Abstract
BACKGROUND
Perinatal brachial plexus palsy (PBPP) is a flaccid paralysis of the arm at birth that affects different nerves of the brachial plexus supplied by C5 to T1 in 0.42 to 5.1 infants per 1000 live births.
OBJECTIVES
To identify antenatal factors associated with PBPP and possible preventive measures, and to review the natural history as compared with the outcome after primary or secondary surgical interventions.
METHODS
A literature search on randomized controlled trials, systematic reviews and meta-analyses on the prevention and treatment of PBPP was performed. EMBASE, Medline, CINAHL and the Cochrane Library were searched until June 2005. Key words for searches included ‘brachial plexus’, ‘brachial plexus neuropathy’, ‘brachial plexus injury’, ‘birth injury’ and ‘paralysis, obstetric’.
RESULTS
There were no prospective studies on the cause or prevention of PBPP. Whereas birth trauma is said to be the most common cause, there is some evidence that PBPP may occur before delivery. Shoulder dystocia and PBPP are largely unpredictable, although associations of PBPP with shoulder dystocia, infants who are large for gestational age, maternal diabetes and instrumental delivery have been reported. The various forms of PBPP, clinical findings and diagnostic measures are described. Recent evidence suggests that the natural history of PBPP is not all favourable, and residual deficits are estimated at 20% to 30%, in contrast with the previous optimistic view of full recovery in greater than 90% of affected children. There were no randomized controlled trials on nonoperative management. There was no conclusive evidence that primary surgical exploration of the brachial plexus supercedes conservative management for improved outcome. However, results from nonrandomized studies indicated that children with severe injuries do better with surgical repair. Secondary surgical reconstructions were inferior to primary intervention, but could still improve arm function in children with serious impairments.
CONCLUSIONS
It is not possible to predict which infants are at risk for PBPP, and therefore amenable to preventive measures. Twenty-five per cent of affected infants will experience permanent impairment and injury. If recovery is incomplete by the end of the first month, referral to a multidisciplinary team is necessary. Further research into prediction, prevention and best mode of treatment needs to be done.
Keywords: Brachial plexus, Delivery, Fetus, Newborn, Palsy
Abstract
HISTORIQUE
La paralysie périnatale du plexus brachial (PPPB) est une paralysie flasque du bras à la naissance, touchant divers nerfs du plexus brachial innervés par les racines C5 à T1. Cette paralysie survient dans 0,42 à 5,1 cas pour 1 000 naissances vivantes.
OBJECTIFS
Repérer les facteurs anténatals associés à la PPPB et les mesures préventives possibles et examiner l’évolution naturelle par rapport à l’issue après des interventions chirurgicales primaires ou secondaires.
MÉTHODOLOGIE
Une recherche dans les publications des essais aléatoires et contrôlés, des examens systématiques et des méta-analyses sur la prévention et le traitement de la PPPB a été exécutée. Cette recherche a été effectuée dans les bases de données EMBASE, Medline, CINAHL et Cochrane Library jusqu’à juin 2005. Les mots clés utilisés étaient brachial plexus, brachial plexus neuropathy, brachial plexus injury, birth injury et paralysis, obstetric.
RÉSULTATS
Il n’existait pas d’études prospectives sur la cause ou la prévention de la PPPB. Bien que les traumatismes survenant à la naissance en seraient le plus souvent responsables, certaines données probantes laissent supposer que la PPPB peut survenir avant l’accouchement. La dystocie des épaules et la PPPB sont en grande partie imprévisibles, bien qu’on ait documenté une association entre la PPPB et la dystocie des épaules, la macrosomie fœtale, le diabète de la mère et l’accouchement instrumenté. Les diverses formes de PPPB, les observations cliniques et les mesures diagnostiques sont décrites. D’après des données probantes récentes, l’évolution naturelle de la PPPB n’est pas toujours favorable, et on évalue que les déficits résiduels oscillent entre 20 % et 30 %, contrairement au point de vue optimiste antérieur de récupération complète chez plus de 90 % des enfants touchés. Aucun essai aléatoire et contrôlé ne porte sur la prise en charge non chirurgicale. Aucune donnée concluante n’indiquait que l’exploration chirurgicale primaire du plexus brachial était plus efficace qu’une prise en charge conservatrice pour améliorer le devenir. Cependant, d’après les résultats d’études non aléatoires, les enfants atteints de graves lésions s’en sortent mieux après une reconstruction chirurgicale. Les reconstructions chirurgicales secondaires étaient moins efficaces que l’intervention primaire, mais elles pouvaient tout de même améliorer la fonction du bras des enfants présentant une atteinte marquée.
CONCLUSIONS
Il est impossible de prévoir quels nourrissons sont vulnérables à la PPPB et donc sensibles à des mesures préventives. Vingt-cinq pour cent des nourrissons touchés souffriront d’une atteinte et d’une lésion permanentes. Si la récupération est incomplète à la fin du premier mois, l’aiguillage vers une équipe multidisicplinaire s’impose. Des recherches supplémentaires sur les prévisions, la prévention et le meilleur mode de traitement sont nécessaires.
Perinatal brachial plexus palsy (PBPP), also known as obstetric brachial plexus palsy, is defined as a flaccid paresis of the arm at birth with a larger passive than active range of motion. In the literature, the term obstetric brachial plexus palsy is mostly used, which carries implications of causality. For that reason, the term ‘perinatal brachial plexus paralysis’ would be more appropriate. ‘Erb palsy’ is only one subtype of PBPP (1). PBPP can be classified as upper, middle, lower or complete, and the different types are summarized in Table 1.
TABLE 1.
Different types of perinatal brachial plexus palsy
| Brachial plexus | Cranial nerves | Findings | Narakas group |
|---|---|---|---|
| Upper | C5, C6 | Weakness of shoulder external rotation or abduction of arm and elbow flexion/supination | I – Erb/Duchenne |
| Middle | C5, C6, C7 | Same as upper plus elbow flexion/supination paralysis and loss of wrist extension | II – Erb/Duchenne |
| Lower | C8, T1 | Floppy hand with claw-like deformity | Klumpke’s (rare) |
| Complete | C5, C6, C7, C8, T1 | Flail arm | III
IV = III + Horner’s |
The objectives of the present paper were to review antenatal factors associated with PBPP, to review possible preventive measures and their medicolegal implications, to highlight that this condition is not as benign as previously thought, and to review the natural evolution as compared with primary or secondary surgical intervention.
METHODS
An attempt was made to identify all published randomized controlled trials, systematic reviews and meta-analyses evaluating causation, association, prevention and treatment of PBPP. Other types of clinical studies and review articles were also considered. Electronic bibliographic databases including EMBASE, Medline, CINAHL, Database of Reviews of Effects and Cochrane Library electronic databases were searched using Ovid (Ovid Technologies Inc, USA) up until June 2005. Subject headings included in the search were: ‘brachial plexus’, ‘brachial plexus neuropathy’, ‘brachial plexus injury’, ‘birth injury’ and ‘paralysis, obstetric’. Subject headings were modified for each database and were expanded to include narrower terms. The search strategy was designed to limit citations to those considering paediatric populations. Bibliographies of identified publications were examined for further relevant studies. The search was not limited by language or publication status.
INCIDENCE
The incidence of PBPP has been reported to be between 0.42 and 5.1 per 1000 live births. The reports of the largest populations found incidences between 0.42 and 1.5 per 1000 live births (1–3).
PATHOGENESIS
Various types of nerve injury
There are different degrees of nerve injury classified according to the severity of damage to the axon (4,5). The mildest form, neurapraxia, is a temporary conduction block due to damage to the myelin sheath at the site of injury, and function usually returns within weeks. In a more severe form, axonotmesis, nerve fibres are disrupted, but the myelin sheath remains intact. The function often returns within months, although the recovery may not be complete. In neurotmesis, the nerves have been totally disrupted and the fibres have to regenerate to function again. In this case, the recovery is seldom complete. The nerve root may be avulsed from the spinal cord, often with the dorsal root ganglion displaced outside the spinal canal, with no chance for recovery.
Risk factors
PBPP has generally been thought to be the result of excessive lateral traction on the brachial plexus at the time of delivery. However, there is some evidence that PBPP may also be the result of an in utero insult (6), or may be related to the process of labour itself, rather than to the management of the delivery (7,8). Gilbert et al (2) reviewed 1611 cases of PBPP and found that 47% of cases did not involve shoulder dystocia or downward traction on the brachial plexus. The retrospective nature of that study and the difficulty in objectively defining shoulder dystocia may have led to ascertainment bias and to under-reporting of shoulder dystocia. They also noted that 60 cases of PBPP were associated with delivery by cesarean section and suggested fetal malpresentation before or during labour as the possible cause. McFarland et al (9) were the first to note that cesarean section did not totally eliminate the risk for PBPP. The occurrence of PBPP after delivery by cesarean section has now been well described. In the largest survey ever, Evans-Jones et al (1) reported on 776,618 live births with an incidence of PBPP of 0.42 per 1000. There were no predisposing factors in 6% of the cases and only 63% had shoulder dystocia.
If PBPP were the result of excessive lateral traction on the brachial plexus as the anterior shoulder passes under the symphysis pubis, then the injured shoulder should always be the anterior one. However, Walle et al (10) reviewed 170 cases of PBPP and found that one-third of all injuries involved the posterior arm, which could be explained by impaction of the posterior shoulder on the maternal sacral promontory. In a historical review on Erb palsy, Sandmire and DeMott (11) suggested that maternal propulsive forces are the most likely cause of PBPP.
Recognized obstetric risk factors for PBPP, including shoulder dystocia (OR 340; 95% CI 47 to 897), birth weight greater than 4.5 kg (OR 17.9; 95% CI 10.3 to 31.3), maternal diabetes (OR 3.2; 95% CI 1.6 to 6.2), midpelvic instrumental (OR 18.3; 95% CI 5.7 to 59.3) or vacuum delivery (OR 17.2; 95% CI 5.1 to 58.2), prolonged second-stage labour (OR 8.3; 95% CI 4.0 to 17.3) and a previous infant with PBPP, increase the risk 220-fold (2,9,12–17). Macrosomic infants with birth weights greater than 4.5 kg born to mothers with diabetes and delivered by instrumental vaginal delivery have been reported to be at highest risk for PBPP (2). However, although the risk is high at 7.8%, 92% of the infants in this group were delivered vaginally without complications (9), meaning that 92% of the mothers would have undergone unnecessary surgery if all macrosomic infants of diabetic mothers had been delivered by cesarean section (18). Furthermore, in only 10% to 19% of cases of PBPP do the historic factors have a predictive value (16,19) and, thus, just a small number of these injuries are potentially preventable.
The strongest predictor of PBPP is birth weight (9,12) and yet, antenatally, fetal weight has limited value in predicting who will develop PBPP (20). Although early induction of labour for suspected fetal macrosomia in nondiabetic women results in a lower birth weight than in a control group, there is no reduction in the rate of cesarean section, shoulder dystocia or permanent brachial plexus injuries (21,22). In diabetic women, intensified management of gestational diabetes succeeded in reducing the rates of cesarean section and shoulder dystocia, but the rate of PBPP remained the same (23). When combining the two strategies (ie, controlling fetal weight by intense management of maternal diabetes and delivery by cesarean section if the estimated fetal weight was greater than 4.25 kg), the occurrence of shoulder dystocia in the 1377 patients was 1.5% as compared with 2.8% in the control group (OR 1.9; CI 1.0 to 3.5), but the incidence of PBPP remained unchanged (24). There was an increased rate of delivery by cesarean section without a decrease in birth traumas. Although that study may have had a risk for type II error, the majority of PBPP occurred in infants with a birth weight less than 4.0 kg and without maternal diabetes. This indicates that, although risk factors for PBPP should alert the clinician, the complexity of the problem does not make it suitable for rigid treatment protocols, and management decisions are best individualized on a case-by-case basis with expert clinical judgment.
It appears that PBPP in the absence of shoulder dystocia occurs by a different mechanism (25,26) and is a distinct entity. In infants with PBPP, but without shoulder dystocia, birth weight is smaller and the rate of persistence at one year of age is significantly higher (ie, 41.2% versus 8.7%) and affects the posterior shoulder more commonly, suggesting an in utero mechanism, possibly from pressure of the shoulder against the bony maternal pelvis (27).
In summary, although up to two-thirds of the cases of permanent PBPP involve shoulder dystocia, excessive lateral traction at the time of the delivery does not explain all cases.
DIAGNOSIS
Clinical findings
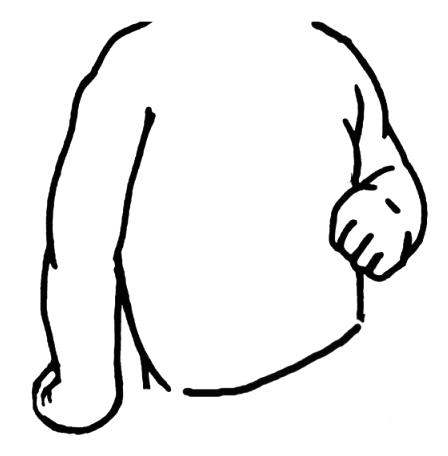
The upper trunk of the brachial plexus (C5 and C6) is most frequently injured, resulting in Erb palsy, which is characterized by the ‘waiter’s tip’ posture (28) (Figure 1). This is due to the weakness of the abductors and external rotators of the shoulder, flexors and supinators of the elbow and radial extensors of the wrist, all supplied by the upper trunk, with preservation of internal rotators and adductors, elbow extensors and wrist flexors, all supplied by the middle trunk and C7. Associated phrenic nerve damage may cause diaphragmatic paralysis. In addition to being the most common form of PBPP, Erb palsy carries the best prognosis of recovery. Total plexus palsy (C5 to T1) presents with total paralysis of the arm and hand (28,29) and is often associated with Horner’s syndrome, and has a less favourable outcome (30). Klumpke’s palsy (C8 to T1), involving only the lower trunk, is most uncommon in modern obstetric practice, accounting for only 0.6% of all PBPP (31). These injuries may cause claw hands, drop wrists and severe loss of hand function (Table 1).
Figure 1).
Erb palsy of the right side with typical ‘waiter’s tip’ posture
The initial evaluation of the neonate with suspected PBPP should include a thorough history and physical examination with particular emphasis on signs of concurrent injuries and consideration of differential diagnoses. Effective communication with the infant’s parents on these findings and their implications are paramount (32).
One should observe for signs of respiratory compromise, suggesting phrenic nerve injury. The upper extremities are inspected for asymmetry in size, temperature, position and posture. Detecting the presence of fractures of the clavicles, humeri and ribs, or shoulder dislocation is important. The initial examination includes an assessment of range of motion, observation for spontaneous motor activity and presence and symmetry of deep tendon and Moro reflexes. Response to sensory stimulation is evaluated. The head and neck should be examined for Horner’s syndrome, signs suggesting damage to other regional nerves and the presence of torticollis (33).
Ancillary diagnostic aids
Diagnostic imaging may assist in the initial evaluation. A chest x-ray and ultrasound of the diaphragm may reveal evidence of phrenic nerve palsy (34,35). X-rays of the cervical spine, clavicle and upper extremity can assist in identifying fractures and congenital malformations (13,36). If a lesion of the central nervous system is suspected, evaluation with magnetic resonance imaging is appropriate (37–39).
Differential diagnosis
The differential diagnosis of congenital brachial plexus palsy includes fracture of the clavicle or humerus (resulting in pseudoparalysis) (40), osteomyelitis (41), sepsis of the glenohumeral joint, arthrogryposis, brachial neuropathy secondary to sepsis (42), lesions of the spinal cord (43) and cerebral palsy (44).
TREATMENT
Prevention
Shoulder dystocia continues to represent a largely unpredictable obstetric emergency. Cesarean section has been associated with a lower risk for PBPP (12,45). However, 3695 elective cesarean sections would be needed to prevent one permanent case of PBPP among those nondiabetic mothers who carry a fetus with an estimated weight of greater than 4.5 kg (18). It is therefore unlikely that prospective studies of either shoulder dystocia or PBPP can possibly ever be performed. Because there is currently no accepted method to objectively quantify ‘excessive’ lateral traction, the mere occurrence of PBPP should not be taken as clear evidence of medical negligence.
Natural history
Understanding the natural history of PBPP is important in providing a prognosis and evaluating potential therapies, especially brachial plexus exploration and surgical repair. Reported rates of recovery vary significantly (46–53). Pondaag et al (54) recently published a systematic review on this topic, including studies done up until 2001. They concluded that there were insufficient data to describe the natural history of PBPP. They identified potential causes for the variation in outcome data, including referral and selection bias, retrospective study design, heterogeneity of plexus injuries, and variable use of evaluation measures and definitions of outcome. Studies with the highest methodological quality found residual deficits in 20% to 33% (46,55,56) of cases compared with previous reports suggesting 90% of patients showing full recovery (54). Recent evidence suggests that the natural history of PBPP is not all favourable.
Bisinella and Birch (8) studied children with PBPP registered with the British Paediatric Surveillance Unit between 1998 and 1999. Seventy-four of these children were referred for tertiary care and prospectively followed for 24 to 36 months. Recovery to ‘normal or near normal’ levels occurred in 52.7% of patients. Nine patients (12.2%) underwent brachial plexus surgery and 20 patients (27%) required surgical correction of a secondary shoulder deformity. Evans-Jones et al (1) reported on 276 confirmed cases from the British Paediatric Surveillance Unit from the same time period. At six months, 52% of children with PBPP had recovered fully. Of those remaining, 46% failed to recover complete function in any muscle group in the affected arm and 2% showed no recovery. Hoeksma et al (56) reported a historical cohort study involving 56 children with PBPP of whom only 66% demonstrated complete neurological recovery.
Long-term sequelae may include muscular weakness, abnormal posture, bony deformity (57), shoulder and elbow contractures (58), dislocation (59,60) and shortening of the involved arm (61). Surprisingly, severe loss of sensory function and long-term chronic pain are uncommon (62). Accidental contact burns (63) and self-mutilation (64) have been reported in children with total plexus injuries. Failure to use the involved arm in spite of good motor recovery has been attributed to developmental apraxia (65) or to agnosia (66). Developmental and behavioural problems have been described in children with PBPP, particularly in those with more severe injuries (67).
PBPP is therefore not a benign condition, and the previous optimism for spontaneous recovery is not supported by the current literature. A high level of dissatisfaction has been reported among parents of children with PBPP, particularly with respect to receiving inaccurate and misleading information from health care professionals regarding the prognosis (32).
Prognosis and assessment
Children who show early improvement tend to go on to full recovery, with no limb discrepancy in function. It is expected that patients with neurapraxia will show complete recovery by one month of age (68). Those who recover later tend to have incomplete recovery of function. Despite having an incomplete recovery, many children will go on to have useful function of the limb without primary exploration and reconstruction (52,53). Others do better with surgery.
The essence of decision-making is to identify those infants who will have a better outcome with primary surgery than with conservative management. The patient with early full recovery clearly does not need surgical exploration and repair. Conversely, those with Horner’s syndrome and total palsy do require surgical intervention (30,68–71). For the other children with incomplete recovery it is more difficult to predict whether they will benefit from primary surgery (68,69). Unfortunately, this decision needs to be made early to optimize the potential for a good surgical result. Prediction of prognosis and early surgical decision-making is multifaceted, relying on history, electrodiagnostics, radiographic studies and, most important, physical examination.
Gilbert et al (72,73) pioneered much of the prognostic data by analyzing large series of patients. In their reviews, biceps and deltoid function had the most predictive value. Narakas divided patients into three groups: those who started recovery before three weeks did fine, those who started recovery between three weeks and two months often required secondary surgeries, and those who began recovery after two months required early exploration (71).
The modified Mallet Classification, Toronto Test Score and Active Movement Scale (64) are reliable instruments for assessing upper extremity function in patients with PBPP. A commonly used assessment tool is the Active Movement Scale of The Hospital for Sick Children (74,75). Fifteen different movements in the affected arms are assessed and scored on an eight-point scale. The infant is enticed to move using a variety of play stimuli. The eight gradations allow for discrimination of movements. This tool also allows for comparison of children who have surgery with those who are treated conservatively, and it allows for comparison of pre- and postoperative results. Using the Active Movement Scale, elbow flexion and extension, wrist extension, and finger and thumb extension are scored. If children do not reach a preset score by three months of age, surgery is recommended. Children who pass at three months and are followed conservatively are retested at nine months for improvement of elbow flexion using the ‘cookie test’. With the child sitting, a cookie is given. If the child can get the cookie to his or her mouth with the elbow held at the side and less than 45° of neck flexion, the elbow flexion is deemed adequate and surgery is not indicated. Failure to get the cookie to the mouth is an indication for primary exploration and repair of the brachial plexus.
Electrodiagnosis is often used to identify the level and extent of the lesion (76–78). There remains controversy as to whether nerve conduction studies and needle electromyography, typically performed at three months of age, are helpful in predicting recovery or selecting patients for reconstruction (51,79–82). Needle electromyography tends to overestimate recovery, due mostly to technical difficulties in examining infants and to the density of motor fibres in infants (51). As long as the factors unique to paediatric electromyography are taken into account, experienced paediatric electrophysiologists can help to select patients for surgical exploration (83).
Computed tomography myelography and magnetic resonance imaging may be used in surgical planning, although their value is controversial (38–40,84,85). Clinical examination remains the most valuable means of assessing the severity and location of injury (77,83).
Nonoperative management
Prognosis is uncertain in the first four weeks (56). Any infant who does not recover completely after four weeks should be referred to a centre with special expertise in managing PBPP. These children benefit from the care of specialized multidisciplinary teams who can provide ongoing evaluation using standardized assessments and guidance for potential therapies.
The goals of conservative therapy are to prevent contractures, dislocations, muscular imbalance and maladaptive patterns of movement, as well as maintaining range of motion and optimizing functional use of the affected extremity.
Published evaluations of conservative therapy are limited to predominantly retrospective studies. Ter Steeg et al (86) published a review of this literature. Although methods of therapy have changed over time, prospective clinical trials of nonoperative treatment are absent from the literature. Currently, patients with PBPP receive range of motion and strengthening exercises, facilitation of functional patterns of movement, sensory awareness activities, and, when appropriate, static and dynamic splinting (87). Parents are encouraged to carry out the majority of exercises at home. Therapies including the use of botulinum toxin to weaken overpowering of the antagonist muscles and neuromuscular electrical stimulation to strengthen reconverting muscles have been suggested but require further investigation in clinical trials (88–91). Parents should be instructed in how to take care of the affected arm with respect to minimizing discomfort in the immediate neonatal period. We found no evidence that prolonged immobilization improves recovery or prevents further injuries.
Primary surgical exploration of the brachial plexus
Primary reconstructive techniques include neurolysis, nerve grafting and neurotization. Neurolysis involves resecting scar tissue from around the nerve (extraneural neurolysis) and from within the nerve (intraneural neurolysis). Nerve grafting is anatomical reconstruction from proximal donors to distal targets through a nerve graft while neurotization is nonanatomical reconstruction using either plexus donors (intraplexal neurotization) or nonplexus donors (extraplexal neurotization). Examples of extraplexal neurotization donors include the spinal accessory nerve, phrenic nerve, intercostal nerves, contralateral C7 and hypoglossal nerves.
The surgical procedure is a lengthy one requiring an entire day of operating. The dissection can be tedious and fraught with potential dangers in the scarred posterior triangle. Reconstruction involves neuroma resection and interpositional nerve grafting using sural nerves, intercostal nerves and spinal accessory nerves. The nerves are anastomosed using fibrin tissue glue. This not only shortens the operating time, but also prevents a foreign body reaction to the stitch and ensures microsurgical alignment. Further details of primary surgery for PBPP have been described by Marcus and Clarke (92).
In the recent systematic review by McNeely and Drake (93), there was no conclusive evidence for benefit of brachial plexus surgery over conservative management of PBPP, resulting in a grade C recommendation. Evidence from studies published in languages other than English and those published since McNeely’s review do not substantially change this level of recommendation (73,94–101). The methodological quality of studies examining the natural history of PBPP has been brought into question, limiting the usefulness of their results as controls when evaluating surgical outcomes (54). The lack of randomized studies or cohort studies with adequate control groups, variable inclusion criteria and the absence of blinded functional outcome measures has hindered the establishment of strong evidence (102). However, there has been one large series with encouraging long-term results (103).
Secondary surgery
Secondary reconstructions are inferior to proper primary intervention (44,104). The role of secondary surgery is to augment the function that a child has regained either through primary surgery or through natural healing. It behooves the clinician to ensure that any transfer, fusion, tenodesis or osteotomy improves the overall functions of daily living for the patient.
Reconstructive surgery is available for the shoulder, elbow, forearm, wrist and hand. A recent review by Haerle and Gilbert (103) of case series of secondary reconstruction noted improvement of range of motion for patients with residual deficits with and without primary reconstruction.
One-fifth to one-third of children who have had primary plexus reconstruction require secondary shoulder surgery to treat an internal rotation contracture with weakness of abduction and external rotation (104–106). The three main types of surgery for the shoulder include contracture releases, tendon transfers and osteotomy. In general, the type of surgery will depend on the state of the glenohumeral joint, which is assessed by both physical examination and radiological studies (107,108).
The most common elbow problems are weakness of flexion, static flexion contracture and weakness of extension. Depending on the type of problem, botulinum toxin injections, tendon transfers or osteotomies may be beneficial for recovery.
In the wrist, the most common problem is weakness of extension with resulting wrist drop and functionally leading to an inability to open the fingers in extension to grasp objects. Therapy begins with splinting of the wrist into extension, with the aim of improving overall function of the hand. If splinting results in improvement, more permanent solutions, such as tendon transfer, tenodesis or fusion arthroplasty, can be performed (30). In the child with complete palsy, the forearms may develop a supination contracture, whereas the child with an upper plexus injury may present with a pronation deformity.
Postoperative therapy
Postoperative rehabilitation is crucial. Following plexus reconstruction, reinervation is slow and may be incomplete. Contractures, dislocations and other deformities, as well as agnosia, may result from neurological deficit (73,109,110). These may be prevented or minimized by a rehabilitation program aimed at maintaining range of motion, and strengthening and promoting age-related functional skills. Rehabilitation also has an important role in maximizing functional recovery following secondary reconstructive surgery (40).
Children described as having complete neurological recovery have been shown to encounter subsequent impairment, including limitation in range of motion, contractures, bony deformities and developmental apraxia (57,65,86). Therefore, long-term follow-up to monitor progress and to minimize functional impairment is important (111).
Therapists have a crucial role in assessing recovery of function and to provide ongoing modification of goal-oriented therapy. Consistent serial examinations should be performed repeatedly throughout a patient’s development using a validated scale, such as the Active Movement Scale (Table 2) (74,77,92,112). Other assessment tools have been developed, including the classification system of Mallet, which is useful to identify functional or maladaptive patterns of movement in older children; however, it has not been thoroughly validated. PBPP results in functional impairments affecting individuals across broad domains of disability (113,114). Children with PBPP would benefit from the development of validated outcome measures that consider the domains of activity, participation, and contextual and environmental factors, in addition to measures of impairment, as outlined by the International Classification of Functioning, Disability and Health (115).
TABLE 2.
The Hospital for Sick Children Active Movement Scale
| Observation | Muscle grade |
|---|---|
| Gravity eliminated | |
| No contraction | 0 |
| Contraction, no motion | 1 |
| Motion one-half range or less | 2 |
| Motion greater than one-half range | 3 |
| Full motion | 4 |
| Against gravity | |
| Motion one-half range or less | 5 |
| Motion greater than one-half range | 6 |
| Full motion | 7 |
Reproduced from reference 75
CONCLUSIONS
PBPP is usually due to injuries sustained in the perinatal period.
Despite the fact that a number of risk factors have been associated with PBPP, the neonates who will demonstrate PBPP cannot be predicted.
Of affected infants, 75% recover completely within the first month of life.
Permanent impairment and disability are experienced by 25% of affected infants.
If a physical examination demonstrates an incomplete recovery by the end of the first month, referral to a multidisciplinary brachial plexus team should be made. The team should include neurologists or physiatrists, rehabilitation therapists and plastic surgeons.
There are no randomized controlled trials to evaluate nonsurgical management as compared with primary brachial plexus surgery.
Primary exploration and reconstruction surgery, if performed early, can improve the outcome in those with the most severe injuries.
Secondary soft tissue and bony reconstruction surgery may improve function in children with significant impairments.
Further studies are needed to prevent injury, improve prediction of the natural recovery, establish more precise criteria for surgery and measure the outcome.
REFERENCES
- 1.Evans-Jones G, Kay SP, Weindling AM, et al. Congenital brachial palsy: Incidence, causes, and outcome in the United Kingdom and Republic of Ireland. Arch Dis Child Fetal Neonatal Ed. 2003;88:F185–9. doi: 10.1136/fn.88.3.F185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gilbert WM, Nesbitt TS, Danielsen B. Associated factors in 1611 cases of brachial plexus injury. Obstet Gynecol. 1999;93:536–40. doi: 10.1016/s0029-7844(98)00484-0. [DOI] [PubMed] [Google Scholar]
- 3.Donnelly V, Foran A, Murphy J, McParland P, Keane D, O’Herlihy C. Neonatal brachial plexus palsy: An unpredictable injury. Am J Obstet Gynecol. 2002;187:1209–12. doi: 10.1067/mob.2002.127723. [DOI] [PubMed] [Google Scholar]
- 4.Seddon H. Surgical Disorders of the Peripheral Nerves. New York: Churchill Livingstone; 1975. [Google Scholar]
- 5.Sunderland S. Nerve Injuries and Their Repair: A Critical Appraisal. Edinburgh: Churchill Livingstone; 1991. [Google Scholar]
- 6.Alfonso I, Papazian O, Shuhaiber H, Yaylali I, Grossman JA. Intrauterine shoulder weakness and obstetric brachial plexus palsy. Pediatr Neurol. 2004;31:225–7. doi: 10.1016/j.pediatrneurol.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 7.Pollack RN, Buchman AS, Yaffe H, Divon MY. Obstetrical brachial palsy: Pathogenesis, risk factors, and prevention. Clin Obstet Gynecol. 2000;43:236–46. doi: 10.1097/00003081-200006000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Bisinella GL, Birch R. Obstetric brachial plexus lesions: A study of 74 children registered with the British Paediatric Surveillance Unit (March 1998–March 1999) J Hand Surg [Br] 2003;28:40–5. doi: 10.1054/jhsb.2002.0841. [DOI] [PubMed] [Google Scholar]
- 9.McFarland LV, Raskin M, Daling JR, Benedetti TJ. Erb/Duchenne’s palsy: A consequence of fetal macrosomia and method of delivery. Obstet Gynecol. 1986;68:784–8. [PubMed] [Google Scholar]
- 10.Walle T, Hartikainen-Sorri AL. Obstetric shoulder injury. Associated risk factors, prediction and prognosis. Acta Obstet Gynecol Scand. 1993;72:450–4. doi: 10.3109/00016349309021133. [DOI] [PubMed] [Google Scholar]
- 11.Sandmire HF, DeMott RK. Erb’s palsy causation: A historical perspective. Birth. 2002;29:52–4. doi: 10.1046/j.1523-536x.2002.00156.x. [DOI] [PubMed] [Google Scholar]
- 12.Ecker JL, Greenberg JA, Norwitz ER, Nadel AS, Repke JT. Birth weight as a predictor of brachial plexus injury. Obstet Gynecol. 1997;89:643–7. doi: 10.1016/s0029-7844(97)00007-0. [DOI] [PubMed] [Google Scholar]
- 13.Peleg D, Hasnin J, Shalev E. Fractured clavicle and Erb’s palsy unrelated to birth trauma. Am J Obstet Gynecol. 1997;177:1038–40. doi: 10.1016/s0002-9378(97)70010-3. [DOI] [PubMed] [Google Scholar]
- 14.Benedetti TJ, Gabbe SG. Shoulder dystocia. A complication of fetal macrosomia and prolonged second stage of labor with midpelvic delivery. Obstet Gynecol. 1978;52:526–9. [PubMed] [Google Scholar]
- 15.al-Qattan MM, el-Sayed AA, al-Kharfy TM, al-Jurayyan NA. Obstetrical brachial plexus injury in newborn babies delivered by caesarean section. J Hand Surg [Br] 1996;21:263–5. doi: 10.1016/s0266-7681(96)80112-4. [DOI] [PubMed] [Google Scholar]
- 16.Gherman RB, Ouzounian JG, Goodwin TM. Brachial plexus injury: An in utero injury? Am J Obstet Gynecol. 1999;180:1303–7. doi: 10.1016/s0002-9378(99)70633-2. [DOI] [PubMed] [Google Scholar]
- 17.al-Qattan MM, al-Kharfy TM. Obstetric brachial plexus injury in subsequent deliveries. Ann Plast Surg. 1996;37:545–8. doi: 10.1097/00000637-199611000-00015. [DOI] [PubMed] [Google Scholar]
- 18.Rouse DJ, Owen J, Goldenberg RL, Cliver SP. The effectiveness and cost of elective cesarean delivery for fetal macrosomia diagnosed by ultrasound. JAMA. 1996;276:1480–6. [PubMed] [Google Scholar]
- 19.Perlow JH, Wigton T, Hart J, Strassner HT, Nageotte MP, Wolk BM. Birth trauma. A five-year review of incidence and associated perinatal factors. J Reprod Med. 1996;41:754–60. [PubMed] [Google Scholar]
- 20.Bryant DR, Leonardi MR, Landwehr JB, Bottoms SF. Limited usefulness of fetal weight in predicting neonatal brachial plexus injury. Am J Obstet Gynecol. 1998;179:686–9. doi: 10.1016/s0002-9378(98)70065-1. [DOI] [PubMed] [Google Scholar]
- 21.Irion O, Boulvain M. Induction of labor for suspected fetal macrosomia (Cochrane Review) Cochrane Database Syst Rev. 2000;2:CD000938. doi: 10.1002/14651858.CD000938. [DOI] [PubMed] [Google Scholar]
- 22.Gonen O, Rosen DJ, Dolfin Z, Tepper R, Markov S, Fejgin MD. Induction of labor versus expectant management in macrosomia: A randomized study. Obstet Gynecol. 1997;89:913–7. doi: 10.1016/s0029-7844(97)00149-x. [DOI] [PubMed] [Google Scholar]
- 23.Langer O, Rodriguez DA, Xenakis EM, McFarland MB, Berkus MD, Arrendondo F. Intensified versus conventional management of gestational diabetes. Am J Obstet Gynecol. 1994;170:1036–47. doi: 10.1016/s0002-9378(94)70097-4. [DOI] [PubMed] [Google Scholar]
- 24.Conway DL, Langer O. Elective delivery of infants with macrosomia in diabetic women: Reduced shoulder dystocia versus increased cesarean deliveries. Am J Obstet Gynecol. 1998;178:922–5. doi: 10.1016/s0002-9378(98)70524-1. [DOI] [PubMed] [Google Scholar]
- 25.Jennett RJ, Tarby TJ. Brachial plexus palsy: An old problem revisited again. II. Cases in point. Am J Obstet Gynecol. 1997;176:1354–7. doi: 10.1016/s0002-9378(97)70357-0. [DOI] [PubMed] [Google Scholar]
- 26.Brown B, Karmin I, Lapinski R, Lescale K. Dual mechanism responsible for brachial plexus injuries. Am J Obstet Gynecol. 1997;176:S137. (Abst) [Google Scholar]
- 27.Gherman RB, Ouzounian JG, Miller DA, Kwok L, Goodwin TM. Spontaneous vaginal delivery: A risk factor for Erb’s palsy? Am J Obstet Gynecol. 1998;178:423–7. doi: 10.1016/s0002-9378(98)70413-2. [DOI] [PubMed] [Google Scholar]
- 28.Erb W. Ueber eine eigenthumliche localisation von Lahmun gen implexus brachialis. Verhandlungen des naturhisorischen vereins von Heidelberg. 1874;2:130–7. [Google Scholar]
- 29.Duchenne G. De l’électrisation localisée et de son application à la pathologie et la thérapeutique. Paris: JB Ballière et fils; 1872. pp. 357–62. [Google Scholar]
- 30.Al-Qattan MM, Clarke HM, Curtis CG. The prognostic value of concurrent Horner’s syndrome in total obstetric brachial plexus injury. J Hand Surg [Br] 2000;25:166–7. doi: 10.1054/jhsb.1999.0351. [DOI] [PubMed] [Google Scholar]
- 31.al-Qattan MM, Clarke HM, Curtis CG. Klumpke’s birth palsy. Does it really exist? J Hand Surg [Br] 1995;20:19–23. doi: 10.1016/s0266-7681(05)80008-7. [DOI] [PubMed] [Google Scholar]
- 32.Bellew M, Kay SP. Early parental experiences of obstetric brachial plexus palsy. J Hand Surg [Br] 2003;28:339–46. doi: 10.1016/s0266-7681(03)00081-0. [DOI] [PubMed] [Google Scholar]
- 33.Suzuki S, Yamamuro T, Fujita A. The aetiological relationship between congenital torticollis and obstetrical paralysis. Int Orthop. 1984;8:175–81. doi: 10.1007/BF00269913. [DOI] [PubMed] [Google Scholar]
- 34.Anagnostakis D, Economou-Mavrou C, Moschos A, Vlachos P, Liakakos D. Diaphragmatic paralysis in the newborn. Arch Dis Child. 1973;48:977–9. doi: 10.1136/adc.48.12.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Al-Qattan MM, Clarke HM, Curtis CG. The prognostic value of concurrent phrenic nerve palsy in newborn children with Erb’s palsy. J Hand Surg [Br] 1998;23:225. doi: 10.1016/s0266-7681(98)80179-4. [DOI] [PubMed] [Google Scholar]
- 36.al-Qattan MM, Clarke HM, Curtis CG. The prognostic value of concurrent clavicular fractures in newborns with obstetric brachial plexus palsy. J Hand Surg [Br] 1994;19:729–30. doi: 10.1016/0266-7681(94)90246-1. [DOI] [PubMed] [Google Scholar]
- 37.Birchansky S, Altman N. Imaging the brachial plexus and peripheral nerves in infants and children. Semin Pediatr Neurol. 2000;7:15–25. doi: 10.1016/s1071-9091(00)80006-6. [DOI] [PubMed] [Google Scholar]
- 38.Abbott R, Abbott M, Alzate J, Lefton D. Magnetic resonance imaging of obstetrical brachial plexus injuries. Child Nerv Syst. 2004;20:720–5. doi: 10.1007/s00381-004-1003-6. [DOI] [PubMed] [Google Scholar]
- 39.Zhou L, Yousem DM, Chaudhry V. Role of magnetic resonance neurography in brachial plexus lesions. Muscle Nerve. 2004;30:305–9. doi: 10.1002/mus.20108. [DOI] [PubMed] [Google Scholar]
- 40.Kay SP. Obstetrical brachial palsy. Br J Plast Surg. 1998;51:43–50. doi: 10.1054/bjps.1997.0166. [DOI] [PubMed] [Google Scholar]
- 41.Solebo JO, Keane MR, Obaro RO, Browne LM. Osteomyelitis of head of humerus presenting as Erbs palsy in a neonate. Eur J Pediatr. 2004;163:262. doi: 10.1007/s00431-004-1411-3. [DOI] [PubMed] [Google Scholar]
- 42.Sadleir LG, Connolly MB. Acquired brachial-plexus neuropathy in the neonate: A rare presentation of late-onset group-B streptococcal osteomyelitis. Dev Med Child Neurol. 1998;40:496–9. doi: 10.1111/j.1469-8749.1998.tb15401.x. [DOI] [PubMed] [Google Scholar]
- 43.Medlock MD, Hanigan WC. Neurologic birth trauma. Intracranial, spinal cord, and brachial plexus injury. Clin Perinatol. 1997;24:845–57. [PubMed] [Google Scholar]
- 44.Birch R. Obstetric brachial plexus palsy. J Hand Surg [Br] 2002;27:3–8. doi: 10.1054/jhsb.2001.0722. [DOI] [PubMed] [Google Scholar]
- 45.Michelow BJ, Clarke HM, Curtis CG, Zuker RM, Seifu Y, Andrews DF. The natural history of obstetrical brachial plexus palsy. Plast Reconstr Surg. 1994;93:675–81. [PubMed] [Google Scholar]
- 46.Sjoberg I, Erichs K, Bjerre I. Cause and effect of obstetric (neonatal) brachial plexus palsy. Acta Paediatr Scand. 1988;77:357–64. doi: 10.1111/j.1651-2227.1988.tb10660.x. [DOI] [PubMed] [Google Scholar]
- 47.Boome RS, Kaye JC. Obstetric traction injuries of the brachial plexus. Natural history, indications for surgical repair and results. J Bone Joint Surg Br. 1988;70:571–6. doi: 10.1302/0301-620X.70B4.3403599. [DOI] [PubMed] [Google Scholar]
- 48.Gordon M, Rich H, Deutschberger J, Green M. The immediate and long-term outcome of obstetric birth trauma. I. Brachial plexus paralysis. Am J Obstet Gynecol. 1973;117:51–6. doi: 10.1016/0002-9378(73)90727-8. [DOI] [PubMed] [Google Scholar]
- 49.Gjorup L. Obstetrical lesion of the brachial plexus. Acta Neurol Scand. 1966;42(Suppl 18):1–80. [PubMed] [Google Scholar]
- 50.Greenwald AG, Schute PC, Shiveley JL. Brachial plexus birth palsy: A 10-year report on the incidence and prognosis. J Pediatr Orthop. 1984;4:689–92. doi: 10.1097/01241398-198411000-00006. [DOI] [PubMed] [Google Scholar]
- 51.van Dijk JG, Pondaag W, Malessy MJ. Obstetric lesions of the brachial plexus. Muscle Nerve. 2001;24:1451–61. doi: 10.1002/mus.1168. [DOI] [PubMed] [Google Scholar]
- 52.DiTaranto P, Campagna L, Price AE, Grossman JA. Outcome following nonoperative treatment of brachial plexus birth injuries. J Child Neurol. 2004;19:87–90. doi: 10.1177/08830738040190020101. [DOI] [PubMed] [Google Scholar]
- 53.Smith NC, Rowan P, Benson LJ, Ezaki M, Carter PR. Neonatal brachial plexus palsy. Outcome of absent biceps function at three months of age. J Bone Joint Surg Am. 2004;86:2163–70. [PubMed] [Google Scholar]
- 54.Pondaag W, Malessy MJ, van Dijk JG, Thomeer RT. Natural history of obstetric brachial plexus palsy: A systematic review. Dev Med Child Neurol. 2004;46:138–44. doi: 10.1017/s0012162204000258. [DOI] [PubMed] [Google Scholar]
- 55.Jackson ST, Hoffer MM, Parrish N. Brachial-plexus palsy in the newborn. J Bone Joint Surg Am. 1988;70:1217–20. [PubMed] [Google Scholar]
- 56.Hoeksma AF, ter Steeg AM, Nelissen RG, van Ouwerkerk WJ, Lankhorst GJ, de Jong BA. Neurological recovery in obstetric brachial plexus injuries: An historical cohort study. Dev Med Child Neurol. 2004;46:76–83. doi: 10.1017/s0012162204000179. [DOI] [PubMed] [Google Scholar]
- 57.Hoeksma AF, ter Steeg AM, Dijkstra P, Nelissen RG, Beelen A, de Jong BA. Shoulder contracture and osseous deformity in obstetrical brachial plexus injuries. J Bone Joint Surg Am. 2003;85:316–22. doi: 10.2106/00004623-200302000-00020. [DOI] [PubMed] [Google Scholar]
- 58.Hoffer MM. The shoulder in neonatal brachial palsy. Clin Orthop Relat Res. 1999;368:101–4. [PubMed] [Google Scholar]
- 59.Hoffer MM, Phipps GJ. Surgery about the elbow for brachial palsy. J Pediatr Orthop. 2000;20:781–5. doi: 10.1097/00004694-200011000-00016. [DOI] [PubMed] [Google Scholar]
- 60.Al-Qattan MM. Tendon transfer to reconstruct wrist extension in children with obstetric brachial plexus palsy. J Hand Surg [Br] 2003;28:153–7. doi: 10.1016/s0266-7681(02)00301-7. [DOI] [PubMed] [Google Scholar]
- 61.McDaid PJ, Kozin SH, Thoder JJ, Porter ST. Upper extremity limb-length discrepancy in brachial plexus palsy. J Pediatr Orthop. 2002;22:364–6. [PubMed] [Google Scholar]
- 62.Anand P, Birch R. Restoration of sensory function and lack of long-term chronic pain syndromes after brachial plexus injury in human neonates. Brain. 2002;125:113–22. doi: 10.1093/brain/awf017. [DOI] [PubMed] [Google Scholar]
- 63.Al-Qattan MM. Accidental contact burns of the upper limb in children with obstetric brachial plexus injury. Burns. 1999;25:669–72. doi: 10.1016/s0305-4179(99)00043-1. [DOI] [PubMed] [Google Scholar]
- 64.Al-Qattan MM. Self-mutilation in children with obstetric brachial plexus palsy. J Hand Surg [Br] 1999;24:547–9. doi: 10.1054/jhsb.1999.0222. [DOI] [PubMed] [Google Scholar]
- 65.Brown T, Cupido C, Scarfone H, Pape K, Galea V, McComas A. Developmental apraxia arising from neonatal brachial plexus palsy. Neurology. 2000;55:24–30. doi: 10.1212/wnl.55.1.24. [DOI] [PubMed] [Google Scholar]
- 66.Eng GD, Binder H, Getson P, O’Donnell R. Obstetrical brachial plexus palsy (OBPP) outcome with conservative management. Muscle Nerve. 1996;19:884–91. doi: 10.1002/(SICI)1097-4598(199607)19:7<884::AID-MUS11>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 67.Bellew M, Kay SP, Webb F, Ward A. Developmental and behavioural outcome in obstetric brachial plexus palsy. J Hand Surg [Br] 2000;25:49–51. doi: 10.1054/jhsb.1999.0331. [DOI] [PubMed] [Google Scholar]
- 68.Waters PM. Comparison of the natural history, the outcome of microsurgical repair, and the outcome of operative reconstruction in brachial plexus birth palsy. J Bone Joint Surg Am. 1999;81:649–59. doi: 10.2106/00004623-199905000-00006. [DOI] [PubMed] [Google Scholar]
- 69.Al-Qattan MM. The outcome of Erb’s palsy when the decision to operate is made at 4 months of age. Plast Reconstr Surg. 2000;106:1461–5. doi: 10.1097/00006534-200012000-00003. [DOI] [PubMed] [Google Scholar]
- 70.Gilbert A. Indications and strategy. In: Gilbert A, editor. Brachial Plexus Injuries. London: Martin Dunitz; 2001. pp. 205–10. [Google Scholar]
- 71.Clarke HM, Curtis CG. Examination and prognosis. In: Gilbert A, editor. Brachial Plexus Injuries. London: Martin Dunitz; 2001. pp. 159–72. [Google Scholar]
- 72.Gilbert A, Brockman R, Carlioz H. Surgical treatment of brachial plexus birth palsy. Clin Orthop. 1991;264:39–47. [PubMed] [Google Scholar]
- 73.Gilbert A, Tassin JL. Surgical repair of the brachial plexus in obstetric paralysis. Chirurgie. 1984;110:70–5. [PubMed] [Google Scholar]
- 74.Curtis C, Stephens D, Clarke HM, Andrews D. The active movement scale: An evaluative tool for infants with obstetrical brachial plexus palsy. J Hand Surg [Am] 2002;27:470–8. doi: 10.1053/jhsu.2002.32965. [DOI] [PubMed] [Google Scholar]
- 75.Clarke HM, Curtis CG. An approach to obstetrical brachial plexus injuries. Hand Clin. 1995;11:563–81. [PubMed] [Google Scholar]
- 76.Smith SJ. The role of neurophysiological investigation in traumatic brachial plexus lesions in adults and children. J Hand Surg [Br] 1996;21:145–7. doi: 10.1016/s0266-7681(96)80088-x. [DOI] [PubMed] [Google Scholar]
- 77.Yilmaz K, Caliskan M, Oge E, Aydinli N, Tunaci M, Ozmen M. Clinical assessment, MRI, and EMG in congenital brachial plexus palsy. Pediatr Neurol. 1999;21:705–10. doi: 10.1016/s0887-8994(99)00073-9. [DOI] [PubMed] [Google Scholar]
- 78.Smith S. Electrodiagnosis. In: Birch R, Bonney G, Wynn Parry CB, editors. Surgical Disorders of the Peripheral Nerves. Edinburgh: Churchill Livingstone; 1998. pp. 467–90. [Google Scholar]
- 79.Slooff AC. Obstetric brachial plexus lesions and their neurosurgical treatment. Microsurgery. 1995;16:30–4. doi: 10.1002/micr.1920160109. [DOI] [PubMed] [Google Scholar]
- 80.Van Dijk JG, Malessy MJ, Stegeman DF. Why is the electromyogram in obstetric brachial plexus lesions overly optimistic? Muscle Nerve. 1998;21:260–1. doi: 10.1002/(sici)1097-4598(199802)21:2<260::aid-mus20>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 81.Vredeveld JW, Blaauw G, Slooff B, Richards R, Rozeman SC. The findings in paediatric obstetric brachial palsy differ from those in older patients: A suggested explanation. Dev Med Child Neurol. 2000;42:158–61. doi: 10.1017/s0012162200000281. [DOI] [PubMed] [Google Scholar]
- 82.Heise CO, Lorenzetti L, Marchese AJ, Gherpelli JL. Motor conduction studies for prognostic assessment of obstetrical plexopathy. Muscle Nerve. 2004;30:451–5. doi: 10.1002/mus.20121. [DOI] [PubMed] [Google Scholar]
- 83.Bisinella GL, Birch R, Smith SJ. Neurophysiological prediction of outcome in obstetric lesions of the brachial plexus. J Hand Surg [Br] 2003;28:148–52. doi: 10.1016/s0266-7681(02)00281-4. [DOI] [PubMed] [Google Scholar]
- 84.Francel PC, Koby M, Park TS, et al. Fast spin-echo magnetic resonance imaging for radiological assessment of neonatal brachial plexus injury. J Neurosurg. 1995;83:461–6. doi: 10.3171/jns.1995.83.3.0461. [DOI] [PubMed] [Google Scholar]
- 85.Chow BC, Blaser S, Clarke HM. Predictive value of computed tomographic myelography in obstetrical brachial plexus palsy. Plast Reconstr Surg. 2000;106:971–7. doi: 10.1097/00006534-200010000-00001. [DOI] [PubMed] [Google Scholar]
- 86.ter Steeg AM, Hoeksma AF, Dijkstra PF, Nelissen RG, De Jong BA. Orthopaedic sequelae in neurogically recovered obstetrical brachial plexus injury. Case study and literature review. Disabil Rehabil. 2003;25:1–8. [PubMed] [Google Scholar]
- 87.Mulloy EM, Ramos LE. Special rehabilitation considerations in the management of obstetrical brachial plexus injuries. Hand Clin. 1995;11:619–22. [PubMed] [Google Scholar]
- 88.Rollnik JD, Hierner R, Schubert M, et al. Botulinum toxin treatment of cocontractions after birth-related brachial plexus lesions. Neurology. 2000;55:112–4. doi: 10.1212/wnl.55.1.112. [DOI] [PubMed] [Google Scholar]
- 89.Hierner R, Rollnik JD, Berger AC, Dengler R. Botulinum toxin type a for the treatment of biceps/triceps co-contraction in obstetrical brachial plexus lesions: Preliminary results after a follow-up of 18 months. Eur J Plast Surg. 2001;24:2–6. [Google Scholar]
- 90.Desiato MT, Risina B. The role of botulinum toxin in the neuro-rehabilitation of young patients with brachial plexus birth palsy. Pediatr Rehabil. 2001;4:29–36. doi: 10.1080/13638490151068456. [DOI] [PubMed] [Google Scholar]
- 91.Ramos LE, Zell JP. Rehabilitation program for children with brachial plexus and peripheral nerve injury. Semin Pediatr Neurol. 2000;7:52–7. doi: 10.1016/s1071-9091(00)80010-8. [DOI] [PubMed] [Google Scholar]
- 92.Marcus JR, Clarke HM. Management of obstetrical brachial plexus palsy evaluation, prognosis, and primary surgical treatment. Clin Plast Surg. 2003;30:289–306. doi: 10.1016/s0094-1298(02)00100-1. [DOI] [PubMed] [Google Scholar]
- 93.McNeely PD, Drake JM. A systematic review of brachial plexus surgery for birth-related brachial plexus injury. Pediatr Neurosurg. 2003;38:57–62. doi: 10.1159/000068045. [DOI] [PubMed] [Google Scholar]
- 94.Gilbert A, Whitaker I. Obstetrical brachial plexus lesions. J Hand Surg [Br] 1991;16:489–91. doi: 10.1016/0266-7681(91)90100-3. [DOI] [PubMed] [Google Scholar]
- 95.Gilbert A, Razaboni R, Amar-Khodja S. Indications and results of brachial plexus surgery in obstetrical palsy. Orthop Clin North Am. 1988;19:91–105. [PubMed] [Google Scholar]
- 96.Antoniadis G, Konig RW, Mohr K, Kretschmer T, Richter HP. Management of obstetrical brachial plexus palsy – own experience with the primary operative technique. Handchir Mikrochir Plast Chir. 2003;35:98–105. doi: 10.1055/s-2003-40773. [DOI] [PubMed] [Google Scholar]
- 97.Berger AC, Hierner R, Becker MH. Early microsurgical revision of the brachial plexus in traumatic birth injuries. Patient selection and outcome. Orthopade. 1997;26:710–8. doi: 10.1007/s001320050145. [DOI] [PubMed] [Google Scholar]
- 98.Bersnev VP, Margolin EG. The diagnostic and treatment characteristics of birth injury to the brachial plexus in children and adolescents. Zh Vopr Neirokhir Im N N Burdenko. 1995;3:16–9. [PubMed] [Google Scholar]
- 99.Chen QH, Chen DS, Fang YS. Early microsurgical treatment of upper obstetrical brachial plexus injury. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2003;17:400–2. [PubMed] [Google Scholar]
- 100.Petrolati M, Raimondi PL, Cavallazzi RM, Saporiti E. Microsurgical treatment of obstetrical palsies: Our experience in 15 years of surgery. Rivista Italiana di Chirurgia Plastica. 1994;26:299–306. [Google Scholar]
- 101.Xu J, Cheng X, Gu Y. Different methods and results in the treatment of obstetrical brachial plexus palsy. J Reconstr Microsurg. 2000;16:417–20. doi: 10.1055/s-2006-947147. [DOI] [PubMed] [Google Scholar]
- 102.Piatt JH., Jr Birth injuries of the brachial plexus. Pediatr Clin North Am. 2004;51:421–40. doi: 10.1016/S0031-3955(03)00212-8. [DOI] [PubMed] [Google Scholar]
- 103.Haerle M, Gilbert A. Management of complete obstetric brachial plexus lesions. J Pediatr Orthop. 2004;24:194–200. doi: 10.1097/00004694-200403000-00012. [DOI] [PubMed] [Google Scholar]
- 104.Birch R. Medial rotation contracture and posterior dislocation of the shoulder. In: Gilbert A, editor. Brachial Plexus Injuries. London: Martin Dunitz; 2001. pp. 249–64. [Google Scholar]
- 105.Egloff DV, Raffoul W, Bonnard C, Stalder J. Palliative surgical procedures to restore shoulder function in obstetric brachial palsy. Critical analysis of Narakas’ series. Hand Clin. 1995;11:597–606. [PubMed] [Google Scholar]
- 106.Gilbert A. Long-term evaluation of brachial plexus surgery in obstetrical palsy. Hand Clin. 1995;11:583–94. [PubMed] [Google Scholar]
- 107.Pearl ML, Edgerton BW, Kon DS, et al. Comparison of arthroscopic findings with magnetic resonance imaging and arthrography in children with glenohumeral deformities secondary to brachial plexus birth palsy. J Bone Joint Surg Am. 2003;85:890–8. doi: 10.2106/00004623-200305000-00018. [DOI] [PubMed] [Google Scholar]
- 108.van der Sluijs JA, van der Meij M, Verbeke J, Manoliu RA, Wuisman PI. Measuring secondary deformities of the shoulder in children with obstetric brachial plexus lesion: Reliability of three methods. J Pediatr Orthop B. 2003;12:211–4. doi: 10.1097/01.bpb.0000060289.16932.41. [DOI] [PubMed] [Google Scholar]
- 109.Hoffer MM, Phipps GJ. Closed reduction and tendon transfer for treatment of dislocation of the glenohumeral joint secondary to brachial plexus birth palsy. J Bone Joint Surg Am. 1998;80:997–1001. doi: 10.2106/00004623-199807000-00008. [DOI] [PubMed] [Google Scholar]
- 110.Birch R, Bonney G, Wynn Parry C. Surgical Disorders of the Peripheral Nerves. Edinburgh: Churchill Livingstone; 1998. Birth lesions of the brachial plexus; pp. 209–33. [Google Scholar]
- 111.Partridge C, Edwards S. Obstetric brachial plexus palsy: Increasing disability and exacerbation of symptoms with age. Physiother Res Int. 2004;9:157–63. doi: 10.1002/pri.319. [DOI] [PubMed] [Google Scholar]
- 112.Al-Qattan MM. Assessment of the motor power in older children with obstetric brachial plexus palsy. J Hand Surg [Br] 2003;28:46–9. doi: 10.1054/jhsb.2002.0831. [DOI] [PubMed] [Google Scholar]
- 113.Keret D, Mendez AA, Ger E. Social and functional results of brachial plexus birth injury. Harefuah. 1991;121:235–7. [PubMed] [Google Scholar]
- 114.Sundholm LK, Eliasson AC, Forssberg H. Obstetric brachial plexus injuries: Assessment protocol and functional outcome at age 5 years. Dev Med Child Neurol. 1998;40:4–11. doi: 10.1111/j.1469-8749.1998.tb15350.x. [DOI] [PubMed] [Google Scholar]
- 115.World Health Organization. International Classification of Functioning, Disability and Health (ICF) Geneva: World Health Organization; 2001. [Google Scholar]