Summary
Fully-grown mammalian oocytes maintain a prophase I, germinal-vesicle stage arrest in the ovary for extended periods before a mid-cycle luteinizing surge induces entry into the first meiotic division. Cdh1 is an activator of the Anaphase-Promoting Complex (APC), and APCcdh1 is normally restricted to late M - early G1 of the cell cycle. Here we find that APCcdh1 is active in mouse oocytes and is necessary to maintain prophase arrest.
Fully-grown mammalian oocytes remain arrested at prophase I within antral follicles until stimulated to enter the first meiotic division by a mid-cycle surge in luteinising hormone. An oolemma receptor maintains this arrest by raising protein kinase A activity1 which inhibits Maturation-Promoting Factor (CDK1-cyclin B1) by affecting the phosphorylation status of CDK12. Oocytes can resume meiosis spontaneously, manifest by germinal vesicle breakdown (GVB), when released into culture media, but remain arrested if agents such as the phosphodiesterase inhibitor milrinone3, are added to maintain protein kinase A.
Raising cyclin B1 levels in milrinone-arrested oocytes by microinjection of its cRNA coupled to GFP induced GVB. Spatially the cyclin B1-GFP expressed in oocytes mirrored the distribution reported in adult cells 4 (Supplementary Information, Fig S1a). Cytoplasmic cyclin B1 entered the nucleus before GVB and became associated with chromatin afterwards. However, the GVB rate in these oocytes was <15% by 5 h (Fig 1a), and never exceeded 20%, even after 24 h. The proteasomal inhibitor MG132 had a mild stimulatory effect on GVB over 5 h, and when combined with cyclin B1 the rate GVB increased 2-3 fold compared to cyclin B1 alone (Fig 1a and see Supplementary Information, Table S1). The increased rate of GVB was likely caused by increased cyclin B1-GFP since levels doubled with MG132 (Fig 1b).
Figure 1.
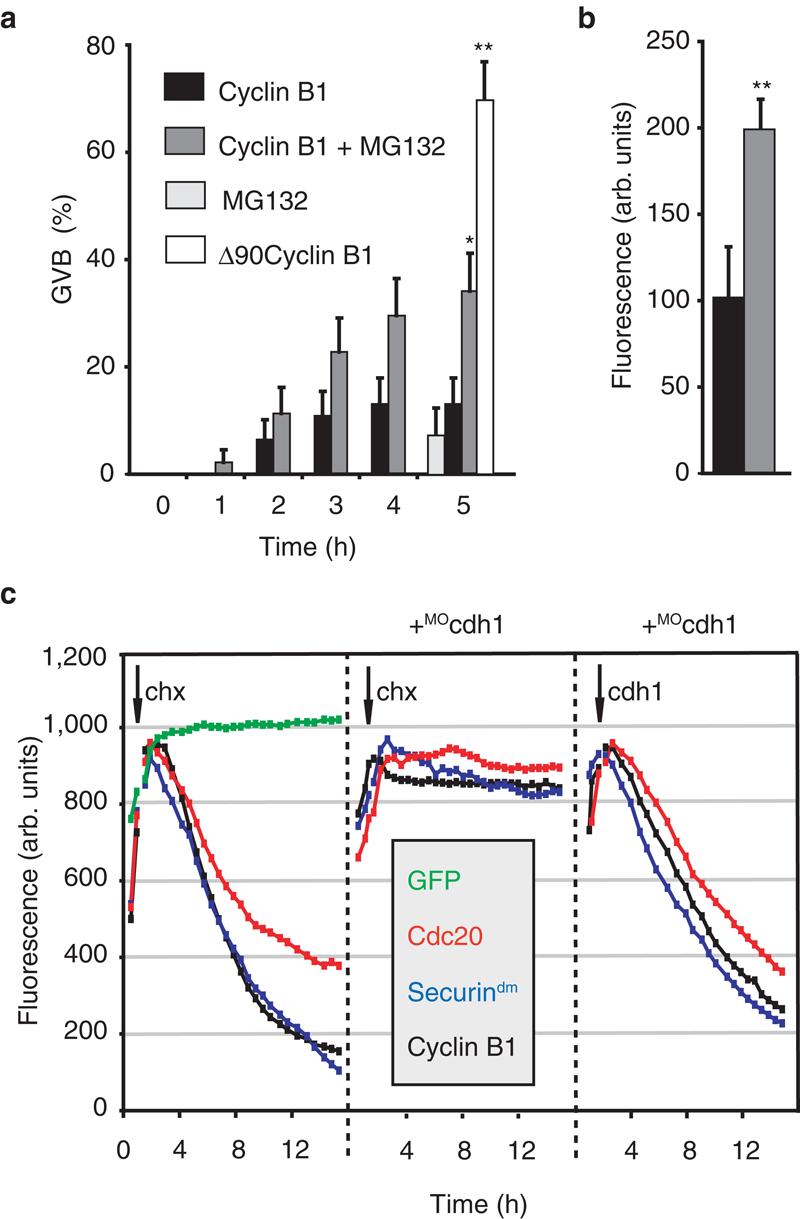
APCcdh1 activity in GV oocytes. (a) GVB rates in oocytes from the time of microinjection (0 h) of cyclin B1-GFP cRNA with (n=44) or without 50μM MG132 (n=46) or with Δ90cyclin B1-GFP (n=50); or incubated with MG132 alone (n=53). Data are means ± standard error. *p<0.05, **p<0.01, significantly different from cyclin B1 alone (Chi-squared test). (b) GFP levels in oocytes at 5 h from microinjection of cyclin B1-GFP with (grey, n=44) or without (black, n=32) MG132, normalised with respect to oocytes cultured without MG132. (c) GFP levels in oocytes microinjected with cRNA to GFP; GFP-coupled cyclin B1; securindm or cdc20, following addition of cycloheximide (chx) or cdh1 cRNA as indicated. Where indicated (+MOcdh1) oocytes had been microinjected with cdh1 morpholino 24 h previously. All media contained 1μM milrinone. Recordings are representative of between 11-18 oocytes per condition.
Cyclin B1 degradation requires polyubiquitination by the Anaphase-Promoting Complex (APC) followed by proteasomal degradation5. In mitosis, the APC needs one of two essential co-activators, cdc20 and cdh1, which are both present in mouse eggs6. APCcdc20 and APCcdh1 both degrade substrates such as cyclin B1 that contain a Destruction-(D)-box. Therefore we repeated the above cyclin B1 experiment using Δ90-cyclin B1, an N-terminal truncation which removes the D-box 7. Δ90-cyclin B1 cRNA induced 70% GVB rates by 5 h (Fig 1a), and 80% by 24 h; rates that are 4-5 fold higher than cyclin B1-GFP and with the MG132 data are consistent with cyclin B1 being degraded in GV oocytes.
Cyclin B1 degradation in oocytes, where MPF is low, is likely due to APCcdh1 because APCcdc20 requires high MPF levels for activity8. Oocytes do contain cdh1 (see Supplementary Information, Fig S1b) therefore to examine if APCcdh1 was active at this time, in addition to cyclin B1, we coupled two further APCcdh1 substrates to GFP, injected their cRNA and measured their stability following protein synthesis inhibition. We used cdc20 itself and a mutant form of securin (securindm) in which its D-Box has been mutated. Both constructs are degraded only by APCcdh1, and not APCcdc20, by virtue of a KEN-box 7,9.
All three APCcdh1 substrates, but not a GFP control, were degraded (Fig 1c and see Supplementary Information, Table S1). We could also observe degradation of endogenous cyclin B1 during the same time period suggesting it is not an artefact of very high protein levels (Supplementary Information, Fig S1e). Consistent with destruction of these proteins through the action of a ubiquitin ligase, cdc20-GFP loss was blocked by methylubiquitin, which terminates polyubiquitination, and MG132 (see Supplementary Information, Table S1).
To rule out cdh1-independent mechanisms for their degradation we examined the ability of these substrates to be degraded in oocytes knocked-down for cdh1. Microinjection of cdh1 morpholino (MOcdh1) for 24 h reduced cdh1 levels by about 90% (see Supplementary Information, Fig S1c) and inhibited degradation of the APCcdh1 substrates (Fig 1c and see Supplementary Information, Table S1). We confirmed the specificity of these observations to cdh1 knockdown by two approaches. First, we observed that cyclin B1-GFP degradation proceeded normally in oocytes incubated for 24 h with a control morpholino (invMOcdh1) which is an inverted base sequence of MOcdh1 (see Supplementary Information, Fig S1f and Table S1). Second, we performed a cdh1 rescue experiment (see Supplementary Information, Fig S1d) and observed degradation of these APCcdh1 substrates following addition of cdh1 cRNA to MOcdh1-treated oocytes (Fig 1c, and see Supplementary Information, Table S1).
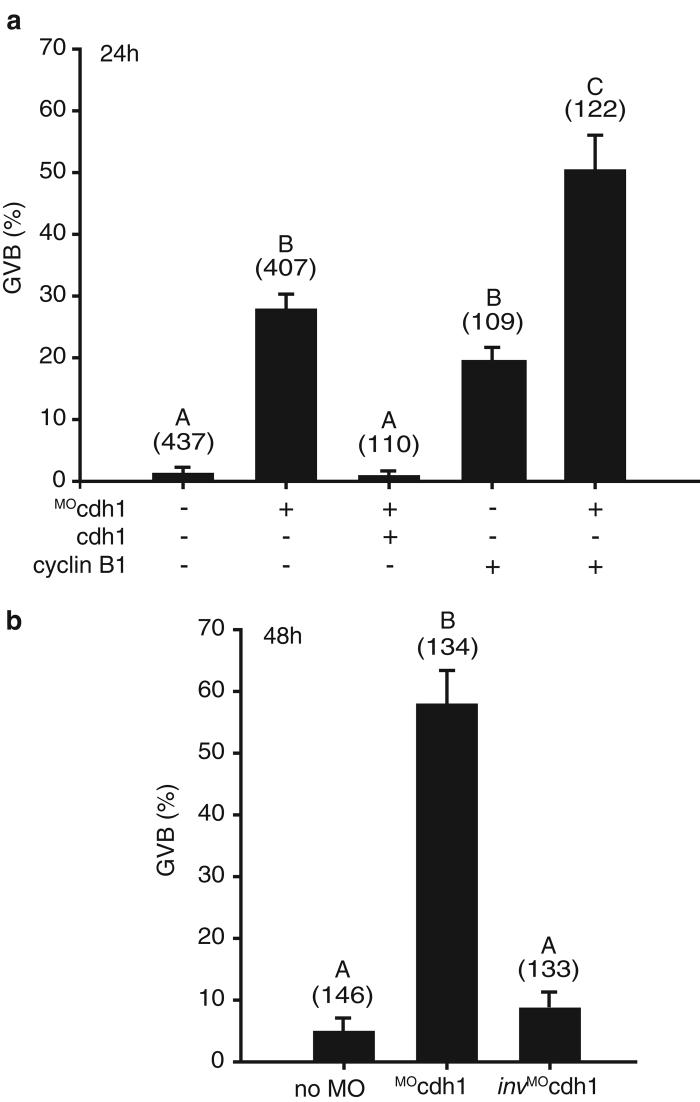
We wanted to establish if the measurable APCcdh1 activity in GV oocytes had any physiological role in the process of GV arrest, especially since it could degrade cyclin B1. In non-injected oocytes 2.5% of oocytes underwent GVB at 24 h and 5% at 48 h (Fig 2). Similar low levels of GVB also occurred in oocytes microinjected with the control invMOcdh1 over 48h demonstrating that milrinone provides a very good block to GVB. However, MOcdh1 induced GVB with ∼30% of oocytes undergoing GVB at 24 h and ∼60% at 48 h, an effect that was reversed by cdh1 rescue. Furthermore MOcdh1 dramatically increased the rate of GVB achieved by microinjection of cyclin B1 cRNA (Fig 1a). Finally we could also observe increased endogenous cyclin B1 levels following introduction of MOcdh1, but not invMOcdh1, in both oocytes that had remained GV-arrested or had undergone GVB, although as expected cyclin B1 was higher for the latter (Supplementary Information, Fig S1e).
Figure 2.
APCcdh1 maintains GV arrest. GVB in oocytes 24 h (a) or 48 h (b) after microinjection of MOcdh1, invMOcdh1, cyclin B1 cRNA or cdh1 cRNA as indicated. The number of oocytes assessed for each condition is in parenthesis. At both timepoints bars with different letters (A, B and C) are significantly different (p<0.01, Chi-squared).
We have described an essential role in prophase I oocytes for APCcdh1 in maintaining arrest. Although it is a ubiquitin ligase associated with late M-early G1 of the mitotic cell cycle, such findings are not without precedence since it also functions in the axonal morphogenesis of post-mitotic brain neurones10. Taken together they suggest that APCcdh1 has been adapted to specialised cell functions outside of mitotic exit.
Supplementary Material
Acknowledgements
The Wellcome Trust supported this work by a project grant (075744) and equipment grant (065354) to KTJ. We thank Michael Aitchison in the preparation of figures.
References
- 1.Mehlmann LM, et al. Science. 2004;306:1947–50. doi: 10.1126/science.1103974. [DOI] [PubMed] [Google Scholar]
- 2.Han SJ, Chen R, Paronetto MP, Conti M. Curr Biol. 2005;15:1670–6. doi: 10.1016/j.cub.2005.07.056. [DOI] [PubMed] [Google Scholar]
- 3.Tsafriri A, Chun SY, Zhang R, Hsueh AJ, Conti M. Dev. Biol. 1996;178:393–402. doi: 10.1006/dbio.1996.0226. [DOI] [PubMed] [Google Scholar]
- 4.Clute P, Pines J. Nat. Cell Biol. 1999;1:82–7. doi: 10.1038/10049. [DOI] [PubMed] [Google Scholar]
- 5.Murray AW. Cell. 2004;116:221–34. doi: 10.1016/s0092-8674(03)01080-8. [DOI] [PubMed] [Google Scholar]
- 6.Chang HY, Levasseur M, Jones KT. J Cell Sci. 2004;117:6289–96. doi: 10.1242/jcs.01567. [DOI] [PubMed] [Google Scholar]
- 7.Madgwick S, et al. Dev Biol. 2004;275:68–81. doi: 10.1016/j.ydbio.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 8.Kramer ER, Scheuringer N, Podtelejnikov AV, Mann M, Peters JM. Mol Biol Cell. 2000;11:1555–69. doi: 10.1091/mbc.11.5.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zur A, Brandeis M. Embo J. 2001;20:792–801. doi: 10.1093/emboj/20.4.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Konishi Y, Stegmüller J, Matsuda T, Bonni S, Bonni A. Science. 2004;303:1026–30. doi: 10.1126/science.1093712. [DOI] [PubMed] [Google Scholar]
- 11.Zernicka-Goetz M, et al. Development. 1997;124:1133–7. doi: 10.1242/dev.124.6.1133. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.