Summary
When shunts are selectively used during carotid endarterectomy, the adequacy of collateral cerebral blood flow (CBF) after the carotid artery is clamped is determined by monitors based on different physiologic measurements. In this series of three patients, we used electroencephalography (EEG) to measure neuronal electrical activity and transcranial Doppler ultrasonography (TCD) to measure CBF velocity. In each of our cases, the EEG was unchanged from preclamp values, while TCD CBF velocity was dramatically reduced. All three patients had transient neuropsychometric or neurologic changes after surgery, which resolved.
Keywords: Carotid endarterectomy, Surgery, TCD, EEG monitoring, Cerebral ischemia
A number of cerebral monitors have been developed to measure different components of cerebral “well-being” under general anesthesia: neuronal functioning with electroencephalography (EEG), cerebral blood flow (CBF) velocity with transcranial Doppler ultrasonography (TCD), cerebral tissue oxygen saturation with near infrared spectroscopy (NIRS), measurement of stump pressure, and oxygen saturation of the venous blood with sampling from jugular bulb catheters. A number of these are used to determine the adequacy of collateral circulation when the carotid artery is clamped during carotid endarterectomy (CEA). Inadequate collateral circulation is an indication for placement of a shunt. However, what does it mean if there is discordance between these monitors in terms of cerebral functioning?
We report three cases in which patients had general anesthesia and discordance between the EEG and TCD findings while undergoing CEA without shunting. All three were found to have transient clinical evidence of brain injury. The injury in one patient was determined based on his performance using a battery of neuropsychometric tests. It was present 1 day after surgery and resolved by 1 month. In the other patients, it was based on a standard neurologic examination. The new neurologic deficits were present immediately after surgery. These deficits began to resolve within 15 minutes, and were totally resolved by the next day.
CASE REPORTS
Case 1
An asymptomatic 85-year-old man with a 90% stenosis of the left internal carotid artery presented for elective CEA. Four months prior to surgery, he had a normal cardiac stress test. His past medical history included hip replacement, cholecystectomy, and hernia repair. He was a former smoker. Current medications included celecoxib, lansoprazole, and aspirin.
He also volunteered to participate in an IRB approved study using a battery of neuropsychological tests to evaluate subtle brain injury following CEA. The tests included Boston Naming, Controlled Oral Word Association, Rey Complex Figure and its immediate recall, Buschke Selective Reminding, Grooved Pegboard, and the Halstead-Reitan Trail Making Parts A and B. These tests were designed to assess different cognitive domains. They were administered preoperatively and postoperatively at 1 day, and 1 month. The change in neuropsychometric test performance was calculated by subtracting the baseline score from the postoperative score and Z-scores calculated from the SD of the difference in scores for a control group of patients having spine surgery (1). The patient’s preoperative score was within normal limits.
The patient received no preoperative medication. The procedure was performed under general anesthesia. In the operating room, the patient was monitored with a pulse oximeter, electrocardiogram, invasive blood pressure with a radial artery catheter, an 8 channel-EEG (Neurotrac II, Ambler, PA) (4 on each side), and TCD (Multigon Industries, Model 500M, Yonkers, NY) with a 2 MHz probe insonated on the left middle cerebral artery (MCA) at a depth of 55 mm. The TCD probe was held in position using the Spencer Marc 600 headholder (Spencer Technologies, Seattle, WA). After denitrogenation with 100% O2, anesthesia was induced with propofol 100 mg, lidocaine 100 mg, fentanyl 100 μg, midazolam 2 mg, and succinylcholine 120 mg. An 8.0 endotracheal tube was placed atraumatically into the trachea. Anesthesia was maintained with N2O in O2 (2:1 v:v) and isoflurane (expired concentration 0.5% to 0.7% throughout the case). A phenylephrine infusion (40 μg/ml) was started to maintain a systolic blood pressure above 180 mmHg during clamping. The total occlusion time was 20 minutes.
During the occlusion of the left carotid artery there were no EEG changes (Fig. 1d). However, TCD velocity went to zero during the same time period (Figs. 1a, 1b). No shunt was inserted. No emboli were observed when the carotid artery clamp was removed and flow returned to precross-clamp values (Fig. 1c). Otherwise, the surgical procedure was uneventful. The patient was extubated and transported to the ICU in stable condition. No neurologic deficits were present on physical examination immediately after surgery. However, on postoperative day one, the patient’s overall neuropsychometric test performance revealed subtle cognitive decline, which was absent at the 1 month follow-up time period. Cognitive decline was defined as two SD or more decline in performance compared to a control group of similar age as previously defined (1).
FIG. 1.
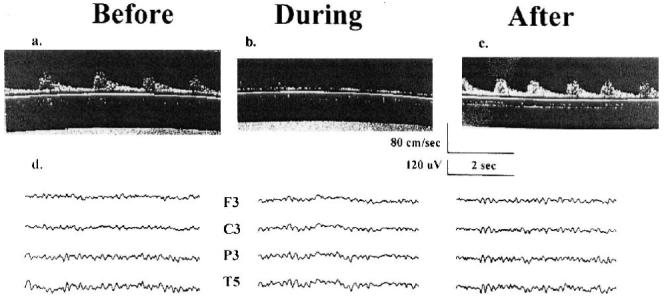
Transcranial Doppler ultrasonography and electroencephalogram. A, B and C are three transcranial Doppler (TCD) ultrasonography records from the patient reported in Case 1 with the TCD probe placed over the left temporal bone and insonated on the middle cerebral artery. “Before,” “during,” and “after” refer to three different records of cerebral blood flow (CBF) velocity taken at different times during surgery: before the carotid artery is clamped, while the carotid artery is clamped, and after the clamp has been removed. The scales for time in seconds and CBF velocity in cm/sec are shown below C. D shows three electroencephalographic (EEG) records taken at the three times described above. Ten electrodes are applied using an Electro-Cap (Electro-Cap International Inc., Eaton, OH). The electrodes are arranged in a referential montage using the International 10-20 positioning system with four over each hemisphere (Frontal [F], Central [C], Parietal [P], and Temporal [T]). The “reference” electrode placed centrally at Cz (“Z” refers to midline), and a “ground” electrode at Fz. Only the EEG traces for the left hemisphere are shown. The scales for time in seconds and EEG amplitude in μV are shown below C.
Case 2
A 62-year-old asymptomatic woman was found to have bilateral carotid bruits on a routine physical exam and 95% bilateral carotid stenosis by MRA. Her medical history consisted of coronary artery disease with a CABG 4 years prior, insulin-dependent diabetes mellitus, hyperlipidemia, and hypertension. Her medications included clopidogrel, cerivastatin, losartan, metoprolol, insulin, omeprazole, and furosemide. She never smoked. Three months prior to this admission she underwent a right CEA uneventfully. She presented now for elective left CEA.
She received no preoperative medications. Monitoring was identical to the case described above, as was anesthesia induction and maintenance. The MCA was insonated at a depth of 52 mm. The total occlusion time was 58 minutes. No shunt was inserted. As seen in the previous case, no EEG changes were seen, despite significant TCD changes consistent with the absence of flow in the middle cerebral artery for the entire period of cross-clamping. No emboli were observed when the carotid artery clamp was removed. The surgery was otherwise uneventful. Immediately following surgery and anesthesia, the patient was noted to have right arm monoplegia. However, over the next 15 minutes, strength gradually improved to 4/5 proximal more than distal strength. Over the next 24 hours, she had complete return of motor function in the right upper extremity. She was discharged home on the first postoperative day. She was thought to have had subcortical ischemia because the EEG was normal and the TCD had absent cerebral blood flow velocity, similar to what was seen in the previous case.
Case 3
A 64-year-old asymptomatic man was found to have bilateral carotid artery stenosis, 80% on the right and 60% to 80% on the left. His medical history consisted of noninsulin-dependent diabetes mellitus, hypercholesterolemia, and hypertension. His medications included aspirin, quinapril, simvastatin, and loratadine. He stopped smoking 10 years ago. He presented now for elective right CEA.
He received no preoperative medications. Monitoring was identical to the case described above, as was anesthesia induction and maintenance. The MCA was insonated at a depth of 39 mm. The total occlusion time was 48 minutes. No shunt was inserted. As was seen in the previous case, no EEG changes were seen despite significant TCD changes consistent with the absence of flow in the middle cerebral artery for the entire period of cross-clamping. No emboli were observed when the carotid artery clamp was removed. The surgery was otherwise uneventful. Immediately following surgery and anesthesia, the trachea was extubated and the patient was noted to have left arm monoplegia. However, over the next 15 minutes, strength gradually improved to 5/5 proximal more than distal strength. He was discharged home on the first postoperative day. He was thought to have had subcortical ischemia because the EEG was normal and the TCD had absent cerebral blood flow velocity, similar to what was seen in the previous case.
DISCUSSION
These three patients experienced transient cerebral dysfunction. Each patient was asymptomatic and had no neurologic abnormalities by routine examination. In addition, there were no alterations of the EEG upon clamping the carotid artery intraoperatively. However, the TCD signal was markedly attenuated during clamping and returned to preclamp values upon unclamping the carotid artery. Emboli were not seen after unclamping the carotid artery. The patient described in Case 1 experienced cognitive decline at one day postoperatively, when performance compared to baseline was assessed using a neuropsychometric battery. This decline proved to be transient when the patient was evaluated at 1 month and performed normally. We consider these changes in cognitive performance significant.
Moller et al. showed that about 26% of elderly patients developed cognitive dysfunction one week after noncardiac surgery compared to an incidence of 3.4% in a control group of similar age but not having surgery (2). The cognitive performance of the surgical group improved after 3 months to an incidence of about 10% compared to an incidence of 2.8% in the control group (2). Moller also defined abnormality as approximately two SDs different from performance in the control group (2). His conclusion was that cognitive dysfunction occurs because of surgery or anesthesia and long-term deterioration increases with age. Moller’s conclusions and ours differ because we used a control group composed of elderly patients having spine surgery with a similar anesthetic. Therefore, the differences in cognitive performance between patients in our two groups were due to the nature of the surgery beyond the effects of anesthesia. We also defined abnormal cognitive performance as two or more SDs of decreased performance compared to our control group (1).
The patients in Cases 2 and 3, on the other hand, experienced a decline based on the immediate neurologic examination after waking up from anesthesia. These signs improved within 15 minutes and were completely resolved by 24 hours in patient 2, and within 15 minutes in patient 3. Neurologic signs may become unmasked in patients with previous cerebral injury as they awaken from the affects of anesthesia (3). While this may be the case with our patients, we had no evidence of previous brain injury.
Cerebral complications from CEA are due to either focal or global ischemia: the former due to emboli from the operative site, the latter from impaired collateral circulation following carotid artery cross-clamping. The most frequent cause of new neurologic findings is felt to be microemboli (4). However, hypoperfusion during carotid artery cross-clamping will also produce injury due to inadequate collateral blood flow. If hypoperfusion persists, cerebral injury will result. Rampil et al. demonstrated that if significant EEG changes of ischemia persist for approximately 10 minutes or more after the carotid artery is cross-clamped in patients without preexisting neurologic injury, these patients will suffer a postoperative stroke after surgery (5). These two causes of cerebral injury occur at different times during surgery. Emboli arise when the carotid artery is unclamped (6) and hypoperfusion during the carotid artery cross-clamping period (5).
The purpose of cerebral monitoring, when the carotid artery is cross-clamped, is to determine the adequacy of hemispheric blood flow via collaterals. The EEG evaluates the neuronal integrity of many neurons from the cortex and assumes that changes in the EEG after the carotid artery is clamped reflect decreased cortical cerebral blood flow (7). However, the EEG fails to provide an assessment of subcortical electrical activity and its blood flow. There is increasing evidence that these subcortical areas affect cognitive performance (8). Therefore, we may miss cognitive changes from ischemia in this area.
Conversely, the TCD evaluates changes in CBF velocity in a large conductance vessel, namely the MCA, and assumes that adequate CBF velocity in the MCA predicts adequate perfusion in the cortex. While TCD measures changes in CBF velocity, it fails to evaluate cerebral cortical function. Different criteria have been developed to determine whether a decrease in CBF velocity is associated with a high probability of hemispheric ischemia (9). Transcranial Doppler ultrasonography monitoring can also determine the presence of microemboli. These have been shown to occur frequently when the carotid artery is unclamped. While most of these emboli are gaseous, those that are particulate in nature have been associated with cerebral injury (6,10).
Both EEG and TCD allow for continuous monitoring of cerebral “well-being” and have been used to reduce perioperative complications of CEA (Fig. 1). A number of studies have been performed comparing monitoring with EEG and TCD (11-13). Monitoring using the EEG and TCD have been shown to be statistically correlated with each other. However, each looks at different aspects of the brain. In Figure 2A, we illustrate adequate collateral cerebral circulation when the carotid artery is clamped at the distal end of the common carotid artery, and at the two branches, the internal and external carotid arteries. Both the anterior communicating and posterior communicating arteries supply blood to maintain the EEG and CBF within normal ranges (Fig. 2A). When these two pathways of collateral CBF are inadequate, then both the EEG and TCD become abnormal (Fig. 2B). However, if one pathway of collateral CBF is inadequate to provide enough CBF in both the anterior and middle cerebral arteries, then either the CBF velocity in the middle cerebral artery (Fig. 2C) or the CBF in the anterior cerebral artery (Fig. 2D) may be inadequate to maintain CBF velocity as measured by TCD (Fig. 2C) or cortical CBF as measured by EEG (Fig. 2D). We hypothesize that the former represents the situation with our cases (Fig. 2C). Cognitive and neurologic changes arise from subcortical ischemia.
FIG. 2.
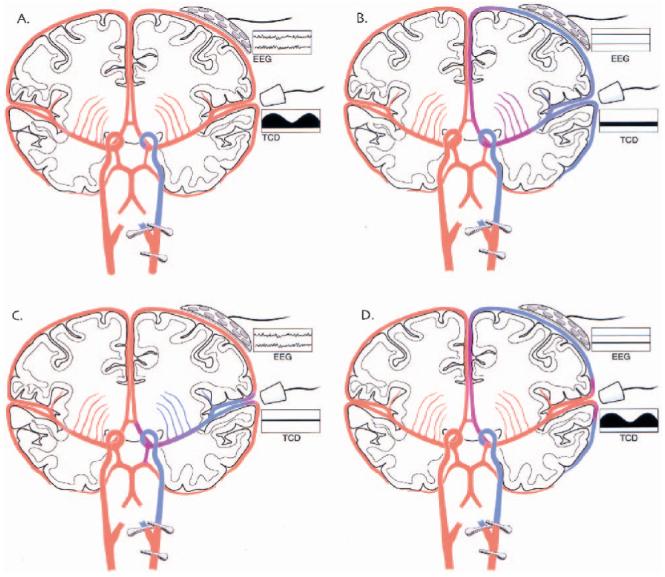
Coronal cross-section of the cerebral hemispheres showing: adequacy of cerebral perfusion, location of EEG and TCD monitors and their records. These four diagrams show coronal cross-sections of both cerebral hemispheres. The common carotid artery and its divisions have been clamped in all of the diagrams. The EEG and TCD monitors and their respective records are labeled. The adequacy of cerebral blood flow (CBF) after the carotid artery is clamped is color-coded. “Red” is CBF that is adequate to maintain normal neuronal functioning and “blue” is inadequate CBF for the same purpose. Shades of color between these two extremes are meant to illustrate a gradient of CBF. The EEG and TCD records are meant to depict “normal” EEG and TCD records in A and “abnormal” records in B. One record from each type of monitor is “normal” and “abnormal” in C and D. Cerebral blood flow velocity is abnormal in C and EEG, in D.
If the processed EEG is to be used as a guide for shunting, it needs to identify accurately those patients who are critically ischemic due to temporary unilateral occlusion of their carotid artery system. Of course, the EEG is measuring cortical activity, which reflects CBF from multiple sources. The reliability of EEG monitoring depends on three major factors: the number of channels (with 16 being ideal, but seldom possible in the majority of operating rooms), the experience level of the observer, and the depth of anesthesia (14,15). Sometimes EEG changes arise from causes other than cerebral ischemia, as we have already reported in one example (16). Similarly, while TCD is used to measure CBF changes in the operating room during CEA because it is a continuous noninvasive monitor of CBF in the MCA, it is also subject to misinterpretation and may lack sensitivity and specificity (17).
While one might conclude from these case reports that one monitoring modality is more sensitive than the other, an alternative conclusion is that these monitoring devices evaluate different components of cerebral functioning, and should be used together (11). The cortex is richly supplied with pial-pial collaterals from the anterior and posterior cerebral arteries. It is conceivable that these collaterals provide enough blood flow to allow normal EEG generation. Conversely, MCA blood flow may be inadequate to perfuse subcortical structures that are associated with cognitive functions and result in cerebral dysfunction (8).
Both types of monitors have led to false conclusions. There are studies showing severe or moderate EEG or somatosensory evoked potential changes without a significant decrease in CBF velocity in the MCA by TCD (18). Because of data similar to this, a number of physicians have defined a critical reduction of CBF velocity as an indication for shunt insertion to decrease the probability of cerebral injury (19). Although these data need confirmation in a larger series, preliminary analysis suggests that EEG and TCD monitoring should be viewed as complementary and not competing technologies as measures of cerebral integrity.
Acknowledgments
Dr. Heyer is supported in part by grants from the Charles A. Dana Foundation, and the NIH (1R01AG17604).
Dr. Connolly is supported in part by a grant from the American Federation for Aging Research, and the NIH (KO8 NS2038) and RO1 NS40409.
REFERENCES
- 1.Heyer EJ, Sharma R, Rampersad A, et al. A controlled prospective study of neuropsychological dysfunction following carotid endarterectomy. Arch Neurol. 2002;59:217–222. doi: 10.1001/archneur.59.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moller JT, Cluitmans P, Rasmussen LS, et al. Long-term postoperative cognitive dysfunction in the elderly: ISPOCD1 study. Lancet. 1998;351:857–861. doi: 10.1016/s0140-6736(97)07382-0. [DOI] [PubMed] [Google Scholar]
- 3.Thal GD, Szabo MD, LopezBresnahan M, et al. Exacerbation or unmasking of focal neurologic deficits by sedatives. Anesthesiology. 1996;85:21–25. doi: 10.1097/00000542-199607000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Krul JM, van Gijn J, Ackerstaff RG, et al. Site and pathogenesis of infarcts associated with carotid endarterectomy. Stroke. 1989;20:324–328. doi: 10.1161/01.str.20.3.324. [DOI] [PubMed] [Google Scholar]
- 5.Rampil IJ, Holzer JA, Quest DO, et al. Prognostic value of computerized EEG analysis during carotid endarterectomy. Anesth Analg. 1983;62:186–192. [PubMed] [Google Scholar]
- 6.Gaunt ME, Martin PJ, Smith JL, et al. Clinical relevance of intraoperative embolization detected by transcranial Doppler ultrasonography during carotid endarterectomy: a prospective study of 100 patients. Br J Surg. 1994;81:1435–1439. doi: 10.1002/bjs.1800811009. [DOI] [PubMed] [Google Scholar]
- 7.Sundt TM, Jr., Sharbrough FW, Piepgras DG, et al. Correlation of cerebral blood flow and electroencephalographic changes during carotid endarterectomy: with results of surgery and hemodynamics of cerebral ischemia. Mayo Clin Proc. 1981;56:533–543. [PubMed] [Google Scholar]
- 8.de Groot JC, de Leeuw FE, Oudkerk M, et al. Cerebral white matter lesions and cognitive function: the Rotterdam Scan Study. Ann Neurol. 2000;47:145–151. doi: 10.1002/1531-8249(200002)47:2<145::aid-ana3>3.3.co;2-g. [DOI] [PubMed] [Google Scholar]
- 9.McDowell H, Jr, Gross GM, Halsey JH. Carotid endarterectomy monitored with transcranial Doppler. Ann Surg. 1992;215:514–518. doi: 10.1097/00000658-199205000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muller M, Reiche W, Langenscheidt P, et al. Ischemia after carotid endarterectomy: comparison between transcranial Doppler sonography and diffusion-weighted MR imaging. AJNR Am J Neuroradiol. 2000;21:47–54. [PMC free article] [PubMed] [Google Scholar]
- 11.Arnold M, Sturzenegger M, Schaffler L, et al. Continuous intraoperative monitoring of middle cerebral artery blood flow velocities and electroencephalography during carotid endarterectomy: a comparison of the two methods to detect cerebral ischemia. Stroke. 1997;28:1345–1350. doi: 10.1161/01.str.28.7.1345. [DOI] [PubMed] [Google Scholar]
- 12.Jansen C, Moll FL, Vermeulen FE, et al. Continuous transcranial Doppler ultrasonography and electroencephalography during carotid endarterectomy: a multimodal monitoring system to detect intraoperative ischemia. Ann Vasc Surg. 1993;7:95–101. doi: 10.1007/BF02042666. [DOI] [PubMed] [Google Scholar]
- 13.Limoni P, Comani V. Carotid endarterectomy with TCD and EEG monitoring. Stroke. 1993;24:1762–1763. [PubMed] [Google Scholar]
- 14.Craft RM, Losasso TJ, Perkins WJ, et al. EEG monitoring for cerebral ischemia during carotid endarterectomy (CEA): how much is enough? Anesthesiology. 1994;81:A214. [Google Scholar]
- 15.Kresowik TF, Worsey MJ, Khoury MD, et al. Limitations of electroencephalographic monitoring in the detection of cerebral ischemia accompanying carotid endarterectomy. J Vasc Surg. 1991;13:439–443. doi: 10.1067/mva.1991.26500. [DOI] [PubMed] [Google Scholar]
- 16.Heyer E, Adams D, Moses C, et al. Erroneous conclusion from processed EEG with changing anesthetic depth. Anesthesiology. 2000;90:603–607. doi: 10.1097/00000542-200002000-00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aaslid R. Developments and principles of transcranial Doppler. In: Newell DW, Aaslid R, editors. Transcranial Doppler. Raven Press; New York: 1992. pp. 1–8. [Google Scholar]
- 18.Thiel A, Russ W, Zeiler D, et al. Transcranial Doppler sonography and somatosensory evoked potential monitoring in carotid surgery. Eur J Vasc Surg. 1990;4:597–602. doi: 10.1016/s0950-821x(05)80814-x. [DOI] [PubMed] [Google Scholar]
- 19.Halsey JH, Jr., The International Transcranial Doppler Collaborators Risks and benefits of shunting in carotid endarterectomy. Stroke. 1992;23:1583–1587. doi: 10.1161/01.str.23.11.1583. [DOI] [PubMed] [Google Scholar]


