Abstract
Background
Although subtle cognitive injury as revealed by neuropsychological testing occurs in a substantial number of patients following carotid endarterectomy (CEA), there is controversy about whether this finding is a result of the surgery or the anesthesia.
Objectives
To examine the changes in neuropsychological test performance in patients following CEA vs a control group of patients older than 60 years following spine surgery, so as to determine whether neuropsychological dysfunction after CEA is a result of surgery or anesthesia.
Methods
Patients undergoing CEA (n=80) and lumbar spine surgery (n=25) were assessed with a battery of neuropsychological tests preoperatively and on postoperative days 1 and 30. The neuropsychological performance of patients in the control group was used to normalize performance for patients in the CEA group, by calculating z scores using the mean and SD of the change scores in the control group. Significant cognitive dysfunction was defined as performance that exceeded 2 SDs above the mean performance of patients in the control group.
Results
Postoperative days 1 and 30 total deficit scores were significantly worse in the CEA group compared with the controls. When individual test results were examined, the CEA group performed significantly worse than the controls on the Rey Complex Figure test and Halstead-Reitan Trails B on day 1, and on the Rey Complex Figure on day 30. Overall, cognitive dysfunction was seen in 22 patients (28%) in the CEA group on day 1 and in 11 (23%) of 48 patients on day 30.
Conclusions
Subtle cognitive decline following CEA occurs and persists for at least several weeks after surgery. This decline was absent in a control group.
Carotid endarterectomy (CEA) is an effective means of preventing stroke in appropriately selected patients.1-4 Although the incidence of perioperative stroke is low, subtle cognitive changes as revealed by neuropsychological (NP) testing occur in a high percentage of patients following CEA. Several studies have demonstrated improvement,5-10 others show no change,11,12 and still others demonstrate a decline13-15 in postoperative NP performance. Although decline in NP performance may be related to intraoperative hemispheric cerebral ischemia,16,17 microemboli,18 or subclinical microinfarcts,13 some decline may be due to the effects of general anesthesia,19,20 particularly in older patients.21 Most previous studies have not controlled for the effects of perioperative variables, such as anesthesia, stress, and pain.
To better characterize the cognitive injury that occurs following CEA, we tested patients before and after surgery using a battery of NP tests to evaluate several cognitive domains to reveal subtle cognitive injury. Furthermore, we defined a group of patients undergoing spine surgery (age, >60 years) to serve as an appropriate control group to account for the effects of these perioperative variables.22 This prospective study explores the changes in NP test performance 1 day and 1 month following CEA.
RESULTS
Demographic and intraoperative variables are shown in Table 1 for all patients. Performance on all the NP tests was normally distributed at baseline for patients in the CEA group, including Trails A when 1 outlier was excluded. Because we expected patients with abnormalities of the central nervous system to perform worse than those without injury, it is not surprising that “symptomatic” CEA patients (those who had suffered a previous stroke or transient ischemic attack [TIA]) performed worse at baseline on all 3 NP examinations compared with the control subjects.26 In contrast, “asymptomatic” CEA patients performed significantly worse only on Trails A at baseline (P=.03). Therefore, we might also expect symptomatic patients to demonstrate a greater decline in NP performance after surgery than asymptomatic patients. However, a subgroup analysis of patients undergoing CEA showed no significant difference in NP outcome between symptomatic and asymptomatic patients. There was no significant relationship between educational level and NP outcome scores. Three patients in the CEA group had postoperative strokes and were excluded from the analysis. One patient had a postoperative myocardial infarction and was not excluded from the analysis. No significant difference in NP performance was seen between those who had a saphenous vein patch and those who did not.
Table 1. Demographics and Intraoperative Variables in Carotid Endarterectomy (CEA) and Spine Groups*.
| Demographic or Variable | CEA (n = 80) | Spine (n = 25) |
|---|---|---|
| Age, y | 70.5 (9.2) | 73.4 (7.6) |
| Sex (male/female) | 71/29 | 76/24 |
| Handedness (right/left) | 94/6 | 96/4 |
| Height, cm | 171.8 (9.7) | 173.9 (12.4) |
| Weight, kg | 78.8 (12.5) | 83.2 (18.7) |
| Education, y† | 14.3(3.4) | 16.4(3.9) |
| Hypertension | 50 | 56 |
| Diabetes mellitus | 28 | 8 |
| Previous stroke or transient ischemic attack† | 48 | 8 |
| Previous myocardial infarction | 39 | 24 |
| Previous CEA | 14 | 4 |
| Duration of surgery, min | 132 (32) | 146 (72) |
| Fentanyl citrate, μg/kg† | 1.9(1.4) | 2.8(1.4) |
| Midazolam hydrochloride, μg/kg† | 0.03 (0.01) | 0.03 (0.02) |
Values are mean (SD) or percentage.
P<.05; CEA and spine groups are significantly different in terms of years of education (P = .01), previous stroke or TIA (P = .0003), and amount of fentanyl (P = .009).
Postoperative day 1 total deficit scores were significantly worse in the CEA group compared with the control patients (Table 2, P=.003). When individual test results were examined (Table 2), the CEA patients performed significantly worse than control patients on the Rey Complex Figure (P=.006) and Trails B (P=.01). There were no significant differences in performance between patients undergoing right and left CEAs on any of the NP tests. Postoperative day 30 total deficit scores (Table 2) were significantly worse in the CEA group (P=.03) compared with the control patients. When individual test results were examined at the 1-month follow-up (Table 2), the CEA group still performed significantly worse on the Rey Complex Figure (P=.01). Of the 37 CEA patients with significant decline on either the Rey Complex Figure or Trails B, only 16% (6 patients) demonstrated a significant decline on both tests, while 84% (31 patients) demonstrated a significant decline on one or the other (16 [43%] on Rey Complex Figure and 15 [41%] on Trails B).
Table 2. Neuropsychological Test Scores*.
| CEA |
Spine |
P Value |
||||
|---|---|---|---|---|---|---|
| Test Deficit Score | Day 1 | Day 30 | Day 1 | Day 30 | Day 1 | Day 30 |
| COW | 0.5 ± 0.1 | 0.6 ± 0.2 | 0.5 ± 0.2 | 0.6 ± 0.3 | .93 | .91 |
| Rey | 1.4 ± 0.2 | 1.4 ± 0.2 | 0.6 ± 0.2 | 0.6 ± 0.2 | .006 | .01 |
| Trails A | 0.9 ± 0.2 | 0.4 ± 0.2 | 0.6 ± 0.2 | 0.5 ± 0.3 | .24 | .60 |
| Trails B | 1.1 ± 0.2 | 0.7 ± 0.2 | 0.5 ± 0.1 | 0.5 ± 0.3 | .01 | .67 |
| Total deficit score | 3.9 ± 0.4 | 3.1 ± 0.4 | 2.2 ± 0.3 | 1.8 ± 0.4 | .003 | .03 |
Values are mean ± SEM. CEA indicates carotid endarterectomy; COW, controlled oral word. Comparisons are by t test. Baseline values for Rey Complex Figure were not different between CEA and spine groups: 28.6 ± 6.9 and 30.7 ± 4.0 for CEA and spine groups, respectively, where values are mean ± SD.
To determine a cutoff for significant cognitive dysfunction following CEA, postoperative day 1 total deficit scores were examined in the control group (n=25). Total deficit scores higher than 2 SDs (>5.46 points) above the mean of the control group (higher points indicated poorer performance) represented significant cognitive dysfunction (Figure, A). Of the 80 CEA patients, 22 (28%) had significant cognitive dysfunction on postoperative day 1. Only 1 control patient had a postoperative day 1 total deficit score higher than 2 SDs above the mean.
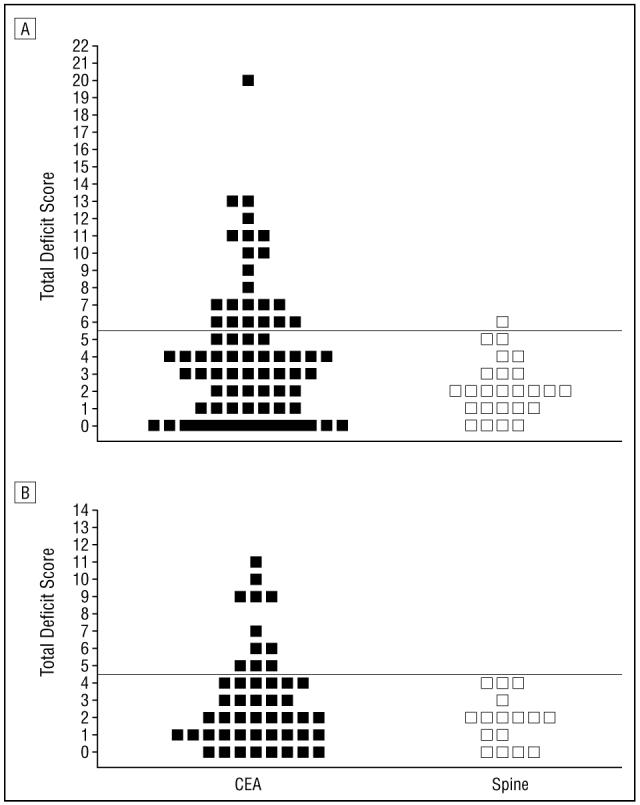
A, Total deficit scores at day 1: Higher total deficit scores represent a greater decline in performance. The cutoff for significant cognitive injury (5.46 points) at day 1 is 2 SDs above the mean of the control group (2.16±1.65) and is depicted by the horizontal line. Based on this criterion, 22 patients (28%) have significant cognitive decline following CEA, compared with a control group of patients undergoing spine surgery. In the control group, lumbar spine surgeries included 21 (86%) undergoing laminectomy, 3 (12%) undergoing discectomy, and 1 (2%) undergoing microdiscectomy. B, Total deficit scores at day 30: The cutoff for significant cognitive injury (4.65 points) is 2 SDs above the mean of the control group (1.81±1.42) and is depicted by the horizontal line. Based on this criterion, 11 (23%) of 48 patients have significant cognitive decline 1 month after CEA, compared with a control group of patients undergoing spine surgery.
Total deficit scores higher than 4.65 points represented significant cognitive dysfunction at day 30 after surgery (Figure, B). Of the 48 CEA patients tested at day 30, 11 (23%) demonstrated significant cognitive decline, compared with the controls. Because 32 patients (40%) in the CEA group declined to complete the study at 1 month, we compared baseline performance scores between them and the 48 patients who returned. There was no significant difference in test performance on all 4 individual NP tests at baseline and at 1 day after surgery between these 2 subgroups of patients. The 2 subgroups differed with respect to 2 perioperative variables: the 48 patients who returned had a significantly higher incidence of previous stroke or TIA (P<.02) but a lower incidence of previous myocardial infarction (P<.004), compared with the patients who did not return. Six patients injured at day 30 were also injured at day 1(P<.03, χ2 test). No control patients had postoperative day 30 total deficit scores higher than 2 SDs above the mean. Despite the loss of 8 control patients at the 1-month follow-up, there was no significant difference in their test performance on all 4 individual NP tests at baseline and 1 day after surgery, compared with the 17 who returned. The control patients who returned were significantly shorter in stature than the patients who did not return (P<.02).
Of the 22 patients in the CEA group with significant cognitive dysfunction 1 day after surgery, 6 (27%) remained injured 1 month later. Ten patients who were injured 1 day after surgery declined their 1-month follow-up examination. Of the 58 patients who did not demonstrate significant cognitive dysfunction 1 day after surgery, 5 (9%) demonstrated significant cognitive dysfunction at 1 month. Nineteen patients who were uninjured 1 day after surgery declined follow-up.
Patients in the CEA group with significant cognitive dysfunction were compared with patients in the group without dysfunction. These 2 subgroups did not significantly differ with respect to perioperative variables (age, sex, educational level, or handedness), medical history (stroke or TIA, CEA, diabetes mellitus, or hypertension), surgical variables (side of surgery, contralateral carotid stenosis, duration of carotid cross-clamping, or duration of surgery), or anesthetic variables (fentanyl or midazolam dosage or intraoperative temperature). However, patients with a history of myocardial infarction were more likely to develop cognitive dysfunction following CEA than those without a history (P<.01).
SUBJECTS AND METHODS
SUBJECTS
Eighty-three patients undergoing elective CEA and 25 patients (age, >60 years) undergoing lumbar spine surgery were recruited to participate in this institutional review board-approved study. We included all patients who were able to perform the NP evaluation in English, and excluded all patients with either a postoperative stroke or pain scores greater than 4 at their first follow-up examination. After written informed consent was obtained, patients were assessed with a battery of NP tests at 3 time points: before surgery (n=108), 1 day after surgery (n=105), and 30 days after surgery (n=65). All examinations were performed at least 3 hours after any analgesic or sedative medication had been administered. Preoperative and postoperative magnetic resonance imaging and computed tomographic scanning were not performed. Although intraoperative transcranial Doppler ultrasonography was performed on some patients, it was not used to determine the need for shunting or to count the number of emboli coming from the surgical field.
ANESTHESIA
No patients were premedicated. All patients received general anesthesia, with routine hemodynamic and temperature monitoring recorded continually every minute. Sedation before induction consisted of fentanyl citrate and midazolam hydrochloride. Anesthetic agents for induction included 1 or more of the following: propofol, etomidate, or thiopental sodium, and succinylcholine chloride, cisatracurium besylate, or rocuronium bromide; for maintenance: isoflurane or sevoflurane with nitrous oxide in oxygen in a 2:1 ratio, and cisatracurium or rocuronium; and for emergence: neostigmine bromide and glycopyrrolate for reversal of neuromuscular blockade, and labetalol hydrochloride or esmolol hydrochloride for hemodynamic control. For patients undergoing CEA, a radial arterial catheter for measuring blood pressure continuously and an 8-channel encephalographic monitor (Neurotrac II; Moberg Medical, Inc, Ambler, Pa) were used. Significant encephalographic change on clamping the carotid artery was defined as a 50% or greater decrease in amplitude in the alpha or beta frequencies and a similar increase in the delta or theta frequencies, or complete loss of all cerebral electrical activity. Anesthesia for patients having spine surgery was essentially identical to that for patients having CEA, except for higher mean dosages of fentanyl in patients undergoing spine surgery (Table 1).
SURGERY
All laminectomies were performed by a member of the neurosurgical spine service (P.C.M., J.G.M., or D.O.Q.), and all CEAs were performed by members of either the neurovascular service (R.A.S., D.O.Q., or E.S.C.) or the vascular service (G.J.T.). The surgery for CEA consisted of positioning the patient supine with the head in an extended midline position. An incision was made along a skin crease from just below the angle of the mandible to near the midline through skin, subcutaneous tissue, and platysma. The common, internal, and external carotid arteries were exposed and controlled. All patients undergoing CEA received 5000 to 6000 U of heparin sodium bolus. No patients were shunted. In patients operated on by one of us (G.J.T.), a saphenous vein patch was used and protamine sulfate reversal was given selectively.
Patients under going spine surgery were placed in the prone position. Following a midline incision, dissection through the paraspinal muscles was accomplished unilaterally in patients undergoing discectomy and bilaterally in those undergoing laminectomy. The microscope was used for visualization of disk removal in 1 patient. Patients were returned to the supine position before extubation. No patients, in either the CEA or control groups, received blood transfusions.
All patients were extubated in the operating room and recovered for 1 to 3 hours in a postoperative care unit. After CEA, patients were transferred to the intensive care unit, where they stayed for 1 night. No patients having lumbar spine surgery required a stay in the intensive care unit. All patients remained in the hospital for 1 to 3 days for postoperative pain scoring and NP testing.
NP EVALUATION
Patients were assessed with a battery of NP tests. All examinations were administered by the same research assistant (R.S.), trained to administer and score these NP tests under the supervision of neuropsychologists (Y.S. and R.M.L.). Four raw scores were generated from the battery of 3 NP tests, which were chosen to represent a limited range of cognitive domains. Halstead-Reitan Trails parts A and B evaluated visual conceptual and visuomotor tracking by timing how long it took a subject to connect consecutively numbered circles with a single line (part A) and then connect the same number of consecutively numbered and lettered circles by alternating between the 2 sequences (part B). The Controlled Oral Word Association Test evaluated verbal fluency and provided information on the function of the dominant hemisphere (“left”). Patients were asked to generate as many words as they could beginning with 3 target letters (C, F, and L) in 1 minute. Their raw score was the sum of the words produced for all 3 letters. The copy portion of the Rey Complex Figure test was administered to assess perceptual and visuospatial organization and provided information on the function of the nondominant hemisphere (“right”). Patients were asked to copy the Rey Complex Figure to the best of their ability.23 A standardized scoring system24 was used to evaluate the presence of specific design features and the accuracy of their location. Although we would have preferred a more extensive battery, our population of older patients better tolerated this one because of its short administration time.
The degree of pain was assessed at the same time NP testing was performed, using an 11-point Numeric Pain Intensity Scale adapted from a previous study.25 Patients with pain scores greater than 4 were excluded at the first follow-up, because of the confounding effect of pain on NP test performance.22
STATISTICAL MEASURES
For each NP test, the change in performance was calculated by subtracting the baseline score from the postoperative score. A normative data set for these changes was derived from the control population for each NP test. The mean change and SD for the control population were then used to calculate a z score for each test: z score=(change score-mean change scorespine)/SD of change scorespine. To illustrate cognitive decline, a point system was used to transform negative z scores into points for each NP test. Points were assigned as follows: z scores ≥-0.5=0 points; <-0.5 to -1.0=1 point; <-1.0 to -1.5=2 points; <-1.5 to -2.0=3 points; <-2.0 to -2.5=4 points; <-2.5 to -3.0=5 points; and <-3.0=6 points. This test deficit score measured how far each patient’s performance in the CEA group deviated from the mean performance of the control group. For each patient, the total number of test deficit scores was summed to produce the total deficit score. This total deficit score represents overall performance on the NP battery, while test deficit scores represent performance on individual NP tests. Group differences were compared by using the Satterthwaite modification to the unpaired t test because of differences in the variance of the measurement between groups. The Kolmogorov-Smirnov test was used to test whether the distribution of test and z scores between the control and CEA groups for each NP test was normal.
COMMENT
Using total deficit scores to measure NP performance, patients who underwent CEA sustained a significant decline on postoperative days 1 and 30, compared with a control group of patients having spine surgery. This decline was measured using a battery of 3 NP tests to evaluate language, attention, and perceptual and visuospatial organization. This persistent decline in performance was not seen in a previous uncontrolled study.14
Numerous investigations spanning several decades have explored whether CEA affects postoperative NP performance.27 One universal limitation of these studies was the absence of an appropriate control group to account for the effects of general anesthesia and surgery on NP performance.19,20 Some studies used nonoperative controls,6,7,12,28,29 others used operative controls,5,7,9,13 and still others used no control group at all.8,14,15,30,31
Our 2 groups were well matched in perioperative variables, medical history, anesthetic variables, and perioperative pain levels. The only difference was that patients undergoing spine surgery required higher doses of fentanyl during surgery and were in more pain after surgery. However, increased doses of fentanyl would bias the data against the control group, causing them to perform worse on the NP tests, and we excluded patients with pain scores higher than 4 from our analysis.22 Therefore, it is likely that factors specific to CEA, such as hemispheric cerebral ischemia or microemboli, may be responsible for the decline in NP performance.
When results of individual tests 1 day after surgery were examined, the CEA group patients performed significantly worse than did controls on the Rey Complex Figure and Trails B tests. A significant decline on both of these tests occurred in only 6 patients (16%). This finding may support the hypothesis that different mechanisms of ischemia, focal vs hemispheric, are responsible for the postoperative cognitive decline following CEA. At 30 days after surgery, the CEA group performed significantly worse than did controls on only the Rey Complex Figure test.
Besides using a control group to determine the effects of anesthesia and surgery, NP performance in a control group was used to account for changes in NP performance that were due to the “practice effect,” which arises when patients are repeatedly tested. Using the mean and SD of the change scores in the control group, z scores were calculated to measure how individuals in the CEA group performed compared with patients in the control group. By using a surgical control group, we avoided using less rigorous statistical methods, such as defining deficit based on a percentage decline, used in a previous study.14 In addition, the severity of significant injury on individual NP test results can be quantified using test deficit scores, and overall injury can be quantified using total deficit scores.
For this study, significant cognitive injury was defined as a decline in performance that exceeded 2 SDs above the mean of the spine surgery cohort. Using this criterion, the NP performance of 24 (96%) of the control patients was within the normal (uninjured) range on postoperative day 1. All control patients performed within the normal range 1 month after surgery. Although most patients in the CEA group showed no change or improved NP performance after surgery, 22 (28%) demonstrated a significant decline in NP performance 1 day after surgery, and 11 (23%) of 48 were injured 1 month after surgery. There was also no difference in the incidence of cognitive injury with respect to the side of the CEA, using the Rey Complex Figure test as a nondominant hemisphere task and the oral word test as a dominant hemisphere task.
Although the thrust of this article was to highlight the incidence of patients who developed cognitive deficits, some patients undergoing CEA have experienced improved postoperative NP performance.14 This improvement may have occurred because of relief of flow failure after CEA. Future studies may investigate cerebral blood flow before and after surgery and attempt to correlate relief of flow failure with improved NP performance after surgery.
Although we were concerned about the reduced number of patients at the second follow-up, there were no obvious differences in NP test performance on either baseline or first follow-up examinations among the CEA and control groups. The patients in the CEA group who returned for the second follow-up were more likely to have had a previous stroke or TIA and were less likely to have had a myocardial infarction. Except for shorter stature, the control patients who returned for the second follow-up were no different from the control patients who returned for the first follow-up only.
The battery of NP tests used in this study consistently reveals subtle cognitive decline following CEA, which persists for at least several weeks after surgery. These changes were apparently unrelated to anesthetic or perioperative factors. They represent subtle neurological deficits that arose as a result of the surgery itself, unrelated to overt clinical stroke.
Acknowledgments
This study was supported in part by grants from the American Federation for Aging Research (Dr Connolly) and the Charles A. Dana Foundation (Dr Heyer), New York, NY, and by research grants KO8 NS2038 and RO1 NS40409 (Dr Connolly) and 1R01 AG17604-01A2 (Dr Heyer) from the National Institutes of Health, Bethesda, Md.
We are grateful for the excellent statistical support provided by Robert R. Sciacca, Eng ScD.
REFERENCES
- 1.North American Symptomatic Carotid Endarterectomy Trial Collaborators Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991;325:445–453. doi: 10.1056/NEJM199108153250701. [DOI] [PubMed] [Google Scholar]
- 2.Hobson RW, II, Weiss DG, Fields WS, et al. Efficacy of carotid endarterectomy for asymptomatic carotid stenosis. N Engl J Med. 1993;328:221–227. doi: 10.1056/NEJM199301283280401. [DOI] [PubMed] [Google Scholar]
- 3.Executive Committee for the Asymptomatic Carotid Atherosclerosis Study Endarterectomy for asymptomatic carotid artery stenosis. JAMA. 1995;273:1421–1428. [PubMed] [Google Scholar]
- 4.European Carotid Surgery Trialists’ Collaborative Group MRC European Carotid Surgery Trial: interim results for symptomatic patients with severe (70-99%) or with mild (0-29%) carotid stenosis. Lancet. 1991;337:1235–1243. [PubMed] [Google Scholar]
- 5.Kelly MP, Garron DC, Javid H. Carotid artery disease, carotid endarterectomy, and behavior. Arch Neurol. 1980;37:743–748. doi: 10.1001/archneur.1980.00500610023002. [DOI] [PubMed] [Google Scholar]
- 6.De Leo D, Serraiotto L, Pellegrini C, Magni G, Franceschi L, Deriu GP. Outcome from carotid endarterectomy: neuropsychological performances, depressive symptoms and quality of life: 8-month follow-up. Int J Psychiatry Med. 1987;17:317–325. doi: 10.2190/1grb-rkbh-nb2a-ppwr. [DOI] [PubMed] [Google Scholar]
- 7.Bornstein RA, Benoit BG, Trites RL. Neuropsychological changes following carotid endarterectomy. Can J Neurol Sci. 1981;8:127–132. doi: 10.1017/s031716710004302x. [DOI] [PubMed] [Google Scholar]
- 8.Bennion RS, Owens ML, Wilson SE. The effect of unilateral carotid endarterectomy on neuropsychological test performance in 53 patients. J Thorac Cardiovasc Surg. 1985;26:21–26. [PubMed] [Google Scholar]
- 9.Hemmingsen R, Mejsholm B, Vorstrup S, Lester J, Engell HC, Boysen G. Carotid surgery, cognitive function, and cerebral blood flow in patients with transient ischemic attacks. Ann Neurol. 1986;20:13–19. doi: 10.1002/ana.410200104. [DOI] [PubMed] [Google Scholar]
- 10.Antonelli Incalzi R, Gemma A, Landi F, et al. Neuropsychologic effects of carotid endarterectomy. J Clin Exp Neuropsychol. 1997;19:785–794. doi: 10.1080/01688639708403760. [DOI] [PubMed] [Google Scholar]
- 11.Casey JE, Ferguson GG, Kimura D, Hachinski VC. Neuropsychological improvement versus practice effect following unilateral carotid endarterectomy in patients without stroke. J Clin Exp Neuropsychol. 1989;11:461–470. doi: 10.1080/01688638908400906. [DOI] [PubMed] [Google Scholar]
- 12.Parker JC, Granberg BW, Nichols WK, Jones JG, Hewett JE. Mental status outcomes following carotid endarterectomy: a six-month analysis. J Clin Neuropsychol. 1983;5:345–353. doi: 10.1080/01688638308401182. [DOI] [PubMed] [Google Scholar]
- 13.Vanninen E, Vanninen R, Aikia M, et al. Frequency of carotid endarterectomy-related subclinical cerebral complications. Cerebrovasc Dis. 1996;6:272–280. [Google Scholar]
- 14.Heyer E, Adams D, Todd G, et al. Neuropsychometric changes in patients after carotid endarterectomy. Stroke. 1998;29:1110–1115. doi: 10.1161/01.str.29.6.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lind C, Wimmer A, Magometschnigg H, et al. Effects of carotid endarterectomy on various neuropsychologic parameters: a neuropsychologic longitudinal study [in German] Langenbecks Arch Chir. 1993;378:345–352. doi: 10.1007/BF01876438. [DOI] [PubMed] [Google Scholar]
- 16.Brinkman SD, Braun P, Ganji S, Morrell RM, Jacobs LA. Neuropsychological performance one week after carotid endarterectomy reflects intra-operative ischemia. Stroke. 1984;15:497–503. doi: 10.1161/01.str.15.3.497. [DOI] [PubMed] [Google Scholar]
- 17.Cushman L, Brinkman SD, Ganji S, Jacobs LA. Neuropsychological impairment after carotid endarterectomy correlates with intraoperative ischemia. Cortex. 1984;20:403–412. doi: 10.1016/s0010-9452(84)80008-8. [DOI] [PubMed] [Google Scholar]
- 18.Gaunt ME, Martin PJ, Smith JL, et al. Clinical relevance of intraoperative embolization detected by transcranial Doppler ultrasonography during carotid endarterectomy: a prospective study of 100 patients. Br J Surg. 1994;81:1435–1439. doi: 10.1002/bjs.1800811009. [DOI] [PubMed] [Google Scholar]
- 19.Townes BD, Dikmen SS, Bledsoe SW, Hornbein TF, Martin DC, Janesheski JA. Neuropsychological changes in a young, healthy population after controlled hypotensive anesthesia. Anesth Analg. 1986;65:955–959. [PubMed] [Google Scholar]
- 20.Jones MJ. The influence of anesthetic methods on mental function. Acta Chir Scand Suppl. 1989;550:169–175. [PubMed] [Google Scholar]
- 21.Moller JT, Cluitmans P, Rasmussen LS, et al. for the International Study of PostOperative Cognitive Dysfunction investigators Long-term postoperative cognitive dysfunction in the elderly. Lancet. 1998;351:857–861. doi: 10.1016/s0140-6736(97)07382-0. [DOI] [PubMed] [Google Scholar]
- 22.Heyer EJ, Sharma R, Winfree CJ, et al. Severe pain confounds neuropsychological test performance. J Clin Exp Neuropsychol. 2000;22:633–639. doi: 10.1076/1380-3395(200010)22:5;1-9;FT633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lezak MD. Neuropsychological Assessment. 3rd ed. Oxford University Press; New York, NY: 1995. [Google Scholar]
- 24.Meyers J, Meyers K. Rey Complex Figure Test and Recognition Trial Professional Manual. Psychological Assessment Resources Inc; Odessa, Fla: 1995. [Google Scholar]
- 25.Downie WW, Leatham PA, Rhind VM, Wright V, Branco JA, Anderson JA. Studies with pain rating scales. Ann Rheum Dis. 1978;37:378–381. doi: 10.1136/ard.37.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thal GD, Szabo MD, Lopez-Bresnahan M, Crosby G. Exacerbation or unmasking of focal neurologic deficits by sedatives. Anesthesiology. 1996;85:21–25. doi: 10.1097/00000542-199607000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Lunn S, Crawley F, Harrison MJ, Brown MM, Newman SP. Impact of carotid endarterectomy upon cognitive functioning: a systematic review of the literature. Cerebrovasc Dis. 1999;9:74–81. doi: 10.1159/000015901. [DOI] [PubMed] [Google Scholar]
- 28.Rasmussen LS, Christiansen M, Johnsen J, Gronholdt ML, Moller JT. Subtle brain damage cannot be detected by measuring neuron-specific enolase and S-100β protein after carotid endarterectomy. J Cardiothorac Vasc Anesth. 2000;14:166–170. doi: 10.1016/s1053-0770(00)90012-0. [DOI] [PubMed] [Google Scholar]
- 29.Sirkka A, Salenius JP, Portin R, Nummenmaa T. Quality of life and cognitive performance after carotid endarterectomy during long-term follow-up. Acta Neurol Scand. 1992;85:58–62. doi: 10.1111/j.1600-0404.1992.tb03996.x. [DOI] [PubMed] [Google Scholar]
- 30.Williams M, McGee TF. Psychological study of carotid occlusion and endarterectomy. Arch Neurol. 1964;10:293–297. doi: 10.1001/archneur.1964.00460150063006. [DOI] [PubMed] [Google Scholar]
- 31.Owens M, Pressman M, Edwards AE, et al. The effect of small infarcts and carotid endarterectomy on postoperative psychologic test performance. J Surg Res. 1980;28:209–216. doi: 10.1016/0022-4804(80)90117-1. [DOI] [PubMed] [Google Scholar]


