Abstract
Recent improvements in brain slice technology have made this biological preparation increasingly useful for examining pathophysiology of brain diseases in a tissue context. Brain slices maintain many aspects of in vivo biology, including functional local synaptic circuitry with preserved brain architecture, while allowing good experimental access and precise control of the extracellular environment, making them ideal platforms for dissection of molecular pathways underlying neuronal dysfunction. Importantly, these ex vivo systems permit direct treatment with pharmacological agents modulating these responses and thus provide surrogate therapeutic screening systems without recourse to whole animal studies. Virus or particle mediated transgenic expression can also be accomplished relatively easily to study the function of novel genes in a normal or injured brain tissue context.
In this review we will discuss acute brain injury models in organotypic hippocampal and co-culture systems and the effects of pharmacological modulation on neurodegeneration. The review will also cover the evidence of developmental plasticity in these ex vivo models, demonstrating emergence of injury-stimulated neuronal progenitor cells, and neurite sprouting and axonal regeneration following pathway lesioning. Neuro-and axo-genesis are emerging as significant factors contributing to brain repair following many acute and chronic neurodegenerative disorders. Therefore brain slice models may provide a critical contextual experimental system to explore regenerative mechanisms in vitro.
I. BRAIN SLICES AS MODELS FOR NEURODEGENERATIVE DISEASES
i. Introduction
Brain slice models offer unique advantages over other in vitro platforms in that they can replicate many aspects of the in vivo context. Slices largely preserve the tissue architecture of the brain regions that they originated from and maintain neuronal activities with intact functional local synaptic circuitry. With no need for lengthy animal surgery to model neuropathology of brain injury or laborious monitoring of multiple physiological parameters following in vivo manipulation, the usefulness of brain slices in basic research, as well as in the drug discovery process, has been increasing in recent years. A number of pharmacological and genetic manipulations that affect the neurochemical behaviors of the brain in vivo have been shown to be reproduced in brain slices [37, 131, 135]. As slice-based assay systems provide good experimental access and allow precise control of extracellular environments, it facilitates research establishing clear correlations between molecular changes with neuropathological outcomes. In addition, it is possible to adopt these ex vivo models for the screening of therapeutic molecules or novel genes. With development of disease-relevant slice models that simulate essential features of in vivo neurodegenerative pathologies, a larger panel of treatments could be efficiently evaluated in living tissues in a normal or injured brain tissue context without complication from brain penetration or metabolic stability. The first section in this review will summarize a few different preparations of slice systems, functional and biochemical evidence that supports their utility for the studies of adult brain, and injury models that represent major pathology of neurodegenerative disorders.
ii. Organotypic Slice Cultures
Over the years, slice culture systems have been successfully established from a variety of brain regions including hippocampus, striatum, cortex, spinal cord and cerebellum [15, 87, 115, 123, 142]. Furthermore, a number of tissue slice co-cultures have been developed, which allow the assessment of inter-neuronal responses across brain regions. The utilities of these co-cultures have been well established in examples of entorhino-hippocampal, cortico-spinal and cortico-striatal preparations [11, 60, 105, 122, 176].
Several methods have been developed to maintain thin slices of brain alive in a long-term culture. Two earlier methods described by Gahwiler [62] or Linder [96] were based on the use of either roller tubes or Maximov-type chambers. Though more contextual than the dissociated primary neuronal cultures, organotypic cultures prepared by the conventional “roller tube” technique have been shown to be difficult to prepare and known to bear a large experimental variability due to thinning of tissues to a nonphysiological monolayer of cells. In more recent years, a membrane interface culture method was described by Muller and colleagues [155], which provides an easier access to the slice culture preparation. While all of these techniques generate brain slices that maintain viability during a long term culture and that successfully preserve much of the tissue architectures, most studies now utilize various modifications of the membrane interface methods.
The principle of membrane interface culture methods is to maintain brain slices on a porous membrane filter at the interface between medium and a humidified atmosphere. The medium provides adequate nutrition to tissues through the membrane via capillary action. A detailed procedure for slice preparations and culturing tips have been previously elaborated by Bergold and Casaccia-Bonnefil and Noraberg et al. [14, 118].
Organotypic slice cultures have been typically prepared from brains of animals at postnatal days 3-9. Brain tissues from these young ages show a high degree of plasticity and resistance to mechanical trauma during the slice preparation, which is important in obtaining viable and healthy cultures routinely. At this age, basic synaptic connections have been established, particularly in the CA1 area of the hippocampus, but mature synapses have not yet been established in the brain, which normally develop during the following 2–3 weeks in vivo [45, 129]. Interestingly, hippocampal slices from 0 to 1 day old neonates are highly viable during culture, but they gradually lose their morphological characteristics, thus being unsuitable for long-term culture [39]. This may suggest that less developed cells are more likely to dedifferentiate [93, 171], compared to cultures from older neonates.
While perinatal cultures undergo a series of neuronal reorganizations in vitro to adopt a number of features of mature neuronal synapses, utilization of cultures derived from mature hippocampal slices might provide unique opportunities to study neuronal responses in vitro as in the adult brain. In fact, one of the elements shown in immature slice cultures is spontaneous epileptiform activity often observed after long-term culture (over 3 weeks in culture), which results from the formation of an aberrant mossy fiber pathway in vitro. This is in contrast to the mature slice cultures, where spontaneous epileptic activity has rarely been observed [179]. Two recent reports have elegantly demonstrated the establishment of functional hippocampal cultures from mature brains [58, 179]. In both studies, three to four week-old rats were used to isolate hippocampal tissues, an age at which developmental maturation of the hippocampal formation reaches an adult level [129]. Preparation of slice cultures from older animals is technically more challenging and we and others noticed that degeneration during a long-term culture period occurs more often in these young adult slices compared to neonatal slice cultures. In our experience, this could be minimized by lowering the culture temperature to 34°C.
Multi-layered pyramidal neurons in the CA1 area of immature hippocampus tend to spread horizontally during culturing to form a wider band of neurons. In comparison, mature slice cultures maintain a densely packed neuronal layer with fine dendritic arborization and dendritic spines for weeks in culture [179]. These slices retained intact synaptic networks, as manifested by a demonstration of evoked excitatory post-synaptic potentials (EPSP) upon stimulation of Schaffer collaterals as well as synaptic facilitation and depression [58]. Moreover, our data from a neurochemical study, illustrating massive ischemic injury-induced efflux of neurotransmitters, further substantiates the presence of active synapses in these slice cultures. Therefore, considering the balance of merits and challenges associated with this preparation, mature slice cultures could have specific utilities in either confirmatory studies following initial outcome in the neonatal culture or evaluation of critical pathways relevant to the aged brain.
iii. Development and Plasticity of Brain Slice Cultures
Typical hippocampal slice cultures obtained from rats at postnatal days 3-9 can be maintained in vitro for weeks, during which developmental maturation of hippocampal tissues continues ex vivo, as represented by characteristic morphological alterations. The existing basic synaptic connections at the early ages become progressively elaborated to form mature synaptic networks, which largely mimic the endogenous developmental changes in the brain during the first few weeks after birth. High synaptic density, dendritic arborization, presence of active synaptic vesicles, and astrocytic maturation are a few examples of synaptic maturation in the slice cultures [9, 23, 46, 111, 179]. Confocal microscopic analysis by De Simoni and colleagues [45] showed that organotypic slices cultured for 1, 2, or 3 weeks in vitro were developmentally equivalent to brain slices dissected at postnatal day 14, 17 or 21, respectively, in the number of primary branches, the total length of neurons, outgrowth of apical dendrites and spine density. In addition to structural development, long-term slice cultures have been shown to adopt patterns of gene regulation, protein expression, and synaptic activity of the adult hippocampus [9, 45, 137]. Binding studies with [3H] AMPA have demonstrated that the ligand binding sites in slice cultures are most concentrated in the dendritic fields of CA1 and CA3 [9], which is characteristic of the adult hippocampus [16], while GluR mRNAs were detected in the neuronal cell bodies. In addition, in the study using whole cell patch-clamp recordings, there were no significant differences between organotypic and age-matched acute slices in the frequency of action potentials, total miniature synaptic activities, and GABAergic miniature currents [45].
It is important to note, however, that there are also some properties in organotypic cultures that differ from endogenous brain maturation in vivo. Tracing of single neurons by labeling with a fluorescent dye showed that neurons in organotypic slices were more complex, shown by an increased number of branches and higher order dendrites [45]. Consistent with this, a significant increase in the frequency of glutamatergic miniature synaptic currents was detected in organotypic slices, which probably reflects an increased number of total synapses. How are these differences induced in organotypic cultures? During slice preparation, axons of many neurons are inevitably cut. Subsequently, these traumatic reactions are stabilized over the course of the culture period and damaged axons have a chance to recover and reroute the processes to form new neural connections. This is in contrast to acute slice systems, where there are few changes in neuroanatomy, as they are used within hours after the preparation.
The observation of in vitro neuronal rearrangements in organotypic slices leads to a few important points to note. First, this provides evidence that organotypic slice cultures can be used as a valuable tool in studying post-lesion outgrowth since evidence shows that damaged axons can regenerate in a supportive environment [47, 156, 176]. Second, the contribution of excitotoxicity and synaptic firing to acute neuronal injuries, such as ischemia and traumatic injury, and its impact on the outcome of long-term recovery have to be examined with caution, since their involvement might have been over-accentuated in this model, compared to the native system. In a recent study, Adcock et al. (2004) have examined the development of Purkinje cells in organotypic cerebellar slice cultures [2]. It is known that the development of the dendritic tree of a neuron is regulated by the synaptic input from afferent fibers and activity-dependent signaling by neurotrophic factors. Surprisingly, they found that in ex vivo brain slices development of the Purkinje cell dendritic tree was normal in the absence of excitatory neurotransmission and BDNF signaling. From these observations, they concluded that many aspects of Purkinje cell dendritic development are achieved by an intrinsic growth program. However, it cannot be excluded that a higher basal synaptic activity in the organotypic cerebellum and resultant increases in autocrine/paracrine inputs might have played a role in leading to the normal development of dendritic arbors in the absence of afferent stimulatory inputs.
iv. Functional Analysis of Neuronal Viability and Neurodegeneration
To strengthen the usefulness of organotypic slice cultures in a wide range of studies examining neuronal fate, several methods have been utilized assessing neuronal death and survival. The two most common measures, propidium iodide (PI) uptake and lactate dehydrogenase (LDH) release, are based on changes in the cellular membrane permeability upon injury. Compared to healthy neurons, injured cells have compromised membrane integrity, allowing otherwise membrane-impermeable molecules to pass through. PI is taken up by damaged cells and intercalated into DNA. This renders the dye to become fluorescent, which can be easily quantified either using imaging software, if specific regions are to be analyzed, or by a plate reader to determine total fluorescence [37]. Assessment of LDH activity in the culture medium has been widely used as a quantitative measure of cell death in primary cultures [86]. Similarly, increased LDH efflux is also detected in injured organotypic slices [21, 117]. Assessment of cell death by mean PI uptake and LDH efflux into the medium has been found to be highly correlated with manual cell counting of live vs. dead cells [115, 117], and therefore can offer efficient measures of neurotoxicity in the organotypic slices.
Fluoro-Jade is a specific and selective marker to identify neurons undergoing degeneration [146]. It has been shown to stain not only cell bodies, but also dendrites, axon fibers, and terminals of degenerating neurons in brain tissues and organotypic slices [18, 55, 66, 88, 117]. When excitotoxic responses were evaluated using corticostriatal slices, exposure to kainic acid resulted in a concentration-dependent increase in Fluoro-Jade stained neurons in both the striatal and cortical parts of the slice cultures. The staining was in good correlation with an increase in PI uptake, while little Fluoro-Jade staining was found in control cultures, corresponding to a low PI uptake in these cultures [88].
One of the commonly used viability assays in dissociated cultures is to measure the metabolic conversion of yellow MTT dye by active mitochondria to form insoluble purple precipitates in live cells. While this assay has not been routinely used to assess viability of slice cultures, it offers a different measure of viability than PI uptake or LDH release in that the MTT assay does not rely on membrane integrity of cells, but rather assesses their metabolic activity. In addition, unlike the LDH assay, one can obtain region-specific information related to differential cellular susceptibility to toxicity. Connelly and his colleagues have examined the utility of this assay to assess viability of organotypic slices from brain stem, hippocampus and spinal cord [39]. They observed accumulation of formazan precipitate in multiple cellular layers of the slices, which could be easily visualized by bright field or phase contrast microscopy. As slices degenerated with time, delineation between purple regions (representing live cells) and unstained regions (representing dead cells) was clear, enabling the quantification of slice viability based on percent tissue area containing dark precipitates. They further suggested that slices could be subjected to the MTT assay after obtaining other experimental parameters such as LDH release or electrical recordings. In this way, comparative correlations could be obtained between different assays and ultimately these multi-parameter outcomes would provide an accurate assessment of the vital status of brain slices.
In addition to general viability, a higher level of neuronal function can be verified by measuring intact synaptic activity. Mature hippocampal slices exhibit diverse synaptic events, including post-tetanic potentiation, long-term potentiation and long-term depression [13, 58, 167]. These activities implicitly measure functional intactness of neural connections beyond survival, and thus further establish working brain tissues in vitro. We will discuss this in more detail in a separate section below.
v. Pharmacological Assessment in Organotypic Slice Models of CNS Disorders
Many features of long-term slice cultures such as developmental maturation, neuronal activity, cytoarchitectural maintenance as well as improvement of slice technology make them a useful tool for investigation of pathophysiology of brain diseases. Ischemic injury represented by oxygen-glucose deprivation (OGD) is one of the widely-adopted models to study acute stroke pathology. Organotypic hippocampal slices subjected to a brief OGD injury exhibit region-selective death of pyramidal neurons in the CA1 region, similar to that observed in vivo, while sparing neighboring neurons in the CA3 and CA4 and granular neurons in the dentate gyrus [37, 58, 89]. Ischemia-induced changes in several biochemical parameters such as neurotransmitter release, post-translational modification of the synaptic protein phosphosynapsin, and involvement of caspase pathways, have been well characterized [37, 58, 80].
Neuronal death following ischemic stroke is known to involve an acute phase of necrosis in the ischemic core as well as a slowly propagating neurodegeneration in the penumbral regions [81]. Disruption of oxygen supply leads to rapid energy failure and membrane depolarization and subsequently causes increases in intracellular calcium and excitatory amino acid secretion. On the other hand, neurons in the ischemic penumbra are subject to energy-dependent programmed cell death cascades, involving several key apoptotic players such as caspases, Bcl-2, and apoptosis inducing factor-1 [101, 153]. Importantly, cell death in the organotypic cultures also seems to present both types of pathology. It has been demonstrated that pharmacological modulators such as excitotoxic inhibitors, channel blockers, and antiapoptotic reagents that could afford neuroprotection in vivo are also active in preventing neuronal death following OGD injury in slice models (see a review by Noraberg et al. [118]). In a recent report, activation of caspase 3 was visually illustrated by confocal microscopy using live cultures of organotypic slices, demonstrating a region-selective increase in active caspase 3 signals, which temporally preceded the cell death [37]. In addition, when the experimental window of observation was extended up to 5-6 days after OGD, there appeared a histological indication of cellular recovery in a small population of cells. Five days after OGD, while a majority of the pyknotic nuclear staining persisted, membrane blebbing observed in earlier days was significantly diminished and nuclear condensation appeared to be restored as shown by thionin staining [37]. Although functional outcomes beyond the morphological recovery were not assessed in this study, it can be speculated that these initial changes may be linked to the endogenous neurological recovery following ischemic injury in preclinical models [109, 182], supporting the utility of long-term slice cultures for regenerative research.
The application of slice cultures has also been well explored in research of Alzheimer’s disease (AD). The hippocampus is known to play a central role in learning and memory and its malfunction has been implicated to be largely responsible for the cognitive deficits manifested in Alzheimer’s patients. Although still controversial, it is generally believed that beta-amyloid peptide (Aβ) “overdose” is a cause of AD pathology and, consistent with this view, diverse studies have attempted to characterize the effects of Aβ treatment in organotypic hippocampal slices. First, a number of laboratories have looked at the susceptibility of hippocampal neurons to Aβ-induced neurotoxicity in organotypic slice cultures. A study by Bruce et al. have shown that the treatment of mature cultures (equivalent postnatal day 35) with beta-amyloid resulted in a time-dependent and a concentration-dependent increase in neuronal injury, leading to about 25% neuronal death at 48 hr of continuous Aβ exposure and approximately 40% at 72 hr [20]. Importantly, immature organotypic brain slices were shown to be resistant to Aβ-induced neurotoxicity, thus substantiating the correlation between neuronal susceptibility and aging in this model. Secondly, organotypic hippocampal slices are known to deposit plaque-like Aβ aggregates [72]. Initially, immunohistochemical analysis of slices following 2–3 days of treatment with Aβ showed Aβ immunoreactivity in the cellular compartment, mostly in microglia. By day 7 the slices treated with Aβ showed diffuse and seemingly extracellular plaque-like deposits, which continued to progress over another week. The Aβ deposits were also shown to be associated with reactive glia. Furthermore, the deposition of Aβ plaques was increased by incubation with TGF-beta family proteins, which ultimately accompanied degenerative morphological changes in the CA1 region. These results in hippocampal slice cultures are consistent with those obtained from in vivo studies, which demonstrate enhanced Aβ deposition by TGF-beta treatment [61]. Recently it was shown that TGF-beta2 can target Aβ to neurons in organotypic hippocampal slices and it was causally associated with increased ApoE release from the slice cultures [71]. Further, a low-density lipoprotein receptor-related protein (LRP) antagonist, RAP, was able to effectively block TGF-beta2-mediated targeting of Aβ to CA1 hippocampal neurons in both organotypic hippocampal cultures and brain infused with Aβ, supporting the utility of these slices in modeling the chronic brain diseases.
Lastly, hippocampal slice cultures demonstrate both structural and activity-dependent synaptic plasticity and Aβ has been shown to impair this plastic response [90]. With an advantage of long-term survival, organotypic hippocampal cultures can be utilized to evaluate the functions of novel genes or pharmacological agents in LTP formation and synaptic efficacy, which may provide insights for molecular mechanisms of memory formation and facilitation of cognitive function.
Glutamate-mediated excitotoxicity has been known to be involved in the pathogenesis of many neurodegenerative diseases. Corse and colleagues have developed organotypic slice cultures of lumbar spinal cord as a model of glutamate pathology in amyotrophic lateral sclerosis (ALS) [42]. ALS is a condition caused by a progressive degeneration of the motor neurons in the cortex and spinal cord, and chronic glutamate-toxicity is postulated as one of the major contributing mechanisms [150]. In the study by Corse et al. neurons in spinal cord cultures demonstrate a long-lasting viability up to 3 months and a well-preserved organotypic morphology including dorsal and ventral grey regions and central canals [42]. To replicate the chronic defects of a glutamate system observed in ALS patients, the slices were challenged with threo-hydroxyaspartate, which selectively inhibits glutamate transport, thereby resulting in the rise of extracellular glutamate concentration and a marked loss of ventral motor neurons. In this model of motor neuron degeneration, treatments with certain neurotrophic factors and anti-excitotoxic agents were shown to be neuroprotective [42, 100]. Interestingly, BDNF was not neuroprotective in these cultures, although spinal motor neurons express TrkB receptors [181]. In view of the lack of efficacy of BDNF treatment in clinical trials of ALS [12, 120], the organotypic glutamate toxicity model may provide a predictive preclinical model for ALS in forecasting the outcome of clinical trials.
One of the distinct advantages of organotypic slice models over other in vitro systems is that the slice cultures maintain the cytoarchitecture of the tissue being studied, allowing interaction of multiple cell types in the brain, namely neurons, astrocytes and microglia. While astrocytes represent the major non-neuronal cells in the brain, microglia also comprise approximately 15 % of the adult brain population. The interplay between these glial cells and neurons has critical roles during normal development as well as during neuropathogenesis. Although the origin of microglia has been a matter of controversy, results from various in vivo and in vitro studies converge on the hypothesis that brain microglia come from both infiltrating blood monocytes and resident progenitor cells. Some of the strong evidence supporting the presence of endogenous proliferating microglia are provided by studies using slice systems. Eliason et al. have shown that neocortex organotypic slice cultures subject to electrolytic lesions increased the number of microglial cells detected 1-8 days following lesions [53]. As slice cultures are deprived of any blood circulation, it is evident that this increase is attributed to progenitor cells residing in the brain tissue. Similar microglial activation around damage sites has also been well described in several rat brain injury models [1, 147]. Therefore, results from the study by Eliason et al. demonstrate that slice systems can genuinely recapitulate important in vivo cellular responses in vitro.
Recent advancements in slice cultures further extend the utility of these models in research of neuronal demyelination and oligodendrocyte degeneration. A brief application of N-methyl-d-aspartate to organotypic hippocampal slices induced neuronal death and astrocyte activation along with secondary oligodendrocyte degeneration [128]. Treatment of slice cultures with a chimeric derivative of IL-6 and soluble IL-6 receptor resulted in preserving myelin basic protein (MBP) production. This result is consistent with the protective roles of IL-6 shown in vivo. Using IL-6 knock out animals, Cafferty et al. have shown that IL-6 is a critical component in preconditioning injury responses of the sciatic nerve and increased regeneration of dorsal column axons [26]. In another study by Birgbauer et al. the process of demyelination and remyelination was studied using an organotypic cerebellar slice culture system [15]. Cerebellar slices demonstrated significant myelination after 1 week in culture. Treatment of the cultures at 7 days with the bioactive lipid lysolecithin produced marked demyelination, as determined by immunostaining for the myelin components myelin basic protein (MBP), myelin oligodendrocyte glycoprotein (MOG), and 2′, 3′-cyclic nucleotide 3′-phosphodiesterase (CNPase). Importantly, after a transient demyelinating insult with lysolecithin, the cultures recovered with oligodendrocyte differentiation and some limited remyelination was observed. This study supports the potential of using the organotypic cerebellar slice culture system as a model for studying myelination, demyelination, and remyelination in vitro.
vi. Application to Neurodegenerative Diseases
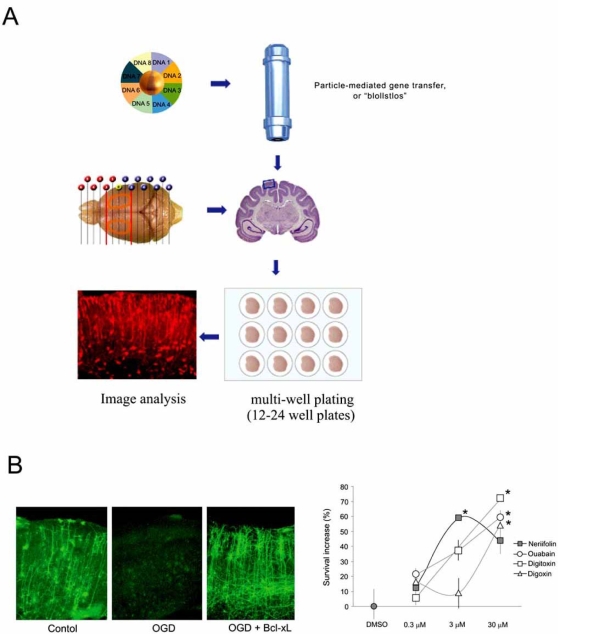
Development of therapeutics targeting neurodegenerative pathways faces major challenges, not least of which is the ability to test candidate reagents with reasonable throughput and with access to directly measure modulation of molecular targets and signal transduction pathways under investigation. The small body of work examining neuro-restorative and regenerative responses in organotypic culture is currently promising and suggests that positional and developmental cues and responses to exogenous stimuli are translated authentically in culture from the intact animal. Although tissues must be taken from young animals in order to develop optimally in culture, and this may not mimic treatment goals in mature brain, core mechanisms of proliferation, differentiation and axonal outgrowth are clearly preserved within the parameters so far measurable. In addition to the considerable throughput now offered by organotypic slice platforms in comparison with whole animal studies [44, 159, 173], the ability to label specific pathways also adds value to evaluating contextual responses. Indeed by anterograde labeling of entorhinal neurons or by using genetically labeled co-cultures, it is possible to easily visualize labeled fibers extending from the entorhinal cortex to within the hippocampus [76]. One of the most compelling reasons for use of brain slices is the facility to image transgenic and transfected cultures using high resolution confocal and time-lapse microscopy. Expression of disease inducing or disease associated genes for Huntingdon’s, Parkinson’s and Alzheimer’s disease are currently in progress and supporting screening of focused small molecule libraries (Fig. 1). Although the throughput is characteristically lower than conventional screens using cell lines or primary neurons, a hit in these slice systems is efficacious in both a tissue and disease context [173]. Furthermore, expression of such fluorescent transgenes allows a focus onto morphological plasticity both at the cellular and sub-cellular levels that may remain uncaptured within intact animals and may be pivotal to the mechanisms of neuronal recovery and development of therapeutic targets.
Fig. (1).
(A) Schematic diagram of brain slice preparation and biolistic transfection in a multi-well platform (from personal communication with D. Lo et al.) (B) Application of brain slice technology for screening of pharmacological and genetic candidates affording neuroprotection (reproduced from [173] with kind permission).
II. REGENERATION IN ORGANOTYPIC CULTURES
i. Introduction
The mature CNS is now recognized to respond adaptively to injury [54, 108, 140, 141]. Stem cells are stimulated to produce neuronal and glial progenitors and these cells appear to be recruited from germinal zones to sites of injury. Existing neurons are also able to mount a regenerative sprouting response to chemical and structural injury. In best cases, these responses can generate a partial neuroanatomical repair capable of integrating new cells into existing neuronal circuitry [57, 113]. However, the inherent complexity of the brain makes it very difficult to analyze the functional quality of the new tissue and the mechanisms by which it originated. Alternatively, organotypic brain slice cultures simulating contextual brain injury provide an in vitro platform with better access for study.
Since the prevailing view until recently has been that the mature CNS possessed very little intrinsic plasticity [95], it is perhaps not surprising that experimentation using ex vivo models of brain injury have seldom been extended to examine their regenerative properties. Recently, this situation is changing and below we will describe the current understanding of regenerative capacities in cultured brain tissue and where possible the authenticity of the response as it translates in vitro experimental findings to whole animal studies.
ii. Neurogenesis and Gliogenesis
The hippocampus is perhaps the best studied brain structure in organotypic culture. In vivo neurogenesis occurs in the adult hippocampus of several vertebrate species, including humans [84]. New granule neurons are born in the sub-ventricular zone and migrate to the dentate gyrus where they differentiate. Ischemic and chemical injury appears to transiently up-regulate the numbers of newborn cells, however, their ability to be sustained and contribute to repair is largely unknown [162]. Several laboratories have now examined the capacity of cultured postnatal hippocampal tissue to generate new glial cells and neurons [35, 92, 118, 132]. Most obviously, there is an extensive proliferation of astrocytes (mostly reactive type II), microglia and fibroblastic cells immediately following explantation leading to a type II astrocytic glial cover to the sectioned hippocampal surface; the majority of type I astrocytes and fibroblasts appear confined to outgrowth zones at the substratum culture interface. No evidence for oligodendrocyte proliferation has been detected, potentially indicating the early ontogeny of the oligodendroglial lineage within the hippocampus or the lack of stimulation of oligodendroglial precursor proliferation under simple culture conditions. In the rat, neurogenesis within the dentate gyrus appears to develop normally with new-born BrdU/ NeuN positive cells appearing spontaneously and incorporating according to a similar pattern to that which is detectable in the intact animal [132]. These cells appear to be proliferative for at least 4 weeks following labeling in slice culture. In order to better visualize neural stem cells, a knock-in of the EGFP reporter gene into the bHLH transcription factor Ngn2 locus has been used; this factor is responsible for neuronal determination within developing stem cells [116, 158]. Using mouse organotypic hippocampal tissue cultured for 2.5 weeks it has been possible to track migration paths of neuronal precursors over a period of 45 days. These studies demonstrated that the migratory pattern of Ngn2 labeled cells closely correlates with the postnatal pattern of development established in vivo, where cells leave the secondary dentate matrix within a strong rostro-ventral gradient and persist in the subgranular zone of the dentate gyrus. Ultimately, these cells co-express BrdU and doublecortin, an early neuronal marker [70].
These data clearly demonstrate the birth and maturation of neurons in hippocampal slice cultures for substantial periods of time and that these cells differentiate regionally and phenotypically appropriately. In addition to these parallels, treatment with growth factors such as EGF (epidermal growth factor) can increase numbers of proliferating neuronal precursors in slice cultures [132] and EGF has also been shown to upregulate the numbers of neuronal precursor cells in vivo [113]. In situ, EGF receptors are also likely to respond to TGF-alpha (endogenous ligand) to promote neurogenesis [41]. Indeed, TGF-alpha signaling via the EGF receptor pathway appears to cooperate with stimuli from both sonic hedgehog and soluble amyloid precursor protein to promote neuronal stem cell proliferation [27]. IGF1 (insulin like growth factor 1), FGF2 (basic fibroblast growth factor) and CNTF (ciliary neurotrophic factor) have also been demonstrated to play an important role in neurogenic stem cell turn over and CNTF does so in part by up-regulation of Notch1 expression [69]. In vivo, it is likely that several of these factors are contributed by astrocytes and that metalloproteinase enzymes activate the pathways by cleavage of membrane located growth factors [17]. Factors including BDNF (brain derived neurotrophic factor) and VEGF (vascular endothelial growth factor) also play important roles in promoting survival of differentiating neurons [38, 74]. Ischemia has been shown to up-regulate neurogenic mechanisms and several expression profiling studies implicate significant numbers of genes in mobilizing the response, reviewed by [97]. Hypoxic treatment also appears to regulate neurogenesis and survival in the cultured hippocampus and dentate gyrus [33]. Hypoxic conditions applied to rat hippocampal slice cultures followed by reoxygenation also activates the ERK and JNK pathways as ischemia does in vivo [56, 184]. Application of profiling and gene knockdown technologies to the organotypic slice under conditions designed to stimulate neurogenesis should allow dissection of molecular pathways critical to stem cell proliferation and differentiation in a simplified but contextual manner.
Based on demonstrated plasticity, the hippocampal slice system appears a validated, disease relevant and authentic system to study endogenous regenerative responses. However, less information is available regarding neurogenic and gliogenic responses in organotypic cultures harvested from other brain regions or their modulation by injury and chemical stimuli. Slice cultures from the embryonic neocortex are an exception as they demonstrate both gliogenesis and neurogenesis and retain authentic anatomy and the ability to be cultured for prolonged periods [10, 75].
iii. Axogenesis
Several organotypic culture systems have been used to analyze the regenerative capacities of severed axons. Recently, the entorhino-hippocampal pathway has been studied in a number of different laboratories. The entorhinal cortex connects the hippocampal and neocortical formations, is critical in supporting learning and memory and is significantly affected during Alzheimer’s disease [170]. In vivo, the ability of axotomized entorhinal axons to regrow declines with developmental age from a relatively robust response to a barely detectable one by the second postnatal week. This diminished developmental regenerative capacity is mirrored in entorhinal-hippocampal co-cultures where severed entorhinal axons lose their capacity to regrow following axotomy after 2 weeks in vitro. However, this gradual loss in regenerative capacity makes the pathway an excellent system with which to investigate factors and mechanisms to promote axonal regeneration. In addition, the ability to encapsulate the entire pathway in horizontal slices and to juxtapose different ages of entorhinal and hippocampal tissues in cocultures makes this a very flexible system for study (see below).
The ability of young hippocampal tissue to support the regrowth of severed of E18 entorhinal axons in culture has been examined. This tissue appears permissive for regeneration and results in an axonal projection to the hippocampal tissue terminating in the outer molecular layer of the hippocampus, the appropriate in vivo target [76]. However, it has also been established that there is a limited ability to modify the regenerative connections made from the postnatal entorhinal cortex by juxtaposition of different hippocampal target fields due to the apparent early specification of entorhinal projection neurons [166]. Because of this limited capacity for regeneration following lesion within the perforant pathway the period at six days of in vitro culture provides an optimal basis for screening agents that enhance the growth of axons. To date, several growth factors, including NT-4, GDNF and FGF appear to be effective in promoting axonal regrowth, resulting in significant increases in detectable fibers reaching their targets [130].
The presence of myelin inhibitors of axonal regeneration is also well established within the perforant pathway and myelin proteins are readily detectable in organotypic cultures [22]. The ability of functionally neutralizing antibodies directed against a major component of myelin to promote perforant pathway regeneration has recently been investigated. In these studies, treatment with IN-1 antibodies that functionally block the Nogo-A ligand [22] enhanced entorhinal axon regrowth; however, the potential functional integration of regenerated fibers remains to be tested [105]. The mossy fiber tract constitutes another major axonal pathway in the hippocampus and plays a central role in learning and memory. Mossy fibers normally exhibit very specific patterns of innervation to the stratum lucidum and following transection appear substantially able to regenerate according to their native pattern [24]. Mossy fiber tract regeneration has therefore been examined in an organotypic context to study the influence of endogenous and exogenous inhibitors. In addition to components of myelin, chondroitin and keratan sulphate proteoglycans are known to strongly inhibit regrowth of axons in vitro [106]. Digestion of chondroitin sulphate molecules following spinal cord injury can also substantively enhance sprouting and regeneration. However, the role played by both these inhibitors in the brain is unclear and dramatic changes in the pattern of mossy fiber outgrowth following transection can be produced by keratinase but not by chondroitinase treatment [106]. Overall, interplay between inhibitory factors and permissive guidance cues are likely to dictate regenerative outcome; although this study suggests, at least in the hippocampus, keratinase may be the more important factor in guiding outgrowth. Importantly, this organotypic system demonstrates that it can quantitatively differentiate between related inhibitory molecules. Further study will hopefully elucidate whether the smaller quantities of proteoglycans that accumulate following brain injury play a positive role in establishing anatomically correct regenerative responses by guiding axonal regrowth appropriately or whether, as in the spinal cord, they provide barriers to regeneration, by blocking sprouting.
In addition to the mossy fiber pathway, transection of the Schaffer collateral pathway is also possible in hippocampal slices [104]. This lesion usually responds by reactive sprouting in the CA3 region within 3 days of the injury and restoration of synaptic transmission within 2 weeks between the CA3 and CA1 regions. Apparently, these responses can be modulated dramatically by exposure to glutamate receptor antagonists [110, 175]. In particular, NMDA receptor antagonists induce significant collateral sprouting, whilst non-NMDA glutamate antagonism prevents any induction of a sprouting response. The mechanisms are unknown but may depend more on modulation of neurotrophic factor signaling than excitatory activity, since they are not affected by profound sodium channel inhibition. Extensive fiber growth following injury, as described for the mossy fiber pathway above, suggests that outgrowth inhibitory factors may not play a major growth repulsive role in organotypic culture. However, it will be important to establish if in vitro responses to physical and chemical stimulation, including up regulation of inhibitory factors, are mounted in the same qualitative and quantitative manner as they appear in vivo. Elucidation of these differences will be critical in translating the authenticity of ex vivo and in vitro studies.
III. EVALUATION OF INDICATORS OF NEURONAL FUNCTION
i. Introduction
Following the various morphological indicators of neuronal survival and outgrowth as discussed in the previous sections, it remains important to address the functional status of the surviving tissue. The best indicator of functioning neuronal tissue is generally considered to be electrophysiological measurement of synaptic activity, although other endpoints such as Ca2+ indicator dyes [107, 136, 165, 168], Cl- sensitive dyes [63], intrinsic light transmittance or cell volume [4], ion sensitive electrodes [148], and microdialysis [138] have been used in some studies. The following discussion will focus on the various electrophysiological measures of neuronal function in slices.
Electrical recordings of synaptic efficacy and plasticity have been used for many years to measure neuronal function in slices. Standard measures of synapse function include determining input-output curves (synapse strength), paired-pulse behavior (pre- and post-synaptic contributions) and long-term potentiation (LTP) and long-term depression (LTD) of the synapses under study. Hippocampal slices are the most common preparation, although cortico-striatal [29, 30], spinal cord LTP [133, 163, 177], and spinal cord LTD [36, 144] have also been used. In acute slices, extracellular stimulating and recording electrodes can be placed in any area containing high densities of pre- and post-synaptic neurons. In this way, the summed activity from many cells can be recorded simultaneously. Recordings of single cells can also be recorded using either intracellular or patch-clamp techniques, thus allowing more experimental control of the system. This latter technique is generally utilized for recording in organotypic slices, as field (population) potentials are more difficult to detect in these preparations. These standard methods of recording synaptic efficacy and plasticity have been reviewed elsewhere [99, 143, 180], and thus comprehensive review of these subjects will not be covered here. Instead, the application of these techniques to neurodegenerative disorders will be discussed.
ii. Alzheimer’s Disease
Hippocampal slices offer a well-validated model for studying the impact of chronic neurodegenerative mechanisms on synaptic transmission and plasticity. A variety of different mouse models of Alzheimer’s disease have been generated, many of which are designed to alter the levels of Aβ. Hippocampal slices have been analyzed from many of these mice, and most studies have shown that synaptic transmission and/or long-term potentiation (LTP) are deficient when compared to wild-type littermate controls [19, 34, 59, 77, 91]. These deficits often worsen with the age of the mice, and in some cases have been demonstrated to precede amyloid plaque formation [77]. In a few cases, these alterations have been described to occur from changes in synaptic or dendritic structure [79, 178], as few of the mouse models display neurodegeneration. It is also becoming more widely accepted, however, that Aβ oligomers directly impair synaptic function, as Aβ oligomer application directly onto slices from wild-type animals can impair LTP [169, 172, 174] and facilitate the induction of LTD [85], presumably mimicking the acute effects of such peptides in vivo. Recent work [94] has described the extraction of a 56 kD Aβ from Tg2576 mice that when applied to slices can inhibit LTP. This protein is apparently a dodecamer of Aβ, possibly via formation of 4 trimer molecules.
Impaired LTP or synaptic transmission in some mouse models of AD can be reversed by pharmacological inhibition of gamma-secretase [73, 172], one of the enzymes responsible for producing Aβ. In addition, rescue of the synaptic deficits in these animals has been reversed by crossing the AD mouse line being studied with knock-out strains of either presenilin 1 (a required protein for gamma-secretase) or BACE1, the other enzyme responsible for cleaving the parent amyloid precursor protein into Aβ [48, 121]. In addition, many other mechanisms have been demonstrated to enhance synaptic transmission and/or plasticity in models showing such deficits, thus leading to multiple hypotheses regarding potential cognitive enhancers in AD [40, 67, 118, 169].
iii. Anoxia/Oxygen-Glucose Deprivation (OGD)
The presence of synaptically evoked population spikes is often utilized as a functional indicator of neuronal viability, as this requires survival of both the pre- and post-synaptic cells and their synaptic connections [7, 50, 98, 149]. Cell body survival alone may not result in functional tissue, as axons and synaptic transmission must also be spared to score as functional using this criteria [157]. Deprivations that injure tissue result in a decreased number of slices that are able to generate a population spike or a decrease in the amplitude of a population spike from an individual slice. Previously [7], glutamate receptor antagonists (NMDA and/or non-NMDA) have been shown to be neuroprotective in this model regardless of the readout, thus indicating its similarity to other models of ischemia.
Most commonly, acutely isolated, adult tissue is used for these studies, which allows one to avoid the developmental differences often inherent when using cultured tissue from young/newborn animals. Another advantage to using acute, non-cultured slices is that the signals recorded extracellularly are large, as the tissue retains its denser and thicker characteristics compared to cultured slices. This does, however, limit outcome measures to short term readouts (hours, not days), which lends itself more to the study of excitotoxic mechanisms than to apoptotic ones.
In most studies, hippocampus, corticostriatum [28, 124] spinal cord [112, 164] or other brain tissue is chopped or vibratomed into ∼400 μm thick slices, which are then transferred into either a Haas-type interface recording chamber or a submerged chamber. In the hippocampus, electrophysiology is typically done using a bipolar stimulating electrode on the surface of the Schaffer collaterals, mossy fibers or perforant path, and recording from area CA1, CA3 or dentate gyrus, respectively, is performed. Data is taken from one or more slices at a time, and anoxia/ischemia or excitotoxins are applied to the slices through the recording chamber solution and atmosphere. Alternatively, many (up to 10) slices are placed into two recording chambers, which are run in parallel. Each slice is tested for viability by examining evoked population spikes (EPSPs), after which ischemic agents are applied through the recording chamber. After a recovery period (typically 60-90 min after the insult), each previously–viable slice is again tested for viability. Experimental data are then expressed as the change in amplitude of the individual responses or the percentage of slices which remained viable, respectively.
Hippocampal slices can be subjected to various metabolic stresses [3, 7, 50, 107, 149]. Anoxic insults are usually produced by switching the gas mixture arriving at the recording chamber from 95:5 O2:CO2 to 95:5 N2:CO2 for a period up to 30 minutes. In vitro oxygen-glucose deprivation (OGD) is defined as the exposure of slices to simultaneous anoxic and aglycemic conditions. In some experiments, slices are exposed to a high (∼10 mM) concentration of glutamate, NMDA or other excitatory neurotransmitter in the ACSF. Glutamate concentrations utilized are higher than that occurring in ischemia, which clearly indicates that the glutamate uptake mechanisms in slices are functioning under these conditions and that this activity helps maintain viable neurons when exposed to glutamate (but not for ligands such as NMDA which lack an active uptake mechanism). In these types of experiments, potential neuroprotective agents can be included in the ACSF before, during and/or after an insult. The timing of inhibitor application in relation to the metabolic stressor/insult may affect the neuroprotective ability of some classes of compounds, reflecting the state of ionic gradients in the tissue.
A variety of different electrophysiological end points have been utilized. Many studies examine population spikes (action potentials) or field EPSPs, as these represent the aggregate activity of many neurons/synapses. Thus, if damage results in a partial survival of the tissue (pre- or postsynaptically), this method will detect a smaller response [9, 119, 149]. Conversely, more detailed information from individual neurons may be obtained by patch-clamp recording with the resulting excitatory postsynaptic potentials or currents (EPSPs/EPSCs) being the endpoint. Additionally, spontaneous synaptic activity has been recorded by many investigators, but this has found limited utility in acute slice experiments due to a low level of unstimulated activity in control tissue, high variability, and ‘crude’ quantification (spike and burst counts) that is often needed [6, 8, 51, 102, 103]. Several studies of synaptic transmission in vitro and in vivo have found alterations (reductions) after ischemic events, and this is speculated to serve some protective function for the brain [5, 64, 183]. One report of increased activity after an OGD event, however, was attributed to a potentiation-like phenomenon occurring in the slices [31], presumably due to a large release of glutamate during the event. This does not, however, appear to be a general phenomenon that has applicability to most slice models.
The hippocampal slice is a model system in which the study of the mechanisms underlying ischemic damage can be investigated in a controlled manner. In most studies [7, 139, 161] a severe insult is applied, which results in an average control slice survival of <10%. This severe damage is caused by large amounts of glutamate release, as the tissue can be fully protected by applying a combination of NMDA (MK-801) and AMPA (GYKI) antagonists, as has been shown in a variety of different studies (reviewed by [43, 145]). If more moderate insults are delivered, moderate damage can result, which is blocked fully by MK-801 alone. The level of damage occurring in slices can be adjusted easily by altering the duration of the anoxia/ischemia used and the temperature of the slice chamber. This glutamate release is likely through both neuronal and glial sources, with the exact fraction differing depending on the exact circumstances of the experiments.
Several studies have indicated that glutamate can rise to neurotoxic levels during severe ischemic episodes by way of reverse uptake through the glial glutamate transporter EAAT2 [139, 161]. One could conclude that blocking this reversed uptake might be neuroprotective, but this has not been demonstrated as of yet. In contrast, gene deletion or antisense knockdown of EAAT2 has been shown to exacerbate damage induced by transient focal ischemia in rodents, [114, 134]. In addition, dysfunction of high affinity glutamate transporters, either experimentally or via natural mutation, has been linked to a number of neurodegenerative conditions, including amyotrophic lateral sclerosis (ALS), epilepsy and retinal degeneration (reviewed by [52]).
A moderate anoxic or OGD insult when applied to slices is an attempt to model moderate/penumbral ischemic insults that can occur in vivo. In contrast to the ischemic core, which is probably modeled better by the severe insult method, surrounding brain areas with continued partial blood flow (termed the penumbra) are less seriously affected, and thus suffer significantly less cellular damage than in the ischemic core. As might be predicted, less glutamate is released into the extracellular space in the penumbra [68, 125]. The electrochemical gradients in the penumbra are not as severely disrupted as in the core, and therefore glutamate is not significantly released through reversed operation of the glial glutamate transporters. Instead, most of the glutamate emanates from neuronal sources within the penumbra [68]. This version of the anoxic/OGD slice model should be able to identify more subtle mechanisms of glutamate release that could be important therapeutically.
Most experimental protocols involve exposing slices to anoxic or OGD periods. Mechanistic differences between anoxic and OGD conditions have been reported to result in differing activity of neuroprotective agents [157]. Anoxia has been shown to cause an ‘anoxic depolarization’ loss of function, increase in intracellular Ca2+ and subsequent death of cells in an NMDA independent manner [3, 154]. In contrast, OGD induces glutamate release, Ca2+ influx, and subsequent cell death via well described processes [49, 160].
Although we will not extensively review the literature here, a few studies have used biochemical measures of cell death in slices as an endpoints [18, 37, 75, 82, 135]. These studies usually seek to use methods of detecting apoptosis or other cell death features used in dissociated cell cultures and apply them to hippocampal slices. Due to the difficulties of imaging in thick acute slices, most work has been performed in organotypic slice cultures where imaging is technically more feasible.
Previous studies have indicated that synaptic responses in regions sensitive to ischemia (like CA1) can potentiate after an ischemic insult in a long-term potentiation-like (iLTP) phenomenon [31, 32]. In striatal spiny neurons/corticostriatal synapses, iLTP is NMDAr, mGluR, Ca2+ influx and MAP-kinase dependent, similar to that of traditionally stimulated LTP. Striatal cholinergic interneurons, however, are resistant to ischemia and iLTP, but do undergo stimulated LTP. Similarly, anoxic LTP has been demonstrated to occur in area CA1, where it is AMPAr/NMDAr mediated, like stimulated LTP [78]. Both forms of LTP exhibit NMDAr upregulation, Ca2+ influx dependence, decreased paired pulse facilitation, and demonstrate occlusion with each other. Thus, the presence of ischemia/anoxia induced LTP may be a surrogate for neurons sensitive to these types of insults.
iv. Parkinson’s Disease
Neurodegeneration in striatal dopaminergic neurons is a well known cause of the in vivo effects brought about in Parkinson’s disease. This neurotoxicity has been reproduced in striatal slice models (acute and organotypic slices) using L-DOPA, MPP+, rotenone and paraquat, although physiological endpoints have generally not been used [25, 65, 151, 152]. One interesting recent exception to this, however, is the demonstration that L-DOPA-treated animals which develop involuntary movements (resembling human dyskinesias) have a selective impairment in LTD [126]. This is in contrast to control animals and to L-DOPA treated animals that did not develop involuntary movements. Both LTP and LTD are key components of synaptic plasticity in the corticostriatal system, and thus loss of part of this system will have obvious negative consequences. The details of the physiological affects of dopamine and its receptors in striatum has been nicely reviewed by the major authors contributing to this field, and so will not be reiterated here [127].
CONCLUSION
Growing numbers of studies have been conducted over the past two decades on the development and optimization of various acute and organotypic brain slice models, which has led to the implementation of validated platforms for studies of both normal and diseased brain functions. Hitherto successful applications of neural slice cultures span widely from the developmental studies of neuronal architecture and synaptic circuitry to the pathological modeling of stroke, epilepsy, AD, Parkinson’s and Huntington’s diseases. In addition to being an in vitro system with a number of advantages over animal models, such as easy access and precise control of the extracellular environment, the slice platform has become even more powerful with technical advancements in high-quality imaging and the direct translation of neuronal viability to its functional outcome. The functional endpoints of synaptic activities and plasticity in the acute and cultured slices make possible measurements of both short and long-term alterations in synaptic efficacy with reasonable throughput, relative to animal models. The endpoints measured in slices are potentially directly applicable to brain function, as the cellular contacts and architecture are largely intact, especially in the acute slices. An additional dimension available with brain slices is the ability to prepare slices from normal, drug treated, and/or genetically modified animals, plus allowing treatment of the tissue with additional agents once its taken in vitro. The combination of these treatments creates an extremely flexible and powerful suite of techniques for a variety of research applications. A recent study using GFP-expressing embryonic stem cells further highlights the versatility of long-term neural slice cultures, in which a therapeutic potential of cellular replacement strategies was tested in the nigro-striatal slices [83]. Tissue-specific migration, phenotypic differentiation and synaptic incorporation of embryonic neuronal precursor cells onto mature neurons within engrafted slices make this in vitro brain system highly valuable in testing the feasibility of cell transplantation approaches and the functional outcome of circuitry reconstruction in three dimensional brain tissues.
While tackling the challenges of identifying effective and tractable drug targets, development of novel therapeutics for many neurological diseases relies on the ability to test candidate reagents in the most contextual and surrogate models of human diseases. Though brain has naturally been an exceptionally difficult organ to mimic in isolation, the advancements in brain slices and its accompanying technological innovations has provided a tremendous potential to address questions with a speed that previously could not have been achieved. Further increases in our understanding of this field, along with the continual emergence of studies successfully translating results from in vitro to in vivo, will have great value in defining additional molecular mechanisms of neurodegeneration and regeneration as well as pharmacological interventions by novel therapies.
REFERENCES
- 1.Acarin L, Gonzalez B, Castellano B, Castro AJ. Quantitative analysis of microglial reaction to a cortical excitotoxic lesion in the early postnatal brain. Exp Neurol. 1997;147:410–417. doi: 10.1006/exnr.1997.6593. [DOI] [PubMed] [Google Scholar]
- 2.Adcock KH, Metzger F, Kapfhammer JP. Purkinje cell dendritic tree development in the absence of excitatory neurotransmission and of brain-derived neurotrophic factor in organotypic slice cultures. Neuroscience. 2004;127:137–145. doi: 10.1016/j.neuroscience.2004.04.032. [DOI] [PubMed] [Google Scholar]
- 3.Aitken PG, Balestrino M, Somjen GG. NMDA antagonists: lack of protective effect against hypoxic damage in CA1 region of hippocampal slices. Neurosci Lett. 1988;89:187–192. doi: 10.1016/0304-3940(88)90379-5. [DOI] [PubMed] [Google Scholar]
- 4.Andrew RD, MacVicar BA. Imaging cell volume changes and neuronal excitation in the hippocampal slice. Neuroscience. 1994;62:371–383. doi: 10.1016/0306-4522(94)90372-7. [DOI] [PubMed] [Google Scholar]
- 5.Aoyagi A, Saito H, Abe K, Nishiyama N. Early impairment and late recovery of synaptic transmission in the rat dentate gyrus following transient forebrain ischemia in vivo. Brain Res. 1998;799:130–137. doi: 10.1016/s0006-8993(98)00465-x. [DOI] [PubMed] [Google Scholar]
- 6.Arias RL, Bowlby MR. Pharmacological characterization of antiepileptic drugs and experimental analgesics on low magnesium-induced hyperexcitability in rat hippocampal slices. Brain Res. 2005;1047:233–244. doi: 10.1016/j.brainres.2005.04.052. [DOI] [PubMed] [Google Scholar]
- 7.Arias RL, Tasse JR, Bowlby MR. Neuroprotective interaction effects of NMDA and AMPA receptor antagonists in an in vitro model of cerebral ischemia. Brain Res. 1999;816:299–308. doi: 10.1016/s0006-8993(98)01051-8. [DOI] [PubMed] [Google Scholar]
- 8.Armand V, Rundfeldt C, Heinemann U. Effects of retigabine (D-23129) on different patterns of epileptiform activity induced by low magnesium in rat entorhinal cortex hippocampal slices. Epilepsia. 2000;41:28–33. doi: 10.1111/j.1528-1157.2000.tb01501.x. [DOI] [PubMed] [Google Scholar]
- 9.Bahr BA. Long-term hippocampal slices: a model system for investigating synaptic mechanisms and pathologic processes. J Neurosci Res. 1995;42:294–305. doi: 10.1002/jnr.490420303. [DOI] [PubMed] [Google Scholar]
- 10.Baker RE, Dijkhuizen PA, Van Pelt J, Verhaagen J. Growth of pyramidal, but not non-pyramidal, dendrites in long-term organotypic explants of neonatal rat neocortex chronically exposed to neurotrophin-3. Eur J Neurosci. 1998;10:1037–1044. doi: 10.1046/j.1460-9568.1998.00118.x. [DOI] [PubMed] [Google Scholar]
- 11.Baratta J, Marienhagen JWA, Ha D, Yu J, Robertson RT. Cholinergic innervation of cerebral cortex in organotypic slice cultures: Sustained basal forebrain and transient striatal cholinergic projections. Neuroscience. 1996;72:1117–1132. doi: 10.1016/0306-4522(95)00603-6. [DOI] [PubMed] [Google Scholar]
- 12.Beck M, Flachenecker P, Magnus T, Giess R, Reiners K, Toyka KV, Naumann M. Autonomic dysfunction in ALS: a preliminary study on the effects of intrathecal BDNF. Amyotroph Lateral Scler Other Motor Neuron Disord. 2005;6:100–103. doi: 10.1080/14660820510028412. [DOI] [PubMed] [Google Scholar]
- 13.Behnisch T, Francesconi W, Sanna PP. HIV secreted protein Tat prevents long-term potentiation in the hippocampal CA1 region. Brain Res. 2004;1012:187–189. doi: 10.1016/j.brainres.2004.03.037. [DOI] [PubMed] [Google Scholar]
- 14.Bergold PJ, Casaccia-Bonnefil P. Preparation of organotypic hippocampal slice cultures using the membrane filter method. Methods Mol Biol. 1997;72:15–22. doi: 10.1385/0-89603-394-5:15. [DOI] [PubMed] [Google Scholar]
- 15.Birgbauer E, Rao TS, Webb M. Lysolecithin induces demyelination in vitro in a cerebellar slice culture system. J Neurosci Res. 2004;78:157–166. doi: 10.1002/jnr.20248. [DOI] [PubMed] [Google Scholar]
- 16.Blackstone CD, Moss SJ, Martin LJ, Levey AI, Price DL, Huganir RL. Biochemical characterization and localization of a non-N-methyl-D-aspartate glutamate receptor in rat brain. J Neurochem. 1992;58:1118–1126. doi: 10.1111/j.1471-4159.1992.tb09370.x. [DOI] [PubMed] [Google Scholar]
- 17.Blobel CP. ADAMs: key components in EGFR signalling and development. Nat. Rev. Mol. Cell Biol. 2005;6:32–43. doi: 10.1038/nrm1548. [DOI] [PubMed] [Google Scholar]
- 18.Bonde C, Noraberg J, Zimmer J. Nuclear shrinkage and other markers of neuronal cell death after oxygen-glucose deprivation in rat hippocampal slice cultures. Neurosci Lett. 2002;327:49–52. doi: 10.1016/s0304-3940(02)00382-8. [DOI] [PubMed] [Google Scholar]
- 19.Brown JT, Richardson JC, Collingridge GL, Randall AD, Davies CH. Synaptic transmission and synchronous activity is disrupted in hippocampal slices taken from aged TAS10 mice. Hippocampus. 2005;15:110–117. doi: 10.1002/hipo.20036. [DOI] [PubMed] [Google Scholar]
- 20.Bruce AJ, Malfroy B, Baudry M. Beta -Amyloid toxicity in organotypic hippocampal cultures: Protection by EUK-8, a synthetic catalytic free radical scavenger. Proc. Natl. Acad. Sci. USA. 1996;93:2312–2316. doi: 10.1073/pnas.93.6.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bruce AJ, Sakhi S, Schreiber SS, Baudry M. Development of kainic acid and N-methyl-D-aspartic acid toxicity in organotypic hippocampal cultures. Exp Neurol. 1995;132:209–219. doi: 10.1016/0014-4886(95)90026-8. [DOI] [PubMed] [Google Scholar]
- 22.Buchli AD, Schwab ME. Inhibition of Nogo: a key strategy to increase regeneration, plasticity and functional recovery of the lesioned central nervous system. Ann Med. 2005;37:556–567. doi: 10.1080/07853890500407520. [DOI] [PubMed] [Google Scholar]
- 23.Buchs PA, Stoppini L, Muller D. Structural modifications associated with synaptic development in area CA1 of rat hippocampal organotypic cultures. Brain Res. Dev. Brain Res. 1993;71:81–91. doi: 10.1016/0165-3806(93)90108-m. [DOI] [PubMed] [Google Scholar]
- 24.Butler CD, Schnetz SA, Yu EY, Davis JB, Temple K, Silver J, Malouf AT. Keratan sulfate proteoglycan phosphacan regulates mossy fiber outgrowth and regeneration. J Neurosci. 2004;24:462–473. doi: 10.1523/JNEUROSCI.3040-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bywood PT, Johnson SM. Mitochondrial complex inhibitors preferentially damage substantia nigra dopamine neurons in rat brain slices. Exp Neurol. 2003;179:47–59. doi: 10.1006/exnr.2002.8044. [DOI] [PubMed] [Google Scholar]
- 26.Cafferty WBJ, Gardiner NJ, Das P, Qiu J, McMahon SB, Thompson SWN. Conditioning injury-induced spinal axon regeneration fails in interleukin-6 knock-out mice. J Neurosci. 2004;24:4432–4443. doi: 10.1523/JNEUROSCI.2245-02.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caille I, Allinquant B, Dupont E, Bouillot C, Langer A, Muller U, Prochiantz A. Soluble form of amyloid precursor protein regulates proliferation of progenitors in the adult sub-ventricular zone. Development. 2004;131:2173–2181. doi: 10.1242/dev.01103. [DOI] [PubMed] [Google Scholar]
- 28.Calabresi P, Ascone CM, Centonze D, Pisani A, Sancesario G, D’Angelo V, Bernardi G. Opposite membrane potential changes induced by glucose deprivation in striatal spiny neurons and in large aspiny interneurons. J Neurosci. 1997;17:1940–1949. doi: 10.1523/JNEUROSCI.17-06-01940.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Calabresi P, Mercuri NB, Bernardi G. Synaptic and intrinsic control of membrane excitability of neostriatal neurons. II. An in vitro analysis. J Neurophysiol. 1990;63:663–675. doi: 10.1152/jn.1990.63.4.663. [DOI] [PubMed] [Google Scholar]
- 30.Calabresi P, Mercuri NB, De Murtas M, Bernardi G. Involvement of GABA system in the feedback regulation of glutamate- and GABA-mediated synaptic potentials in rat neostriatum. J. Physiol. (Lond.) 1991;440:581–599. doi: 10.1113/jphysiol.1991.sp018726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calabresi P, Saulle E, Centonze D, Pisani A, Marfia GA, Bernardi G. Post-ischaemic long-term synaptic potentiation in the striatum: a putative mechanism for cell type-specific vulnerability. Brain. 2002;125:844–860. doi: 10.1093/brain/awf073. [DOI] [PubMed] [Google Scholar]
- 32.Calabresi P, Saulle E, Marfia GA, Centonze D, Mulloy R, Picconi B, Hipskind RA, Conquet F, Bernardi G. Activation of metabotropic glutamate receptor subtype 1/protein kinase C/mitogen-activated protein kinase pathway is required for postischemic long-term potentiation in the striatum. Mol Pharmacol. 2001;60:808–815. [PubMed] [Google Scholar]
- 33.Cavaliere F, Dinkel K, Reymann K. The subventricular zone releases factors which can be protective in oxygen/glucose deprivation-induced cortical damage: an organotypic study. Exp Neurol. 2006;7201:66–74. doi: 10.1016/j.expneurol.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 34.Chapman PF, White GL, Jones MW, Cooper-Blacketer D, Marshall VJ, Irizarry M, Younkin L, Good MA, Bliss TVP, Hyman BT, Younkin SG, Hsiao KK. Impaired synaptic plasticity and learning in aged amyloid precursor protein transgenic mice. Nat Neurosci. 1999;2:271–276. doi: 10.1038/6374. [DOI] [PubMed] [Google Scholar]
- 35.Chechneva O, Dinkel K, Schrader D, Reymann KG. Identification and characterization of two neurogenic zones in interface organotypic hippocampal slice cultures. Neuroscience. 2005;136:343–355. doi: 10.1016/j.neuroscience.2005.07.058. [DOI] [PubMed] [Google Scholar]
- 36.Chen J, Sandkuhler J. Induction of homosynaptic long-term depression at spinal synapses of sensory a delta-fibers requires activation of metabotropic glutamate receptors. Neuroscience. 2000;98:141–148. doi: 10.1016/s0306-4522(00)00080-4. [DOI] [PubMed] [Google Scholar]
- 37.Cho S, Liu D, Fairman D, Li P, Jenkins L, McGonigle P, Wood A. Spatiotemporal evidence of apoptosis-mediated ischemic injury in organotypic hippocampal slice cultures. Neurochem Int. 2004;45:117–127. doi: 10.1016/j.neuint.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 38.Cicinelli P, Marconi B, Zaccagnini M, Pasqualetti P, Filippi MM, Rossini PM. Imagery-induced cortical excitability changes in stroke: a transcranial magnetic stimulation study. Cereb Cortex. 2006;16:247–253. doi: 10.1093/cercor/bhi103. [DOI] [PubMed] [Google Scholar]
- 39.Connelly CA, Chen LC, Colquhoun SD. Metabolic activity of cultured rat brainstem, hippocampal and spinal cord slices. J Neurosci Methods. 2000;99:1–7. doi: 10.1016/s0165-0270(00)00205-3. [DOI] [PubMed] [Google Scholar]
- 40.Cooke SF, Bliss TV. Long-term potentiation and cognitive drug discovery. Curr. Opin. Investig. Drugs. 2005;6:25–34. [PubMed] [Google Scholar]
- 41.Cooper O, Isacson O. Intrastriatal transforming growth factor alpha delivery to a model of Parkinson’s disease induces proliferation and migration of endogenous adult neural progenitor cells without differentiation into dopaminergic neurons. J Neurosci. 2004;24:8924–8931. doi: 10.1523/JNEUROSCI.2344-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Corse AM, Bilak MM, Bilak SR, Lehar M, Rothstein JD, Kuncl RW. Preclinical testing of neuroprotective neurotrophic factors in a model of chronic motor neuron degeneration. Neurobiol Dis. 1999;6:335–346. doi: 10.1006/nbdi.1999.0253. [DOI] [PubMed] [Google Scholar]
- 43.Crepel V, Congar P, Aniksztejn L, Gozlan H, Hammond C, Ben-Ari Y. Synaptic plasticity in ischemia: role of NMDA receptors. Prog. Brain Res. 1998;116:273–285. doi: 10.1016/s0079-6123(08)60443-4. [DOI] [PubMed] [Google Scholar]
- 44.Danzer SC, Crooks KRC, Lo DC, McNamara JO. Increased expression of brain-derived neurotrophic factor induces formation of basal dendrites and axonal branching in dentate granule cells in hippocampal explant cultures. J Neurosci. 2002;22:9754–9763. doi: 10.1523/JNEUROSCI.22-22-09754.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Simoni A, Griesinger CB, Edwards FA. Development of rat CA1 neurones in acute versus organotypic slices: role of experience in synaptic morphology and activity. J Physiol. 2003;550:135–147. doi: 10.1113/jphysiol.2003.039099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.del Rio JA, Heimrich B, Soriano E, Schwegler H, Frotscher M. Proliferation and differentiation of glial fibrillary acidic protein-immunoreactive glial cells in organotypic slice cultures of rat hippocampus. Neuroscience. 1991;43:335–347. doi: 10.1016/0306-4522(91)90298-3. [DOI] [PubMed] [Google Scholar]
- 47.del Rio JA, Sole M, Borrell V, Martinez A, Soriano E. Involvement of Cajal-Retzius cells in robust and layer-specific regeneration of the entorhino-hippocampal pathways. Eur J Neurosci. 2002;15:1881–1890. doi: 10.1046/j.1460-9568.2002.02027.x. [DOI] [PubMed] [Google Scholar]
- 48.Dewachter I, Reverse D, Caluwaerts N, Ris L, Kuipe C, Van den Haute C, Spittaels K, Umans L, Serneels L, Thiry E, Moechars D, Mercken M, Godaux E, Van Leuven F. Neuronal deficiency of presenilin 1 inhibits amyloid plaque formation and corrects hippocampal long-term potentiation but not a cognitive defect of amyloid precursor protein [v717i] transgenic mice. J Neurosci. 2002;22:3445–3453. doi: 10.1523/JNEUROSCI.22-09-03445.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- 50.Dong W.-Q, Schurr A, Reid KH, Shields CB, West CA. The rat hippocampal slice preparation as an in vitro model of ischemia. Stroke. 1988;19:498–502. doi: 10.1161/01.str.19.4.498. [DOI] [PubMed] [Google Scholar]
- 51.Dost R, Rundfeldt C. The anticonvulsant retigabine potently suppresses epileptiform discharges in the low Ca++ and low Mg++ model in the hippocampal slice preparation. Epilepsy Res. 2000;38:53–66. doi: 10.1016/s0920-1211(99)00065-0. [DOI] [PubMed] [Google Scholar]
- 52.Dunlop J, Zaleska MM, Eliasof S, Moyer JA. Excitatory amino acid transporters as emerging targets for central nervous system therapeutics. Emerg Therapeut Targ. 1999;3:1–28. [Google Scholar]
- 53.Eliason DA, Cohen SA, Baratta J, Yu J, Robertson RT. Local proliferation of microglia cells in response to neocortical injury in vitro. Brain Res. Dev. Brain Res. 2002;137:75–79. doi: 10.1016/s0165-3806(02)00413-3. [DOI] [PubMed] [Google Scholar]
- 54.Emsley JG, Mitchell BD, Kempermann G, Macklis JD. Adult neurogenesis and repair of the adult CNS with neural progenitors, precursors, and stem cells. Prog Neurobiol. 2005;75:321–341. doi: 10.1016/j.pneurobio.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 55.Eyupoglu IY, Savaskan NE, Brauer AU, Nitsch R, Heimrich B. Identification of neuronal cell death in a model of degeneration in the hippocampus. Brain Res. Brain Res. Protoc. 2003;11:1–8. doi: 10.1016/s1385-299x(02)00186-1. [DOI] [PubMed] [Google Scholar]
- 56.Fahlman CS, Bickler PE, Sullivan B, Gregory GA. Activation of the neuroprotective ERK signaling pathway by fructose-1,6-bisphosphate during hypoxia involves intracellular Ca2+ and phospholipase C. Brain Res. 2002;958:43–51. doi: 10.1016/s0006-8993(02)03433-9. [DOI] [PubMed] [Google Scholar]
- 57.Falk A, Frisen J. New neurons in old brains. Ann Med. 2005;37:480–486. doi: 10.1080/07853890500371890. [DOI] [PubMed] [Google Scholar]
- 58.Finley M, Fairman D, Liu D, Li P, Wood A, Cho S. Functional validation of adult hippocampal organotypic cultures as an in vitro model of brain injury. Brain Res. 2004;1001:125–132. doi: 10.1016/j.brainres.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 59.Fitzjohn SM, Morton RA, Kuenzi F, Rosahl TW, Shearman M, Lewis H, Smith D, Reynolds DS, Davies CH, Collingridge GL, Seabrook GR. Age-related impairment of synaptic transmission but normal long-term potentiation in transgenic mice that overexpress the human app695swe mutant form of amyloid precursor protein. J Neurosci. 2001;21:4691–4696. doi: 10.1523/JNEUROSCI.21-13-04691.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Franke H, Schelhorn N, Illes P. Dopaminergic neurons develop axonal projections to their target areas in organotypic cocultures of the ventral mesencephalon and the striatum/prefrontal cortex. Neurochem Int. 2003;42:431–439. doi: 10.1016/s0197-0186(02)00134-1. [DOI] [PubMed] [Google Scholar]
- 61.Frautschy SA, Yang F, Calderon L, Cole GM. Rodent models of Alzheimer’s disease: rat A beta infusion approaches to amyloid deposits. Neurobiol Aging. 1996;17:311–321. doi: 10.1016/0197-4580(95)02073-x. [DOI] [PubMed] [Google Scholar]
- 62.Gahwiler BH. Organotypic monolayer cultures of nervous tissue. J Neurosci Methods. 1981;4:329–342. doi: 10.1016/0165-0270(81)90003-0. [DOI] [PubMed] [Google Scholar]
- 63.Galeffi F, Sah R, Pond BB, George A, Schwartz-Bloom RD. Changes in intracellular chloride after oxygen-glucose deprivation of the adult hippocampal slice: effect of diazepam. J Neurosci. 2004;24:4478–4488. doi: 10.1523/JNEUROSCI.0755-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gao TM, Howard EM, Xu ZC. Transient neurophysiological changes in CA3 neurons and dentate granule cells after severe forebrain ischemia in vivo. J Neurophysiol. 1998;80:2860–2869. doi: 10.1152/jn.1998.80.6.2860. [DOI] [PubMed] [Google Scholar]
- 65.Gille G, Hung ST, Reichmann H, Rausch WD. Oxidative stress to dopaminergic neurons as models of Parkinson’s disease. Ann. N. Y. Acad. Sci. 2004;1018:533–540. doi: 10.1196/annals.1296.066. [DOI] [PubMed] [Google Scholar]
- 66.Gilliams-Francis KL, Quaye AA, Naegele JR. PARP cleavage, DNA fragmentation, and pyknosis during excitotoxininduced neuronal death. Exp Neurol. 2003;184:359–372. doi: 10.1016/j.expneurol.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 67.Gong B, Vitolo OV, Trinchese F, Liu S, Shelanski M, Arancio O. Persistent improvement in synaptic and cognitive functions in an Alzheimer mouse model after rolipram treatment. J Clin Investig. 2004;114:1624–1634. doi: 10.1172/JCI22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gonzales C, Zaleska MM, Djali S, Heyburn JW, Dawson LA. Differences in glutamate transporter GLT-1 function in the ischemic core vs. penumbral zones during transient middle cerebral artery occlusion. Soc Neurosci. 2001:2307. Abst. [Google Scholar]
- 69.Hagg T. Molecular regulation of adult CNS neurogenesis: an integrated view. Trends Neurosci. 2005;28:589–595. doi: 10.1016/j.tins.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 70.Hand R, Bortone D, Mattar P, Nguyen L, Heng JI, Guerrier S, Boutt E, Peters E, Barnes AP, Parras C, Schuurmans C, Guillemot F, Polleux F. Phosphorylation of Neurogenin2 specifies the migration properties and the dendritic morphology of pyramidal neurons in the neocortex. Neuron. 2005;48:45–62. doi: 10.1016/j.neuron.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 71.Harris-White ME, Balverde Z, Lim GP, Kim P, Miller SA, Hammer H, Galasko D, Frautschy SA. Role of LRP in TGFbeta2-mediated neuronal uptake of Abeta and effects on memory. J Neurosci Res. 2004;77:217–228. doi: 10.1002/jnr.20149. [DOI] [PubMed] [Google Scholar]
- 72.Harris-White ME, Chu T, Balverde Z, Sigel JJ, Flanders KC, Frautschy SA. Effects of transforming growth factor-beta (isoforms 1-3) on amyloid-beta deposition, inflammation, and cell targeting in organotypic hippocampal slice cultures. J Neurosci. 1998;18:10366–10374. doi: 10.1523/JNEUROSCI.18-24-10366.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hartman RE, Izumi Y, Bales KR, Paul SM, Wozniak DF, Holtzman DM. Treatment with an amyloid-β antibody ameliorates plaque load, learning deficits, and hippocampal long-term potentiation in a mouse model of alzheimer’s disease. J Neurosci. 2005;25:6213–6220. doi: 10.1523/JNEUROSCI.0664-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hashimoto K, Shimizu E, Iyo M. Critical role of brain-derived neurotrophic factor in mood disorders. Brain Res. Brain Res. Rev. 2004;45:104–114. doi: 10.1016/j.brainresrev.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 75.Haydar TF, Bambrick LL, Krueger BK, Rakic P. Organotypic slice cultures for analysis of proliferation, cell death, and migration in the embryonic neocortex. Brain Res. Brain Res. Protoc. 1999;4:425–437. doi: 10.1016/s1385-299x(99)00033-1. [DOI] [PubMed] [Google Scholar]
- 76.Hechler D, Nitsch R, Hendrix S. Green-fluorescentprotein-expressing mice as models for the study of axonal growth and regeneration in vitro. Brain Res. Brain Res. Rev. 2006;52:160–169. doi: 10.1016/j.brainresrev.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 77.Hsia AY, Masliah E, McConlogue L, Yu G, Tatsuno G, Hu K, Kholodenko D, Malenka RC, Nicoll RA, Mucke L. Plaque-independent disruption of neural circuits in Alzheimer’s disease mouse models. Proc. Natl. Acad. Sci. USA. 1999;96:3228–3233. doi: 10.1073/pnas.96.6.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hsu K.-S, Huang C.-C. Characterization of the anoxia-induced long-term synaptic potentiation in area CA1 of the rat hippocampus. Br J Pharmacol. 1997;122:671–681. doi: 10.1038/sj.bjp.0701409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jacobsen JS, Wu C.-C, Redwine JM, Comery TA, Arias R, Bowlby M, Martone R, Morrison JH, Pangalos MN, Reinhart PH, Bloom FE. Early-onset behavioral and synaptic deficits in a mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA. 2006;103:5161–5166. doi: 10.1073/pnas.0600948103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jung YJ, Park SJ, Park JS, Lee KE. Glucose/oxygen deprivation induces the alteration of synapsin I and phosphosynapsin. Brain Res. 2004;996:47–54. doi: 10.1016/j.brainres.2003.09.069. [DOI] [PubMed] [Google Scholar]
- 81.Kametsu Y, Osuga S, Hakim AM. Apoptosis occurs in the penumbra zone during short-duration focal ischemia in the rat. J. Cereb. Blood Flow Metab. 2003;23:416–422. doi: 10.1097/01.WCB.0000052281.23292.7C. [DOI] [PubMed] [Google Scholar]
- 82.Kasischke K, Buchner M, Ludolph AC, Riepe MW. Nuclear shrinkage in live mouse hippocampal slices. Acta Neuropathol. 2001;101:483–490. doi: 10.1007/s004010000317. [DOI] [PubMed] [Google Scholar]
- 83.Kearns SM, Scheffler B, Goetz AK, Lin DD, Baker HD, Roper SN, Mandel RJ, Steindler DA. A method for a more complete in vitro Parkinson’s model: Slice culture bioassay for modeling maintenance and repair of the nigrostriatal circuit. J Neurosci Methods. 2006;157:1–9. doi: 10.1016/j.jneumeth.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 84.Kempermann G. Why new neurons? Possible functions for adult hippocampal neurogenesis. J Neurosci. 2002;22:635–638. doi: 10.1523/JNEUROSCI.22-03-00635.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kim J.-H, Anwyl R, Suh Y.-H, Djamgoz MBA, Rowan MJ. Use-dependent effects of amyloidogenic fragments of β-amyloid precursor protein on synaptic plasticity in rat hippocampus in vivo. J Neurosci. 2001;21:1327–1333. doi: 10.1523/JNEUROSCI.21-04-01327.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Koh JY, Choi DW. Quantitative determination of glutamate mediated cortical neuronal injury in cell culture by lactate dehydrogenase efflux assay. J Neurosci Methods. 1987;20:83–90. doi: 10.1016/0165-0270(87)90041-0. [DOI] [PubMed] [Google Scholar]
- 87.Krassioukov AV, Ackery A, Schwartz G, Adamchik Y, Liu Y, Fehlings MG. An in vitro model of neurotrauma in organotypic spinal cord cultures from adult mice. Brain Res. Brain Res. Protoc. 2002;10:60–68. doi: 10.1016/s1385-299x(02)00180-0. [DOI] [PubMed] [Google Scholar]
- 88.Kristensen BW, Noraberg J, Jakobsen B, Gramsbergen JB, Ebert B, Zimmer J. Excitotoxic effects of non-NMDA receptor agonists in organotypic corticostriatal slice cultures. Brain Res. 1999;841:143–159. doi: 10.1016/s0006-8993(99)01833-8. [DOI] [PubMed] [Google Scholar]
- 89.Laake JH, Haug FM, Wieloch T, Ottersen OP. A simple in vitro model of ischemia based on hippocampal slice cultures and propidium iodide fluorescence. Brain Res. Brain Res. Protoc. 1999;4:173–184. doi: 10.1016/s1385-299x(99)00021-5. [DOI] [PubMed] [Google Scholar]
- 90.Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, Morgan TE, Rozovsky I, Trommer B, Viola KL, Wals P, Zhang C, Finch CE, Krafft GA, Klein WL. Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins. Proc. Natl. Acad. Sci. USA. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Larson J, Lynch G, Games D, Seubert P. Alterations in synaptic transmission and long-term potentiation in hippocampal slices from young and aged PDAPP mice. Brain Res. 1999;840:23–35. doi: 10.1016/s0006-8993(99)01698-4. [DOI] [PubMed] [Google Scholar]
- 92.Laskowski A, Schmidt W, Dinkel K, Martinez-Sanchez M, Reymann KG. bFGF and EGF modulate trauma-induced proliferation and neurogenesis in juvenile organotypic hippocampal slice cultures. Brain Res. 2005;1037:78–89. doi: 10.1016/j.brainres.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 93.Laywell ED, Kearns SM, Zheng T, Chen KA, Deng J, Chen HX, Roper SN, Steindler DA. Neuron-to-astrocyte transition: phenotypic fluidity and the formation of hybrid asterons in differentiating neurospheres. J Comp Neurol. 2005;493:321–333. doi: 10.1002/cne.20722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lesne S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, Gallagher M, Ashe KH. A specific amyloid-β protein assembly in the brain impairs memory. Nature. 2006;440:352–356. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- 95.Lichtenwalner RJ, Parent JM. Adult neurogenesis and the ischemic forebrain. J. Cereb. Blood Flow Metab. 2006;26:1–20. doi: 10.1038/sj.jcbfm.9600170. [DOI] [PubMed] [Google Scholar]
- 96.Lindner G, Grosse G. [Morphometric studies of the rat hippocampus after static and dynamic cultivation] Z. Mikrosk. Anat. Forsch. 1982;96:485–496. [PubMed] [Google Scholar]
- 97.Lippoldt A, Reichel A, Moenning U. Progress in the identification of stroke-related genes: emerging new possibilities to develop concepts in stroke therapy. CNS Drugs. 2005;19:821–832. doi: 10.2165/00023210-200519100-00002. [DOI] [PubMed] [Google Scholar]
- 98.Lipton P, Whittingham TS. Reduced ATP concentration as a basis for synaptic transmission failure during hypoxia in the in vitro guinea-pig hippocampus. J Physiol. 1982;325:51–65. doi: 10.1113/jphysiol.1982.sp014135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lisman J, Spruston N. Postsynaptic depolarization requirements for LTP and LTD: a critique of spike timing-dependent plasticity. Nat Neurosci. 2005;8:839–841. doi: 10.1038/nn0705-839. [DOI] [PubMed] [Google Scholar]
- 100.Maragakis NJ, Jackson M, Ganel R, Rothstein JD. Topiramate protects against motor neuron degeneration in organotypic spinal cord cultures but not in G93A SOD1 transgenic mice. Neurosci Lett. 2003;338:107–110. doi: 10.1016/s0304-3940(02)01386-1. [DOI] [PubMed] [Google Scholar]
- 101.Mattson MP, Duan W, Pedersen WA, Culmsee C. Neurodegenerative disorders and ischemic brain diseases. Apoptosis. 2001;6:69–81. doi: 10.1023/a:1009676112184. [DOI] [PubMed] [Google Scholar]
- 102.McBain CJ, Boden P, Hill RG. Rat hippocampal slices ‘in vitro’ display spontaneous epileptiform activity following long-term organotypic culture. J Neurosci Methods. 1989;27:35–49. doi: 10.1016/0165-0270(89)90051-4. [DOI] [PubMed] [Google Scholar]
- 103.Merlin LR, Taylor GW, Wong RKS. Role of metabotropic glutamate receptor subtypes in the patterning of epileptiform activities in vitro. J Neurophysiol. 1995;74:896–900. doi: 10.1152/jn.1995.74.2.896. [DOI] [PubMed] [Google Scholar]
- 104.Mielke JG, Comas T, Woulfe J, Monette R, Chakravarthy B, Mealing GA. Cytoskeletal, synaptic, and nuclear protein changes associated with rat interface organotypic hippocampal slice culture development. Brain Res. Dev. Brain Res. 2005;160:275–286. doi: 10.1016/j.devbrainres.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 105.Mingorance A, Fontana X, Sole M, Burgaya F, Urena JM, Teng FY, Tang BL, Hunt D, Anderson PN, Bethea JR, Schwab ME, Soriano E, del Rio JA. Regulation of Nogo and Nogo receptor during the development of the entorhinohippocampal pathway and after adult hippocampal lesions. Mol. Cell Neurosci. 2004;26:34–49. doi: 10.1016/j.mcn.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 106.Mingorance A, Sole M, Muneton V, Martinez A, Nieto-Sampedro M, Soriano E, del Rio JA. Regeneration of lesioned entorhino-hippocampal axons in vitro by combined degradation of inhibitory proteoglycans and blockade of Nogo-66/NgR signaling. FASEB J. 2006;20:491–493. doi: 10.1096/fj.05-5121fje. [DOI] [PubMed] [Google Scholar]
- 107.Mitani A, Yanase H, Namba S, Shudo M, Kataoka K. In vitro ischemia-induced intracellular Ca2+ elevation in cerebellar slices: a comparative study with the values found in hippocampal slices. Acta Neuropathol. (Berl.) 1995;89:2–7. doi: 10.1007/BF00294252. [DOI] [PubMed] [Google Scholar]
- 108.Mitchell BD, Emsley JG, Magavi SS, Arlotta P, Macklis JD. Constitutive and induced neurogenesis in the adult mammalian brain: manipulation of endogenous precursors toward CNS repair. Dev Neurosci. 2004;26:101–117. doi: 10.1159/000082131. [DOI] [PubMed] [Google Scholar]
- 109.Modo M, Stroemer RP, Tang E, Veizovic T, Sowniski P, Hodges H. Neurological sequelae and long-term behavioural assessment of rats with transient middle cerebral artery occlusion. J Neurosci Methods. 2000;104:99–109. doi: 10.1016/s0165-0270(00)00329-0. [DOI] [PubMed] [Google Scholar]
- 110.Mulholland PJ, Prendergast MA. Transection of intrinsic polysynaptic pathways reduces N-methyl-D-aspartate neurotoxicity in hippocampal slice cultures. Neurosci Res. 2003;46:369–376. doi: 10.1016/s0168-0102(03)00102-0. [DOI] [PubMed] [Google Scholar]
- 111.Muller D, Buchs PA, Stoppini L. Time course of synaptic development in hippocampal organotypic cultures. Brain Res. Dev. Brain Res. 1993;71:93–100. doi: 10.1016/0165-3806(93)90109-n. [DOI] [PubMed] [Google Scholar]
- 112.Nakai T, Milusheva E, Baranyi M, Uchihashi Y, Satoh T, Vizi ES. Excessive release of [3H]noradrenaline and glutamate in response to simulation of ischemic conditions in rat spinal cord slice preparation: effect of NMDA and AMPA receptor antagonists. Eur J Pharmacol. 1999;366:143–150. doi: 10.1016/s0014-2999(98)00917-0. [DOI] [PubMed] [Google Scholar]
- 113.Nakatomi H, Kuriu T, Okabe S, Yamamoto S, Hatano O, Kawahara N, Tamura A, Kirino T, Nakafuku M. Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell. 2002;110:429–441. doi: 10.1016/s0092-8674(02)00862-0. [DOI] [PubMed] [Google Scholar]
- 114.Namura S, Maeno H, Takami S, Jiang XF, Kamichi S, Wada K, Nagata I. Inhibition of glial glutamate transporter GLT-1 augments brain edema after transient focal cerebral ischemia in mice. Neurosci Lett. 2002;324:117–120. doi: 10.1016/s0304-3940(02)00193-3. [DOI] [PubMed] [Google Scholar]
- 115.Newell DW, Barth A, Papermaster V, Malouf AT. Glutamate and non-glutamate receptor mediated toxicity caused by oxygen and glucose deprivation in organotypic hippocampal cultures. J Neurosci. 1995;15:7702–7711. doi: 10.1523/JNEUROSCI.15-11-07702.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nieto M, Schuurmans C, Britz O, Guillemot F. Neural bHLH genes control the neuronal versus glial fate decision in cortical progenitors. Neuron. 2001;29:401–413. doi: 10.1016/s0896-6273(01)00214-8. [DOI] [PubMed] [Google Scholar]
- 117.Noraberg J, Kristensen BW, Zimmer J. Markers for neuronal degeneration in organotypic slice cultures. Brain Res. Brain Res. Protoc. 1999;3:278–290. doi: 10.1016/s1385-299x(98)00050-6. [DOI] [PubMed] [Google Scholar]
- 118.Noraberg J, Poulsen FR, Blaabjerg M, Kristensen BW, Bonde C, Montero M, Meyer M, Gramsbergen JB, Zimmer J. Organotypic hippocampal slice cultures for studies of brain damage, neuroprotection and neurorepair. Curr. Drug Target - CNS Neurol. Disord. 2005;4:435–452. doi: 10.2174/1568007054546108. [DOI] [PubMed] [Google Scholar]
- 119.O’Byrne M, Tipton K, McBean G, Kollegger H. Assessment of neurotoxicity and neuroprotection. J. Neural Transm. Suppl. 1997;50:153–164. doi: 10.1007/978-3-7091-6842-4_15. [DOI] [PubMed] [Google Scholar]
- 120.Ochs G, Penn RD, York M, Giess R, Beck M, Tonn J, Haigh J, Malta E, Traub M, Sendtner M, Toyka KV. A phase I/II trial of recombinant methionyl human brain derived neurotrophic factor administered by intrathecal infusion to patients with amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Other Motor Neuron Disord. 2000;1:201–206. doi: 10.1080/14660820050515197. [DOI] [PubMed] [Google Scholar]
- 121.Ohno M, Sametsky EA, Younkin LH, Oakley H, Younkin SG, Citron M, Vassar R, Disterhoft JF. BACE1 deficiency rescues memory deficits and cholinergic dysfunction in a mouse model of alzheimer’s disease. Neuron. 2004;41:27–33. doi: 10.1016/s0896-6273(03)00810-9. [DOI] [PubMed] [Google Scholar]
- 122.Oishi Y, Baratta J, Robertson RT, Steward O. Assessment of factors regulating axon growth between the cortex and spinal cord in organotypic co-cultures: effects of age and neurotrophic factors. J Neurotrauma. 2004;21:339–356. doi: 10.1089/089771504322972121. [DOI] [PubMed] [Google Scholar]
- 123.Ostergaard K, Finsen B, Zimmer J. Organotypic slice cultures of the rat striatum: an immunocytochemical, histochemical and in situ hybridization study of somatostatin, neuropeptide Y, nicotinamide adenine dinucleotide phosphate-diaphorase, and enkephalin. Exp. Brain Res. 1995;103:70–84. doi: 10.1007/BF00241966. [DOI] [PubMed] [Google Scholar]
- 124.Pedersen JZ, Bernardi G, Centonze D, Pisani A, Rossi L, Rotilio G, Calabresi P. Hypoglycemia, hypoxia, and ischemia in a corticostriatal slice preparation: electrophysiologic changes and ascorbyl radical formation. J. Cereb. Blood Flow Me-tab. 1998;18:868–875. doi: 10.1097/00004647-199808000-00006. [DOI] [PubMed] [Google Scholar]
- 125.Phyllis JW, Smith-Barbour M, Perkins LM, O’Regan MH. Characterization of glutamate, aspartate and GABA release from ischaemic rat cerebral cortex. Brain Res. Bull. 1994;34:457–466. doi: 10.1016/0361-9230(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 126.Picconi B, Centonze D, Hakansson K, Bernardi G, Greengard P, Fisone G, Cenci MA, Calabresi P. Loss of bidirectional striatal synaptic plasticity in L-DOPA-induced dyskinesia. Nat Neurosci. 2003;6:501–506. doi: 10.1038/nn1040. [DOI] [PubMed] [Google Scholar]
- 127.Picconi B, Pisani A, Barone I, Bonsi P, Centonze D, Bernardi G, Calabresi P. Pathological Synaptic Plasticity in the Striatum: Implications for Parkinson’s Disease. Neurotoxicology. 2005;26:779–783. doi: 10.1016/j.neuro.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 128.Pizzi M, Sarnico I, Boroni F, Benarese M, Dreano M, Garotta G, Valerio A, Spano P. Prevention of neuron and oligodendrocyte degeneration by interleukin-6 (IL-6) and IL-6 receptor/IL-6 fusion protein in organotypic hippocampal slices. Mol. Cell Neurosci. 2004;25:301–311. doi: 10.1016/j.mcn.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 129.Pokorny J, Yamamoto T. Postnatal ontogenesis of hippocampal CA1 area in rats. II. Development of ultrastructure in stratum lacunosum and moleculare. Brain Res. Bull. 1981;7:121–130. doi: 10.1016/0361-9230(81)90076-9. [DOI] [PubMed] [Google Scholar]
- 130.Prang P, Del Turco D, Kapfhammer JP. Regeneration of entorhinal fibers in mouse slice cultures is age dependent and can be stimulated by NT-4, GDNF, and modulators of G-proteins and protein kinase C. Exp Neurol. 2001;169:135–147. doi: 10.1006/exnr.2001.7648. [DOI] [PubMed] [Google Scholar]
- 131.Pringle AK, Sundstrom LE, Wilde GJ, Williams LR, Iannotti F. Brain-derived neurotrophic factor, but not neurotrophin-3, prevents ischaemia-induced neuronal cell death in organotypic rat hippocampal slice cultures. Neurosci Lett. 1996;211:203–206. doi: 10.1016/0304-3940(96)12745-2. [DOI] [PubMed] [Google Scholar]
- 132.Raineteau O, Rietschin L, Gradwohl G, Guillemot F, Gahwiler BH. Neurogenesis in hippocampal slice cultures. Mol. Cell Neurosci. 2004;26:241–250. doi: 10.1016/j.mcn.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 133.Randic M, Jiang MC, Cerne R. Long-term potentiation and long-term depression of primary afferent neurotransmission in the rat spinal cord. J Neurosci. 1993;13:5228–5241. doi: 10.1523/JNEUROSCI.13-12-05228.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rao VL, Dogan A, Todd KG, Bowen KK, Kim BT, Roth-stein JD, Dempsey RJ. Antisense knockdown of the glial glutamate transporter GLT-1, but not the neuronal glutamate transporter EAAC1, exacerbates transient focal cerebral ischemia-induced neuronal damage in rat brain. J Neurosci. 2001;21:1876–1883. doi: 10.1523/JNEUROSCI.21-06-01876.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ray AM, Owen DE, Evans ML, Davis JB, Benham CD. Caspase inhibitors are functionally neuroprotective against oxygen glucose deprivation induced CA1 death in rat organotypic hippocampal slices. Brain Res. 2000;867:62–69. doi: 10.1016/s0006-8993(00)02230-7. [DOI] [PubMed] [Google Scholar]
- 136.Regehr WG, Connor JA, Tank DW. Optical imaging of calcium accumulation in hippocampal pyramidal cells during synaptic activation. Nature. 1989;341:533–536. doi: 10.1038/341533a0. [DOI] [PubMed] [Google Scholar]
- 137.Rivera S, Gold SJ, Gall CM. Interleukin-1 beta increases basic fibroblast growth factor mRNA expression in adult rat brain and organotypic hippocampal cultures. Brain Res. Mol. Brain Res. 1994;27:12–26. doi: 10.1016/0169-328x(94)90179-1. [DOI] [PubMed] [Google Scholar]
- 138.Robert F, Parisi L, Bert L, Renaud B, Stoppini L. Microdialysis monitoring of extracellular glutamate combined with the simultaneous recording of evoked field potentials in hippocampal organotypic slice cultures. J Neurosci Methods. 1997;74:65–76. doi: 10.1016/s0165-0270(97)02261-9. [DOI] [PubMed] [Google Scholar]
- 139.Rossi DJ, Oshima T, Attwell D. Glutamate release in severe brain ischaemia is mainly by reversed uptake. Nature. 2000;403:316–321. doi: 10.1038/35002090. [DOI] [PubMed] [Google Scholar]
- 140.Rossini PM, Calautti C, Pauri F, Baron JC. Post-stroke plastic reorganisation in the adult brain. Lancet Neurol. 2003;2:493–502. doi: 10.1016/s1474-4422(03)00485-x. [DOI] [PubMed] [Google Scholar]
- 141.Rossini PM, Dal Forno G. Neuronal post-stroke plasticity in the adult. Restor Neurol Neurosci. 2004;22:193–206. [PubMed] [Google Scholar]
- 142.Rytter A, Cronberg T, Asztely F, Nemali S, Wieloch T. Mouse hippocampal organotypic tissue cultures exposed to in vitro “ischemia” show selective and delayed CA1 damage that is aggravated by glucose. J. Cereb. Blood Flow Metab. 2003;23:23–33. doi: 10.1097/01.WCB.0000034361.37277.1B. [DOI] [PubMed] [Google Scholar]
- 143.Sajikumar S, Navakkode S, Frey JU. Protein synthesis-dependent long-term functional plasticity: methods and techniques. Curr Opin Neurobiol. 2005;15:607–613. doi: 10.1016/j.conb.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 144.Sandku‥hler J, Chen JG, Cheng G, Randic M. Low frequency stimulation of afferent Ad-fibers induces long-term depression of primary afferent synapses with substantia gelatinosa neurons in the rat. J Neurosci. 1997;17:6483–6491. doi: 10.1523/JNEUROSCI.17-16-06483.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sattler R, Tymianski M. Molecular mechanisms of glutamate receptor-mediated excitotoxic neuronal cell death. Mol Neurobiol. 2001;24:107–129. doi: 10.1385/MN:24:1-3:107. [DOI] [PubMed] [Google Scholar]
- 146.Schmued LC, Albertson C, Slikker W., Jr. Fluoro-Jade: a novel fluorochrome for the sensitive and reliable histochemical localization of neuronal degeneration. Brain Res. 1997;751:37–46. doi: 10.1016/s0006-8993(96)01387-x. [DOI] [PubMed] [Google Scholar]
- 147.Schroeter M, Jander S, Witte OW, Stoll G. Heterogeneity of the microglial response in photochemically induced focal ischemia of the rat cerebral cortex. Neuroscience. 1999;89:1367–1377. doi: 10.1016/s0306-4522(98)00398-4. [DOI] [PubMed] [Google Scholar]
- 148.Schubert P, Keller F, Nakamura Y, Rudolphi K. The use of ion-sensitive electrodes and fluorescence imaging in hippocampal slices for studying pathological changes of intracellular Ca2+ regulation. J. Neural Transm. Suppl. 1994;44:73–85. doi: 10.1007/978-3-7091-9350-1_6. [DOI] [PubMed] [Google Scholar]
- 149.Schurr A, Payne RS, Heine MF, Rigor BM. Hypoxia, excitotoxicity, and neuroprotection in the hippocampal slice preparation. J Neurosci Methods. 1995;59:129–38. doi: 10.1016/0165-0270(94)00203-s. [DOI] [PubMed] [Google Scholar]
- 150.Shaw PJ, Ince PG. Glutamate, excitotoxicity and amyotrophic lateral sclerosis. J Neurol. 1997;244(Suppl. 2):S3–14. doi: 10.1007/BF03160574. [DOI] [PubMed] [Google Scholar]
- 151.Sherer TB, Betarbet R, Testa CM, Seo BB, Richardson JR, Kim JH, Miller GW, Yagi T, Matsuno-Yagi A, Greenamyre JT. Mechanism of toxicity in rotenone models of Parkinson’s disease. J Neurosci. 2003;23:10756–10764. doi: 10.1523/JNEUROSCI.23-34-10756.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Shimizu K, Matsubara K, Ohtaki K.-i, Shiono H. Paraquat leads to dopaminergic neural vulnerability in organotypic midbrain culture. Neurosci Res. 2003;46:523–532. doi: 10.1016/s0168-0102(03)00163-9. [DOI] [PubMed] [Google Scholar]
- 153.Snider BJ, Gottron FJ, Choi DW. Apoptosis and necrosis in cerebrovascular disease. Ann N Y Acad Sci. 1999;893:243–253. doi: 10.1111/j.1749-6632.1999.tb07829.x. [DOI] [PubMed] [Google Scholar]
- 154.Somjen GG, Aitken PG, Balestrino M, Herreras O, Kawasaki K. Spreading depression-like depolarization and selective vulnerability of neurons. A brief review. Stroke. 1990;21:179–183. [PubMed] [Google Scholar]
- 155.Stoppini L, Buchs PA, Muller D. A simple method for organotypic cultures of nervous tissue. J Neurosci Methods. 1991;37:173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- 156.Stoppini L, Parisi L, Oropesa C, Muller D. Sprouting and functional recovery in co-cultures between old and young hippocampal organotypic slices. Neuroscience. 1997;80:1127–1136. doi: 10.1016/s0306-4522(97)00132-2. [DOI] [PubMed] [Google Scholar]
- 157.Stys PK. Anoxic and ischemic injury of myelinated axons in CNS white matter: from mechanistic concepts to therapeutics. J Cereb Blood Flow Metab. 1998;18:2–25. doi: 10.1097/00004647-199801000-00002. [DOI] [PubMed] [Google Scholar]
- 158.Sun Y, Nadal-Vicens M, Misono S, Lin MZ, Zubiaga A, Hua X, Fan G, Greenberg ME. Neurogenin promotes neurogenesis and inhibits glial differentiation by independent mechanisms. Cell. 2001;104:365–376. doi: 10.1016/s0092-8674(01)00224-0. [DOI] [PubMed] [Google Scholar]
- 159.Sundstrom L, Morrison B, 3rd, Bradley M, Pringle A. Organotypic cultures as tools for functional screening in the CNS. Drug Discov Today. 2005;10:993–1000. doi: 10.1016/S1359-6446(05)03502-6. [DOI] [PubMed] [Google Scholar]
- 160.Szatkowski M, Attwell D. Triggering and execution of neuronal death in brain ischaemia: two phases of glutamate release by different mechanisms. Trends Neurosci. 1994;17:359–365. doi: 10.1016/0166-2236(94)90040-x. [DOI] [PubMed] [Google Scholar]
- 161.Szatkowski M, Barbour B, Attwell D. Non-vesicular release of glutamate from glial cells by reversed electrogenic glutamate uptake. Nature. 1990;348:443–446. doi: 10.1038/348443a0. [DOI] [PubMed] [Google Scholar]
- 162.Takasawa K, Kitagawa K, Yagita Y, Sasaki T, Tanaka S, Matsushita K, Ohstuki T, Miyata T, Okano H, Hori M, Matsumoto M. Increased proliferation of neural progenitor cells but reduced survival of newborn cells in the contralateral hippocampus after focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 2002;22:299–307. doi: 10.1097/00004647-200203000-00007. [DOI] [PubMed] [Google Scholar]
- 163.Terman GW, Eastman CL, Chavkin C. Mu opiates inhibit long-term potentiation induction in the spinal cord slice. J Neurophysiol. 2001;85:485–494. doi: 10.1152/jn.2001.85.2.485. [DOI] [PubMed] [Google Scholar]
- 164.Uchihashi Y, Bencsics A, Umeda E, Nakai T, Sato T, Vizi ES. Na+ channel block prevents the ischemia-induced release of norepinephrine from spinal cord slices. Eur J Pharmacol. 1998;346:145–150. doi: 10.1016/s0014-2999(98)00049-1. [DOI] [PubMed] [Google Scholar]
- 165.Uchino S, Nakamura T, Nakamura K, Nakajima-IiJima S, Mishina M, Kohsaka S, Kudo Y. Real-time, two-dimensional visualization of ischaemia-induced glutamate release from hippocampal slices. Eur J Neurosci. 2001;13:670–678. doi: 10.1046/j.1460-9568.2001.01430.x. [DOI] [PubMed] [Google Scholar]
- 166.Valcanis H, Tan SS. Layer specification of transplanted interneurons in developing mouse neocortex. J Neurosci. 2003;23:5113–5122. doi: 10.1523/JNEUROSCI.23-12-05113.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Vanderklish P, Saido TC, Gall C, Arai A, Lynch G. Proteolysis of spectrin by calpain accompanies theta-burst stimulation in cultured hippocampal slices. Brain Res Mol Brain Res. 1995;32:25–35. doi: 10.1016/0169-328x(95)00057-y. [DOI] [PubMed] [Google Scholar]
- 168.Velazquez JLP, Frantseva MV, Carlen PL. In vitro ischemia promotes glutamate-mediated free radical generation and intracellular calcium accumulation in hippocampal pyramidal neurons. J Neurosci. 1997;17:9085–9094. doi: 10.1523/JNEUROSCI.17-23-09085.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Vitolo OV, Sant’Angelo A, Costanzo V, Battaglia F, Arancio O, Shelanski M. Amyloid β-peptide inhibition of the PKA/CREB pathway and long-term potentiation: Reversibility by drugs that enhance cAMP signaling. Proc. Natl Acad Sci USA. 2002;99:13217–13221. doi: 10.1073/pnas.172504199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.von Gunten A, Kovari E, Bussiere T, Rivara CB, Gold G, Bouras C, Hof PR, Giannakopoulos P. Cognitive impact of neuronal pathology in the entorhinal cortex and CA1 field in Alzheimer’s disease. Neurobiol Aging. 2006;27:270–277. doi: 10.1016/j.neurobiolaging.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 171.Walder S, Zhang F, Ferretti P. Up-regulation of neural stem cell markers suggests the occurrence of dedifferentiation in regenerating spinal cord. Dev Genes Evol. 2003;213:625–630. doi: 10.1007/s00427-003-0364-2. [DOI] [PubMed] [Google Scholar]
- 172.Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ. Naturally secreted oligomers of amyloid β protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- 173.Wang JKT, Portbury S, Thomas MB, Barney S, Ricca DJ, Morris DL, Warner DS, Lo DC. Cardiac glycosides provide neuroprotection against ischemic stroke: Discovery by a brain slice-based compound screening platform. Proc Natl Acad Sci USA. 2006;103:10461–10466. doi: 10.1073/pnas.0600930103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wang Q, Walsh DM, Rowan MJ, Selkoe DJ, Anwyl R. Block of long-term potentiation by naturally secreted and synthetic amyloid β-peptide in hippocampal slices is mediated via activation of the kinases c-Jun N-terminal kinase, cyclin-dependent kinase 5, and p38 mitogen-activated protein kinase as well as metabotropic glutamate receptor type 5. J Neurosci. 2004;24:3370–3378. doi: 10.1523/JNEUROSCI.1633-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wang XM, Bausch SB. Effects of distinct classes of N-methyl-D-aspartate receptor antagonists on seizures, axonal sprouting and neuronal loss in vitro: suppression by NR2B-selective antagonists. Neuropharmacology. 2004;47:1008–1020. doi: 10.1016/j.neuropharm.2004.07.036. [DOI] [PubMed] [Google Scholar]
- 176.Woodhams PL, Atkinson DJ. Regeneration of entorhinodentate projections in organotypic slice cultures: mode of axonal regrowth and effects of growth factors. Exp Neurol. 1996;140:68–78. doi: 10.1006/exnr.1996.0116. [DOI] [PubMed] [Google Scholar]
- 177.Woolf C, Thompson S, King A. Prolonged primary afferent induced alterations in dorsal horn neurones, an intracellular analysis in vitro and in vivo. J Physiol Paris. 1989;83:255–266. [PubMed] [Google Scholar]
- 178.Wu C.-C, Chawla F, Games D, Rydel RE, Freedman S, Schenk D, Young WG, Morrison JH, Bloom FE. Selective vulnerability of dentate granule cells prior to amyloid deposition in PDAPP mice: digital morphometric analyses. Proc Natl Acad Sci USA. 2004;101:7141–7146. doi: 10.1073/pnas.0402147101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Xiang Z, Hrabetova S, Moskowitz SI, Casaccia-Bonnefil P, Young SR, Nimmrich VC, Tiedge H, Einheber S, Karnup S, Bianchi R, Bergold PJ. Long-term maintenance of mature hippocampal slices in vitro. J Neurosci Methods. 2000;98:145–154. doi: 10.1016/s0165-0270(00)00197-7. [DOI] [PubMed] [Google Scholar]
- 180.Xu J, Kang J. The mechanisms and functions of activity-dependent long-term potentiation of intrinsic excitability. Rev Neurosci. 2005;16:311–323. doi: 10.1515/revneuro.2005.16.4.311. [DOI] [PubMed] [Google Scholar]
- 181.Yan Q, Matheson C, Lopez OT, Miller JA. The biological responses of axotomized adult motoneurons to brain-derived neurotrophic factor. J Neurosci. 1994;14:5281–5291. doi: 10.1523/JNEUROSCI.14-09-05281.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Yi Li JC, Chun Ling Zhang, Lei Wang, Dunyue Lu, Mark Katakowski, Qi Gao, Li Hong Shen, Jing Zhang, Mei Lu, Michael Chopp. Gliosis and brain remodeling after treatment of stroke in rats with marrow stromal cells. Glia. 2005;49:407–417. doi: 10.1002/glia.20126. [DOI] [PubMed] [Google Scholar]
- 183.Youssef FF, Addae JI, McRaea A, Stoneb TW. Long-term potentiation protects rat hippocampal slices from the effects of acute hypoxia. Brain Res. 2001;907:144–150. doi: 10.1016/s0006-8993(01)02594-x. [DOI] [PubMed] [Google Scholar]
- 184.Zhou L, Del Villar K, Dong Z, Miller CA. Neurogenesis response to hypoxia-induced cell death: map kinase signal transduction mechanisms. Brain Res. 2004;1021:8–19. doi: 10.1016/j.brainres.2004.05.115. [DOI] [PubMed] [Google Scholar]