evolutionary maintenance of such elevations in S. beldingi results in a hormonal profile in young animals that appears to facilitate learning according to ecological and developmental factors.
Fig. 3.
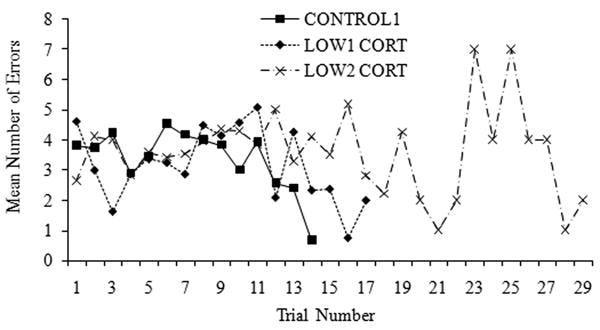
Mean numbers of errors made in the spatial maze in each trial by juveniles in each cortisol condition in Group 1. Experimental treatment of groups is described in Methods and in Figure 2. Measures of variance are omitted for clarity. Note that in the LOW2 CORT group, only one juvenile is represented in trials 20-29.
Acknowledgments
I thank Jared Bruck, Jason Bruck, Matthew Heintz, Caroline Pitt and Wendy Tidhar for assistance in data collection, Jocelyn Bryant for assistance with hormone assays, and Martha McClintock, Nancy Peters, Trevor Price and the Mateo Lab for comments on previous versions of the manuscript. I also thank Dan Leger for providing some of the S. beldingi alarm-call recordings used here. This work was supported by the NIMH and adhered to animal-care standards set forth by the NIH as well as the Animal Behavior Society's Guidelines for the Treatment of Animals in Behavioral Research and Teaching. It was approved by the University of Chicago's and University of California-Santa Barbara's IACUC. Collecting permits were obtained from the California Department of Fish and Game and permission to work on federal lands was granted by the United States Forest Service.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Belthoff JR, Dufty AM. Corticosterone, body condition and locomotor activity: a model for dispersal in screech-owls. Animal Behaviour. 1998;55:405–415. doi: 10.1006/anbe.1997.0625. [DOI] [PubMed] [Google Scholar]
- Bodnoff SR, Humphrey AG, Lehmann JG, Diamone DM, Rose GM, Meaney MJ. Enduring effects of chronic corticosterone treatment on spatial learning, synaptic plasticity and hippocampal neuropathology in young and mid-aged rats. Journal of Neuroscience. 1995;15:61–69. doi: 10.1523/JNEUROSCI.15-01-00061.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casolini P, Cigliana G, Alema GS, Ruggieri V, Angelucci L, Catalani A. Effect of increased maternal corticosterone during lactation on hippocampal corticosteroid receptors, stress response and learning in offspring in the early stages of life. Neuroscience. 1997;79:1005–1012. doi: 10.1016/s0306-4522(96)00668-9. [DOI] [PubMed] [Google Scholar]
- Catalani A, Casolini P, Scaccianoce S, Patacchioli FR, Spinozzi P, Angelucci L. Maternal corticosterone during lactation permanently affects brain corticosteroid receptors, stress response and behaviour in rat progeny. Neuroscience. 2000;100:319–325. doi: 10.1016/s0306-4522(00)00277-3. [DOI] [PubMed] [Google Scholar]
- Catalani A, Marinelli M, Scaccianoce S, Nicolai R, Muscolo LAA, Porcu A, Koranyi L, Piazza PV, Angelucci L. Progeny of mothers drinking corticosterone during lactation has lower stress-induced corticosterone secretion and better cognitive performance. Brain Research. 1993;624:209–215. doi: 10.1016/0006-8993(93)90079-3. [DOI] [PubMed] [Google Scholar]
- Claflin DL, Hennessy MB, Jensen SJ. Sex-specific effects of corticosterone on hippocampally mediated learning in young rats. Physiology and Behavior. 2005;85:159–166. doi: 10.1016/j.physbeh.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Lupien SJ, McEwen BS. Support for a bimodal role for Type II adrenal steroid receptors in spatial memory. Neurobiology of Learning and Memory. 1999;72:39–46. doi: 10.1006/nlme.1998.3898. [DOI] [PubMed] [Google Scholar]
- Dachir S, Kadar T, Robinzon B, Levy A. Cognitive deficits induced in young rats by long-term corticosterone administration. Behavioral and Neural Biology. 1993;60:103–109. doi: 10.1016/0163-1047(93)90173-f. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, de Kock S, Schild V, Veldhuis HD. Antiglucocorticoid RU 38486 attenuates retention of a behaviour and disinhibits the hypothalamic-pituitary-adrenal axis at different brain sites. Neuroendocrinology. 1988;47:109–115. doi: 10.1159/000124900. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Oitzl MS, Joels M. Stress and cognition: Are corticosteroids good or bad guys? Trends in Neurosciences. 1999;22:422–426. doi: 10.1016/s0166-2236(99)01438-1. [DOI] [PubMed] [Google Scholar]
- Duncko R, Cornwell B, Cui LH, Merikangas KR, Grillon C. Acute exposure to stress improves performance in trace eyeblink conditioning and spatial learning tasks in healthy men. Learning and Memory. 2007;14:329–335. doi: 10.1101/lm.483807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson D, Sapolsky R. Mineralocorticoid receptor overexpression differentially modulates specific phases of spatial and nonspatial memory. Journal of Neuroscience. 2007;27:8046–8052. doi: 10.1523/JNEUROSCI.1187-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson BM, Shettleworth SJ. Place versus response learning revisited: Tests of blocking on the radial maze. Behavioral Neuroscience. 2005;119:567–586. doi: 10.1037/0735-7044.119.2.567. [DOI] [PubMed] [Google Scholar]
- Hauser MD. How infant vervet monkeys learn to recognize starling alarm calls: The role of experience. Behaviour. 1988;105:187–201. [Google Scholar]
- Heath J. Corticosterone levels during nest departure of juvenile American kestrels. Condor. 1997;99:806–811. [Google Scholar]
- Herbert J, Goodyer IM, Grossman AB, Hastings MH, de Kloet ER, Lightman SL, Lupien SJ, Roozendaal B, Seckl JR. Do corticosteroids damage the brain? Journal of Neuroendocrinology. 2006;18:393–411. doi: 10.1111/j.1365-2826.2006.01429.x. [DOI] [PubMed] [Google Scholar]
- Hodes GE, Shors TJ. Distinctive stress effects on learning during puberty. Hormones and Behavior. 2005;48:163–171. doi: 10.1016/j.yhbeh.2005.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holekamp KE. Natal dispersal in Belding's ground squirrels (Spermophilus beldingi) Behavioral Ecology and Sociobiology. 1984;16:21–30. [Google Scholar]
- Jenkins SH, Eshelman BD. Spermophilus beldingi. Mammalian Species. 1984;221:1–8. [Google Scholar]
- Kesner RP, Bolland BL, Dakis M. Memory for spatial locations, motor responses, and objects: triple dissociation among the hippocampus, caudate nucleus, and extrastriate visual cortex. Experimental Brain Research. 1993;93:462–470. doi: 10.1007/BF00229361. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS. The acute effects of corticosteroids on cognition: Integration of animal and human model studies. Brain Research Reviews. 1997;24:1–27. doi: 10.1016/s0165-0173(97)00004-0. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Wilkinson CW, Briere S, Menard C, Kin NMKNY, Nair NPV. The modulatory effects of corticosteroids on cognition: studies in young human populations. Psychoneuroendocrinology. 2002;27:401–416. doi: 10.1016/s0306-4530(01)00061-0. [DOI] [PubMed] [Google Scholar]
- Mateo JM. The development of alarm-call response behaviour in free-living juvenile Belding's ground squirrels. Animal Behaviour. 1996;52:489–505. doi: 10.1006/anbe.1996.0446. [DOI] [PubMed] [Google Scholar]
- Mateo JM. Developmental and geographic variation in stress hormones in wild Belding's ground squirrels (Spermophilus beldingi) Hormones and Behavior. 2006;50:718–725. doi: 10.1016/j.yhbeh.2006.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateo JM. Ecological and physiological correlates of anti-predator behaviors of Belding's ground squirrels (Spermophilus beldingi) Behavioral Ecology and Sociobiology. 2007;62:37–49. doi: 10.1007/s00265-007-0436-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateo JM, Cavigelli SA. A validation of extraction methods for non-invasive sampling of glucocorticoids in free-living ground squirrels. Physiological and Biochemical Zoology. 2005;78:1069–1084. doi: 10.1086/432855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateo JM, Holmes WG. Development of alarm-call responses in Belding's ground squirrels: The role of dams. Animal Behaviour. 1997;54:509–524. doi: 10.1006/anbe.1996.0446. [DOI] [PubMed] [Google Scholar]
- Mateo JM, Holmes WG. How rearing history affects alarm-call responses of Belding's ground squirrels (Spermophilus beldingi, Sciuridae) Ethology. 1999a;105:207–222. [Google Scholar]
- Mateo JM, Holmes WG. Plasticity of alarm-call response development in Belding's ground squirrels (Spermophilus beldingi, Sciuridae) Ethology. 1999b;105:193–206. [Google Scholar]
- Pfeffer K, Fritz J, Kotrschal K. Hormonal correlates of being an innovative greylag goose, Anser anser. Animal Behaviour. 2002;63:687–695. [Google Scholar]
- Piersma T, Reneerkens J, Ramenofsky M. Baseline corticosterone peaks in shorebirds with maximal energy stores for migration: A general preparatory mechanism for rapid behavioral and metabolic transitions? General and Comparative Endocrinology. 2000;120:118–126. doi: 10.1006/gcen.2000.7543. [DOI] [PubMed] [Google Scholar]
- Pravosudov VV. Long-term moderate elevation of corticosterone facilitates avian food-caching behaviour and enhances spatial memory. Proceedings of the Royal Society of London. Series B: Biological Sciences. 2003;270:2599–2604. doi: 10.1098/rspb.2003.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan U, Coss RG. Recognition of heterospecific alarm vocalizations by bonnet macaques (Macaca radiata) Journal of Comparative Psychology. 2000;114:3–12. doi: 10.1037/0735-7036.114.1.3. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Cahill L, McGaugh JL. Interaction of emotionally activated neuromodulatory systems in regulating memory storage. In: Ishikawa K, McGaugh JL, Sakata H, editors. Brain Processes and Memory. Amsterdam: Elsevier Sciences; 1996. pp. 39–54. [Google Scholar]
- Roozendaal B, McGaugh JL. Amygdaloid nuclei lesions differentially affect glucocorticoid-induced memory enhancement in an inhibitory avoidance task. Neurobiology of Learning and Memory. 1996;65:1–8. doi: 10.1006/nlme.1996.0001. [DOI] [PubMed] [Google Scholar]
- Roskoden T, Linke R, Schwegler H. Transient early postnatal corticosterone treatment of rats leads to accelerated acquisition of a spatial radial maze task and morphological changes in the septohippocampal region. Behavioural Brain Research. 2005;157:45–53. doi: 10.1016/j.bbr.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Saldanha CJ, Schlinger BA, Clayton NS. Rapid effects of corticosterone on cache recovery in mountain chickadees (Parus gambeli) Hormones and Behavior. 2000;37:109–115. doi: 10.1006/hbeh.2000.1571. [DOI] [PubMed] [Google Scholar]
- Sandi C, Loscertales M, Guaza C. Experience-dependent facilitating effect of corticosterone on spatial memory formation in the water maze. European Journal of Neuroscience. 1997;9:637–642. doi: 10.1111/j.1460-9568.1997.tb01412.x. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Meaney MJ. Maturation of the adrenocortical stress response: Neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Research Reviews. 1986;11:65–76. doi: 10.1016/s0006-8993(86)80190-1. [DOI] [PubMed] [Google Scholar]
- Sherman PW, Morton ML. Demography of Belding's ground squirrels. Ecology. 1984;65:1617–1628. [Google Scholar]
- Shors TJ, Weiss C, Thompson RF. Stress-induced facilitation of classical conditioning. Science. 1992;257:537–539. doi: 10.1126/science.1636089. [DOI] [PubMed] [Google Scholar]
- Shriner WM. Antipredator responses to a previously neutral sound by free-living adult golden-mantled ground squirrels, Spermophilus lateralis (Sciuridae) Ethology. 1999;105:747–757. [Google Scholar]
- Siegel S, Castellan NJ. Nonparametric Statistics for the Behavioral Sciences. New York: McGraw-Hill; 1988. [Google Scholar]
- Takahashi LK. Organizing action of corticosterone on the development of behavioral inhibition in the preweanling rat. Developmental Brain Research. 1994;81:121–127. doi: 10.1016/0165-3806(94)90074-4. [DOI] [PubMed] [Google Scholar]