Abstract
The aims of the present study were to assess the effects of long-term estrogen replacement therapy (ERT) on size and indices of bone turnover in periarticular osteophytes in ovariectomized cynomolgus monkeys and to compare dynamic indices of bone turnover in osteophyte bone with those of subchondral bone (SCB) and epiphyseal/metaphyseal cancellous (EMC) bone. One hundred sixty-five adult female cynomolgus macaques were bilaterally ovariectomized and randomly divided into three age- and weight-matched treatment groups for a 36-month treatment period. Group 1 (OVX control) received no treatment, Group 2 (SPE) received soy phytoestrogens, and Group 3 (ERT) received conjugated equine estrogens in the diet; all monkeys were labeled with calcein before necropsy. A midcoronal, plastic-embedded section of the right proximal tibia from 20 randomly selected animals per treatment group was examined histologically. Forty-nine of the sections (OVX control, n=16; SPE, n=16; ERT, n=17) contained lateral abaxial osteophytes, and static and dynamic histomorphometry measurements were taken from osteophyte bone, SCB from the lateral tibial plateau, and EMC bone. Data were analyzed using the ANOVA and Kruskal-Wallis test, correlation and regression methods, and the Friedman and Wilcoxon signed rank test. There was no significant effect of long-term ERT on osteophyte area or on any static or dynamic histomorphometry parameters. The bone volume, trabecular number, and trabecular thickness in osteophyte bone were considerably higher than in EMC bone; whereas, trabecular separation was considerably lower in osteophyte bone. In all three treatment groups, BS/BV was significantly lower in osteophyte bone vs. EMC bone and significantly higher in osteophyte bone vs. lateral SCB. We conclude that osteophyte area and static and dynamic histomorphometry parameters within periarticular tibial osteophytes in ovariectomized cynomolgus monkeys are not significantly influenced by long-term ERT, but that site differences in static and dynamic bone histomorphometry parameters exist, particularly between EMC and osteophyte bone.
Keywords: animal models, bone histomorphometry, estrogen, osteoarthritis, primate
Introduction
Although periarticular osteophytes are commonly present in osteoarthritis (OA), their role in the pathogenesis of OA is unknown. It remains unclear whether osteophytes are an epiphenomenon caused by OA or if they are part of a reactive process in an unstable joint. The clinical relevance of osteophytes in the knee joint is also controversial, with some sources reporting a significant correlation between the presence of osteophytes in the tibiofemoral joint and knee joint pain and others reporting no association [1,2]. A recent review article suggests that osteophytes are not “non-functional bystanders” in all cases of OA, but may have “constructive roles” (e.g., increased joint stability or recuperation of the joint space) in at least some affected joints [3]. These authors also suggest that understanding the biology of osteophyte formation can give insight into the disturbed homeostasis of joints with OA and, ultimately, provide directions for OA therapy [3].
Estrogen replacement therapy (ERT) has been shown to decrease the risk of postmenopausal osteoporosis in humans by maintaining bone mass [4]. Similarly, ERT maintains bone mass in ovariectomized animals and decreases bone turnover in both humans and animals [5,6]. In a previous study, our laboratory demonstrated that long-term ERT significantly reduced the severity of articular cartilage lesions of naturally occurring OA in the medial tibial plateaus of ovariectomized cynomolgus macaques compared with untreated ovariectomized controls [7]. Ham et al. also reported that the number of osteophytes (sum of axial and abaxial osteophytes in medial tibial plateau) was lower in the ERT treatment group; however, detailed evaluation of osteophytes was not a focus of that study [7]. A subsequent study involving a subset of these same tibial sections, and designed to evaluate axial and abaxial osteophytes individually, found that ERT did not consistently reduce the prevalence and had no significant effect on the cross-sectional area of periarticular tibial osteophytes compared with the untreated ovariectomized control animals [8].
Dynamic histomorphometry studies are a more sensitive method for examining treatment effects on bone and provide information on the more recent history of the site than do static histomorphometry studies. As far as we are aware, bone turnover in osteophyte bone has not been examined previously in naturally occurring OA, nor has it been compared with turnover in other bony sites. The effects of ERT on bone turnover in osteophytes also are unknown; however, a previous study demonstrated that long-term ERT significantly reduced indices of bone turnover in both the subchondral bone (SCB) of the medial tibial plateau and epiphyseal-metaphyseal cancellous (EMC) bone of the proximal tibia in postmenopausal cynomolgus monkeys compared with untreated ovariectomized controls [9]. Interestingly, the results of that study indicated that the bone turnover indices were higher in the SCB than in the EMC bone, demonstrating that there are site differences in bone turnover rates that may be determined by functional or biomechanical factors [9]. The purposes of the present study were to: 1) evaluate the static histomorphometry indices of osteophyte bone and compare them with the same indices in EMC bone; 2) examine the effects of long-term ERT on dynamic histomorphometry indices in osteophytes; and 3) compare dynamic histomorphometry indices in osteophytes with those of SCB and EMC bone. We hypothesized that ERT would reduce bone turnover in osteophyte bone similar to that of SCB and EMC bone and that bone turnover in osteophytes would be more similar to that of SCB than EMC bone due to the more superficial location of osteophyte bone and SCB and the likelihood that these sites are more strongly influenced by biomechanical forces than is EMC bone.
Materials and Methods
Animals
The animals used in this study were from a study that was designed to evaluate the effects of estrogen deficiency, exogenous estrogen replacement therapy (ERT), and soy phytoestrogen treatment (SPE) on coronary artery atherosclerosis [10]. The original study included 180 feral adult female cynomolgus macaques and has previously been described in detail 7,10].
Study design
The animals were fed a moderately atherogenic diet (40% of calories from fat) for 26 months. At the end of this 26-month period, they were bilaterally ovariectomized to simulate menopause and were randomly divided into three age- and weight-matched treatment groups for a 36-month treatment period, during which all animals were fed a moderately atherogenic diet containing 120 kcal/kg of body weight/day [10]. Group 1 (OVX control, n=60) received no treatment, Group 2 (SPE, n=60) received soy phytoestrogens (at 129 mg/day human equivalent), and Group 3 (ERT, n=60) received conjugated equine estrogens [Premarin; Wyeth- Ayerst Laboratories, Philadelphia, PA] (at 0.625 mg/day human equivalent) in the diet. Plasma hormone levels were measured during the 36-month treatment period to document effective treatment levels [10]. All animals were administered intravenous calcein (10 mg/kg) 21 and 7 days prior to necropsy. At the termination of the study, the mean age of the animals was 12.0 years (range 9.6–15.8 years; SD = 1.1), as estimated by dentition, [11] and the mean body weight was 3.3 kg (range 2.1 –6.2 kg; SD = 0.7) [7].
Necropsy and tissue preparation
At necropsy, the right knee joint from each animal was collected, disarticulated, and fixed in 70% ethanol. After removal of soft tissues, each proximal tibia was serially sectioned at 2-mm intervals using a diamond saw, and a midcoronal section of the right proximal tibia (that included the medial and lateral tibial plateaus) from 20 randomly selected animals in each treatment group (n=60 total) was identified for histomorphometry measurements and was embedded in Bioplastic (Wards Scientific, Rochester, NY). Ten-micrometer thick sections were cut using a sledge microtome and mounted unstained on glass slides using Eukitt mounting material (Calibrated Instruments, Hawthorne, NY). All sections were randomized and the evaluator (EO) was blinded to the treatment group assignments. The dynamic histomorphometry measurements of the subchondral bone (SCB) of the medial tibial plateau and epiphyseal-metaphyseal cancellous bone (EMC) from these same 60 randomly-chosen proximal tibial sections have previously been reported [9].
Periarticular tibial abaxial osteophyte identification and cross-sectional area
A total of 57 out of 60 sections of proximal tibia (n=19 per treatment group) were available for evaluation due to problems in producing high-quality histological sections from three of the tissue blocks. The histological criteria used to identify and measure the cross-sectional area of axial and abaxial osteophytes in the proximal tibiae of cynomolgus monkeys has been described previously [8]. Briefly, abaxial osteophytes were defined as outgrowths of bone and cartilage at the joint margins of the proximal tibia, where the normal contour of the bone was altered. Because the margins of the abaxial (peripheral/marginal) osteophytes were much more well defined than those of the axial osteophytes (referred to as central tibial spines in the human literature) and due to the fact that there were considerably more specimens containing abaxial osteophytes in the lateral tibial plateau than in the medial tibial plateau, only results from the 49 sections containing lateral abaxial osteophytes are reported. The SCB plate of the lateral tibial plateau was evaluated histologically and dynamic histomorphometry measurements were taken in this site, which was more closely adjacent to the location of the osteophytes than the medial tibial plateau, from which histomorphometry results from the SCB compartment previously have been published [9].
Using Osteomeasure histomorphometry software (Osteometrics, Decatur, GA), the perimeter of each lateral abaxial osteophyte (OVX control, n=16; SPE, n=16; ERT, n=17) was hand-traced on a digital pad using a 2X objective and one or two measurement squares (12.25 mm2 each), depending on the overall size of the osteophyte, using criteria previously described for determination of osteophyte measurements [8]. Void spaces that were enclosed by bone were also traced, and the void perimeter values were added to the total perimeter in the following formula (BPm=TbPfPm+VdPm); the void area values were subtracted from the total bone area using the following formula (BAr=TbPfAr-VdAr) (Fig. 1; Table 1). Osteophyte cross-sectional area was determined by tracing the entire perimeter of each osteophyte; this value was used in place of total area (T.Ar.) in the calculation of the static and dynamic parameters (Table 1).
Fig. 1.
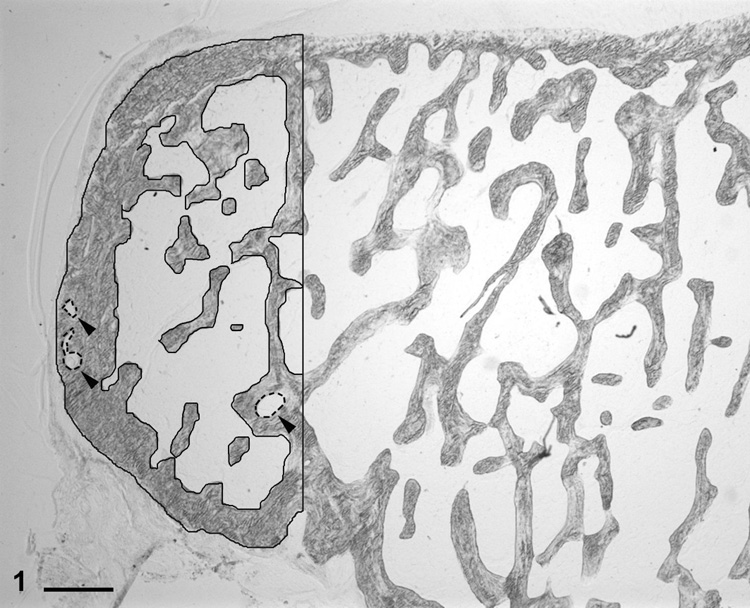
Proximal tibia, lateral joint (abaxial) compartment; cynomolgus monkey. Histological appearance of a well-defined periarticular osteophyte. The solid black tracings represent the bone perimeter and the dashed line tracings (arrowheads) represent void perimeters. Unstained section. Bar = 500 µm.
Table 1.
Primary measurements, formulas for derived indices, and abbreviations based on the recommendations of the American Society for Bone and Mineral Research Nomenclature Committee.
| Parameter | Units | Name | Formula |
|---|---|---|---|
| Primary measurements | |||
| Ir.L.Wi | µm | Interlabel width | |
| Ir.L.t | days | Interlabel interval | |
| dL.Pm | mm | Double-labeled perimeter | |
| sL.Pm | mm | Single-labeled perimeter | |
| B.Pm | mm | Bone perimeter | |
| B.Ar | mm2 | Bone area | |
| T.Ar | mm2 | Total area of measurement field | |
| Derived dynamic indices | |||
| MAR | µm/day | Mineral apposition rate | (π/4)*Ir.L.Wi/Ir.L.t |
| MS/BS | % | Mineralizing surface | 100*(dL.Pm+0.5*sL.Pm)/B.Pm |
| BFR/BS | µm3/µm2/year | Bone formation rate, surface referent | 3.65*MAR*MS/BS |
| BS/BV | mm/mm2 | Bone surface/volume | (4/ π)*B.Pm/B.Ar |
| BFR/BV | %/year | Bone formation rate, bone referent | 0.1*(BFR/BS)*(BS/BV) |
| dL.S/BS | % | Double-labeled surface | 100*dL.Pm/B.Pm |
| Derived static indices | |||
| Tb.N | mm−1 | Trabecular number | (2/π)*B.Pm/T.Ar |
| Tb.Th | µm | Trabecular thickness | 2000/(BS/BV) |
| Tb.Sp | µm | Trabecular seperation | 1000/Tb.N-Tb.Th |
| BV/TV | % | Bone volume | 100*B.Ar/T.Ar |
Dynamic bone histomorphometry measurements for lateral abaxial osteophytes, lateral subchondral bone (SCB), and epiphyseal/metaphyseal cancellous bone (EMC)
Measurements of calcein labels within each lateral abaxial osteophyte were made using a 10X objective under fluorescent light on all bone surfaces within the osteophyte area outlined by the perimeter tracing as described above. The primary measurements, formulas for derived indices, and abbreviations are listed in Table 1 and are based on the recommendations of the American Society for Bone and Mineral Research nomenclature committee [12].
The perimeter and border tracings of the SCB of the lateral tibial plateau were made using Osteomeasure histomorphometry software and a digitizing pad as previously described for the medial tibial plateau [9]. Briefly, SCB was defined as the bone between the calcified cartilage-bone junction and the marrow space. Calcein labels in subchondral bone were measured under fluorescent microscopy using a 10X objective in a 3.5 × 3.5 mm field beginning 2 mm lateral to the central long axis of the tibia. Epiphyseal-metaphyseal cancellous (EMC bone) perimeter and border tracings were taken in a 3.5 × 3.5 mm field 3 mm below (deep to) the lower limit of the SCB, centered on the central long axis of the tibia, using a 2X objective under light microscopy as previously described [9]. Measurements of calcein labels within EMC bone were taken using a 10X objective under fluorescent light in the same 3.5 × 3.5 mm field. The EMC label data had previously been collected,[9] and the measurements from sections in which lateral abaxial osteophytes were present were used for comparisons in the present study.
Statistical analyses
The summary statistics are reported as medians and ranges. The distribution of each of the study measurements was evaluated in order to determine the appropriate statistical test. For variables with distributions that were skewed to high or low values, the logarithmic transformation was used. If the log transformation did not help normalize the distribution or if the parameter had a sizeable number of zero values, a non-parametric test was performed. As a result, treatment effects were evaluated using either the analysis of variance (ANOVA) or the Kruskal-Wallis non-parametric procedure. When an overall significant treatment effect (p<0.05) was observed, the treatment groups were compared two at a time using the Tukey method to adjust for multiple comparisons or the non-parametric Wilcoxon rank sum test. Following the Wilcoxon test, the Bonferroni method for post-hoc testing was applied and a p-value of 0.017 (0.05/3=0.017), rounded to 0.02 was used for determining statistical significance between pairs of groups. For normally distributed variables, the Pearson correlation assessed the degree of association between measurements. Regression analysis was used when treatment group was included as a covariate. Treatment was removed from the regression equation if it was not significant. For non-normally distributed measurements, the Spearman correlation was used to evaluate the relationship between indices. Static or dynamic histomorphometry parameters from each of the three bone compartments (lateral abaxial osteophyte bone, lateral SCB, and EMC bone) were compared using the Friedman non-parametric test. Pair-wise comparisons between sites were evaluated using the Wilcoxon signed rank test with a p-value of <0.02 to adjust for multiple comparisons. All statistical analyses were performed using SAS version 8 or 9.1 (SAS Institute Inc., Cary, NC).
Results
Osteophyte cross-sectional area
The median values of the lateral abaxial osteophyte cross-sectional area across the three treatment groups were: OVX, 1.02 mm2 (range: 0.39–6.72); SPE, 1.16 mm2 (range: 0.81 –2.49); and ERT, 0.96 mm2 (range: 0.49–5.49). There were no statistically significant effects of treatment on abaxial osteophyte area (p=0.490).
Osteophyte static bone parameters
There were no significant treatment effects on any of the static parameters (bone volume/total volume, BV/TV; trabecular number, TbN; trabecular thickness, TbTh; and trabecular separation, TbSp) in the lateral abaxial osteophyte bone (p > 0.1) (Table 2, left column for each parameter).
Table 2.
Static bone histomorphometry parameters of lateral abaxial osteophytes and epiphyseal/metaphyseal cancellous bone by treatment group.
| BV/TV (%) |
TbN (mm−1) |
TbTh (µm) |
TbSp (µm) |
|||||
|---|---|---|---|---|---|---|---|---|
| lat abax o.p. | EMC | lat abax o.p. | EMC | lat abax o.p. | EMC | lat abax o.p. | EMC | |
| OVX | 56.08 | 10.00 | 3.98 | 1.25 | 151.70 | 78.80 | 106.49 | 688.90 |
| n=16 | (26.57–87.87) | (5.23–17.55) | (1.47–6.20) | (0.76–1.95) | (103.03–263.18) | (59.10–139.98) | (29.08–500.84) | (442.91–1255.14) |
| SPE | 65.52 | 13.72 | 3.70 | 1.78 | 150.46 | 76.50 | 89.16 | 481.89 |
| n=16 | (37.31–81.65) | (7.34–20.05) | (2.53–5.39) | (1.00–2.18) | 101.04–230.52) | (62.77–107.88) | (40.28–200.59) | (366.91–927.99) |
| ERT | 58.75 | 12.87 | 4.11 | 1.64 | 144.60 | 72.63 | 99.09 | 527.25 |
| n=17 | (40.30–82.59) | (7.19–20.03) | (3.16–5.77) | (1.06–2.80) | (91.86–234.21) | (54.80–88.46) | (36.23–172.32) | (285.33–869.71) |
| p-value | 0.946 | 0.015* | 0.256 | 0.002** | 0.475 | 0.289 | 0.803 | 0.001** |
Values expressed as median values (range)
lat abax o.p. = lateral abaxial osteophyte; EMC = epiphyseal/metaphyseal cancellous bone
OVX = ovariectomized control group; SPE = soy phytoestrogen group; ERT = estrogen replacement group
BV/TV = bone volume; TbN, TbTh, TbSp = trabecular number, thickness, separation, respectively
significant difference between OVX and SPE treatment groups
significant difference between OVX and both SPE and ERT treatment groups
EMC static bone parameters & comparisons with osteophytes
The bone volume per total volume (BV/TV) in the EMC bone was significantly lower (p=0.015) in the OVX group compared to the SPE-treated group and the trabecular number (TbN) of the EMC bone was significantly lower (p=0.002) in the OVX animals compared with both the SPE- and ERT-treated animals (Table 2). Correspondingly, the trabecular separation (TbSp) of the EMC bone was significantly higher (p=0.001) in the OVX animals compared with both the SPE- and ERT-treated animals (Table 2). Although trabecular thickness (TbTh) in the EMC bone was lowest in the ERT-treated group, the differences among treatment groups were not statistically significant (Table 2).
The bone volume in the osteophyte bone was considerably higher than in the EMC bone, with median bone volume/total volume (BV/TV) values in osteophyte bone approximately 4.5–5.5 times greater than those of the EMC bone across all treatment groups (Table 2). Median trabecular number (TbN) and trabecular thickness (TbTh) values were also approximately 2–3 times higher in osteophyte bone than in EMC bone (Table 2). Correspondingly, the median values of trabecular separation (TbSp) were approximately 5–6 times lower in osteophyte bone than in EMC bone (Table 2).
Correlations between osteophyte area and static parameters
There was a significant negative correlation between cross-sectional area and both trabecular number (TbN) (r=−0.71, p <0.001) and bone volume/total volume (BV/TV) (r=−0.69, p <0.001) in osteophytes. In other words, as the cross-sectional area of osteophytes increased, the number of trabeculae and the area of tissue occupied by bone decreased. There was also a significant positive correlation between cross-sectional area and trabecular separation (TbSp) (r=0.76, p <0.001) in osteophytes (i.e., as osteophyte area increased, the separation of trabeculae within the osteophyte also increased). However, there was no significant correlation between osteophyte area and osteophyte trabecular thickness (TbTh), nor were there significant correlations between osteophyte area and any of the static histomorphometry parameters of the EMC bone.
Correlations between static parameters in osteophyte bone and EMC bone
Correlation/regression analysis was also used to examine the relationship(s) between the static parameters of the lateral abaxial osteophyte bone and those of EMC bone. Treatment effect was not significant in any of these correlations and was removed from the equations. There was a significant positive correlation (r=0.35, p=0.013) between trabecular thickness (TbTh) in the osteophyte bone and TbTh of EMC bone; however, there were no significant correlations between the other static parameters (TbN, TbSp, and BV/TV) in the two anatomic sites.
Osteophyte dynamic bone parameters
The median values for all of the dynamic parameters of the osteophyte bone evaluated were lowest in the ERT group, highest in the SPE group, and intermediate in the OVX group (with the exception of BS/BV in which the values were nearly identical across treatment groups); however, differences among the treatment groups were not statistically significant (p >0.1) for any of these parameters (Table 3).
Table 3.
Dynamic histomorphometry indices of lateral abaxial tibial osteophytes.
| BFR/BS (µm3/µm2/year) |
dLS/BS (%) |
MS/BS (%) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| lat abax o.p. | EMC | lat SCB | lat abax o.p. | EMC | lat SCB | lat abax o.p. | EMC | lat SCB | |
| OVX | 6.70 | 11.06 | 3.69 | 2.31 | 1.79 | 0.45 | 7.50 | 3.45 | 8.09 |
| n=16 | (0.00–215.21) | (0.00–69.70) | (0.00–171.48) | (0.00–17.99) | (0.00–10.26) | (0.00–19.32) | (0.00–32.51) | (0.00–17.33) | (0.00–20.30) |
| SPE | 29.71 | 10.77 | 4.51 | 4.05 | 0.87 | 3.11 | 9.32 | 2.43 | 5.93 |
| n=16 | (0.00–719.93) | (0.00–118.66) | (0.00–242.20) | (0.00–20.25) | (0.00–6.51) | (0.00–11.94) | (0.00–29.72) | (0.00–11.32) | (0.00–13.38) |
| ERT | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.55 | 0.28 | 0.28 |
| n=17 | (0.00–151.25) | (0.00–30.40) | (0.00–42.65) | (0.00–14.00) | (0.00–2.67) | (0.00–6.93) | (0.00–24.87) | (0.00–15.31) | (0.00–12.78) |
| p-value | 0.101 | 0.052 | 0.074 | 0.154 | 0.043† | 0.103 | 0.248 | 0.188 | 0.006** |
| MAR (µm/day) | BFR/BV (%/year) | BS/BV (mm/mm2) | |||||||
| lat abax o.p. | EMC | lat SCB | lat abax o.p. | EMC | lat SCB | lat abax o.p. | EMC | lat SCB | |
| OVX | 0.52 | 0.74 | 0.24 | 9.59 | 29.04 | 2.48 | 13.31 | 25.49 | 7.82 |
| n=16 | (0.00–3.84) | (0.00–2.14) | (0.00–2.50) | (0.00–375.83) | (0.00–191.41) | (0.00–196.44) | (7.60–19.41) | (14.28–34.42) | (4.81–12.39) |
| SPE | 0.76 | 0.89 | 0.27 | 31.22 | 25.50 | 3.48 | 13.32 | 25.82 | 7.73 |
| n=16 | (0.00–6.64) | (0.00–2.87) | (0.00–5.80) | (0.00–878.76) | (0.00–219.90) | (0.00–148.21) | (8.68–19.79) | (18.53–31.85) | (3.71–10.49) |
| ERT | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 13.83 | 27.53 | 8.26 |
| n=17 | (0.00–1.67) | (0.00–0.62) | (0.00–2.83) | (0.00–321.15) | (0.00–92.62) | (0.00–38.64) | (8.54–21.77) | (22.60–36.48) | (4.13–11.72) |
| p-value | 0.107 | 0.018* | 0.089 | 0.133 | 0.065 | 0.069 | 0.475 | 0.384 | 0.664 |
data expressed as median values (range)
OVX = ovariectomized control group; SPE = soy phytoestrogen group; ERT = estrogen replacement group
lat abax o.p. = lateral abaxial osteophyte; EMC = epiphyseal-metaphyseal cancellous bone; lat SCB = lateral subchondral bone
dLS/BS = double-labeled surface; MS/BS = mineralizing surface; MAR = mineral apposition rate
BFR/BV = bone formation rate, bone referent; BS/BV = bone surface/volume
significant result; after adjusting for multiple comparisons, the pair-wise comparisons were not statistically significant
significant difference between SPE and ERT treatment groups
significant difference between ERT and both OVX and SPE treatment groups
Dynamic histomorphometry indices in EMC and lateral SCB
EMC BONE
With the exception of BS/BV, all of the dynamic parameters in the EMC bone were the lowest in the ERT group and, with the exception of BS/BV and MAR, all of the dynamic parameters in the EMC bone were highest in the OVX group. The mineral apposition rate (MAR) was significantly higher in the SPE-treated animals than in the ERT-treated animals (p=0.018) (Table 3). In addition, the double-labeled surface (dLS/BS) in EMC bone was highest in the OVX animals and lowest in the ERT-treated animals (p=0.043); however, after adjusting for multiple comparisons, the pair-wise comparisons were not statistically significant (p > 0.02). In all three treatment groups, BS/BV was significantly lower in osteophyte bone than it was in EMC bone (p <0.001).
LATERAL SCB
In the SCB of the lateral tibial plateau, the calcein labels were present primarily along the bone margins (including void spaces); however, there were labels present along the junction of uncalcified cartilage and calcified cartilage in one of the specimens. With the exception of BS/BV (in which the values were similar across the three treatment groups), the median values for all of the dynamic parameters in the lateral SCB were lowest in the ERT group. This trend was similar to that previously reported for the medial SCB [9]. The mineralizing surface (MS/BS) in the lateral SCB was significantly lower in the ERT-treated animals compared with both OVX and SPE-treated animals (p=0.006) (Table 3). With the exception of BS/BV and MS/BS, the median values for all of the dynamic parameters in the lateral SCB were highest in the SPE group (Table 3). In all three treatment groups, BS/BV was significantly higher in osteophyte bone than it was in the lateral SCB (p <0.001).
Correlations between lateral abaxial osteophyte area and dynamic parameters
There were no significant correlations (p >0.05) between osteophyte cross-sectional area and any of the dynamic parameters of the osteophyte bone or of the EMC bone. There were also no consistent correlations between osteophyte area and the dynamic parameters of the lateral SCB.
Correlations among dynamic parameters
There was a significant positive correlation (r=0.35, p=0.015) between BS/BV in osteophyte bone and BS/BV in EMC bone; however, none of the other dynamic parameters were significantly correlated between osteophyte bone and EMC bone. In contrast, with the exception of BS/BV, all of the dynamic parameters in the lateral SCB were significantly correlated (p <0.01 for all) with their corresponding parameters in the osteophyte bone and EMC bone. The correlations ranged from 0.39 to 0.47. Treatment effect was significant (p <0.05) for only the following dynamic parameters: MAR and dLS/BS in EMC bone and MS/BS in lateral SCB. For each of these three parameters, the ERT group had the lowest median value (Table 3).
Discussion
The formation of periarticular osteophytes is considered to be an integral component of OA pathogenesis, as highlighted in a recent review article on the topic [3]. Despite their importance in the radiographic and histologic diagnosis of OA, there is relatively little known about the behavior of periarticular osteophytes and their role in the pathophysiology of OA. The present study is the first to compare the bone composing osteophytes to that of the bone in other anatomic sites within the same knee joint in naturally occurring OA. Using well-defined histological criteria and histomorphometry techniques, the cross-sectional area and bone turnover in periarticular osteophytes of the proximal tibia were examined in detail in a highly relevant animal model (cynomolgus monkeys) in which the effects of long-term ERT on OA severity and bone turnover (SCB and EMC bone) had previously been demonstrated to be significant (SPE and ERT) [7,9].
The fact that there was no effect of hormonal treatment on cross-sectional area in osteophytes had been reported previously;[8] however, histomorphometry parameters of the bone composing osteophytes were not evaluated in that study. Perhaps not surprisingly, although long-term ERT significantly increased trabecular number and decreased trabecular separation in EMC bone compared with ovariectomized control animals, there were neither significant treatment effects, nor trends to suggest effects of ERT on static histomorphometry parameters in osteophytes. These findings suggest that other factors, such as biomechanical influences, most likely play a more important role in the structure of osteophytes than do hormonal influences. This may be due, at least in part, to the proximity of osteophyte bone to the joint surface compared with EMC bone.
In addition to differences in response to long-term hormonal therapy, the structure of osteophyte bone was different from that of EMC bone, having a much higher bone volume with thicker, more numerous, and more closely spaced trabeculae. Possible explanations for these differences include the fact that the bone in these two sites is subjected to different mechanical forces and the fact that the bone structure of osteophytes developed more recently than the EMC bone. Interestingly, as osteophytes increased in size (cross-sectional area), their structure became more similar to that of EMC bone, with decreasing bone volume, trabecular number, and separation, but no change in trabecular thickness.
Most of the indices of bone turnover in SCB, osteophyte bone, and EMC bone were lowest in the ERT group, highest in the SPE group, and intermediate in the OVX group; however, with only one exception (MS/BS in SCB), treatment differences were significant or approached significance only for parameters in EMC bone. There were trends in the dynamic histomorphometry data, however, to suggest that a larger sample size may have identified significant treatment effects in both osteophyte bone and (as has been demonstrated previously for the medial tibial plateau) the SCB in the lateral tibial plateau (Table 3). Interestingly, unlike a previous study of tissues from these same animals in which bone turnover in the SCB of the medial tibial plateau was higher than that in EMC bone,[9] the reverse was true in the present study, in which SCB turnover in the lateral tibial plateau was examined. In fact, the mean values for nearly all of the dynamic parameters were 1.5 to 2 times lower in the SCB of the lateral tibial plateau (Table 3) compared with the medial tibial plateau [9]. Furthermore, although the bone surface/volume (BS/BV) in the SCB of the medial tibial plateau was approximately 70–85% that of the SCB of the lateral tibial plateau, the amount of double label (dLS/BS) in the SCB of the medial tibial plateau was 1.5 times greater than that of the SCB of the lateral tibial plateau (Table 3) [9]. In addition, the mean thickness of the SCB in the medial tibial plateau in these animals has previously been determined to be nearly twice that of the lateral tibial plateau in all three treatment groups [8]. The most likely explanation for all of these findings is that the medial joint compartment is more severely affected by OA than the lateral compartment in this model. This probably relates to biomechanical factors, since the medial joint compartment receives a greater load than the lateral compartment in most species [13,14]. In cynomolgus monkeys, the medial joint compartment precedes the lateral compartment in the development of OA lesions, possibly due at least in part to these differences in joint loading [15].
The results of this study provide evidence that bone turnover in osteophytes is more similar to SCB than EMC bone. For example, with the exception of BS/BV, all dynamic parameters in osteophyte bone were significantly correlated with the corresponding parameter in the lateral SCB, whereas the only significant correlation between osteophyte bone and EMC bone was BS/BV. Because these correlations occurred regardless of treatment group in most cases, these similarities may be due to the anatomic location of these two sites, with both being more superficially located and, thus, more likely to be influenced by biomechanical factors, than is EMC bone.
There are relatively few studies that have investigated bone turnover by histomorphometry in periarticular osteophytes, particularly in non-rodent species, with which the present data may be compared; however, several studies have focused on osteophyte development following transection of the cranial cruciate ligament in dogs [16,17]. One of these was a short-term study that reported that osteophyte growth started shortly after surgery and bone formation reached a peak at 30–40 days; however, all dogs that underwent surgery had active bone formation/growth within the osteophytes at the time of euthanasia (13 to 57 days post-op) [16]. Although dynamic histomorphometry indices in the SCB were not examined in detail, a much reduced amount of fluorochrome labeling (evaluated subjectively) in this compartment compared with the osteophyte bone led these authors to conclude that SCB proliferation of bone did not play a role in the development of osteophytes. Gilbertson reported similar results regarding early osteophyte formation; however, that study also included subjective evaluation of bone labels in dogs up to 48 weeks post operatively, at which time areas of active bone formation still were evident [17]. Subchondral deposition of new bone as well as bone remodeling also was noted, but was reduced compared with the bone of osteophytes and occurred later in the time course of osteophyte development Increased bone turnover activity also was noted in the trabecular bone of the distal end of the femur, beginning approximately three weeks after surgery. Although they demonstrate that osteophytes develop early after the induction of significant joint instability, neither of these studies is directly relevant to the present study, which focused on bone turnover in naturally occurring osteophytes and directly compared bone turnover data among the three sites of interest.
In summary, long-term hormonal therapy has no significant effect on bone volume or on static or dynamic histomorphometry parameters in osteophytes. Bone turnover in osteophytes appears to more closely resemble that in SCB than in EMC bone, possibly due to anatomic and biomechanical factors.
Acknowledgments
The authors thank Josh Parker and Drs. Kim Ham and Laura Eikmeier for technical assistance. We also thank Dr. Thomas Clarkson for providing the knee joints for this study, Dr. Mary Anthony for providing the details of the clinical trial, and Jean Gardin for assistance with tissue collection. This study was supported by National Institutes of Health Grants RR14099 and RR18719.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: All authors have no conflict of interest.
References
- 1.Boegård T, Rudling O, Petersson IF, Jonsson K. Correlation between radiographically diagnosed osteophytes and magnetic resonance detected cartilage defects in the tibiofemoral joint. Ann Rheum Dis. 1998;57:401–407. doi: 10.1136/ard.57.7.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sengupta M, Zhang YQ, Niu JB, Guermazi A, Grigorian M, Gale D, Felson DT, Hunter DJ. High signal in knee osteophytes is not associated with knee pain. Osteoarthritis Cartilage. 2006;15:413–417. doi: 10.1016/j.joca.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 3.van der Kraan PM, van den Berg WB. Osteophytes: relevance and biology. Osteoarthritis Cartilage. 2006;15:237–244. doi: 10.1016/j.joca.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Compston JE. Sex steroids and bone. Physiol Rev. 2001;81:419–447. doi: 10.1152/physrev.2001.81.1.419. [DOI] [PubMed] [Google Scholar]
- 5.Doren M, Samsioe G. Prevention of postmenopausal osteoporosis with oestrogen replacement therapy and associated compounds: update on clinical trials since 1995. Hum Reprod Update. 2000;6:419–426. doi: 10.1093/humupd/6.5.419. [DOI] [PubMed] [Google Scholar]
- 6.Jerome CP, Carlson CS, Register TC, Bain FT, Jayo MJ, Weaver DS, Adams MR. Bone functional changes in intact, ovariectomized, and ovariectomized, hormone-supplemented adult cynomolgus monkeys (Macaca fascicularis) evaluated by serum markers and dynamic histomorphometry. J Bone Miner Res. 1994;9:527–540. doi: 10.1002/jbmr.5650090413. [DOI] [PubMed] [Google Scholar]
- 7.Ham KD, Loeser RF, Lindgren BR, Carlson CS. Effects of long-term estrogen replacement therapy on osteoarthritis severity in cynomolgus monkeys. Arthritis Rheum. 2002;46:1956–1964. doi: 10.1002/art.10406. [DOI] [PubMed] [Google Scholar]
- 8.Olson EJ, Lindgren BR, Carlson CS. Effects of long-term estrogen replacement therapy on the prevalence and area of periarticular tibial osteophytes in surgically postmenopausal cynomolgus monkeys. Bone. 2007;41:282–289. doi: 10.1016/j.bone.2007.04.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ham KD, Carlson CS. Effects of estrogen replacement therapy on bone turnover in subchondral bone and epiphyseal metaphyseal cancellous bone of ovariectomized cynomolgus monkeys. J Bone Miner Res. 2004;19:823–829. doi: 10.1359/JBMR.040309. [DOI] [PubMed] [Google Scholar]
- 10.Clarkson TB, Anthony MS, Moran TM. Inhibition of postmenopausal atherosclerosis progression: a comparison of the effects of conjugated equine estrogens and soy phytoestrogens. J Clin Endocrinol Metab. 2001;86:41–47. doi: 10.1210/jcem.86.1.7151. [DOI] [PubMed] [Google Scholar]
- 11.Bowen WH, Koch G. Determination of age in monkeys (Macaca irus) on the basis of dental development. Lab Anim. 1970;4:113–124. doi: 10.1258/002367770781036481. [DOI] [PubMed] [Google Scholar]
- 12.Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR. Bone histomorphometry: Standardization of nomenclature, symbols, and units. Report of the ASBMR histomorphometry nomenclature committee. J Bone Miner Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- 13.Amin S, LaValley MP, Guermazi A, Grigoryan M, Hunter DJ, Clancy M, Niu J, Gale DR, Felson DT. The relationship between cartilage loss on magnetic resonance imaging and radiographic progression in men and women with knee osteoarthritis. Arthritis Rheum. 2005;52:3152–3159. doi: 10.1002/art.21296. [DOI] [PubMed] [Google Scholar]
- 14.Pollo FE, Otis JC, Backus SI, Warren RF, Wickiewicz TL. Reduction of medial compartment loads with valgus bracing of the osteoarthritic knee. AJSM. 2002;30:414–421. doi: 10.1177/03635465020300031801. [DOI] [PubMed] [Google Scholar]
- 15.Carlson CS, Loeser RF, Jayo MJ, Weaver DS, Adams MR, Jerome CP. Osteoarthritis in cynomolgus macaques: a primate model of naturally occurring disease. J Orthop Res. 1994;12:331–339. doi: 10.1002/jor.1100120305. [DOI] [PubMed] [Google Scholar]
- 16.Marshall JL. Periarticular osteophytes. Initiation and formation in the knee of the dog. Clin Orthop Relat Res. 1969;62:37–47. [PubMed] [Google Scholar]
- 17.Gilbertson EMM. Development of periarticular osteophytes in experimentally induced osteoarthritis in the dog. A study using microradiographic, microangiographic, and fluorescent bone-labelling techniques. Ann Rheum Dis. 1975;34:12–25. doi: 10.1136/ard.34.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]


