Abstract
BACKGROUND
Heel puncture to obtain bilirubin measurements is painful for infants and distressing for parents. Transcutaneous bilirubin measurement using BiliChek (Respironics, USA) is easily performed in any setting. Reliable transcutaneous testing should decrease the number of painful procedures in otherwise well infants, reduce the volume of phlebotomy losses in ill newborns, and reduce the need for hospital or specialized clinic visits after discharge.
OBJECTIVE
To correlate bilirubin measurements using the transcutaneous device BiliChek with ‘gold standard’ serum measurements in well term infants, and in ill term and preterm infants admitted to the authors’ neonatal intensive care unit.
METHODS
The study consisted of two phases. In phase 1, informed consent was obtained from mothers of 99 healthy, full-term infants not receiving phototherapy to perform both serum and transcutaneous bilirubin measurements at the time of heel puncture for routine neonatal screening. In phase 2, 56 infants in the neonatal intensive care unit had a total of 99 transcutaneous readings performed at the time serum bilirubin measurements were ordered for clinical reasons by the attending staff. The operators of the transcutaneous device, who were unaware of the serum bilirubin levels, performed readings within 1 h of the heel puncture.
RESULTS
Using a Bland-Altman comparison in the well term infants, the transcutaneous measurements were −32.2 μmol/L to +31.2 μmol/L (1.96 SD); however, 79 (79.8%) of the transcutaneous measurements were within 15 μmol/L of the serum measurements. The variation in preterm infants was greater at −69.6 μmol/L to +62.0 μmol/L, and only 49 (49.5%) were within 15 μmol/L. For infants receiving phototherapy, the variation was −76.3 μmol/L to +49.5 μmol/L, but improved to −40.4 μmol/L to +31.0 μmol/L if an area of skin was patched for testing, approximating the group not receiving phototherapy.
CONCLUSIONS
Transcutaneous bilirubin measurements obtained with the BiliChek instrument were accurate for measuring bilirubin levels in term jaundiced infants not receiving phototherapy and in those receiving phototherapy if an area of skin was patched. The instrument was not as sensitive in the small sample of preterm infants, and a larger study is required before recommending the use of this instrument in this population.
Keywords: Hyperbilirubinemia, Jaundice, Phototherapy, Preterm, Transcutaneous measurement
Abstract
HISTORIQUE
La ponction du talon pour mesurer la bilirubine est douloureuse pour les nourrissons et pénible pour les parents. La mesure transcutanée de la bilirubine à l’aide de BiliChek (Respironics, États-Unis) s’effectue facilement, en tout lieu. Un dépistage transcutané fiable devrait réduire le nombre d’interventions douloureuses chez les nourrissons en santé, le volume de pertes par phlébotomie chez les nourrissons malades et le besoin de consultations à l’hôpital ou dans une clinique spécialisée après le congé.
OBJECTIF
Relier les mesures de la bilirubine à l’aide du dispositif transcutané BiliChek aux mesures sériques qui constituent les « normes d’excellence » chez les nourrissons à terme et en santé et les nourrissons malades à terme ou prématurés hospitalisés à l’unité de soins intensifs néonatals des auteurs.
MÉTHODOLOGIE
L’étude était répartie en deux phases. Pendant la première phase, les mères de 99 nourrissons à terme et en santé qui ne recevaient pas de photothérapie ont accordé leur consentement éclairé pour qu’on procède à une mesure sérique et transcutanée du taux de bilirubine au moment de la ponction du talon de leur bébé en vue du dépistage néonatal systématique. Pendant la deuxième phase, 56 nourrissons de l’unité de soins intensifs néonatals ont subi un total de 99 lectures transcutanées effectuées lorsque les mesures de bilirubine sérique étaient commandées pour des raisons cliniques par les médecins en titre. Les utilisateurs de l’instrument transcutané, qui ne connaissaient pas les taux de bilirubine sérique, effectuaient la lecture dans l’heure suivant la ponction du talon.
RÉSULTATS
Au moyen de la méthode de Bland-Altman chez les nourrissons à terme et en santé, les mesures transcutanées étaient de −32,2 μmol/L à +31,2 μmol/L (1,96 ÉT); cependant, 79 (79,8 %) des mesures transcutanées se situaient dans un écart de 15 μmol/L de la mesure sérique. La variation chez les prématurés était plus élevée, entre −69,6 μmol/L et +62,0 μmol/L, et seulement 49 (49,5 %) se situait dans un écart de 15 μmol/L. Chez les nourrissons sous photothérapie, la variation était de −76,3 μmol/L à +49,5 μmol/L, mais passait entre −40,4 μmol/L et +31,0 μmol/L si une région de la peau était masquée pour le dépistage, ce qui se rapprochait du groupe qui ne recevait pas de photothérapie.
CONCLUSION
Les mesures transcutanées de bilirubine obtenues à l’aide du dispositif BiliChek étaient précises pour mesurer les taux de bilirubine chez les nourrissons à terme atteints de jaunisse qui n’étaient pas sous photothérapie et chez ceux qui étaient sous photothérapie et dont la région de la peau était couverte. Le dispositif n’était pas aussi sensible dans le petit échantillon de prématurés, et une étude à plus grande échelle s’impose avant qu’on puisse recommander l’utilisation de cet instrument au sein de cette population.
Jaundice, the clinical presentation of hyperbilirubinemia, occurs in up to 65% of healthy newborn infants during the first week of life (1). Most of these infants experience no adverse effects. There is strong evidence, however, implicating bilirubin in the pathogenesis of kernicterus, and the treatment of significant hyperbilirubinemia is directed at the prevention of bilirubin encephalopathy. Case reports have appeared in the recent literature showing re-emergence of kernicterus in infants found to have extreme levels of hyperbilirubinemia (greater than 500 μmol/L) at the time of readmission to the hospital (2,3). To determine whether the bilirubin level is significantly elevated, serum bilirubin levels must be obtained, requiring heel puncture for blood sampling and laboratory analysis. The trend toward earlier discharge of term and near-term healthy infants, often before peak bilirubin levels have been reached, has further complicated identification and treatment of infants at risk because they must attend a health care facility after discharge to have a blood sample obtained (4). The recently updated guidelines of the American Academy of Pediatrics (5) regarding the monitoring and treatment of hyperbilirubinemia stress the need for routine measurement of bilirubin levels, but do not specify serum or transcutaneous measurements. Increasing evidence has shown that newer transcutaneous bilirubin measurement devices, using analysis of spectral reflectance from the skin, accurately predict bilirubin levels without requiring heel puncture (6–11). The obvious benefits of noninvasive bilirubin measurement include less trauma to the patient, reduced risk of infection, immediately available results and potential cost savings. In addition, transcutaneous bilirubin measurement devices offer an easy, cost-effective way for home care nurses to assess neonatal jaundice accurately (12). Attempts to estimate bilirubin levels by noninvasive methods have been made for many years. Early devices often relied on colourimetric methods and were difficult to interpret in infants with differing skin types (13,14). Despite their potential benefits, newer noninvasive bilirubin measurement devices using spectrophotometric measurements (15,16) have not yet gained widespread acceptance. This may partly reflect concern over the accuracy of earlier techniques of transcutaneous bilirubin measurement due to variability in skin pigmentation, gestational age and birth weight. The newest device available for transcutaneous bilirubin measurement, BiliChek (Respironics, USA), has the advantage of using multiple wavelengths and should be more accurate. We evaluated the accuracy and precision of the noninvasive measurement of bilirubin by BiliChek in healthy term infants, and ill term and preterm infants admitted to the neonatal intensive care unit (NICU) at the IWK Health Centre (Halifax, Nova Scotia).
METHODS
The study consisted of two phases, both of which were approved by the Research Ethics Board of the IWK Health Centre. Phase 1 was conducted in the mother-baby unit and required consent to obtain additional blood work. Phase 2 was conducted in the NICU, and because it was felt that there was negligible risk to the infants, it was carried out as a quality assurance activity. All parents of infants admitted to the NICU were given an information sheet regarding the study and could ‘opt out’ if desired.
All healthy term infants were eligible for phase 1, with the only exclusions being refusal of either newborn screening or consent. Information regarding the purpose of the study was given to all mothers at admission to the postnatal ward. Informed consent was obtained. When heel puncture was performed for routine screening of thyroid-stimulating hormone and phenylketonuria, 250 μL of extra blood was drawn for serum bilirubin analysis. Transcutaneous bilirubin levels were recorded immediately before and after the heel puncture using the BiliChek device placed on the baby’s forehead, as recommended by the manufacturer. All transcutaneous measurements were performed by a single research assistant who was unaware of the serum bilirubin level. A convenience sample of 99 infants was used because no pre-existing data were available regarding the accuracy of the device in this population of term infants.
For phase 2, any infant admitted to the NICU was eligible if a serum bilirubin test was ordered by the attending physician. The transcutaneous bilirubin reading was done by the bedside nurse, who was unaware of the serum level at the time of the test. To assure proper use of the device, the nurses were given teaching and practice sessions before the initiation of the study. The authors aimed for a convenience sample of 100 test pairs.
It was felt that the estimation of serum bilirubin by transcutaneous readings would be clinically acceptable if it fell within two standard deviations of the acceptable laboratory variation (±15 μmol/L) found for serum testing (ie, ±30 μmol/L). Data from both phases were collected by two study investigators (HC and KAJ) and stored in a secure database. All information was kept strictly confidential.
Transcutaneous measurements
The BiliChek device is a hand-held fibreoptic device measuring multiple wavelengths by spectral reflectance. Because this device determines the optical densities of bilirubin, hemoglobin and melanin in the skin (17), it offers a significant improvement over previous methods by accounting for differences in skin pigmentation (18). Five readings are performed and the average is reported in a numerical readout.
Serum measurements
All serum measurements were performed in one laboratory using the Vitros BuBc method (Ortho-Clinical Diagnostics, USA), which measures spectral reflectance at 400 nm and 460 nm. This technique uses a slide containing dry, multilayered analytical element coated on a polyester support. A 10 μL drop of blood is deposited and spread on the slide at 37°C. The unconjugated fraction of the bilirubin interacts with the cationic polymeric mordant to form spectrally enhanced complexes with absorptivities at the specified wavelengths. Using reflection densitometry and appropriate mathematical transformation, readings are linearly related to bilirubin concentration. Results obtained by this method correlate well with previous modifications of the Jendrassik-Grof method (19).
RESULTS
The demographics of the entire study sample and the subgroups are presented in Table 1. The sample was primarily of Caucasian descent, reflecting the general population served by the IWK Health Centre. In the phase 1 subgroup, the median serum bilirubin level of the samples was 144 μmol/L (range 17 μmol/L to 294 μmol/L). Thirty-one (31%) of the samples had a serum bilirubin level less than 85 μmol/L, the level necessary to produce visible jaundice. These samples would not have been drawn for clinical purposes. In the phase 2 subgroup, the median serum bilirubin level was 155 μmol/L (range 7 μmol/L to 321 μmol/L). All samples were drawn at the request of the attending physician; however, eight samples (8%) had a bilirubin level less than 85 μmol/L.
TABLE 1.
Demographics of 155 infants whose bilirubin levels were measured using a transcutaneous device
| Subgroup | Term infants, without phototherapy (n=99) | III term and preterm infants, with or without phototherapy (n=56) | Preterm infants, without phototherapy (n=33) | Term and preterm infants, with phototherapy (n=24) |
|---|---|---|---|---|
| Number of tests | 99 | 99 | 65 | 45 |
| Birth weight, g (mean [SD]) | 3523 (560) | 2175 (904) | 1565 (482) | 2225 (1013) |
| Gestational age, weeks (mean [SD]) | 39.4 (1.4) | 33.4 (3.8) | 30.8 (2.5) | 33.1 (4.2) |
| Ethnicity, n (%) | ||||
| Caucasian | 92 (93) | 50 (89) | 28 (85) | 21 (88) |
| African Canadian | 3 (3) | 1 (2) | 1 (3) | 0 |
| First Nations | 1 (1) | 1 (2) | 1 (3) | 0 |
| Other | 3 (3) | 4 (8) | 3 (9) | 3 (12) |
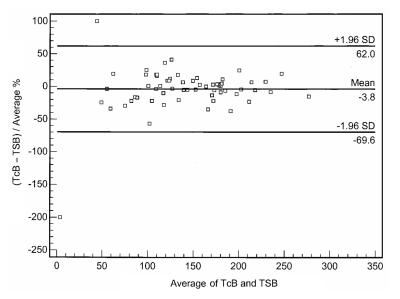
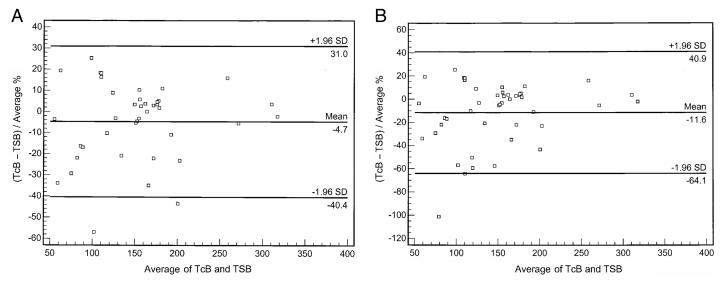
The comparisons between transcutaneous readings and serum levels for each of the subgroups are shown by Bland-Altman plots in Figures 1 to 3. The estimations of serum bilirubin using the device were within acceptable limits for term infants not receiving phototherapy (Figure 1; variation −32.2 μmol/L to +31.2 μmol/L) and infants receiving phototherapy who had an area of skin patched (Figure 2A; variation −40.4 μmol/L to +31.0 μmol/L), but not for all infants receiving phototherapy, patched and not patched (Figure 2B; variation −64.1 μmol/L to +40.9 μmol/L) or for preterm infants (Figure 3; variation −69.6 μmol/L to +62.0 μmol/L).
Figure 1).
Bland-Altman plot comparing transcutaneous bilirubin levels (TcB) with serum bilirubin levels (TSB) in term infants not receiving phototherapy (n=99)
Figure 3).
Bland-Altman plot comparing transcutaneous bilirubin levels (TcB) with serum bilirubin levels (TSB) in preterm infants (n=65)
Figure 2).
Bland-Altman plots comparing transcutaneous bilirubin levels (TcB) with serum bilirubin levels (TSB). A Infants receiving phototherapy with ‘patched’ skin area for testing (n=40). B All infants receiving phototherapy (n=45)
More important for clinical management, however, is the number and percentage of tests that vary enough from the serum sample to affect clinical decision-making. Overestimation of the serum value may lead to unnecessary blood testing for confirmation, while underestimation may prevent appropriate intervention. The number of tests where over- or underestimation occurred are reported separately for term infants and ill term or preterm infants as differing by greater than 15 μmol/L to 20 μmol/L, greater than 20 μmol/L to 25 μmol/L, greater than 25 μmol/L to 30 μmol/L and greater than 30 μmol/L (Table 2). Overall, 128 (65%) of the transcutaneous readings were within 15 μmol/L of the serum sample (comparable with the test-to-test reliability of serum testing), 38 (19%) of the readings overestimated the serum value, while 47 (24%) underestimated the serum value. Nine (9%) of the transcutaneous measurements in term infants varied from the serum value by greater than 30 μmol/L, and seven (7%) of these measurements underestimated the serum value. Seventeen (18%) of the transcutaneous readings varied by greater than 30 μmol/L in the ill term and preterm group, equally represented by over- and underestimation of the serum value by the transcutaneous reading. All values for infants receiving phototherapy without a protected area of skin for transcutaneous measurement varied by greater than 30 μmol/L.
TABLE 2.
Over- and underestimation of serum bilirubin levels as measured by transcutaneous methods
| Term infants (99 tests)
|
Preterm infants (63 tests)
|
III term infants (28 tests)
|
Phototherapy, no patch (7 tests)
|
||||
|---|---|---|---|---|---|---|---|
| Difference, μmol/L | Over, n (%) | Under, n (%) | Over, n (%) | Under, n (%) | Over, n (%) | Under, n (%) | Under, n (%) |
| >30 | 2 (2.0) | 7 (7.0) | 5 (7.9) | 7 (11.1) | 2 (7.1) | 3 (10.7) | 7 (100) |
| ≥25–30 | 5 (5.0) | 3 (3.0) | 1 (1.6) | 1 (1.6) | 0 | 0 | 0 |
| ≥20–25 | 5 (5.0) | 3 (3.0) | 1 (1.6) | 4 (6.3) | 2 (7.1) | 1 (3.6) | 0 |
| ≥15–20 | 6 (6.0) | 4 (4.0) | 8 (12.7) | 4 (6.3) | 1 (3.6) | 3 (10.7) | 0 |
| <15 | 79 (79.8) | 32 (50.8) | 16 (57) | 0 | |||
DISCUSSION
Painless evaluation of neonatal jaundice is highly desirable. Although the degree of jaundice is rarely serious, the potential for irreversible brain damage at extremely high levels of bilirubin exists. We have shown that in healthy term infants being prepared for hospital discharge and in infants receiving phototherapy who have had an area of skin covered where testing can be performed, transcutaneous bilirubin measurements using the BiliChek device are accurate and reliable for the vast majority of infants. The use of the device on the present small sample of preterm infants produced a variation outside an acceptable range to allow clinical decision-making and, therefore, cannot be recommended as the sole measure of bilirubin for these infants at this time. For all infants receiving phototherapy, the accuracy of the measurements was decreased drastically if the area of skin used for measurement had not been protected from the phototherapy lights.
The present study has several limitations that may hamper its generalizability. First, the sample of full-term infants had testing performed by a single well-trained operator, a situation that is not likely to occur in busy well-baby nurseries or in clinical settings. Becoming competent in the use of the instrument, however, is relatively easy and time efficient. Multiple operators could be easily trained in busy clinical settings.
Second, the sample size of the ill term and preterm infants was too small to allow for study of the confounding effects of interactions of level of illness, changes in skin characteristics due to gestational age, variations in bilirubin metabolism due to gestational age and the effect of multiple newly trained operators of the device. Finally, the predominance of Caucasian infants in the study limits the use of these results in populations with a more mixed ethnicity.
The use of transcutaneous bilirubin measurement in full-term infants, including those receiving phototherapy with a properly patched area of skin, can reduce the requirement for painful blood-letting, and should be considered for in-hospital, home visits and clinical settings. This would allow infants with acceptable transcutaneous bilirubin levels to avoid unnecessary testing. The BiliChek device is easily portable and gives rapid results in home or clinical settings where capillary sampling may be unavailable. If an infant is found to have significant hyperbilirubinemia using the transcutaneous device, a confirmatory sample can be taken in a timely manner to facilitate prompt treatment if necessary. As with any diagnostic test, confirmation using a ‘gold standard’ may be necessary when clinical decision-making may be affected. The underestimation of the serum bilirubin level by greater than 30 μmol/L in 7% of the studied infants led us to consider that decision-making might conceivably be altered when the transcutaneous level is greater than 260 μmol/L (ie, serum level as high as 290 μmol/L). Therefore, we recommend confirmation of all transcutaneous readings greater than 260 μmol/L by serum bilirubin measurement. Overestimation of the serum level does occur, and following this recommendation may increase the number of unnecessary confirmatory blood samples.
Transcutaneous bilirubin measurement does not obviate the need to carefully monitor infants who have rapidly rising or early-onset hyperbilirubinemia, evidenced by jaundice in the first 24 h of life or by jaundice in infants who are ill. Transcutaneous readings below the suggested level of 260 μmol/L require confirmation for these infants.
The use of transcutaneous bilirubin measurement in ill term and preterm infants cannot be recommended from the present study.
It is unclear why the estimation of serum bilirubin levels in preterm infants is not as accurate as in their full-term counterparts. The maturity of the epidermis and the lack of substantial subcutaneous fat in preterm infants may play a role. We were unable to look at the effect of increasing gestational age because our sample was too small. Further studies enrolling larger numbers of infants to 24- to 27-week, 28- to 31-week and 32- to 35-week groupings should be considered before using transcutaneous bilirubin measurement for these infants.
Future studies of the use of transcutaneous bilirubin measurement must take into account the fact that the precise serum bilirubin level necessary to cause kernicterus is unknown, in part due to the lack of complete understanding of the interaction of bilirubin with brain tissue. The way that bilirubin deposits in the subcutaneous fat in the skin may, in fact, mimic the level of bilirubin found in brain tissue more closely than does the serum bilirubin level. If this speculation is true, transcutaneous measurement may be more precise and reliable than serum measurement in guiding clinical management. However, caution must be used with this interpretation for infants receiving phototherapy because we have shown that with treatment, the change in skin colour and the bilirubin deposition in the skin improves more rapidly than in the serum.
CONCLUSIONS
The BiliChek device offers painless, accurate bilirubin determinations that can be used to quantify jaundice in healthy newborns in lieu of serum bilirubin determination. Its advantages include a high level of parent and staff acceptance; painless, rapid measurements; a high level of accuracy; easy portability; and cost-effectiveness. It has limitations in that it can underestimate the level of bilirubin in serum samples, which may affect clinical decision-making. The number of serum samples that could be avoided in a group of healthy term infants preparing for discharge is likely much higher than that in ill term or preterm infants because the serum levels at which treatment decisions are made in ill term or preterm infants may be significantly lower than 260 μmol/L. Further large studies with sufficient numbers of infants in selected gestational age groupings should be done to test the accuracy of this measurement device, taking into account the possible confounding effects of differing skin thickness and bilirubin handling in different gestational age and illness severities, before discarding its possible benefits for some subgroups of preterm infants.
Footnotes
FUNDING: IWK Research Services, IWK Health Centre, Halifax, Nova Scotia.
REFERENCES
- 1.Holtrop PC, Maisels MJ. Hyperbilirubinemia. In: Spitzer AR, editor. Intensive Care of the Fetus and Neonate. 1. St Louis: Mosby; 1996. pp. 888–98. [Google Scholar]
- 2.Centers for Disease Control and Prevention. Kernicterus in full-term infants – United States, 1994–1998. MMWR Morb Mortal Wkly Rep. 2001;50:491–4. [PubMed] [Google Scholar]
- 3.Hanko E, Lindemann R, Hansen TW. Spectrum of outcome in infants with extreme neonatal jaundice. Acta Paediatr. 2001;90:782–5. [PubMed] [Google Scholar]
- 4.Faucher DJ, Vallerand D. Management of non-isoimmune hyperbilirubinemia in term neonates with early hospital discharge. Pediatr Res. 1999;45:196A. Abst. [Google Scholar]
- 5.American Academy of Pediatrics, Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004;114:297–316. doi: 10.1542/peds.114.1.297. Erratum in 2004;114:1138. [DOI] [PubMed] [Google Scholar]
- 6.Yamauchi Y, Yamanouchi I. Clinical application of transcutaneous bilirubin measurement. Early prediction of hyperbilirubinemia. Acta Paediatr Scand. 1990;79:385–90. doi: 10.1111/j.1651-2227.1990.tb11481.x. [DOI] [PubMed] [Google Scholar]
- 7.Maisels MJ, Engle WD, Jackson GL, Tayaba R. Noninvasive measurement of serum bilirubin. Pediatr Res. 1999;45:209A. Abst. [Google Scholar]
- 8.Bhutani VK, Gourley GR, Adler S, Kreamer B, Dalin C, Johnson LH. Noninvasive measurement of total serum bilirubin in a multiracial predischarge newborn population to assess the risk of severe hyperbilirubinemia. Pediatrics. 2000;106:E17. doi: 10.1542/peds.106.2.e17. [DOI] [PubMed] [Google Scholar]
- 9.Tayaba R, Gribetz D, Gribetz I, Holzman IR. Noninvasive estimation of serum bilirubin. Pediatrics. 1998;102:E28. doi: 10.1542/peds.102.3.e28. [DOI] [PubMed] [Google Scholar]
- 10.Rubaltelli FF, Gourley GR, Loskamp N, et al. Transcutaneous bilirubin measurement: A multicenter evaluation of a new device. Pediatrics. 2001;107:1264–71. doi: 10.1542/peds.107.6.1264. [DOI] [PubMed] [Google Scholar]
- 11.Tan K, Dong F. Transcutaneous bilirubinometry during and after phototherapy. Acta Pediatr. 2003;92:327–31. [PubMed] [Google Scholar]
- 12.Ruchala PL, Seibold L, Stremsterfer K. Validating assessment of neonatal jaundice with transcutaneous bilirubin measurement. Neonatal Netw. 1996;15:33–7. [PubMed] [Google Scholar]
- 13.Gosset IH. A perspex icterometer for neonates. Lancet. 1960;1:87–8. doi: 10.1016/s0140-6736(60)92902-0. [DOI] [PubMed] [Google Scholar]
- 14.Culley PE, Waterhouse JA, Wood BS. Clinical assessment of depth of jaundice in newborn infants. Lancet. 1960;1:88–9. doi: 10.1016/s0140-6736(60)92903-2. [DOI] [PubMed] [Google Scholar]
- 15.Bilgen H, Ince Z, Ozek E, Bekiroglu N, Ors R. Transcutaneous measurement of hyperbilirubinaemia: Comparison of the Minolta jaundice meter and the Ingram icterometer. Ann Trop Paediatr. 1998;18:325–8. doi: 10.1080/02724936.1998.11747968. [DOI] [PubMed] [Google Scholar]
- 16.Schumacher RE, Thornbery JM, Gutcher GR. Transcutaneous bilirubinometry: A comparison of old and new methods. Pediatrics. 1985;76:10–4. [PubMed] [Google Scholar]
- 17.Onks D, Silverman L, Robertson A. Effect of melanin, oxyhemoglobin and bilirubin on transcutaneous bilirubinometry. Acta Pediatr. 1993;82:19–21. doi: 10.1111/j.1651-2227.1993.tb12507.x. [DOI] [PubMed] [Google Scholar]
- 18.Robertson A, Kazmierczak S, Vos P. Improved transcutaneous bilirubinometry: Comparison of SpectR(X) BiliCheck and Minolta Jaundice Meter JM-102 for estimating total serum bilirubin in a normal newborn population. J Perinatol. 2002;22:12–4. doi: 10.1038/sj.jp.7210592. [DOI] [PubMed] [Google Scholar]
- 19.Wu TW, Dappen GM, Powers DM, Lo DH, Rand RN, Spayd RW. The Kodak Ektachem clinical chemistry slide for measurement of bilirubin in newborns: Principles and performance. Clin Chem. 1982;28:2366–72. [PubMed] [Google Scholar]