Abstract
Separase not only triggers anaphase of meiosis I by proteolytic cleavage of cohesin on chromosome arms; in vitro vertebrate separase also acts as a direct inhibitor of cyclin-dependent kinase 1 (Cdk1) upon liberation from inhibitory securin. Blocking separase-Cdk1 complex formation by microinjection of anti-separase antibodies prevents polar body extrusion in vertebrate oocytes. Importantly, proper meiotic maturation is rescued by chemical inhibition of Cdk1 or expression of Cdk1-binding separase fragments lacking cohesin-cleaving activity.
The cohesin complex holds sister chromatids together and at anaphase is cleaved by the protease separase1,2. Until then separase is kept inactive by mutually exclusive association with cylin-dependent kinase 1 (Cdk1) or securin3,4,5. At metaphase the anaphase-promoting complex or cyclosome (APC/C) mediates degradation of securin and cyclin B1, the regulatory subunit of Cdk1, freeing separase to cleave cohesin6. Biochemically, Cdk1 activity is itself switched off by separase-Cdk1 complex formation4. However it is unclear whether separase acts as a Cdk1 inhibitor in vivo. During meiosis I of vertebrate eggs, fall in cdk1 activity is transient and not associated with complete loss in cyclin B7,8, raising the possibility that separase-dependent inactivation of Cdk1 might be necessary for meiotic maturation.
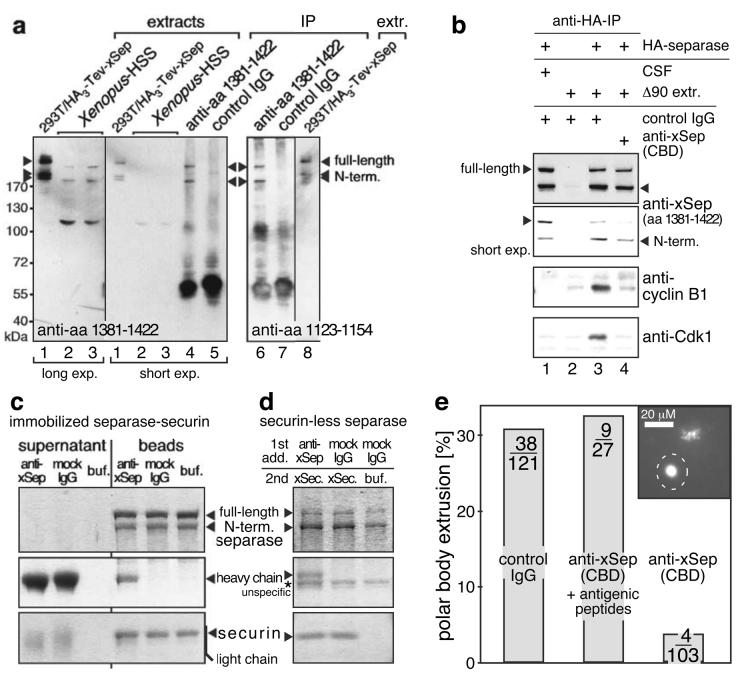
Cdk1 binding has only been demonstrated for human separase4. In addition to phosphorylation of Ser1126 it requires a short sequence showing weak homology to S. cerevisiae Cdc6 and probably binding cyclin B1. To investigate the role of separase-dependent Cdk1 inactivation in meiosis we raised antibodies against the Xenopus sequences corresponding to the two known Cdk1-binding determinants (amino acids 1123-54 plus 1381-1422). Both antibodies recognized recombinant Xenopus separase (Fig. 1A, lanes 1 and 8). The anti-aa 1381-1422 also detected and immuno-precipitated proteins of 240 and 180 kDa from meiotic Xenopus egg extract (lanes 2 to 5). Consistent with these representing full-length and self-cleaved endogenous separase, respectively, the same bands were recognized by anti-aa 1123-54 (lane 6). We incubated recombinant Xenopus separase-securin complexes in anaphase-arrested extracts to degrade securin and then re-isolated separase via N-terminal HA-tags3. Cdk1 co-purified with Xenopus separase demonstrating that human and frog separase share Cdk1-binding despite low conservation of CBDs at sequence level (Fig. 1B, lane 3). A mixture of the two anti-CBD antibodies fully abolished separase-Cdk1 complex formation (lane 4), but did not inhibit cleavage of separase, which is self-imposed and therefore serves as a read-out for proteolytic activity (lanes 1, 3, and 4). Anti-CBD antibodies neither eluted securin from existing separase-securin complexes nor affected binding of recombinant securin to securin-less separase (Fig. 1C and D). We then investigated the effect of the anti-CBD antibodies on progesterone-induced meiotic maturation of surgically removed frog oocytes. Interestingly, microinjection of anti-CBD antibodies dramatically reduced efficiency of polar body (PB) formation as compared to unspecific IgG (8.1 fold) or anti-CBD previously blocked with antigenic separase peptides (8.6 fold; Fig. 1E). Together these experiments indicate that transition from meiosis I to II requires the Cdk1-inhibitory activity of separase.
Figure 1.
Antibodies, which block Cdk1-inhibitory but not proteolytic activity of separase, prevent polar body extrusion. (a) Crude extract of 293T cells expressing HA3-Tev-xSeparase (lanes 1 and 8), high-speed supernatant (HSS) of crushed Xenopus eggs (lanes 2 and 3; 0.5 μl each), or material immunoprecipitated from 10 μl of Xenopus HSS (IP, lanes 4 to 7) were immunoblotted as indicated. (b) Recombinant HA3-Tev-xSeparase-Securin was incubated with unspecific IgG (lanes 1 to 3) or antibodies against Cdk1-binding determinants (CBD; aa 1123-54, 1381-422; lane 4). Following re-isolation from metaphase- (CSF) or anaphase-like (Δ90) meiotic egg extracts Tev-protease eluates were analyzed by immunoblotting. (c) Isolated HA3-Tev-xSeparase-Securin complex on anti-HA beads was challenged by incubation with anti-CBD. Eluted and bead-associated material was analyzed by Commassie-staining. (d) Isolated HA3-Tev-xSeparase on anti-HA beads was incubated with anti-CBD or unspecific IgG before recombinant Δ90-xSecurin was added. After washing, bead-associated material was analyzed by Commassie-staining. (e) Stage VI oocytes were injected with 200 ng of unspecific IgG or anti-xSeparase antibodies (CBD) or anti-CBD blocked by incubation with 100 fold excess of antigenic peptides. Progesterone-matured oocytes were fixed, Hoechst 33258-stained, and inspected for polar bodies (marked on image by dashed circle). Number of polar bodies per total number of analyzed oocytes is indicated.
We also raised an antibody against the CBDs of mouse separase (amino acids 1120-34 and 1340-54), which detected in vitro-translated mouse separase fragment (amino acids 1053-1382; Fig. 2A, lane 1) and proteins of 230 and 170 kDa in murine cell extracts likely representing full-length and self-cleaved endogenous separase (lane 4). Due to 83% sequence identity human separase cross-reacted with anti-mouse separase antibody and could be used for its further characterization (lane 3). The anti-CBD antibody did not inhibit cohesin cleavage by separase (Fig. 2B, lanes 1 and 4) but counteracted separase-Cdk1 complex formation as it partially inhibited Cdk1-dependent inactivation of separase (lanes 2 and 3). As before anti-CBD antibody potently inhibited PB-extrusion relative to unspecific IgG (5.7 fold) upon microinjection into mouse oocytes (Fig. 2C, columns 2 and 4). When we stained anti-CBD-injected oocytes lacking PB with anti-tubulin antibodies the majority (59%) appeared arrested with chromosomes scattered on elongated meiosis I spindles (Fig. 2F). Consistently, chromosome spreading revealed complete separation of all homologues in the majority of anti-CBD-injected oocytes without PB (n=11/19) (Fig. 2G). The homologue non-disjunction in these oocytes (42%) is similar to mock IgG-injected oocytes, where 37% of oocytes fail to extrude PB1 (Fig. 2C) and arrest at metaphase I (with attached homologues, n=15). This is a common maturation failure point9,10 and here it is probably due to microinjection damage. Thus, anti-CBD injection has no or little effect on cohesin cleavage strongly suggesting that meiotic maturation failure is not due to inhibition of separase s proteolytic activity.
Figure 2.
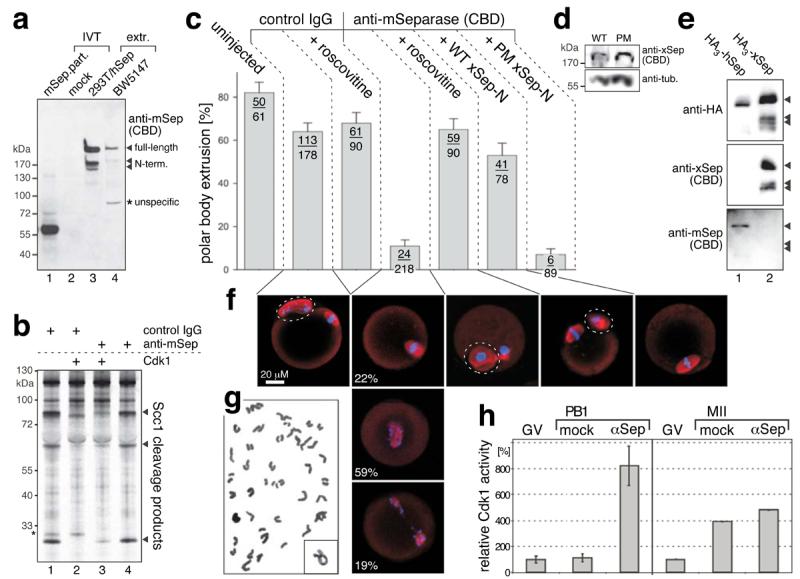
Block of polar body extrusion by anti-separase antibody can be reversed by simultaneous inhibition of Cdk1. (a) In vitro translation (IVT) of mouse separase fragment (aa 1053-1382, lane 1) or negative control (lane 2), and crude extracts of 293T cells expressing HA3-hSeparase (lane 3) or a murine T lymphoma cell line (BW5147, lane 4) were used in Western analysis to characterize an antibody raised against the Cdk1-binding determinants (CDB) of mouse separase (aa 1120-34 and 1340-54). (b) The anti-mSeparase antibody does not impede proteolytic activity but counteracts separase-Cdk1 complex formation. Active human separase was pre-incubated with anti-mSeparase (lanes 3 and 4) or unspecific IgG (which always gave rise to an unexplained, unspecific band denoted by star, lanes 1 and 2), combined with Cdk1 (lanes 2 and 3) or reference buffer (lanes 1 and 4), and assayed for cohesin-cleaving activity. (c) Polar body (PB) extrusion in mouse oocytes injected as indicated with control IgG, anti-mSeparase, and mRNA coding for N-terminal fragments (aa 1 – 1552) of wild type (WT) Xenopus separase or phosphorylation site mutant (PM; Ser1138,1139Ala). Where indicated, roscovitine (100 μM) was added 1 hour before PB formation. Number of polar bodies per total number of analyzed oocytes is indicated (mean +/−SD). (d) Western analysis of mouse oocytes expressing WT or PM fragments of Xenopus separase. Tubulin served as loading control. (e) The anti-mSeparase antibody does not recognize Xenopus separase. Shown are Western blots of affinity purified HA-tagged human (lane 1) and Xenopus separase (lane 2). (f) Spindles (red) and chromosomes (blue) of representative progesterone-treated oocytes imaged by confocal fluorescence microscopy. Dashed circles label PBs. (g) Representative (n=11/19) Giemsa-stained chromosome spread from arrested, anti-CBD-injected oocyte lacking polar body. Inset shows typical bivalent for comparison. (h) Histone H1 assays on 6 pools of 5 control oocytes shortly after PB extrusion plus corresponding anti-CBD injected oocytes (left) and on 2 pools of 15 fully matured control oocytes plus time-matched anti-CBD injected oocytes (right). Shown are mean kinase activities normalized to corresponding oocytes in germinal vesicle (GV) stage +/−SD.
To determine if instead anti-CBD antibodies act by preventing separase-dependent inhibition of Cdk1 we first added the Cdk1 inhibitor roscovitine to anti-CBD-injected oocytes 1 hour before PBs normally extrude. This treatment fully abrogated the effect of anti-CBD on meiotic maturation (Fig. 2C, column 5) while it had no effect on PB-formation in control-injected oocytes (column 3). Separase-Cdk1 complexes cannot be disassembled by treatment with roscovitine or phosphatase making it unlikely that roscovitine indirectly leads to elevated proteolytic activity of separase kept inactive by association with Cdk1 (ref. 4 and data not shown). Next, we injected anti-CBD antibodies together with mRNAs encoding N-terminal separase fragments up to the self-cleavage site and thus lacking the C-terminal protease domain. We used mRNA of Xenopus separase not recognized by anti-mouse CBD-antibody (Fig. 2E). Liberation of endogenous separase from antibody by recombinant expression product could therefore be excluded. Expression of wild type Xenopus separase fragment efficiently reversed the effect of anti-CBD antibody (Fig. 2C, column 6). This was in sharp contrast to separase with phosphorylation site mutations (Ser1138,1139Ala) and compromised Cdk1 binding ability, which was unable to rescue PB-formation despite being expressed at the same level as wild type (column 7 and Fig. 2D). Finally, we performed histone H1 kinase assays on control oocytes, which had just extruded PB1, and time-matched oocytes injected with anti-CBD. While kinase activity in the controls was as low as in germinal vesicle (GV) stage, importantly, it was on average 7.3 fold higher in anti-CBD injected oocytes (Fig. 2H). This difference was transient as fully matured control oocytes displayed only 18% lower kinase activity than corresponding anti-CBD injected oocytes. Together these experiments demonstrate that the Cdk1-inhibitory activity of separase is required for polar body extrusion. Our results might explain why after meiosis I Cdk1 activity drops to its basal (GV) despite persistence of some cyclin B1 (ref. 8).
Interestingly, S. cerevisiae separase also has a well-established meiotic role being essential for spindle disassembly after meiosis I (refs. 11, 12). However, as part of the FEAR network it counteracts Cdk1 indirectly by activating the antagonistic phosphatase Cdc14. Thus, meiotic Cdk1-inactivation seems to be an important conserved function of separase despite apparent difference in mechanisms between phyla.
Supplementary Material
Acknowledgements
We thank Stefan Jentsch for continuous support and Alexander Straßer for technical assistance. This work was supported by grants from the DFG (Emmy-Noether-Program) and the Human Frontier Science Program (CDA) to OS and from The Wellcome Trust (Project 075744) to KTJ.
Footnotes
See Supplementary Information available on the Nature Cell Biology website for Methods.
References
- 1.Ivanov D, Nasmyth K. Cell. 2005;122:849–60. doi: 10.1016/j.cell.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 2.Uhlmann F, Wernic D, Poupart MA, Koonin EV, Nasmyth K. Cell. 2000;103:375–86. doi: 10.1016/s0092-8674(00)00130-6. [DOI] [PubMed] [Google Scholar]
- 3.Stemmann O, Zou H, Gerber SA, Gygi SP, Kirschner MW. Cell. 2001;107:715–26. doi: 10.1016/s0092-8674(01)00603-1. [DOI] [PubMed] [Google Scholar]
- 4.Gorr IH, Boos D, Stemmann O. Mol Cell. 2005;19:135–41. doi: 10.1016/j.molcel.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 5.Huang X, Hatcher R, York JP, Zhang P. Mol Biol Cell. 2005;16:4725–32. doi: 10.1091/mbc.E05-03-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hagting A, et al. J Cell Biol. 2002;157:1125–37. doi: 10.1083/jcb.200111001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohsumi K, et al. J Cell Sci. 1994;107:3005–3013. doi: 10.1242/jcs.107.11.3005. [DOI] [PubMed] [Google Scholar]
- 8.Hampl A, Eppig JJ. Development. 1995;121:925–933. doi: 10.1242/dev.121.4.925. [DOI] [PubMed] [Google Scholar]
- 9.Donahue RP. J Exp Zool. 1968;169:237–250. doi: 10.1002/jez.1401690210. [DOI] [PubMed] [Google Scholar]
- 10.Sorensen RA, Wassarman PM. Dev Biol. 1976;50:531–536. doi: 10.1016/0012-1606(76)90172-x. [DOI] [PubMed] [Google Scholar]
- 11.Buonomo SB, et al. Dev Cell. 2003;4:727–39. doi: 10.1016/s1534-5807(03)00129-1. [DOI] [PubMed] [Google Scholar]
- 12.Marston AL, Lee BH, Amon A. Dev Cell. 2003;4:711–26. doi: 10.1016/s1534-5807(03)00130-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.