Abstract
In this study we compared the effects of propofol, small-dose isoflurane, and nitrous oxide (N2O) on cortical somatosensory evoked potentials (SSEP) and bispectral index (BIS) monitoring in adolescents undergoing spinal fusion. Twelve patients received the following anesthetic maintenance combinations in a randomly determined order: treatment #1: isoflurane 0.4% + N2O 70% + O2 30%; treatment #2: isoflurane 0.6% + N2O 70% + O2 30%; treatment #3: isoflurane 0.6% + air + O2 30%; treatment #4: propofol 120 μg · kg-1 · min-1 + air + O2 30%. Cortical SSEP amplitudes measured during anesthesia maintenance with treatment #3 (isoflurane 0.6%/air) were more than those measured during maintenance with treatment #1 (isoflurane 0.4%/N2O 70%) (P < 0.0001) and treatment #2 (isoflurane 0.6%/N2O 70%) (P < 0.0052). Cortical SSEP amplitudes measured during treatment #4 (propofol 120 μg · kg-1 · min-1/air) were more than treatment #1 (isoflurane 0.4%/N2O 70%) (P < 0.0001), treatment #2 (Iso 0.6%/N2O 70%) (P < 0.0007), and treatment #3 (isoflurane 0.6%/air) (P < 0.0191). In addition, average BIS values measured during treatments 1, 2, 3 and 4 were 62, 62, 61, and 44 respectively. Only treatment #4 (propofol 120 μg · kg-1 · min-1/air) uniformly maintained BIS values less than 60. Our study demonstrates that propofol better preserves cortical SSEP amplitude measurement and provides a deeper level of hypnosis as measured by BIS values than combinations of small-dose isoflurane/N2O or small-dose isoflurane alone.
The effect of anesthetics on cortical somatosensory evoked potential (SSEP) monitoring has been a subject of intense research over the last two decades (1). Volatile anesthetics have been repeatedly shown to decrease cortical amplitude in a dose-dependent fashion (2-5). Nitrous oxide (N2O) reduces cortical amplitude more than volatile anesthetics, and their combined effect is even more dramatic (3-8). Opioids, barbiturates, and propofol have a much less profound effect (9-16). In fact, propofol has been shown to preserve cortical waveform amplitude and latency even with escalating doses (16).
Despite clear evidence that N2O and isoflurane adversely affect cortical SSEP amplitude generation, many anesthesiologists use combinations of these two drugs at variable doses. Perhaps this is because cortical SSEP measurement is possible in the setting of 50%-70% N2O and 0.5 minimal alveolar concentration (MAC) isoflurane for most neurologically intact adults (3-6). However, this anesthetic combination may be unacceptable for small children and patients with abnormal baseline SSEPs from neurological deficits (17-18). Furthermore, these subanesthetic doses may place the patient at risk for intraoperative recall.
The purpose of this study was to compare the effects of four different but commonly used anesthetic combinations on cortical SSEP monitoring and to test the hypothesis that a propofol-based total IV anesthetic (TIVA) technique would induce less change in cortical SSEP amplitude and produce a deeper level of hypnosis as measured by bispectral index (BIS) than combinations of small-dose isoflurane/N2O or small-dose isoflurane/air.
Methods
Twelve healthy adolescents undergoing spinal fusion for idiopathic scoliosis repair were selected for this study (Table 1).
Table 1.
Patient Demographics
| Age (yr) | Gender | Weight (kg) | Operation | |
|---|---|---|---|---|
| Patient #1 | 17 | F | 58 | Anterior-Posterior Fusion |
| Patient #2 | 14 | F | 45 | Posterior Fusion |
| Patient #3 | 17 | M | 61.5 | Posterior Fusion |
| Patient #4 | 14 | M | 56.5 | Posterior Fusion |
| Patient #5 | 16 | M | 63 | Posterior Fusion |
| Patient #6 | 12 | F | 50.4 | Posterior Fusion |
| Patient #7 | 14 | F | 42.3 | Posterior Fusion |
| Patient #8 | 14 | F | 50 | Posterior Fusion |
| Patient #9 | 15 | M | 42.1 | Posterior Fusion |
| Patient #10 | 15 | F | 71 | Posterior Fusion |
| Patient #11 | 14 | F | 50 | Posterior Fusion |
| Patient #12 | 14 | M | 52.5 | Posterior Fusion |
After IRB approval, parental consent, and patient assent, patients underwent anterior-posterior spinal fusion (1) or posterior spinal fusion (11). The following routine monitors were applied: electrocardiogram, noninvasive blood pressure cuff, radial arterial line, pulse oximetry, end-tidal CO2, esophageal temperature probe, nerve stimulator, and urinary catheter. In addition, the Nicolet (Madison, WI) Viking 4 and XLTEK EP 16 monitors were used to measure SSEP data and the BIS (Aspect, XP A2000; Aspect Medical Systems, Newton, MA) monitor was used to measure relative depths of hypnosis.
All patients were premedicated with midazolam (0.5 mg/kg, maximum 20 mg PO) 20 min before arrival in the operating room. Patients underwent either inhaled induction with sevoflurane, N2O, and oxygen, or IV induction with propofol (2-3 mg/kg). Tracheal intubation occurred after muscle relaxation with cisatracurium (0.2 mg/kg).
After induction, each patient received 4 different anesthetic maintenance combinations in a randomly determined order. Each maintenance combination was administered for 20 min before monitoring values and SSEP data were collected. The anesthetic combinations were as follows:
Treatment #1: isoflurane 0.4% + N2O 70% + O2 30%.
Treatment #2: isoflurane 0.6% + N2O 70% + O2 30%.
Treatment #3: isoflurane 0.6% + air + O2 30%.
Treatment #4: propofol 120 μg · kg-1 · min-1 + air + O2 30%.
Remifentanil infusion was added to all of the above anesthetic combinations and titrated to maintain the baseline mean arterial blood pressures throughout the study period. A cisatracurium infusion was used to maintain train-of-four neuromuscular blockade at 0-2/4.
Before switching from one of the isoflurane-based maintenance combinations (treatments 1, 2, 3) to the propofol-based maintenance combination (treatment 4), patients received a propofol bolus (2 mg/kg) to help saturate nonspecific binding sites and promote rapid steady-state serum propofol levels. Serum propofol levels were measured in all patients during maintenance with propofol (treatment 4) and, depending on the sequence of anesthetic combinations that were randomly assigned, again for those patients switching from the propofol treatment to one of the isoflurane combinations (treatments 1, 2, 3) after 20 min of equilibration. Serum propofol levels were analyzed using high-performance liquid chromatography with fluorescence detection.
BIS measurements were recorded for each anesthetic combination after 20 min of equilibration. These values were purely descriptive, and no attempt was made to adjust medications for the sake of achieving a target BIS value.
SSEPs were recorded throughout surgery, except when electrocautery precluded recording. Stimulating electrodes were placed over both posterior tibial nerves at the ankles. Right and left posterior tibial SSEPs were obtained independently. Typically, 500 individual responses were averaged for each evoked potential measurement. Stimulation intensity was 15-20 Ma, and the stimulation rate was 4.3/sec. Recordings were obtained from electrodes placed at scalp locations CP3, CPz, CP4, and Fpz, according to the modified 10-20 system, and on the chin (19). The cortical P38 component of the SSEP was detected using CPz-Fpz and CP3-CP4 derivations for left posterior tibial SSEPs and CPz-Fpz and CP4-CP3 derivations for right posterior tibial SSEPs. Subcortical P31 and N34 components were detected using an Fpz-chin electrode derivation. All recordings were replicated and superimposed to demonstrate reproducibility. The passband was 30 to 1000 Hz (-6 db).
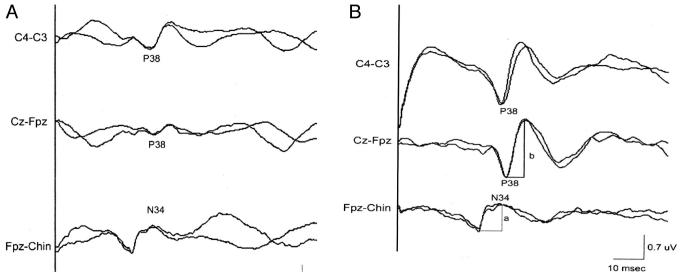
The amplitude of the cortical P38 component was measured as the greater of the P38 voltages CPz-Fpz and CP3-CP4 (or CP4-CP3) derivations. In each of these derivations, the P38 voltage was measured from the P38 peak positivity to the peak of the subsequent negative wave. The amplitude of the N34 negativity was measured with respect to the preceding P31 peak. Latencies measurements were made for the P38 and N34 peaks as illustrated in Figure 1b.
Figure 1.
A, right posterior tibial cortical SSEPs recorded under anesthetic conditions of treatment #1 (isoflurane 0.4% + N2O 70% + O2 30%). B, right posterior tibial cortical SSEPs recorded on the same patient under anesthetic conditions of treatment #4 (propofol 120 μg · kg-1 · min-1 + air + O2 30%). Measurements of N34 amplitude (a) and P38 amplitude (b) are illustrated.
All anesthetic changes and measurements were achieved during the initial stages of surgery. Surgical manipulation of the spinal cord and traction of the spine did not take place at any time during the study period.
Morphine or hydromorphone was administered to each patient during the closing stages of surgery, and titrated to patient comfort upon extubation. A morphine or hydromorphone patient controlled analgesia device was given to each patient in the recovery room for further pain management.
Repeated measurements of analysis of variance (SAS PROC MIXED; SAS, Cary, NC) were conducted to investigate any differences in SSEP (cortical amplitude, cortical latency, subcortical amplitude, subcortical latency) or BIS measurements arising among anesthetic maintenance treatment groups. Differences detected by analysis of variance were further examined by the Tukey-Kramer method, and the P values were adjusted accordingly to maintain an experimental-wide error rate of 0.05. Pearson correlation tests were conducted to detect correlations between heart rate, mean arterial blood pressure, temperature, and remifentanil dose with SSEP or BIS measurements.
Results
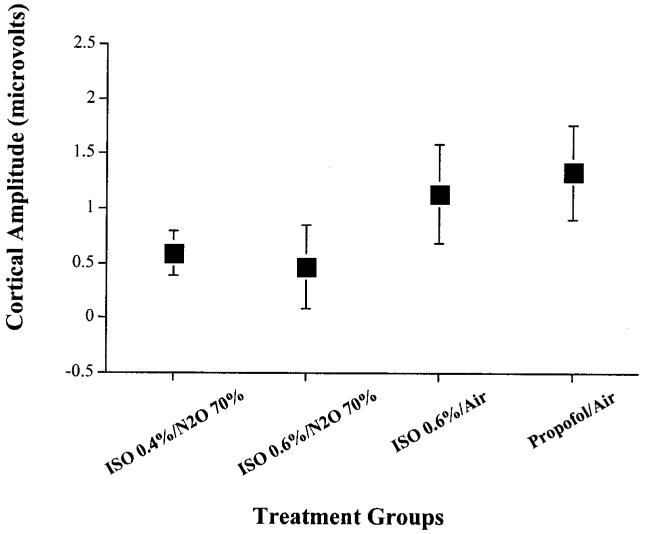
Cortical SSEP amplitudes measured during anesthesia maintenance with treatment #3 (isoflurane 0.6%/air) were greater than those measured during maintenance with treatment #1 (isoflurane 0.4%/N2O 70%) (P < 0.0001) and treatment #2 (isoflurane 0.6%/N2O 70%) (P < 0.0052). Cortical SSEP amplitudes measured during Treatment #4 (propofol 120 μg · kg-1 · min-1/air) were greater than treatment #1 (isoflurane 0.4%/N2O 70%) (P < 0.0001), treatment #2 (isoflurane 0.6%/N2O 70%) (P < 0.0007), and treatment #3 (isoflurane 0.6%/air) (P < 0.0191) (Figures 1 and 2).
Figure 2.
Mean cortical SSEP amplitude measurements for each anesthetic combination. Treatment #4 (propofol 120 μg · kg-1 · min-1 + air + O2 30%) allowed for significantly more robust cortical SSEP amplitude measurement than treatments 1, 2, and 3. The x-axis represents the four different anesthetic maintenance combinations studied: treatment #1, isoflurane 0.4% + N2O 70% + O2 30%; treatment #2, isoflurane 0.6% + N2O 70% + O2 30%; treatment #3, isoflurane 0.6% + air + O2 30%; treatment #4, propofol 120 μg · kg-1 · min-1 + air + O2 30%. The y-axis represents cortical SSEP amplitude size measured in microvolts. Each box plot represents the mean cortical SSEP amplitude value observed for each anesthetic maintenance combination. The lines that extend from the top and bottom of the box-plot represent the standard deviation in cortical SSEP amplitude values observed for each anesthetic maintenance combination.
In one patient, cortical amplitude measurement was not possible during maintenance with treatment #2 (isoflurane 0.6%/N2O 70%). No statistical difference could be found between treatment groups #1 and #2. Among the four treatment groups, no significant differences in cortical latencies, subcortical amplitudes, or subcortical latencies were found.
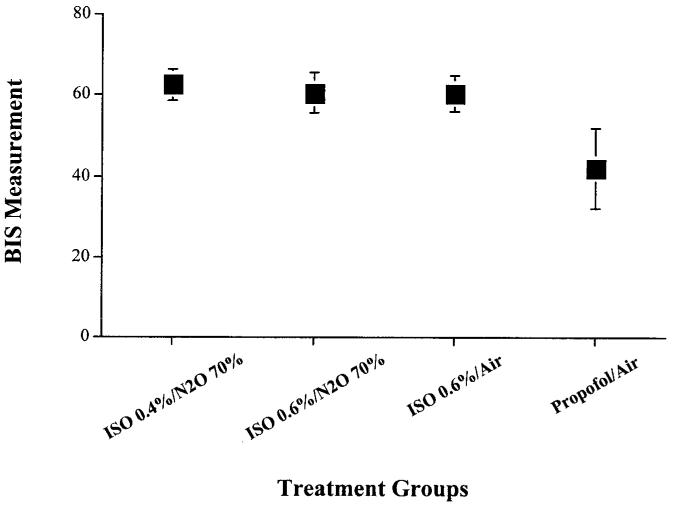
Average BIS values measured during treatments 1, 2, 3, and 4 were 62, 62, 61, and 44 respectively. Only treatment #4 (propofol 120 μg · kg-1 · min-1/air) uniformly maintained BIS values less than 60 (Figure 3).
Figure 3.
Mean bispectral index (BIS) values observed for each anesthetic combination. Only treatment 4 consistently maintained BIS values below 60. The x-axis represents the four different anesthetic maintenance combinations studied: treatment #1, isoflurane 0.4% + N2O 70% + O2 30%; treatment #2, isoflurane 0.6% + N2O 70% + O2 30%; treatment #3, isoflurane 0.6% + air + O2 30%; treatment #4, propofol 120 μg · kg-1 · min-1 + air + O2 30%. The y-axis represents the BIS monitor values recorded for each of the four treatment groups. Each box plot represents the mean BIS value observed for each anesthetic maintenance combination. The lines that extend from the top and bottom of the box plot represent the standard deviation in BIS values observed for each anesthetic maintenance combination.
No significant correlations were found between heart rate, mean arterial blood pressure, temperature, or remifentanil dose with either SSEP or BIS measurements using the Pearson correlation test. Average remifentanil doses (μg · kg-1 · min-1) were 0.31, 0.23, 0.35, and 0.40 for treatments 1, 2, 3, and 4, respectively.
The average serum propofol level for all patients during maintenance with treatment #4 (propofol 120 μg · kg-1 · min-1/air) was 2.04 μg/mL. Those patients randomized to switch from treatment #4 to one of the isoflurane combinations demonstrated an average subsequent serum level of 0.35 μg/mL.
Discussion
SSEP recording uses signal averaging to extract very low voltage physiological signals from a background of higher voltage noise. The efficiency of the recording process is critically dependent on maximizing the signal-to-noise ratio (SNR) of the raw data before averaging. If the SNR of the raw data is too low, satisfactory SSEP monitoring is not possible. The SNR can be maximized by reducing the accompanying noise (e.g., the use of paralytic medications to eliminate electromyographic artifact) and by selecting recording derivations, stimulus variables, and anesthetics that maximize the voltage of the physiologic signals of interest.
Creating the ideal anesthetic environment for cortical SSEP amplitude measurement remains a great challenge for anesthesiologists. Healthy adults with enormous baseline cortical amplitudes can be subjected to a variety of anesthetic conditions and still demonstrate adequately sized wave forms for continuing analysis (3-6). In contrast, general anesthesia often obliterates cortical SSEP responses in patients with very small baseline signals, such as healthy infants and young children, and patients with preexisting neurological disease (17,18). Indeed these patients are often monitored exclusively with subcortical SSEPs, which are less affected by anesthetics. Unfortunately, subcortical signals are not always obtainable or desirable.
This study objectively compared the impact of several common anesthetics on SSEP and BIS monitoring in healthy adolescents undergoing spinal fusion. We chose the class and dose of anesthetics based on community practice and work done by other researchers. The combinations of isoflurane 0.4%-0.6%/N2O 70%, and isoflurane 0.6%/air were examined in light of numerous adult studies documenting adequate SSEP measurement in the setting of isoflurane 0.5 MAC with N2O and 1 MAC without N2O, as well as the amnestic efficacy of 0.4 MAC (0.5%) isoflurane (3-6,20-23). Our choice of propofol 120 μg · kg-1 · min-1/air was based upon adult reports of adequate SSEP measurement and reliable amnesia with propofol doses of 100 μg · kg-1 · min-1 (12-16,24,25).
Because each patient has SSEP and BIS measurements that respond to anesthetics in an individual fashion, comparing groups of patients exposed to different medications may not be entirely valid. The strength of our study lies in its crossover design. Patients in our study served as their own controls, which allowed for direct, clinically relevant SSEP and BIS comparisons.
This crossover design, however, can be flawed if there is incomplete drug elimination between anesthetic treatment group changes. We based our 20-minute intervals for anesthetic equilibration upon the known pharmacokinetics of isoflurane, N2O, and propofol. Given the rapidity of equilibration/elimination for the inhaled anesthetics, we recognized that propofol pharmacokinetics would be the rate-limiting factor for our study. Thus, we wanted to validate the known pharmacokinetic models by objectively measuring serum propofol levels on every patient after 20 minutes of equilibration (2 mg/kg bolus followed by infusion at 120 μg · kg-1 · min-1) and again for those patients whose anesthetic sequence required switching from the propofol treatment to one of the isoflurane combinations. Our results revealed average maintenance serum propofol levels (2.04 μg/mL) consistent with those previously described as adequate for surgery (2-5 μg/mL), and average decrement levels (0.35 μg/mL) 20 minutes after switching to an isoflurane combination far less than those needed for emergence (1.5 μg/mL) (26,27). The propofol blood concentration required to suppress learning by 50% is 0.6 ± 0.1 μg/mL (28,29). Administering propofol only after the inhaled anesthetic combinations were studied would have eliminated this element of “propofol contamination;” however, we believed that randomization of the anesthetic sequences was essential for the study’s validity.
Our results demonstrate that a propofol-based TIVA technique better preserves cortical SSEP amplitude measurement than combinations of small-dose isoflurane/N2O and small-dose isoflurane/air. On average, cortical amplitudes increased by 100% with the use of propofol compared to combinations of small-dose isoflurane/N2O. Isoflurane/air provided markedly better conditions than combinations of small-dose isoflurane/N2O, which confirms the negative additive effect of these drugs. Cortical latencies, subcortical amplitudes, and subcortical latencies did not differ among the four anesthetic groups as predicted by the adult literature. Our results suggest that propofol TIVA may be a better choice than small-dose isoflurane, with or without N2O, for patients with small baseline cortical amplitudes that require monitoring.
Another aim of our study was to compare the relative depth of hypnosis provided by each anesthetic combination. Intraoperative awareness is an important issue for patients and anesthesiologists (30). Many patients who have experienced awareness go on to develop persistent posttraumatic stress disorder, and the ASA Closed Claims Database reveals that intraoperative awareness is a significant source of lawsuits against anesthesiologists (31-33). Thus, efforts to optimize the general anesthetic environment for SSEP measurement must not be made at the expense of patient safety.
At this time, we do not have an ideal method or monitor for measuring depth of hypnosis. None of the patients in this study experienced intraoperative recall as determined by postoperative interview; however, the efficacy of intraoperative verbal/auditory challenges and postoperative interviews and psychological batteries to predict the risk of awareness may be limited. Nordstrom and Sandin (34), for example, have documented 65% of patients subjected to “intentional awareness” during anesthesia experiencing complete amnesia for intraoperative events. In another study by Nordstrom et al. (24), intraoperative recall was found in a patient only after extensive questioning on the eighth postoperative day. Kerssens et al. (35) have recently shown that only one fourth of patients who unequivocally responded to commands during anesthesia with isolated forearm technique were able to recall awareness on postoperative interview. Thus, we used the BIS monitor to objectively compare the depth of hypnosis among the different anesthetic combinations.
The BIS monitor is not a universally accepted monitor of awareness, either explicit or implicit, but it may provide a useful tool for predicting relative depths of hypnosis (35). Values less than 60 are associated with profound hypnosis and a small reported incidence of intraoperative recall. Values more than 60, especially if maintained for long periods of time, may be associated with a significant risk of awareness according to some investigators (29,30,35-38). Our investigation revealed that propofol at 120 μg · kg-1 · min-1 always translated into patient BIS values less than 60, whereas anesthetics consisting of isoflurane alone (0.6%) or combinations of isoflurane (0.4%-0.6%) and N2O 70% did not consistently produce BIS values less than 60.
Within this study, the addition of N2O 70% did not result in a difference in recorded BIS values. This finding is not unexpected given the results of previous investigations. Certain drugs, such as ketamine and N2O, alter electroencephalograph (EEG) variables in ways that make BIS measurement unpredictable. Barr et al. (39) have documented unconsciousness with N2O 70% but no change in BIS. Rampil et al. (40) also confirmed no change in BIS with N2O but documented patients that were fully cooperative and responsive at concentrations of 50%. Thus, in the context of measured BIS values, it may not be possible to estimate the full amnestic impact of adding N2O to a particular regimen. If the BIS monitor is to play any legitimate intraoperative role, we believe clinicians should only use anesthetics that have predictable, verifiable effects on BIS EEG variables.
In conclusion, our study demonstrates that, when added to baseline infusions of opioids and paralytics, a propofol-based hypnotic component can better preserve cortical SSEP amplitude measurement and provide a deeper level of hypnosis as measured by BIS than combinations of small-dose isoflurane/N2O 70% or small-dose isoflurane/air. Our results also dramatically confirm the negative effects of N2O on cortical SSEP amplitude measurement. Furthermore, although small-dose isoflurane/air provided excellent SSEP conditions, our measured BIS values indicate a potential risk for intraoperative recall. Using larger concentrations of isoflurane to increase amnestic potential may be a safer option, but this maneuver may be limited by the dose-dependent decrease in cortical amplitude previously described in the literature. Compared with N2O and isoflurane, propofol appears to be the superior hypnotic drug for cases requiring cortical SSEP measurement because it allows for robust amplitude measurement, and, in light of the work done by Boisseau et al. (16) demonstrating amplitude preservation in the setting of escalating propofol doses, one could potentially use much larger doses of drug to decrease the risk of intraoperative awareness.
Acknowledgments
The authors would like to thank Aspect Medical Systems for the use of their BIS monitor.
Supported, in part, by a grant from the National Institutes of Health (1 RO1 AG17604-02 to EJH).
References
- 1.Banoub M, Tetzlaff JE, Schubert A. Pharmacologic and physiologic influences affecting sensory evoked potentials. Anesthesiology. 2003;99:716–37. doi: 10.1097/00000542-200309000-00029. [DOI] [PubMed] [Google Scholar]
- 2.Sebel PS, Ingram DA, Flynn PJ, et al. Evoked potentials during isoflurane anaesthesia. Br J Anaesth. 1986;58:580–5. doi: 10.1093/bja/58.6.580. [DOI] [PubMed] [Google Scholar]
- 3.McPherson RW, Mahla M, Johnson R, Traystman RJ. Effects of enflurane, isoflurane, and nitrous oxide on somatosensory evoked potentials during fentanyl anesthesia. Anesthesiology. 1985;62:626–33. doi: 10.1097/00000542-198505000-00015. [DOI] [PubMed] [Google Scholar]
- 4.Pathak KS, Ammadio M, Kalamachi A. Effects of halothane, enflurane, and isoflurane on somatosensory evoked potentials during nitrous oxide anesthesia. Anesthesiology. 1987;66:753–7. doi: 10.1097/00000542-198706000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Peterson DO, Drummond JC, Todd MM. Effects of halothane, enflurane, isoflurane, and nitrous oxide on somatosensory evoked potentials in humans. Anesthesiology. 1986;65:35–40. doi: 10.1097/00000542-198607000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Wolfe DE, Drummond JC. Differential effects of isoflurane/nitrous oxide on posterior tibial somatosensory evoked responses of cortical and subcortical origin. Anesth Analg. 1988;67:852–9. [PubMed] [Google Scholar]
- 7.Lam AM, Sharar SR, Mayberg TS, Engl CC. Isoflurane compared with nitrous oxide anaesthesia for intraoperative monitoring of somatosensory evoked potentials. Can J Anaesth. 1994;41:295–300. doi: 10.1007/BF03009907. [DOI] [PubMed] [Google Scholar]
- 8.da Costa VV, Saraiva RA, de Almeida AC, et al. The effect of nitrous oxide on the inhibition of somatosensory evoked potentials by sevoflurane in children. Anesthesia. 2001;56:202–7. doi: 10.1046/j.1365-2044.2001.01543.x. [DOI] [PubMed] [Google Scholar]
- 9.McPherson RW, Sell B, Traystman RJ. Effects of thiopental, fentanyl, and etomidate on upper extremity somatosensory evoked potentials in humans. Anesthesiology. 1986;65:584–9. doi: 10.1097/00000542-198612000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Samra SK, Dy EA, Welch KB, et al. Remifentanil- and fentanyl-based anesthesia for intraoperative monitoring of somatosensory evoked potentials. Anesth Analg. 2001;92:1510–5. doi: 10.1097/00000539-200106000-00031. [DOI] [PubMed] [Google Scholar]
- 11.Koht A, Schutz W, Schmidt G, et al. Effects of etomidate, midazolam, and thiopental on median nerve somatosensory evoked potentials and the additive effects of fentanyl and nitrous oxide. Anesth Analg. 1988;67:435–41. [PubMed] [Google Scholar]
- 12.Kalkman CJ, ten Brink SA, Been HD, Bovill JG. Variability of somatosensory cortical evoked potentials during spinal surgery: effects of anesthetic technique and high-pass digital filtering. Spine. 1991;16:924–9. doi: 10.1097/00007632-199108000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Kalkman CJ, Traast H, Zuurmond WWA, Bovill JG. Differential effects of propofol and nitrous oxide on posterior tibial nerve somatosensory cortical evoked potentials during alfentanil anaesthesia. Br J Anaesth. 1991;66:483–9. doi: 10.1093/bja/66.4.483. [DOI] [PubMed] [Google Scholar]
- 14.Taniguchi M, Nadstawek J, Pechstein U, Schramm J. Total intravenous anesthesia for improvement of intraoperative monitoring of somatosensory evoked potentials during aneurysm surgery. Neurosurgery. 1992;31:891–7. doi: 10.1227/00006123-199211000-00010. [DOI] [PubMed] [Google Scholar]
- 15.Scheepstra GL, de Lange JJ, Booij LH. Median nerve evoked potentials during propofol anaesthesia. Br J Anaesth. 1989;62:92–4. doi: 10.1093/bja/62.1.92. [DOI] [PubMed] [Google Scholar]
- 16.Boisseau N, Madany M, Staccini P, et al. Comparison of the effects of sevoflurane and propofol on cortical somatosensory evoked potentials. Br J Anaesth. 2002;88:785–9. doi: 10.1093/bja/88.6.785. [DOI] [PubMed] [Google Scholar]
- 17.Gilmore RL, Bass NH, Wright EA, et al. Developmental assessment of spinal cord and cortical evoked potentials after tibial nerve stimulation: effects of age and stature on normative data during childhood. Electroencephalogr Clin Neurophysiol. 1985;62:241–51. doi: 10.1016/0168-5597(85)90002-4. [DOI] [PubMed] [Google Scholar]
- 18.Helmers SL, Hall JE. Intraoperative SSEP monitoring in pediatrics. J Pediatr Orthop. 1994;14:592–8. doi: 10.1097/01241398-199409000-00007. [DOI] [PubMed] [Google Scholar]
- 19.American Electroencephalographic Society Guideline thirteen: guidelines for standard electrode position nomenclature. J Clin Neurophysiol. 1994;11:111–3. [PubMed] [Google Scholar]
- 20.Newton DEF, Thornton C, Konieczko KM, et al. Auditory evoked response and awareness: a study in volunteers at sub-MAC concentrations of isoflurane. Br J Anaesth. 1992;69:122–9. doi: 10.1093/bja/69.2.122. [DOI] [PubMed] [Google Scholar]
- 21.Newton DEF, Thornton C, Konieczko KM, et al. Levels of consciousness in volunteers breathing sub-MAC concentrations of isoflurane. Br J Anaesth. 1990;65:609–15. doi: 10.1093/bja/65.5.609. [DOI] [PubMed] [Google Scholar]
- 22.Dwyer R, Bennett HL, Eger EI, II, Heilbron D. Effects of isoflurane and nitrous oxide in subanesthetic concentrations on memory and responsiveness in volunteers. Anesthesiology. 1992;77:888–98. doi: 10.1097/00000542-199211000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Chortkoff BS, Bennett HL, Eger EI., II. Subanesthetic concentrations of isoflurane suppress learning as defined by the category-example task. Anesthesiology. 1993;79:16–22. doi: 10.1097/00000542-199307000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Nordstrom O, Englstrom AM, Persson S, Sandin R. Incidence of awareness in total i.v. anesthesia based on propofol, alfentanil and neuromuscular blockade. Acta Anaesthesiol Scand. 1997;41:978–84. doi: 10.1111/j.1399-6576.1997.tb04823.x. [DOI] [PubMed] [Google Scholar]
- 25.Glass PS, Bloom MJ, Kearse L, et al. Bispectral analysis measures sedation and memory effects of propofol, midazolam, isoflurane, and alfentanil in healthy volunteers. Anesthesiology. 1997;86:836–47. doi: 10.1097/00000542-199704000-00014. [DOI] [PubMed] [Google Scholar]
- 26.Shafer A, Doze VA, Shafer SL, White PF. Pharmacokinetics and pharmacodynamics of propofol infusions during general anesthesia. Anesthesiology. 1988;69:348. doi: 10.1097/00000542-198809000-00011. [DOI] [PubMed] [Google Scholar]
- 27.Hughes MA, Jacobs JR, Glass PSA. Context-sensitive half-time in multi-compartment pharmacokinetic models for intravenous anesthesia. Anesthesiology. 1992;76:334. doi: 10.1097/00000542-199203000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Veselis RA, Reinsel RA, Feshchenko VA, Wronski M. The comparative amnestic effects of midazolam, propofol, thiopental, and fentanyl at equisedative concentrations. Anesthesiology. 1997;87:749–64. doi: 10.1097/00000542-199710000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Leslie K, Sessler DI, Schroeder M, Walters K. Propofol blood concentration and the bispectral index predict suppression of learning during propofol/epidural anesthesia in volunteers. Anesth Analg. 1995;81:1269–74. doi: 10.1097/00000539-199512000-00025. [DOI] [PubMed] [Google Scholar]
- 30.Sandin RH, Enlund G, Samuelson P, Lennmarken C. Awareness during anesthesia: a prospective study. Lancet. 2000;3555:707–11. doi: 10.1016/S0140-6736(99)11010-9. [DOI] [PubMed] [Google Scholar]
- 31.Blacher RS. On awakening paralyzed during surgery: a syndrome of traumatic neurosis. JAMA. 1975;234:67–8. [PubMed] [Google Scholar]
- 32.Lennmarken C, Bildfors K, Enlund G, et al. Victims of awareness. Acta Anaesthesiol Scand. 2002;46:229–31. doi: 10.1034/j.1399-6576.2002.t01-1-460301.x. [DOI] [PubMed] [Google Scholar]
- 33.Domino KB. Closed malpractice claims for awareness during anesthesia. Am Soc Anesthesiologists Newsletter. 1996;60:14–7. [Google Scholar]
- 34.Nordstrom O, Sandin R. Recall during intermittent propofol anaesthesia. Br J Anaesth. 1996;76:699–701. doi: 10.1093/bja/76.5.699. [DOI] [PubMed] [Google Scholar]
- 35.Kerssens C, Klein J, Bonke B. Awareness: monitoring versus remembering what happened. Anesthesiology. 2003;99:570–5. doi: 10.1097/00000542-200309000-00011. [DOI] [PubMed] [Google Scholar]
- 36.Kerssens C, Klein J, van der Woerd A, Bonke B. Auditory information processing during adequate propofol anesthesia monitored by electroencephalogram bispectral index. Anesth Analg. 2001;92:1210–4. doi: 10.1097/00000539-200105000-00024. [DOI] [PubMed] [Google Scholar]
- 37.Kearse L, Rosow C, Zaslavsky A, et al. Bispectral analysis of the EEG predicts conscious processing of information during propofol sedation and hypnosis. Anesthesiology. 1998;88:25–34. doi: 10.1097/00000542-199801000-00007. [DOI] [PubMed] [Google Scholar]
- 38.O’Connor MF, Daves SM, Tung A, et al. BIS monitoring to prevent awareness during general anesthesia. Anesthesiology. 2001;94:520–2. doi: 10.1097/00000542-200103000-00025. [DOI] [PubMed] [Google Scholar]
- 39.Barr G, Jakobsson JG, Owall A, Anderson RE. Nitrous oxide does not alter bispectral index: study with nitrous oxide as sole agent and as an adjunct to i.v. anaesthesia. Br J Anaesth. 1999;82:827–30. doi: 10.1093/bja/82.6.827. [DOI] [PubMed] [Google Scholar]
- 40.Rampil IJ, Kim JS, Lenhardt R, et al. Bispectral EEG index during nitrous oxide administration. Anesthesiology. 1998;89:671–7. doi: 10.1097/00000542-199809000-00017. [DOI] [PubMed] [Google Scholar]