Abstract
Violence and aggression are major causes of death and injury, thus constituting primary public health problems throughout much of the world costing billions of dollars to society. The present review relates our understanding of the neurobiology of aggression and rage to pharmacological treatment strategies that have been utilized and those which may be applied in the future. Knowledge of the neural mechanisms governing aggression and rage is derived from studies in cat and rodents. The primary brain structures involved in the expression of rage behavior include the hypothalamus and midbrain periaqueductal gray. Limbic structures, which include amygdala, hippocampal formation, septal area, prefrontal cortex and anterior cingulate gyrus serve important modulating functions. Excitatory neurotransmitters that potentiate rage behavior include excitatory amino acids, substance P, catecholamines, cholecystokinin, vasopressin, and serotonin that act through 5-HT2 receptors. Inhibitory neurotransmitters include GABA, enkephalins, and serotonin that act through 5-HT1 receptors. Recent studies have demonstrated that brain cytokines, including IL-1β and IL-2, powerfully modulate rage behavior. IL-1-β exerts its actions by acting through 5-HT2 receptors, while IL-2 acts through GABAA or NK1 receptors. Pharmacological treatment strategies utilized for control of violent behavior have met with varying degrees of success. The most common approach has been to apply serotonergic compounds. Others included the application of antipsychotic, GABAergic (anti-epileptic) and dopaminergic drugs. Present and futures studies on the neurobiology of aggression may provide the basis for new and novel treatment strategies for the control of aggression and violence as well as the continuation of existing pharmacological approaches.
Key Words: Aggressive behavior, cytokines, defensive rage, enkephalins, GABA, impulsive behavior, predatory attack, serotonin
INTRODUCTION
Over the past decade, a variety of studies have been carried out designed to determine the effects of selective drugs on the management of human aggressive behavior. One purpose of the present review is to evaluate human studies with respect to what is known of the neurochemistry and neuropharmacology of aggression. In order to achieve this objective, it is appropriate to first consider the behavioral characteristics and basic forms of aggressive behavior, the underlying neural substrates, and principal neurochemical and receptor-neurotransmitter mechanisms linked to their expression and control. These features are considered below in the following section.
OVERVIEW OF THE NEUROBIOLOGY OF AGGRESSIVE BEHAVIOR
Animal Models of Aggressive Behavior
In attempting to understand the processes that are collectively referred to as “aggressive behavior”, it is first useful to provide a definition of this term. One encompassing definition is that it constitutes a type of behavior that either threatens harm or leads to or causes harm, destruction or damage to another organism. Such a definition would suggest that aggression is not a unitary phenomenon, but instead, includes a variety of different behavioral processes. Such forms of aggression may also reflect competition among organisms of the same or of a different species for mates, food, or territory. Various animal research models of aggressive behavior have been utilized [70,83]. These include: fear-induced, maternal, inter-male, irritable, sex-related, territorial, resident-intruder, and predatory aggression. Aside from predatory attack, these models of aggressive behavior share the following common features: they reflect a perceived or real threat, are aversive to the organism, are impulsive, display sympathetic signs, and are defensive in nature. Thus, the general category referred to as defensive (or affective) aggression includes animals (and humans) that display these characteristics. In contrast, predatory attack is quite different from the others, in particular because it requires planning, shows few autonomic signs, and is positively reinforcing to the organism. Based upon this analysis, the remainder of the present sections considers the neurobiological and neuropharmacological properties associated with these two forms of aggressive behavior as determined from studies conducted mainly in the cat.
Affective or defensive rage occurs in nature in response to the presence of, or perceived presence of, a threatening stimulus such as another species within its territory. It is characterized by arching of the back, retraction of the ears, piloerection, pupillary dilatation, impulsivity, marked hissing, and striking of the target species with the forepaw at the threatening object. This form of aggressive behavior can also be elicited by electrical or chemical stimulation of the midbrain periaqueductal gray (PAG) and medial hypothalamus [83,98]. With respect to this model of aggression, it is important to note that these characteristics present in the cat are also often present in humans [69]. In particular, affective defense in humans frequently includes marked sympathetic signs, impulsivity, and attack which is not necessarily directed at a specific individual (in contrast to predatory attack where a specific target is singled out [see discussion below]).
As noted above, predatory attack is quite different from other forms of aggression. In the animal kingdom, it is manifest as hunting behavior. The attack is planned and is preceded by stalking of specific prey object of another species followed by biting of the back of the neck until it kills the prey. In contrast to defensive rage, the cat displays few autonomic signs aside from mild pupillary dilatation. Predatory attack is induced following electrical stimulation of the lateral hypothalamus or ventrolateral aspect of the PAG and ceases immediately following termination of stimulation [83]. In humans, the characteristics of the attack response also parallel those described in the cat. For example, the attack is directed upon a specific target (or individual), contains few sympathetic signs, and is typically premeditated or planned well in advance, which may extend from days to months, or even longer.
Regions and Pathways Mediating Defensive Rage and Predatory Attack
Knowledge of the mechanisms governing the regulation and control of aggression and rage require an understanding of the underlying substrates governing these forms of aggression. The following discussion identifies the sites within the hypothalamus and midbrain from which each of these forms of aggression are elicited and the pathways mediating these behaviors to other regions of the brainstem and related areas of the central nervous system.
Defensive Rage Behavior
Defensive rage behavior can be elicited by electrical stimulation of wide regions along the rostro-caudal axis of the medial hypothalamus. Defensive rage is also elicited by electrical or chemical (i.e., glutamate analog) stimulation of the dorsolateral quadrant of mainly the rostral half of the PAG. Components or fragments of defensive rage can also be elicited from lower regions of the brainstem. These regions include the caudal PAG and pontine tegmentum and presumably lie along the descending pathways mediating this form of aggression [83]. The principal descending pathway from the medial hypothalamus sub serving defensive rage behavior arises from the anterior medial hypothalamus and its primary target is the dorsolateral aspect of the rostral half of the PAG (see Fig. 1A). Other regions of the medial hypothalamus, such as the ventromedial nucleus from which defensive rage can also be elicited, project rostrally to the region of the anterior medial hypothalamus from which the descending pathway to the PAG arises. Moreover, the anterior medial hypothalamus also receives significant inputs from components of the limbic system which modulate aggression and rage behavior. The converging inputs into the anterior medial hypothalamus thus enable this region to serve as a major site of integration for the expression of defensive rage behavior.
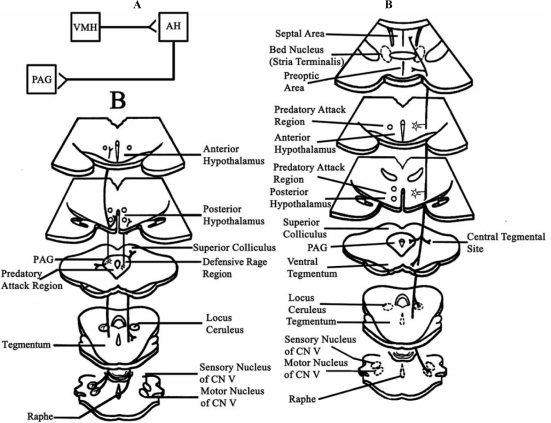
Fig. (1).
(A) Summary of principal hypothalamic pathways mediating defensive rage behavior to the midbrain periaqueductal gray (PAG). The primary output of the medial hypothalamus to PAG arises from the anterior medial hypothalamus (AH) and receives significant input from the ventromedial nucleus of hypothalamus (VMH). (Modified from Fuchs et al. [30] with permission. (B) Diagrams indicating the principal efferent projections of the PAG associated with defensive rage (affective defense) on left side and quiet biting (predatory) attack behavior on right side. Ascending fibers associated with defensive rage are distributed to the medial preoptico-hypothalamus and caudally to the locus ceruleus, tegmental fields, and trigeminal complex. In contrast, the pathway from the PAG associated with predatory attack includes a more limited projection to the posterior lateral hypothalamus, while the caudally directed fibers are directed to the median raphe and central tegmental fields. (From Siegel and Pott, [85] with permission). (C) Diagram illustrates principal ascending and descending projections of the perifornical hypothalamus associated with predatory attack. Note projections to the PAG, tegmental fields, locus ceruleus, and motor nucleus of trigeminal nerve. (From Siegel and Pott [85], with permission).
The second limb of the descending pathway for the expression of defensive rage behavior arises from the region of the dorsolateral PAG, which receives direct inputs from the anterior medial hypothalamus. The efferent projections of this region of the PAG are directed to structures that mediate autonomic and somatomotor components of defensive rage behavior. There are several routes by which autonomic functions are activated from the PAG. One pathway includes a projection to the locus ceruleus, which in turn projects to the intermediolateral cell column of the thoracic and lumbar spinal cord. Converging inputs to these sympathetic regions of spinal cord are also mediated through projections to the solitary nucleus, whose axons then project to the ventrolateral medulla and from there to the intermediolateral cell column of the thoracic and lumbar cord. There are several regions that mediate the somatomotor components of defensive rage behavior. One set of targets includes the motor nuclei of the trigeminal and facial cranial nerves, which are associated with jaw opening essential for the vocalization aspect of the defensive rage response. A second target includes the nuclei of the reticular formation which comprise, in part, reticulospinal fibers directed towards alpha and gamma motor neurons. Those neurons directed to the cervical cord presumably affect movements of the upper limbs and provide the basis for the striking component of the rage response. These two components, which are integrated at the levels of the medial hypothalamus and PAG, comprise the defensive rage response. A separate, ascending projection of the dorsolateral PAG supplies the rostro-caudal extent of the medial hypothalamus, much of which relate to the expression of defensive rage. This projection likely serves as a substrate for a positive feedback mechanism, thus increasing the likelihood that this response can be prolonged under dangerous conditions, which is of survival value to the animal [83]. These anatomical relationships are depicted in Fig. (1B).
It is of interest to note that others have attempted to describe the neuroanatomical sites and pathways associated with aggression in rodents. Initial observations were reported by Panksepp [73] and Woodworth [101] and later by Kruk [53,54] and Lammers et al. [55], who provided a detailed analysis of the response repertoire and sites in rodent brain from which attack can be induced by electrical stimulation and the likely ascending and descending pathways associated with these sites. Stimulation of such sites located mainly in the intermediate hypothalamic area (i.e., just ventrolateral to the position of the fornix), could produce biting attack upon a conspecific, varying in intensity from mild biting of the back of the neck to hard biting of the head and back. The response could also include kicking of the opponent with its hind-paws, clinch fights and attack jumps.
The principal pathways associated with this form of aggression can be summarized briefly as follows: the ascending pathways are directed primarily to the preoptic region and neighboring regions of the septal area. The descending pathways supply mainly the PAG, ventral tegmental area, locus ceruleus, raphe magnus, and solitary nuclear complex. Thus, one can see that there are considerable similarities in the projection patterns between those described in the cat for defensive rage and the rat for the attack pattern described above. It would be of great interest to be able to compare and contrast the pathways associated with other models of aggressive behavior (e.g., resident-intruder and fear-induced). However, such information is lacking in the literature and is in need of systematic investigation.
Predatory Attack Behavior
Predatory attack behavior can be elicited by electrical stimulation most typically of the perifornical lateral hypothalamus, ventrolateral aspect of the PAG, and ventral tegmental area. The principal origin of the descending projections of the hypothalamus is the region of the perifornical lateral hypothalamus from which predatory attack is elicited. This region supplies the ventrolateral aspect of the PAG, ventral tegmental area, central tegmental fields of the midbrain and pons, locus ceruleus, and motor and main sensory nuclei of the trigeminal complex. The projections to the trigeminal complex are significant in that they provide the anatomical substrate for the jaw closing reflex critical for the culmination of biting attack (Fig. 1C). The projections to the brainstem tegmentum presumably provide the initial neuron in a series of descending projections to the lower brainstem and spinal cord essential for other motor aspects of the attack response such as stalking and striking at the prey object.
Anatomical and Functional Relationship Between the Medial and Lateral Hypothalamus
While defensive rage behavior and predatory attack clearly reflect distinctly different forms of aggression that utilize separate and non-overlapping pathways, they also relate to each other in a unique manner. Within the medial hypothalamus and with respect to defensive rage behavior, there are two classes of neurons. One is a projection neuron, which was described above, whose target is the dorsolateral aspect of the PAG and constitutes the descending pathway for this form of aggression. The second is a neuron with a short axon that supplies the lateral hypothalamus. This neuron is GABAergic and inhibits neurons in the lateral hypothalamus associated with predatory attack [37]. Likewise, there are at least two classes of neurons in the lateral hypothalamus with respect to predatory attack. The first is a neuron with a long axon that constitutes the descending pathway for the expression of predatory attack and the second is a GABAergic neuron, with a short axon which supplies the medial hypothalamus and inhibits neurons in the medial hypothalamus associated with defensive rage [14,15] (See Fig. 2). The likely functional significance of the reciprocal inhibitory pathways linking the medial and lateral hypothalamus is as follows: since these two responses are mutually exclusive, the effective expression of one requires the suppression of the other. It is intuitive that a successful act of predation can only be accomplished when a predator quietly approaches the prey object, which requires suppression of hissing and related component responses. Similarly, when defensive rage is required following the presence of a threatening stimulus, elements of predation serve no function and therefore are suppressed in order for the affective components of the response to become manifest. Collectively, the neuroanatomical relationships between the medial and lateral hypothalamus thus provide the essential substrates which are of survival value to the animal.
Fig. (2).
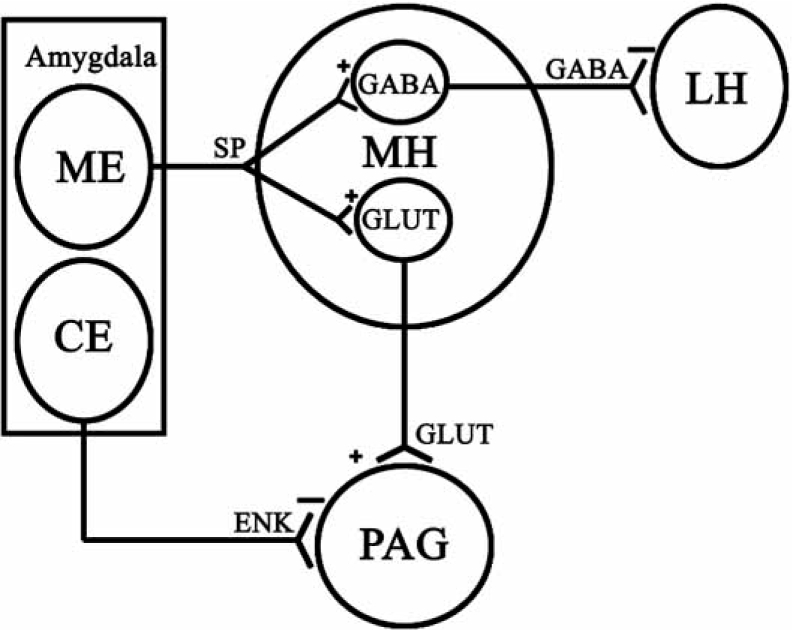
Anatomical and functional bases by which the amygdala modulates defensive rage and predatory attack. The medial amygdala (ME) projects to the medial hypothalamus (MH). Contained within MH includes two classes of neurons – those containing glutamate (GLUT) and those containing GABA. Both classes of neurons receive excitatory input from ME whose functions are mediated by substance P acting through NK1 receptors. GLUT neurons project to the PAG and are excitatory to neurons in this region, the effects of which are mediated through NMDA receptors (see Fig. 3). Thus, the underlying basis for the powerful excitatory effects of ME upon defensive rage includes a disynaptic pathway whose initial synapse is situated in ME and the second synapse in the PAG. In contrast, the same groups of neurons in ME, which potentiate defensive rage, suppress predatory attack. This phenomenon is manifest through activation of GABA neurons in MH, which then project to the lateral hypothalamus (LH) and thus inhibit those neurons are associated with the expression of predatory attack.
Amygdaloid Modulation of Aggression and Rage
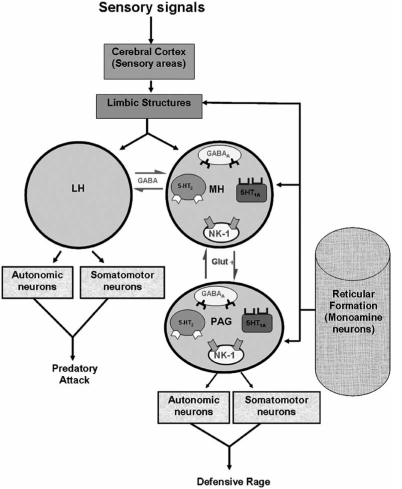
The amygdala is a component of the limbic system, which also includes the hippocampal formation, septal area, prefrontal cortex and cingulate gyrus. This system possesses several common anatomical and functional features that distinguish it from other regions of the brain. These include the following: (1) they receive secondary or tertiary sensory inputs which may vary among limbic structures; (2) they receive inputs from brainstem monoaminergic neurons; and (3) they project directly or indirectly to the hypothalamus and related structures of brainstem. These combined sensory and monoaminergic inputs serve to activate limbic structures to cause powerful modulation of aggression and rage by virtue of their efferent projections to the target structures (Fig. 3). The amygdala was selected for discussion in this section because its modulating effects upon aggression and rage as well as its pathways and related neurotransmitter functions have been identified [83,86].
Fig. (3).
Schematic diagram depicting some of the critical neural mechanisms regulating aggression and contained within limbic structures such as amygdala, hippocampus, and prefrontal cortex are activated by sensory signals that reach them through inputs from sensory regions of cerebral cortex and these limbic neurons are further modulated by monoaminergic neurons situated within the reticular formation of the brainstem. Subsequent changes in levels of excitability within the limbic system alter mediated through efferent pathways of limbic structures such as the fornix and stria terminalis to the hypothalamus, causing changes in excitability levels of hypothalamic neurons, thus directly affecting the neural mechanisms control aggression and rage behavior. The expression of predatory attack is generated in the lateral hypothalamus and the descending pathways which engage both autonomic and somatomotor neurons of the lower brainstem. Likewise, the expression of defensive rage behavior is mediated through neurons in the medial hypothalamus and a glutamatergic descending pathway to the midbrain PAG, which in turn, provides feedback through an ascending neuron onto the medial hypothalamus. The medial and lateral hypothalamus mutually inhibits each other through reciprocal GABAergic neurons. Also depicted in this diagram is that 5-HT1A receptors facilitate and 5-HT2 receptors suppress defensive rage in both MH and PAG, while NK1 receptors in both regions facilitate this form of aggressive behavior.
Excitation of the medial nucleus and medial aspect of the basal complex in the cat of the amygdala potentiates defensive rage behavior elicited from the medial hypothalamus, while excitation of the lateral and central nuclei or lateral aspect of the basal complex suppresses this response [11,80, 82,84]. The potentiating effects of the medial amygdala are mediated over the stria terminalis, which projects to the bed nucleus of the stria terminalis and rostral half of the medial hypothalamus, including the dorsomedial region and shell of the ventromedial nucleus. A primary neurotransmitter of this pathway has been identified and is substance P (SP), acting upon neurokinin-1 (NK1) receptors in the medial hypothalamus [82] (see Fig. 2)). In contrast, excitation of the medial amygdala suppresses predatory attack behavior elicited from the lateral hypothalamus. Suppression is manifest via a disynaptic pathway in which the first limb includes the stria terminalis projection to the medial hypothalamus and the second a GABAergic (inhibitory) neuron projecting from the medial to lateral hypothalamus [37]. The inhibitory effects of the amygdala upon defensive rage behavior are mediated through a descending projection to the midbrain PAG. The neurotransmitter has been shown to be enkephalin acting through μ-opioid receptors in the PAG [79]. In a parallel manner, excitation of the lateral amygdala potentiates predatory attack [8,28]. While the pathway has not been experimentally identified, it is likely to include fibers of the ventral amygdalofugal pathway that project to the lateral hypothalamus [52,99].
Neurochemical Mechanisms of Aggression
Neurotransmitters
An increasing body of knowledge has identified several different classes of neurotransmitters that play a role in regulating aggressive behavior [35,83,86,87]. These include the following small molecule neurotransmitters: acetylcholine, GABA, and biogenic amines (dopamine, norepinephrine and serotonin), and neuropeptides (opioid peptides, substance P, cholecystokinin, vasopressin) (see Table 1). Some of these transmitters potentiate aggressive responses while others have inhibitory properties. In addition, several of these neurotransmitters have been identified with specific neuroanatomical pathways but others have not.
Table 1.
Neurotransmittter-Receptors Modulating Aggressive Behavior
| Neurotransmitter | Receptor | Type of Aggression | Effect |
|---|---|---|---|
| Acetylcholine | Muscarinic | Defensive rage | ↑ |
| Dopamine | D2 | Defensive rage, predatory attack | ↑ |
| Serotonin | 5-HT1A | Defensive rage | ↓ |
| 5-HT2 | Defensive rage | ↑ | |
| Norepinephrine | α2 | Defensive rage | ↑ |
| Defensive rage | ↑ | ||
| Substance P | NK1 | Predatory attack | ↓ |
| Cholecystokinin | CCKB | Defensive rage | ↑ |
| Glutamate | NMDA | Defensive rage | ↑ |
| GABA | GABAA | Defensive rage, predatory attack | ↓ |
| Opioid peptides | μ | Defensive rage | ↓ |
| Vasopressin | V1A | Rodent aggression | ↑ |
| Other compounds: cytokines | |||
| Interleukin-1 | IL-1 Type1 | Defensive rage | ↑ |
| Interleukin-2 | IL-2Rα | Defensive rage (PAG) | ↑ |
| Defensive rage (medial hypothalamus) | ↓ |
↑, Facilitation of aggressive behavior
↓, Suppression of aggressive behavior
Excitatory Neurotransmitters
Studies conducted mainly in rodents and felines have shown that cholinergic agents generally facilitate aggressive responses. These findings are based upon the application of agonists and antagonists systemically that act through muscarinic receptors. Further studies supporting these findings have indicated that cholinergic agents produce their potentiating effects within the region of the medial hypothalamus and can, in fact, induce rage-like responses.
Both dopamine and norepinephrine have similar potentiating effects upon both defensive rage and predatory attack. The mechanism presumably involves activation of catecholaminergic neurons of the brainstem from such regions as the locus ceruleus for norepinephrine and the ventral tegmental area for dopamine, which project to widespread regions of the forebrain, including the hypothalamus and limbic system. Dopamine facilitation is mediated through dopamine D2 and norepinephrine through α2 receptors in the medial hypothalamus. Catecholaminergic facilitation of both defensive rage and predatory attack suggests that these neurotransmitters exert generalized potentiating effects upon whatever ongoing responses are present during the epoch of time when these transmitters are activated. Serotonin distributed from brainstem raphe neurons to the PAG, hypothalamus and limbic system differentially affect defensive rage responses. Activation of 5-hydroxytryptamine (serotonin) type-2 receptors (5-HT2 receptors) in these regions facilitates defensive rage. The suppressing effects of serotonin are indicated below.
As indicated above, there are several pathways associated with the expression or modulation of aggressive or rage behavior whose primary neurotransmitters have been identified. These include glutamate neurons projecting from the medial hypothalamus to the PAG that act through NMDA receptors to mediate the expression of defensive rage, and SP neurons in the medial amygdala that project to the medial hypothalamus, which powerfully facilitate defensive rage and suppress predatory attack (Fig. 2). Glutamate neurons that project from the basal amygdala to the PAG, acting through NMDA receptors, also facilitate defensive rage behavior. Several peptides have also been identified that potentiate defensive rage behavior within the PAG. These include SP, acting through NK1 receptors [34] and cholecystokinin (CCK), acting through CCKB receptors [59]. Within the anterior hypothalamus, it is well known that vasopressin (V) neurons play an important role in temperature regulation and drinking behavior. These neurons also potentiate aggressive responses in rodents, acting through V1A receptors in the anterior hypothalamus [29,100] and vasopressin levels are positively correlated with aggression in humans [23]. The pathway(s) associated with vasopressin functions in aggression have not been identified.
Inhibitory Neurotransmitters
Three neurotransmitters suppress defensive rage behavior. Activation of 5-HT1A receptors in either the PAG or medial hypothalamus by serotonin released from brainstem raphe neurons suppress this form of aggressive behavior [39,78]. Powerful suppression of defensive rage behavior is mediated through μ-opioid receptors in the PAG. As noted above, these receptors are activated by enkephalinergic neurons arising in the central nucleus of amygdala. GABAA receptors in the medial and lateral hypothalamus suppress defensive rage and predatory attack behaviors, respectively. As previously indicated, GABAA receptors in the medial hypothalamus are activated by GABA neurons projecting from the lateral hypothalamus, and likewise, GABA neurons arising in the medial hypothalamus activate these receptors in the lateral hypothalamus (Fig. 2). GABAA receptors in the PAG also suppress defensive rage behavior when activated by GABA neurons, the origin of which has not yet been identified [81].
Genes and Cytokines
Genetic Studies
Two methods have been applied to the study of the role of genetics in aggression and rage behavior. The first involves traditional breeding methods and the second involves the use of genetic engineering to produce “knockout” mice in which specific receptors are absent in the animal. Concerning the first approach, increased levels of aggression are present under the following conditions: (1) in animals selectively bred for heightened sensitivity to cholinergic agonists [77] (2) in animals bred in a manner producing higher levels of brain dopamine levels [76] and (3) in animals bred for selective loss of 5-HT axons [60] Thus, these findings have generally supported the findings obtained from pharmacological and neurochemical approaches summarized above. Concerning the second approach, mice lacking the vasopressin V1b receptor have reduced levels of aggressive behavior [100]. In contrast, mutant mice lacking the 5-HT1B receptor [16] or which display decreased 5-HT turnover in the brain [44,45] have increased levels of aggressive behavior. Paralleling the latter findings, the 5-HT1B gene (HTR1B) has been linked in humans to a diagnosis of antisocial personality disorder and intermittent explosive disorder comorbid with alcoholism [56].
Several authors have employed selective breeding procedures to identify a role for chromosomes in aggressive behavior. For example, Brodkin et al. [10], using an outcross-backcross protocol and genome-wide scan discovered aggression quantitative trait loci on distal chromosome 10 and proximal chromosome X. Of further significance, these authors suggested that the diacylglycerol kinase alpha subunit gene (Dagk1) and the glutamate receptor subunit AMPA3 gene (Gria3) are candidate genes for aggressive behavior. Moreover, Toot et al. [94] used consomic Y-chromosome strains of rats to show that aggressive behavior is linked to the Y-chromosome (see later in ‘Serotonin’ section).
In humans, aggressive behavior is more highly correlated with identical twins than fraternal twins [33,36]. Of further importance, there is evidence for a link between genetics, environment, and aggression. Specifically, Caspi et al. [13] showed that maltreated male children who have a genotype that would be expected to result in increased expression of monoamine oxidase (MAOA), an enzyme that degrades serotonin and norepinephrine, were less likely to express antisocial (including violent) behavior. It should be mentioned, however, that an increase in MAOA would be expected to result in a decrease in serotonin, which as mentioned, is linked with increased aggression. The reasons for this disparity are not known. However, methodological differences across studies should be considered. These include demographic factors, the presence or absence of early life abuse, the possibility that abuse alters serotonin, and type of aggressive behavior measured, among other variables. Biological factors could also play a role. For example, the extent to which serotonin or norepinephrine is altered in a given study linking MAOA and aggression is typically not known. Here, if there were to be preferential reductions in catecholamine levels, then aggression would be expected to be reduced. Moreover, it is not known whether the enzyme is actually altered in subjects. Further to the point, these neurotransmitters affect a wide range of behaviors, which could indirectly influence aggressive behavior. Moreover, a polymorphism of the gene encoding catechol-O-methyltransferase (COMT), which inactivates catecholamines, has been linked with increased aggression in schizophrenic patients [91].
Cytokines and Aggression
Cytokines are pleiotropic cell-cell signaling proteins or glycoproteins produced by various cells types in the periphery and in brain. They were initially described for their roles in innate and acquired immunity. Cytokines also act as neuromodulators, including effects on neurochemical release, neuroendocrine activity, and neurodevelopmental processes, among other effects [25,27]. They also induce marked behavioral and cognitive changes [25,62] and play fundamental roles in other processes, including development, feeding, among other processes. Cytokines exert their biological effects by binding to their receptors, which in turn, activate signaling pathways. Cytokine receptors are present on numerous cell types in periphery and the central nervous system, and may act in autocrine, paracrine, and endocrine manners [25,63]. Thus, a given cytokine can influence activity in the cell from which it is released, as well as local and distant cells. Concerning the paracrine mode of action, some cytokines of peripheral origin may influence brain activity by transport across the blood-brain barrier [2]. Cytokines may also affect brain activity by entry through circumventricular organs, or by indirect routes, including activation of sensory afferents, and stimulation of cytokine release by brain endothelial cells [25,63]. An example of a possible indirect way in which cytokines can modulate aggression was provided from a most recent study concerning the role of C-reactive protein [19]. C-reactive proteins are released from the liver in response to the presence of inflammatory cytokines. In this study, patients were identified from the Buss-Durkee Hostility Inventory on the basis of meeting the criteria for specific personality disorders. The results revealed a positive relationship between aggression scores and plasma C-reactive protein levels. It is possible that the C-reactive protein activates the stress pathways that could influence cytokine production within the brain. Since cytokines and their receptors are present in the brain, they can act as endogenous neuromodulators in regions associated with the expression of aggression and rage, including the hypothalamus and PAG [103].
A further possible linkage between cytokines and aggression is also derived from observations obtained from human studies. Two types of studies have been conducted – one concerning patient populations and the second involved experiments utilizing healthy adult subjects. In studies involving patient populations, cytokine immunotherapy for the treatment of such disorders as AIDS, cancer and hepatitis C have shown that such therapy increased aggressive behavior as determined by measures of anger, hostility and irritability [12,51,67]. In one study, it was demonstrated that experimentally induced enhancement of proinflammatory cytokines was correlated with increases in hostility scores [92]. In another study, a positive correlation was drawn between the presence of hostile marital conflict and increased production of plasma proinflammatory cytokines [49].
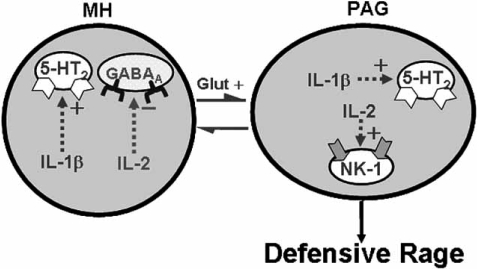
Animal studies have also indicated a relationship between enhancement of cytokine production, immune cell activity and aggression. To cite one example, animals displaying heightened levels of territorial behavior and aggression also revealed higher levels of interferon (IFN)γ and interleukin-2 (IL-2) production from lymphocytes [74]. Several questions are raised from the results of these studies which include the following: (1) Which cytokines relate to the control of aggression and rage? (2) Where in the brain are these cytokines located? and (3) What are the possible mechanisms underlying cytokine modulation of aggressive behavior? Recent studies have provided partial answers to these questions, indicating that IL-1 and IL-2 potently modulate defensive rage behavior and the nature of these effects depend upon the region of the brain where receptors for these cytokines are activated. In one study, it was demonstrated that IL-1β in the medial hypothalamus facilitates defensive rage behavior and that such facilitation is mediated through 5-HT2 receptors [39,40]. In contrast, activation of IL-2 receptors in the same region of the medial hypothalamus suppresses defensive rage behavior. The suppressive effects of IL-2 are mediated through GABAA receptors [4]. Thus, these two cytokines utilize different neurotransmitter mechanisms within the medial hypothalamus to differentially modulate defensive rage behavior. In addition, activation of IL-2 receptors in the PAG potentiates defensive rage behavior, indicating that the locus of activation of this receptor is critical to the nature of its effects upon defensive rage behavior. The potentiating effects of IL-2 in the PAG upon defensive rage are mediated through SP NK1 receptors, a receptor that has known excitatory effects upon defensive rage behavior [3] (Fig. 4). These findings raise an additional question – namely, how can the same cytokine found in different regions of the brain alter defensive rage behavior in opposite ways? An answer to this and related questions concerning how cytokines modulate aggression and rage can only be answered by future studies directed at understanding the signaling pathways associated with these cytokines and their relationship to the neurochemical milieu of the various regions of the forebrain and brainstem associated with the expression and modulation of aggression and rage behavior.
Fig. (4).
Schematic diagram depicts specificity of cytokine modulation of defensive rage behavior. Shown in this diagram are the reciprocal excitatory relationship between the medial hypothalamus (MH) and periaqueductal gray (PAG) in the expression of defensive rage behavior. The reciprocal inhibitory connections between the MH and lateral hypothalamus (LH) are presented in this diagram in order to illustrate the relationships of the defensive rage pathways to those associated with predatory attack. Powerful facilitation of defensive rage from MH is mediated through IL-1 type I and 5-HT2 receptor mechanisms. A similar mechanism exists in the PAG as well. Suppression of defensive rage within MH is mediated through IL-2 and GABAA receptor mechanisms. However, IL-2 in the PAG potentiates defensive rage behavior and this potentiating phenomenon is mediated through IL-2 and substance P – NK1 mechanisms. (Modified from Zalcman and Siegel [103]).
Neurotransmitter Function as Determined from Studies Conducted Utilizing Normal and Patient Populations
Methodological Issues
The studies described above reflect a body of knowledge that has provided a foundation for our understanding of the neurochemical mechanisms underlying aggression and rage behavior. However, our understanding remains incomplete in the absence of a corresponding base of knowledge derived from human studies. Nevertheless, an increasing number of studies accumulated over the past decade conducted in both normal and patient populations have provided support for the findings obtained from animal studies and have suggested several new avenues of investigation. It should be noted that the development of a significant body of data on human subjects which could provide new insights into neurotransmitter functions in the regulation of human aggression is made more difficult by a number of methodological issues inherent in this line of research. A brief description of a number of these issues is presented at this time. One significant problem characteristic of a majority of these studies is that drugs used to study human aggression often times lack receptor specificity, thus confounding interpretation of the data in terms of the relationship of a specific receptorneurotrans-mitter systems to a given form of behavioral modification. Examples of such drugs which lack receptor specificity include lithium [64,65], olanzapine [104] and serotonin reuptake inhibitors, which include, among others, fluoxetine [20,22,23]. A second issue is the nature of experimental and control populations used for these studies. It is virtually impossible to select homogenous populations for each of the groups studied. This may be particularly difficult to accomplish with in-patient populations [64]. Consequently, test measures likely result in greater variability in measurement scores than if the populations were uniform. A third issue is that in many of the studies conducted, analysis of the effects of drugs upon aggressive behavior are secondary to the examination of other behavioral processes, thus reducing the likelihood that such studies included a systematic analysis of aggression and rage behavior. Examples of such difficulties include patients diagnosed with Tourette’s syndrome [90] and borderline personality disorder [104]. Fourth, the methodologies and tools employed by which aggression and rage behavior are measured may vary among investigators. Such variations (such as those associated with different types of rating scales, or those whose findings are based solely upon behavioral observations of the patients [see discussion below]) can increase the discrepancies reported among the results of different studies. A fifth, and related issue, concerns the nature of the form of aggressive behavior examined in many of the reported studies. As determined from both the animal and human literature, at least two forms of aggression can be distinguished, namely affective (or defensive rage) and predatory aggression. Rarely if at all are such distinctions attempted among investigators conducting human studies. In fact, standard scales such as the Buss-Durkee scale leave little room for inclusion of predatory forms of aggression. Yet, as determined from animal studies, the relationship between a given receptor-neurotransmitter system upon an aggressive process is dependent upon the form of aggressive behavior examined.
In spite of these difficulties and limitations, the results of studies conducted on human patient and non-patient populations have provided considerable useful information that has been helpful in generating a better understanding of the relationships between receptor-neurotransmitter systems and aggressive and related forms of behavior. A number of authors have provided excellent reviews of the literature concerning studies of human aggression [32,68]. The main focus of these studies related to the efficacy of drug treatment. In contrast, the primary focus of the last section of this paper, which is not intended to be an exhaustive review of the literature, attempts to provide evidence relating specific neurotransmitter-receptor functions to aggressive behavior. Consequently, the findings described below are arranged according to specific receptor-neurotransmitters wherever possible and summarized below.
Serotonin
Perhaps, of all the receptor-neurotransmitter systems examined in human studies conducted in normal and clinical populations, the findings linking the role of serotonin to aggression have produced the most compelling data in support of a major role of this neurotransmitter in the regulation of aggressive forms of behavior. Various approaches have been utilized for the study of serotonin and aggression. General categories of these approaches include: (1) experimental manipulations of brain serotonin levels, which may include measurements of endocrine responses correlated to serotonin release; and (2) pharmacological manipulation of brain serotonin levels by drugs that either directly or indirectly target the serotonin system. The findings of representative examples of these approaches are summarized below.
One way in which serotonin levels have been altered was to either deplete or enhance plasma tryptophan levels through dietary manipulations. In one study, three variables were manipulated – dietary levels of tryptophan, predisposition to aggression as determined by the Buss-Durkee Hostility Inventory, and induction of aggressive behavior using a “Point Subtraction Aggression Paradigm” [6]. In this paradigm, aggression was expressed in the form of subtraction of money from a fictitious antagonist. The results of this study demonstrated that aggression in the laboratory setting by the more aggressive men was significantly elevated following tryptophan depletion, although tryptophan augmentation was not associated with reduced levels of aggression. The same aggression paradigm was employed to examine the effects of tryptophan depletion in women [66]. Similar to the findings identified in men (described above), women subjected to tryptophan depletion displayed significantly elevated aggression scores. However, unlike the results of the study described above for men, tryptophan enhancement was associated with decreased levels of aggressive responding in women. Thus, these two studies provide evidence tryptophan manipulation can affect aggressive behavior, in which such manipulations appear to be more effective upon women than upon men. Nevertheless, both of these studies provide support for an apparent inverse relationship between serotonin levels and the propensity for the expression of aggressive behavior. In one additional study, it was reported that tryptophan augmentation was associated with significant decreases in quarrelsome behaviors, a finding that in general appears consistent with the overall findings of other studies described above.
In a related study, Hennig et al. [43] were able to identify variations of the tryptophan hydroxylase gene, which, if linked with specific individuals, could explain the lower levels of serotonin in the brain and thus account for heightened expressions of aggressive behavior. Indeed, utilizing healthy male subjects, these authors were able to identify several nucleotide polymorphisms of the tryptophan hydroxylase gene, one of which was then correlated with high levels of the aggressive hostility factor as determined from the Buss-Durkee and related inventory scales. Thus, the findings of this study are clearly consistent with those utilizing other strategies described in this section.
It should also be noted that, most recently, essential fatty acids have been identified as a factor correlated with the expression of impulsive aggressive behavior [31]. In their review of the literature, these authors specifically suggest that essential fatty acids influence hormone receptor density and ion channels, especially those associated with the release and elevations of serotonin levels within brain, thus providing further indirect support for the view linking serotonin levels in the brain and aggressive behavior.
Another approach aimed at linking brain serotonin levels with aggressive behavior involved the use of the drug, fenfluramine, an indirect 5-HT agonist, which enhances the availability of serotonin at the postsynaptic region. Because the release of 5-HT at central synapses induces the release of anterior or posterior pituitary hormones such as prolactin and oxytocin, respectively, the measurement of hormonal levels in blood in response to fenfluramine challenge can be used as an indirect measure of serotonin levels and then correlated with states of aggressive behavior in normal and patient populations. This approach has been developed and adapted by Coccaro and colleagues and their findings as well as those from other laboratories were published in a series of papers over the past 10 years [18,21,24,26,57]. The relationship between fenfluramine and 5-HT was revealed by a significant coefficient of correlation (reflecting an inverse relationship) between prolactin-fenfluramine responses and the serotonin metabolite, 5-HIAA concentrations, in CSF [21,24] thus permitting the authors to conclude that the prolactin-fenfluramine response represents an index of integrated postsynaptic 5-HT activity. These authors further demonstrated that the prolactin-fenfluramine responses correlated inversely with the assault scale of the Buss-Durkee Hostility Inventory Scale. However, it should also be noted that there is evidence suggesting that the relationship between fenfluramine and serotonin may not necessarily be as simple as implied above. Experiments conducted in rat have shown that this relationship is dependent upon the amount of tryptophan provided in the diet [89]. Specifically, these authors observed that following fluvoxamine and fenfluramine administration, 5-HT release was significantly diminished in rats on a low diet of tryptophan, and significantly enhanced after fenfluramine treatment in rats on a high tryptophan diet.
Several other investigators also utilized fenfluramine as an experimental means of altering serotonin levels by comparing such changes to aggression scores in normal populations [18,26]. One study provided further evidence in support of an inverse relationship between fenfluramine induced cortisol responses and scores on the Buss-Durkee Hostility Inventory total score and aggression factor in normal healthy adult males [18]. However, these authors failed to find similar relations with females with respect to cortisol or prolactin responses. Perhaps, the sex differences in this and in other studies may be due in part to differences in experimental populations or to variations in the handling of fenfluramine, especially since plasma levels of this agonist were not measured. Consistent with these findings, Dolan and Anderson [26] utilizing personality disordered offenders, did report an inverse relationship between the prolactin response to fenfluramine challenge and measures of impulsive-antisocial conduct. Thus, it appears that there seems to be a general consensus concerning an inverse relationship between serotonin levels and the expression of aggressive behavior as determined by methods employing fenfluramine as an experimental tool.
A somewhat more extensive approach included a variety of studies that examined the effects of drugs that selectively affect 5HT receptors as well as those whose properties affect other receptor systems as well. These studies are considered in this section. Concerning the effects of administration of selective 5-HT receptor compounds, Several studies have examined the effects of the 5-HT reuptake inhibitor, fluoxetine, upon different groups of patient populations [20,22]. In one study, patients who initially displayed positive correlations between prolactin responses to d-fenfluramine and improvement on an Overt Aggression Scale directed against aggression and irritability were later tested following fluoxetine administration [22]. The results indicated a significant improvement in the overt aggression scale as a result of treatment with fluoxetine, thus providing evidence that the anti-aggressive actions of this drug are dependent upon activation of brain 5-HT synapses. Similar findings were reported by the same investigators following fluoxetine treatment in outpatients with histories of impulsive aggressive behavior and irritability, utilizing the Overt Aggression Scale directed against aggression and irritability relative to patients administered placebos [20]. Moreover, these patients displayed reductions in aggression scores that were sustained for 2-3 months of treatment. Similar findings were also reported following fluoxetine administration to women diagnosed with borderline personality disorder [104]. The issue had been raised that fluoxetine administered in the treatment of depression, alcoholism, eating and obsessive-compulsive disorders may be associated with heightened levels of aggressive behavior. However, a meta-analysis did not provide support for this notion [41].
It should be noted that not all studies utilizing 5-HT reuptake inhibitors have demonstrated similar results. In an early study [75] the antidepressant drug, trazodone, yielded inconsistent results in a study of a small sample of seven patients, in which three patients displayed reductions in aggression and another three showed no clear-cut change in aggressiveness. Perhaps, the inconsistency in this finding may be due to the small sample size, dose levels employed, or possibly to non-specific effects of the drug.
Several other studies have been reported in the literature that has tested relatively selective 5-HT compounds against aggressive behavior. In one study, 12 normal male subjects were selected for study on the basis of high aggression scores on the Buss-Durkee Inventory Scale [17]. Endocrine responses (i.e., prolactin, growth hormone, cortisol, ACTH) were measured following administration of the 5-HT1A antagonist, ipsapirone. It was of interest to note that subjects who displayed blunted endocrine responses in response to ipsapirone treatment (i.e., suggesting reduced serotonergic activity), also displayed higher ratings on aggression. Thus, this finding is clearly consistent with the observations resulting from the studies described above in that, in the present study, heightened levels of aggression are linked to decreases in serotonergic activity in the brain, while, in the those considered above, increased serotonergic activity in the brain is associated with reduced levels of aggression.
The relationship between serotonin and endocrine responses in altering the expression of aggressive behavior extends to gonadal hormones (see review in Birger et al. [5]). For example, Higley and Mehlmen [45] showed that the rate and intensity of aggressive behavior in subjects with reduced 5-HIAA levels in the CSF were exaggerated and that these same subjects also displayed high testosterone concentrations. Of further importance, reduced serotonin activity was linked with impulsivity, while increased testosterone was associated with competitive aggression. Thus, it was suggested that the inverse relationship between 5-HIAA and testosterone levels might result in increases in impulsivity and competitive aggression, which would be expected to increase the severity of aggressive behavior. Consistent with these findings, genetic studies using consomic Y-chromosome strains of rats have provided further support for the serotonin-testosterone-aggression relationship. Toot et al. [94] crossed Spontaneously Hypertensive Rats (SHR) with normotensive Wistar-Kyoto rats WKY) to generate animals whose only genetic difference was the type of Y chromosome that they possessed (SHR Y-chromosome or WKY Y-chromosome). Aggressive behavior, as determined by attack number, as well as wounding and scarring scores in the resident-intruder test, was more pronounced in males with the SHR Y-chromosome than in males with the WKY Y-chromosome [94]. The more aggressive rats also showed increased serum levels of testosterone coincident with reduced serotonin levels in the amygdala compared to the less aggressive rats. Based on these findings, the authors suggested that the between strain differences in aggressive behavior as well as endocrine and neurochemical changes were due to a mutation in a region of the Y-chromosome.
There are also a wide variety of studies that are briefly summarized below because the drugs employed are known or believed to involve at least in part, the serotonin system. The difficulty inherent in relating the results of such studies to serotonin function is that these drugs are known to affect other or multiple receptor systems. In fact, one could argue that the placement of such studies under the heading of different neurotransmitters would be just as logical, but are kept in this section because of the overwhelming evidence linking serotonin activity in the control of aggression and impulsive behavior.
A number of studies have employed lithium for the treatment of disorders such as bipolar disorder and depression. In addition, a number of papers have reported positive effects of the use of lithium in the treatment of aggressive disorders as well [2,7,64,65,88]. Lithium is believed to affect neurotransmitter and receptor systems, in particular by increasing serotonin synthesis and decreasing norepinephrine release. In one case report, it was concluded that lithium administration resulted in a decrease in the frequency of aggressive outbursts in two brain-injured children [2]. In a more extensive, well-controlled double blind study, it was reported that lithium reduced aggression in an in-patient population (relative to placebo treated control patients) as measured by ratings on the Overt Aggression Scale. Although the authors concluded that lithium was safe for short-term treatment of aggression, many of the subjects reported side effects of the drug such as nausea, vomiting and frequent urination, thus complicating interpretation of the data. Similar conclusions were drawn from a study utilizing conduct disordered children in which ratings of aggression decreased significantly over time [64].
A number of other findings described in the literature have also given support to the role played by serotonin in the regulation of aggressive behavior. Several reports have provided evidence that olanzapine, which has a high affinity for serotonin and dopamine receptors, can reduce aggression in female patients with borderline personality disorder [104] and in children with Tourette’s syndrome [90]. Another study reported that application of the drug, quetiapine, which is believed to function as an antagonist at dopamine and 5-HT receptors, was also effective in reducing outbursts and impulsive aggressiveness in patients suffering from borderline personality disorder [1]. Again, it cannot be determined whether the suppressive effects upon aggression were the result of the drug’s actions on dopamine or 5-HT receptors. Moreover, it the drug did affect 5-HT receptors, it cannot be determined which of the 5-HT receptors were blocked. In addition, this drug was not without its side effects in that it caused somnolence, dry mouth and dizziness, thus again complicating interpretation of the data.
GABA
Several basic differences exist between the studies utilizing compounds related to serotonin and those associated with GABA. One major difference is that many more studies related to serotonin have been directed against aggressive behavior in both normal and patient populations than those utilizing compounds related to GABA. A second difference is that, in general, the GABAergic compounds were ones designed for the study of seizures disorders and not for the study of aggression or related emotional disorders. Anti-epileptic drugs are frequently directed against seizures whose primary foci are situated within limbic system structures comprising principal components of the temporal lobe that also powerfully modulate aggressive behavior. Accordingly, it is reasonable to understand how these drugs could also affect aggressive processes. The studies described below summarize the effects of several anticonvulsant drugs directed against GABA receptors upon aggressive and impulsive behaviors.
A number of studies have examined the effects of two closely related anticonvulsant drugs, valproic acid and divalproex, upon impulsivity and aggression in patient populations. Valproic acid is presumed to enhance GABA function in the brain by acting as a transaminase inhibitor, thus preventing its conversion to glutamate in glia. A retrospective study was conducted by Lindenmayer and Kotsaftis [58] which described the findings of 17 separate case reports. For the most part, patients employed in these studies were diagnosed with mental retardation, organic brain syndromes, dementia, schizophrenia, bipolar or borderline personality disorders. Collectively, the case studies reported an approximate 50% reduction in aggressive outbursts following administration of valproic acid. An illustration of one such study is cited here. In this study, [102] four of five patients who suffered traumatic brain injury resulting from automobile injuries and a fifth experienced toxic encephalopathy. In each of these cases, treatment with valproic acid was reported as having been effective in reducing aggressive and destructive behaviors.
Several methodological issues arise from these studies. One major concern here is the absence of double blind designs in the respective studies. In fact, in a recent study in which a double-blind, placebo design was employed, involving children and adolescents with pervasive developmental disorders, no differences were reported between valproate and placebo treated groups over an eight week period [42]. A second concern is that the doses of drug administered ranged widely from study to study. Thirdly, the wide range of patient populations studied creates difficulties in generating overall comparisons of the data. Fourthly, the length of time in which these studies were conducted may not have been optimal and need to be extended. Nevertheless, the data suggested that valproic acid was most effective when used to treat patients with organic brain syndromes, and somewhat less effective with those characterized with dementia, mental retardation and bipolar disorder.
Although the precise mechanism of action of divalproex is not known, it is related to valproic acid and, like valproic acid, its activity results in increased levels of GABA within the brain. In one study, Kavoussi and Coccaro [48] employed 10 patients who were diagnosed with personality disorder. These patients were treated for eight weeks with divalproex sodium and clinical ratings for impulsive aggression and irritability were made bi-weekly, utilizing a modification of the Overt Aggression Scale. In six of the eight patients who completed the study, significant improvement was shown in terms of control of irritability and impulsive aggression, leading these authors to conclude that divalproex sodium can be used for the effective treatment of impulsive aggressive behavior. Consistent with the previous findings, a retrospective study comparing violent and non-violent hospitalized patients treated with Divalproex suggested to the authors that this drug had a calming effect upon the patients studied. Although this study did not employ a double-blind paradigm, the results were consistent with the following study which did employ such a paradigm. In this study, a relatively larger sample size of patients diagnosed with borderline personality disorder, were treated with divalproex for 12 weeks (N=20 drug-treated vs N=32 placebo treated) [46]. The results suggest that both trait impulsivity and state aggression symptoms were reduced following drug treatment in patients with borderline personality disorder.
In another related study, Nickel et al. [72] examined the effects of topiramate upon aggressive behavior in female personality disorder patients. Unlike valproic acid, topiramate appears to have some non-specific properties in that it inhibits excitatory neurotransmission through its actions on kainate and AMPA receptors as well as enhancing GABA activated chloride channels. Therefore, the properties of this drug make it more difficult to ascertain whether its effects are due to its actions on excitatory or inhibitory amino acids. The study, employing double-blind and placebo controls, nevertheless demonstrated that application of this drug resulted in improved scores on various scales measuring anger. In a separate study, flumazenil was employed for the treatment of aggressive behavior in male adults with histories of conduct disorder [93]. This study is included here because flumazenil, which is a benzodiazepine antagonist, is known to reverse the effects of benzodiazepines by competitive inhibition of the benzodiazepine binding site on the GABAA receptor. In contrast to the previous studies described above, the findings of this study failed to reveal any changes in aggressive responding following flumazenil treatment.
One other study should be noted here that concerns the drug, oxcarbazepine. This drug shares one similarity with those described above as it is an anticonvulsant compound. On the other hand, it differs from the others in that it is believed to reduce neuronal activity by inhibiting sodium channels. Although the specific neurotransmitter-receptor systems upon which it acts have not been clearly defined, it has been suggested that oxcarbazepine, as well as the related compound, carbamazepine, may act through GABA receptors [61]. In this placebo controlled, double-blind study, patients were selected on the basis of their meeting the criteria for intermittent explosive disorder. The results, similar to the ones described above, suggested that administration of oxcarbazepine provides benefit to patients who express significant impulsive aggression.
When taken in its totality, and in spite of the lack of specificity in some of the drugs with GABAergic properties, it would appear that the studies described above do, in fact, provide support for the view that activation of GABA receptors are effective in reducing impulsive and affective aggressive behavior.
Dopamine
A number of studies, including retrospective analyses, have been conducted over the past decade which evaluated the efficacy of a number of antipsychotic (or neuroleptic) agents upon aggressive and impulsive behaviors, which was conducted mainly upon patient populations. These include such drugs as clozapine, olan-zapine, risperidone, haloperidol, chlorpromazine, and loxapine. In general, a common feature of these drugs is that they appear to act through dopamine receptors. Typically, drugs such as chlorpromazine, risperidone, and clozapine affect additional receptor systems as well, including other monoamine receptors. Thus, one cannot discount a possible role for 5-HT2 receptors in studies using such drugs as well as by possibly affecting the sensitivity of serotonergic systems (e.g., chlorpromazine). Nevertheless, the effects of these drugs upon aggressive behavior are included in this section because of their common antagonistic effects upon the dopamine system.
Several reports have recently emerged describing the effects of treatment with clozapine upon patient populations [9,50,95-97]. One of these reports is an extensive retrospective analysis involving a review of the effects of treatment of 331 schizophrenic patients [95]. In this analysis, it was reported that, prior to drug treatment, 31.4% of the patients displayed overt physical aggression. Following an average of 47 weeks of clozapine treatment, the authors reported evidence of a reduction in aggressive incidents to 1.1%. The authors further pointed out that the effects were not likely attributed to non-specific factors since there was no evidence of the presence of sedative or general antipsychotic effects upon these patients. Moreover, drug treatment also did not appear to affect other symptoms associated with the respective disorders associated with these patients. However, the data comprising this analysis appeared to lack such controls as a double-blind design and the presence of placebo treated groups. Based upon two other reviews of the literature concerning the effects of clozapine upon aggressive behavior, similar conclusions were drawn with respect to its effectiveness as an anti-aggressive agent [9,96]. Further support for this conclusion was reached following a double-blind study testing the effects of various antipsychotic drugs (i.e., clozapine, olanzapine, risperidone and haloperidol) upon aggressive behavior in patients with schizophrenia [97]. The major conclusions reached from this study were: (1) that clozapine was found to be the most effective drug employed for the treatment of aggressive reactions; (2) that risperidone and olanzapine were also effective in the treatment of aggression in these patients, and (3) that a comparison between the effects of clozapine and haloperidol upon aggression was statistically significant. Data highly consistent with these findings were recently reported by Krakowski et al. [50]. It is of interest to note that the effects of clozapine in reducing aggression were selective relative to its effects upon other psychiatric symptoms.
The effects of administration of risperidone upon different patient populations of individuals who displayed aggressive behavior were determined in two studies. In one of the studies [7] it was reported that children treated with risperidone three months after discharge from inpatient hospitalization displayed significantly improved behavioral ratings as determined by the parents on a New York Parent Rating Scale for Disruptive Behavior. However, the presence of standard control procedures was not apparent from this study. It is of interest to note that in a study involving patients receiving a diagnosis of Alzheimer’s disease demonstrated that risperidone administration was significantly more effective than placebo in treating physical threats and violent behavior.
Not all drugs that act against dopamine receptors have been successful in the treatment of aggressive behavior. In a retrospective study involving intellectually disabled individuals conducted over a 7-year period, it was reported that drugs that included thioridazine or chlorpromazine, or haloperidol, loxapine, or thiothixene, resulted in significant occurrences of relapses over different periods of time. It is possible, however, that the negative findings may be due to or influenced by the dose levels of drugs employed as well as over the frequencies and time periods in which the drugs were administered. One additional study should be noted here. Huizinga et al. [47] sought to test the hypothesis that specific variations in monoamine oxidase A genotype affects the relationship between exposure to adolescent maltreatment and later antisocial behavior. This study revealed the absence of any relationship between adolescent violent victimization and monoamine oxidase A as determined from DNA samples taken from subjects in this study. As indicated previously, dopaminergic and serotonergic systems are antagonistic to each other in the modulation of aggressive and impulsive behavior. Therefore, it is not surprising that positive findings were not observed in this study since monoamine oxidase degrades dopamine, norepinephrine and serotonin. One would thus conclude that drugs that act upon monoamine oxidase would not likely significantly alter aggressive behaviors. Nevertheless, the overall findings of the studies described in this section do suggest that drugs that act as antagonists at dopamine receptors are effective in reducing impulsive and aggressive forms of behavior.
Sites in the Brain Where Drugs May Act to Modulate Aggression and Rage
In the beginning of this paper, we described the primary sites associated with the expression and modulation of aggression and rage. Thus, it is reasonable to conclude that likely sites where drugs would presumably include the hypothalamus and midbrain PAG from which these forms of aggression are expressed. In addition, the various structures comprising the limbic system, which play important roles in modulating aggression and rage, are also likely to be significant targets of these drugs. These regions include the amygdala, hippocampal formation, septal area, anterior aspects of the cingulate gyrus and prefrontal cortex. Of these structures, the amygdala and prefrontal cortex appear to have the most potent modulating properties with respect to the regulation of aggression and rage [83]. The role of the amygdala and its possible neuroanatomical substrates and underlying neurochemical mechanisms were summarized earlier in this paper. Concerning the prefrontal cortex, experimental animal studies clearly point to the likelihood that the prefrontal cortex exerts a powerful inhibitory effect upon aggression and rage [83], for a detailed analysis of this region of the brain). Attention was initially given to the role of the prefrontal cortex in the regulation of emotional behavior from the description of aberrant behavior in the case of Phineas Gage following the passage of an iron rod through his skull, causing massive damage to the frontal region of his brain [38]. In recent years, an increasing body of studies, utilizing imaging methods, have provided evidence of reduced metabolic activity in the prefrontal regions of aggressive patients with personality disorders [71]. Concerning the relationship of transmitter function in the prefrontal cortex of patients displaying impulsive aggression, New et al. [71] provided evidence that patients diagnosed with borderline personality disorder and administered fluoxetine displayed both improvement on the Overt Aggression Scale as well as increases in metabolic rates in the orbitofrontal cortex. Thus, this study provides direct evidence that selective serotonin reuptake inhibitors act, at least in part, through the prefrontal cortex in suppressing impulsive aggression. This study, however, does not preclude the possibility or likelihood that such drugs act through other regions of the brain, notably the hypothalamus and PAG, to modulate aggression and rage.
CONCLUSIONS
In summary, the anatomical substrates and neurochemical mechanisms underlying aggression and rage behavior are becoming more clearly understood. Indeed, this level of knowledge has provided the rationale bases for the application of drugs for the treatment of specific forms of aggressive and impulsive behavior. Most, if not all, of the drugs used for treatment have been applied to the study of affective (defensive) rather than predatory aggression. The primary pathways underlying the expression of affective (defensive) rage behavior arise mainly from the anterior medial hypothalamus and project to the dorsolateral aspect of the PAG. The medial hypothalamus receives significant excitatory input from the medial amygdala, the effects of which are mediated by substance P acting through NK1 receptors. Other excitatory inputs to the medial hypothalamus are mediated through monoamines, acting upon noradrenergic α2, dopaminergic D2, and serotonergic 5HT2 receptors. Inhibitory inputs to the medial hypothalamus include mainly GABAergic neurons from the lateral hypothalamus and possibly elsewhere acting through GABAA postsynaptic receptors, and serotonin, acting through 5-HT1A receptors. The principal neurotransmitter associated with this pathway is glutamate whose functions are mediated by NMDA receptors in the PAG. Other neurotransmitters or neuromodulators that potentiate defensive rage at the level of the PAG include subtance P, acting through NK1 receptors, and cholecystokinin, acting through CCKB receptors. Inhibitory inputs to the PAG include GABAergic neurons, which likely function as interneurons within the neutrophil, powerful enkephalinergic neurons that arise from the central nucleus of amygdala and which project to the PAG, acting through opioid μ receptors, and serotonin, acting through 5-HT1A receptors. The outputs from the PAG which contribute to the expression of rage behavior include pathways directed to both autonomic nuclei of the lower brainstem and to somatomotor nuclei of cranial nerves V and VII, which allow for the vocalization components of this behavior as well as indirectly to lower cervical levels of the spinal cord which contribute to the use of the forepaw in attacking the target animal. An ascending component of the output of the PAG supplies the medial hypothalamus, thus providing a positive feedback to the medial hypothalamus, which permits prolongation of the rage response. Recent studies have revealed the potential importance cytokines, IL-1 and IL-2 in the regulation defensive rage behavior. These cytokines mediate their modulating effects through GABAA, 5-HT1A, 5-HT2, and NK1 receptors in the medial hypothalamus and PAG. Knowledge of the neurotransmitter mechanisms regulating predatory attack behavior is not well understood.
A number of methodological problems are inherent in studies conducted in humans, including the fact that few if any of the subjects utilized for such studies are tested for predatory modes of aggression. Nevertheless, important conclusions have been suggested from these findings. These include the likelihood that drugs that have agonist-like properties or increase levels of serotonin and GABA in the brain reduce levels of aggressiveness and impulsive behavior. Likewise, drugs that are related to dopamine and antagonize the action of this neurotransmitter also have been found to have positive effects upon aggressive and impulsive behavior. Moreover, we know very little about possible interactions between and among neurotransmitter systems. It may be entirely possible or even likely that behavioral changes linked to specific forms of aggression involve reciprocal interactions among several neurotransmitters, manifested by either increases and/or decreases in each of the systems. These possibilities clearly complicate our understanding of the role of neurotransmitters in the regulation of aggressive forms of behavior and point to the need for further studies directed at clarifying the relationships between these neurotransmitter systems. An emerging path of investigation, based mainly on animal studies involving the use of cytokines for the regulation of aggression and rage behavior, is lacking in studies conducted along these lines in humans. Further investigations in this area conducted both on the animal and human level may hopefully reveal new insights into the mechanisms underlying aggression and rage behavior.
ACKNOWLEDGEMENTS
This project was supported by NIH grant NS 07941-35.
REFERENCES
- 1.Bellino S, Paradiso E, Bogetto F. Efficacy and tolerability of quetiapine in the treatment of borderline personality disorder. J Clin Psychiatry. 2006;67:1042–1046. doi: 10.4088/jcp.v67n0705. [DOI] [PubMed] [Google Scholar]
- 2.Bellus SB, Stewart D, Vergo JG, Kost PP, Grace J, Barkstrom SR. The use of lithium in the treatment of aggressive behaviours with two brain-injured individuals in a state psychiatric hospital. Brain Inj. 1996;10:849–860. doi: 10.1080/026990596123954. [DOI] [PubMed] [Google Scholar]
- 3.Bhatt S, Siegel A. Potentiating role of interleukin 2 (IL-2) receptors in the midbrain periaqueductal gray (PAG) upon defensive rage behavior in the cat: Role of neurokinin NK1 receptors. Behav Brain Res. 2006;167:251–260. doi: 10.1016/j.bbr.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 4.Bhatt S, Zalcman S, Hassanain M, Siegel A. Cytokine modulation of defensive rage behavior in the cat: Role of GABA(A) and interleukin2 receptors in the medial hypothalamus. Neuroscience. 2005;133:17–28. doi: 10.1016/j.neuroscience.2005.01.065. [DOI] [PubMed] [Google Scholar]
- 5.Birger M, Swartz M, Cohen D, Alesh Y, Grishpan C, Kotelr M. Aggression: the testosterone-serotonin link. Isr Med Assoc J. 2003;5:653–658. [PubMed] [Google Scholar]
- 6.Bjork JM, Dougherty DM, Moeller FG, Swann AC. Differential behavioral effects of plasma tryptophan depletion and loading in aggressive and nonaggressive men. Neuropsychopharmacology. 2000;22:357–369. doi: 10.1016/S0893-133X(99)00136-0. [DOI] [PubMed] [Google Scholar]
- 7.Blader JC. Pharmacotherapy and postdischarge outcomes of child inpatients admitted for aggressive behavior. J Clin Psychopharmacol. 2006;26:419–425. doi: 10.1097/01.jcp.0000227356.31203.8a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Block CH, Siegel A, Edinger H. Effects of amygdaloid stimulation upon trigeminal sensory fields established during hypothalamicallyelicited quiet biting attack in the cat. Brain Res. 1980;197:39–55. doi: 10.1016/0006-8993(80)90433-3. [DOI] [PubMed] [Google Scholar]
- 9.Briken P, Nika E, Krausz M, Naber D. Atypical neuroleptics in the treatment of aggression and hostility in schizophrenic patients. Fortschr Neurol Psychiatr. 2002;70:139–144. doi: 10.1055/s-2002-20501. [DOI] [PubMed] [Google Scholar]
- 10.Brodkin ES, Goforth SA, Keene AH, Fossella JA, Silver LM. Identification of quantitative trait loci that affect aggressive behavior in mice. J Neurosci. 2002;22:1165–1170. doi: 10.1523/JNEUROSCI.22-03-01165.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brutus M, Shaikh MB, Siegel A, Edinger H. Effects of experimental temporal lobe seizures upon hypothalamically elicited aggressive behavior in the cat. Brain Res. 1986;366:53–63. doi: 10.1016/0006-8993(86)91280-1. [DOI] [PubMed] [Google Scholar]
- 12.Capuron L, Ravaud A, Miller AH, Dantzer R. Baseline mood and psychosocial characteristics of patients developing depressive symptoms during interleukin-2 and/or interferon-alpha cancer therapy. Brain Behav Immun. 2004;18:205–213. doi: 10.1016/j.bbi.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, Taylor A, Poulton R. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297:851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- 14.Cheu JW, Siegel A. GABA receptor mediated suppression of defensive rage behavior elicited from the medial hypothalamus of the cat: role of the lateral hypothalamus. Brain Res. 1998;783:293–304. doi: 10.1016/s0006-8993(97)01357-7. [DOI] [PubMed] [Google Scholar]
- 15.Chi CC, Flynn JP. Neuroanatomic projections related to biting attack elicited from hypothalamus in cats. Brain Res. 1971;35:49–66. doi: 10.1016/0006-8993(71)90594-4. [DOI] [PubMed] [Google Scholar]
- 16.Chiavegatto S, Dawsons VL, Mamounas LA, Koliatsos VE, Dawson TM, Nelson RJ. Brain serotonin dysfunction accounts for aggression in male mice lacking neuronal nitric oxide synthase. PNAS. 2001;98:1277–1281. doi: 10.1073/pnas.031487198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cleare AJ, Bond AJ. Ipsapirone challenge in aggressive men shows an inverse correlation between 5-HT1A receptor function and aggression. Psychopharmacology. 2000;148:344–349. doi: 10.1007/s002130050061. [DOI] [PubMed] [Google Scholar]
- 18.Cleare AJ, Bond AJ. Does central serotonergic function correlate inversely with aggression? A study using D-fenfluramine in healthy subjects. Psychiatry Res. 1997;69:89–95. doi: 10.1016/s0165-1781(96)03052-1. [DOI] [PubMed] [Google Scholar]
- 19.Coccaro EF. Association of C-reactive protein elevation with trait aggression and hostility in personality disordered subjects: a pilot study. J Psychiatry Res. 2006;40:460–465. doi: 10.1016/j.jpsychires.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 20.Coccaro EF, Kavoussi RJ. Fluoxetine and impulsive aggressive behavior in personality-disordered subjects. Arch Gen Psychiatry. 1997;54:1081–1088. doi: 10.1001/archpsyc.1997.01830240035005. [DOI] [PubMed] [Google Scholar]
- 21.Coccaro EF, Kavoussi RJ, Cooper TB, Hauger RL. Central serotonin activity and aggression: Inverse relationship with prolactin response to d-fenfluramine, but not CSF 5-HIAA concentration, in human subjects. Am J Psychiatry. 1997;154:1430–1435. doi: 10.1176/ajp.154.10.1430. [DOI] [PubMed] [Google Scholar]
- 22.Coccaro EF, Kavoussi RJ, Hauger RL. Serotonin function and antiaggressive response to fluoxetine: a pilot study. Biol Psychiatry. 1997;42:546–552. doi: 10.1016/S0006-3223(97)00309-0. [DOI] [PubMed] [Google Scholar]
- 23.Coccaro EF, Kavoussi RJ, Hauger RL, Cooper TB, Ferris CF. Cerebrospinal fluid vasopressin levels: correlates with aggression and serotonin function in personality-disordered subjects. Arch Gen Psychiatry. 1998;55:708–714. doi: 10.1001/archpsyc.55.8.708. [DOI] [PubMed] [Google Scholar]
- 24.Coccaro EF, Kavoussi RJ, Trestman RL, Gabriel SM, Cooper TB, Siever LJ. Serotonin function in human subjects: intercorrelations among central 5-HT indices and aggressiveness. Psychiatry Res. 1997;73:1–14. doi: 10.1016/s0165-1781(97)00108-x. [DOI] [PubMed] [Google Scholar]
- 25.Dantzer R, Aubert A, Bluthe RM, Gheusi G, Cremona S, Laye S, Konsman JP, Parnet P, Kelley KW. Mechanisms of the behavioural effects of cytokines. Adv Exp Med Biol. 1999;461:83–105. doi: 10.1007/978-0-585-37970-8_6. [DOI] [PubMed] [Google Scholar]
- 26.Dolan MC, Anderson IM. The relationship between serotonergic function and the Psychopathy Checklist: Screening Version. J Psychopharmacol. 2003;17:216–222. doi: 10.1177/0269881103017002011. [DOI] [PubMed] [Google Scholar]
- 27.Dunn AJ. Effects of cytokines and infections on brain neurochemistry. In: Ader R, Felten DL, Cohen N, editors. Psychoneuroimmunology. San Diego, CA: Academic Press, Inc.; 2001. pp. 649–666. [Google Scholar]
- 28.Egger MD, Flynn JP. Effects of electrical stimulation of the amygdala on hypothalamically elicited attack behavior in cats. J Neurophysiol. 1963;26:705–720. doi: 10.1152/jn.1963.26.5.705. [DOI] [PubMed] [Google Scholar]
- 29.Ferris CF, Delville Y. Vasopressin and serotonin interactions in the control of agonistic behavior [Review] Psychoneuroendocrinology. 1994;19:593–601. doi: 10.1016/0306-4530(94)90043-4. [DOI] [PubMed] [Google Scholar]
- 30.Fuchs SAG, Edinger HM, Siegel A. The organization of the hypothalamic pathways mediating affective defense behavior in the cat. Brain Res. 1985;330:77–92. doi: 10.1016/0006-8993(85)90009-5. [DOI] [PubMed] [Google Scholar]
- 31.Garland MR, Hallahan B. Essential fatty acids and their role in conditions characterised by impulsivity. Int Rev Psychiatry. 2006;18:99–105. doi: 10.1080/09540260600582009. [DOI] [PubMed] [Google Scholar]
- 32.Goedhard LE, Stolker JJ, Heerdink ER, Nijman HL, Olivier B, Egberts TC. Pharmacotherapy for the treatment of aggressive behavior in general adult psychiatry: a systematic review. J Clin Psychiatry. 2006;67:1013–1024. doi: 10.4088/jcp.v67n0702. [DOI] [PubMed] [Google Scholar]
- 33.Gottesman II, Goldsmith H. Developmental psychopathology of antisocial behavior: inserting genes into its ontogenesis and epigenesis. In: Nelson CA, editor. Threats to optimal development: integrating biological, psychological and social risk factors. Hillsdale, NJ: Lawrence Erlbaum Associates; 1994. pp. 69–104. [Google Scholar]
- 34.Gregg TR, Siegel A. Differential effects of NK1 receptors in the midbrain periaqueductal gray upon defensive rage and predatory attack in the cat. Brain Res. 2003;994:55–66. doi: 10.1016/j.brainres.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 35.Gregg TR, Siegel A. Brain structures and neurotransmitters regulating aggression in cats: implications for human aggression. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:91–140. doi: 10.1016/s0278-5846(00)00150-0. [DOI] [PubMed] [Google Scholar]
- 36.Grove WM, Eckert ED, Heston LL, Bouchard TJ, Jr, Segal NL, Lykken DT. Heritability of substance abuse and antisocial behavior: a study of monozygotic twins raised apart. Biol Psychiatry. 1990;27:293–304. doi: 10.1016/0006-3223(90)90500-2. [DOI] [PubMed] [Google Scholar]
- 37.Han Y, Shaikh MB, Siegel A. Medial amygdaloid suppression of predatory attack behavior in the cat: II. Role of a GABAergic pathway from the medial to the lateral hypothalamus. Brain Res. 1996;716:72–83. doi: 10.1016/0006-8993(95)01587-6. [DOI] [PubMed] [Google Scholar]
- 38.Harlow JM. Passage of an iron rod through the head. Boston Med Surg J. 1848;39:389–393. [PMC free article] [PubMed] [Google Scholar]
- 39.Hassanain M, Bhatt S, Siegel A. Differential modulation of feline defensive rage behavior in the medial hypothalamus by 5-HT1A and 5-HT2 receptors. Brain Res. 2003;981:201–209. doi: 10.1016/s0006-8993(03)03036-1. [DOI] [PubMed] [Google Scholar]
- 40.Hassanain M, Bhatt S, Zalcman S, Siegel A. Potentiating role of interleukin-1 beta (IL-1 beta) and IL- 1 beta type 1 receptors in the medial hypothalamus in defensive rage behavior in the cat. Brain Res. 2005;1048:1–11. doi: 10.1016/j.brainres.2005.04.086. [DOI] [PubMed] [Google Scholar]
- 41.Heiligenstein JH, Beasley CM, Jr, Potvin JH. Fluoxetine not associated with increased aggression in controlled clinical trials. Int Clin Psychopharmacol. 1993;8:277–280. doi: 10.1097/00004850-199300840-00012. [DOI] [PubMed] [Google Scholar]
- 42.Hellings JA, Weckbaugh M, Nickel EJ, Cain SE, Zarcone JR, Jr, Reese RM, Hall S, Ermer DJ, Tsai LY, Schroeder SR, Cook EH. A double-blind, placebo-controlled study of valproate for aggression in youth with pervasive developmental disorders. J Child Adolesc Psychopharmacol. 2005;15:682–692. doi: 10.1089/cap.2005.15.682. [DOI] [PubMed] [Google Scholar]
- 43.Hennig J, Reuter M, Netter P, Burk C, Landt O. Two types of aggression are differentially related to serotonergic activity and the A779C TPH polymorphism. Behav Neurosci. 2005;119:16–25. doi: 10.1037/0735-7044.119.1.16. [DOI] [PubMed] [Google Scholar]
- 44.Higley JD, King ST, Hasert MF, Champoux M, Suomi SJ, Linnoila M. Stability of interindividual differences in serotonin function and its relationship to severe aggression and competent social behavior in rhesus macaque females. Neuropsychopharmacology. 1996;14:67–76. doi: 10.1016/S0893-133X(96)80060-1. [DOI] [PubMed] [Google Scholar]
- 45.Higley JD, Mehlman PT, Poland RE, Taub DM, Vickers J, Suomi SJ, Linnoila M. CSF testosterone and 5-HIAA correlate with different types of aggressive behaviors. Biol Psychiatry. 1996;40:1067–1082. doi: 10.1016/S0006-3223(95)00675-3. [DOI] [PubMed] [Google Scholar]
- 46.Hollander E, Swann AC, Coccaro EF, Jiang P, Smith TB. Impact of trait impulsivity and state aggression on divalproex versus placebo response in borderline personality disorder. Am J Psychiatry. 2005;162:621–624. doi: 10.1176/appi.ajp.162.3.621. [DOI] [PubMed] [Google Scholar]
- 47.Huizinga D, Haberstick BC, Smolen A, Menard S, Young SE, Corley RP, Stallings MC, Grotpeter J, Hewitt JK. Childhood maltreatment, subsequent antisocial behavior, and the role of monoamine oxidase A genotype. Biol Psychiat. 2006;60:677–683. doi: 10.1016/j.biopsych.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 48.Kavoussi RJ, Coccaro EF. Divalproex sodium for impulsive aggressive behavior in patients with personality disorder. J Clin Psychiatry. 1998;59:676–680. doi: 10.4088/jcp.v59n1206. [DOI] [PubMed] [Google Scholar]
- 49.Kiecolt-Glaser JK, Loving TJ, Stowell JR, Malarkey WB, Lemeshow S, Dickinson SL, Glaser R. Hostile marital interactions, proinflammatory cytokine production, and wound healing. Arch Gen Psychiatry. 2005;62:1377–1384. doi: 10.1001/archpsyc.62.12.1377. [DOI] [PubMed] [Google Scholar]
- 50.Krakowski MI, Czobor P, Citrome L, Bark N, Cooper TB. Atypical antipsychotic agents in the treatment of violent patients with schizophrenia and schizoaffective disorder. Arch Gen Psychiatry. 2006;63:622–629. doi: 10.1001/archpsyc.63.6.622. [DOI] [PubMed] [Google Scholar]
- 51.Kraus MR, Schafer A, Faller H, Csef H, Scheurlen M. Psychiatric symptoms in patients with chronic hepatitis C receiving interferon alfa2b therapy. J Clin Psychiatry. 2003;64:708–714. doi: 10.4088/jcp.v64n0614. [DOI] [PubMed] [Google Scholar]
- 52.Krettek JE, Price JL. Amygdaloid projections to subcortical structures within the basal forebrain and brainstem in the rat and cat. J Comp Neurol. 1978;178:225–254. doi: 10.1002/cne.901780204. [DOI] [PubMed] [Google Scholar]
- 53.Kruk MR, Van der Poel AM, De Vos-Frerichs TP. The induction of aggressive behaviour by electrical stimulation in the hypothalamus of male rats. Behaviour. 1979;70:292–322. doi: 10.1163/156853979x00106. [DOI] [PubMed] [Google Scholar]
- 54.Kruk MR, Van der Poel AM, Meelis W, Hermans J, Mostert PG, Mos J, Lohman AHM. Discriminant analysis of the localization of aggression-inducing electrode placements in the hypothalamus of male rats. Brain Res. 1983;260:61–79. doi: 10.1016/0006-8993(83)90764-3. [DOI] [PubMed] [Google Scholar]
- 55.Lammers JHCM, Kruk MR, Meelis W, Van der Poel AM. Hypothalamic substrates for brain stimulation-induced attack, teeth-chattering and social grooming in the rat. Brain Res. 1988;449:311–327. doi: 10.1016/0006-8993(88)91046-3. [DOI] [PubMed] [Google Scholar]
- 56.Lappalainen J, Long JC, Ozaki N, Robin RW, Brown GL, Naukkarinen H, Virkkunen M, Linnoila M, Goldman D. Linkage of antisocial alcoholism to the serotonin 5-HT1B receptor gene in 2 populations. Arch Gen Psychiatry. 1998;55:989–994. doi: 10.1001/archpsyc.55.11.989. [DOI] [PubMed] [Google Scholar]
- 57.Lee R, Garcia F, Van de Kar LD, Hauger RD, Coccaro EF. Plasma oxytocin in response to pharmaco-challenge to D-fenfluramine and placebo in healthy men. Psychiatry Res. 2003;118:129–136. doi: 10.1016/s0165-1781(03)00070-2. [DOI] [PubMed] [Google Scholar]
- 58.Lindenmayer JP, Kotsaftis A. Use of sodium valproate in violent and aggressive behaviors: a critical review. J Clin Psychiatry. 2000;61:123–128. doi: 10.4088/jcp.v61n0207. [DOI] [PubMed] [Google Scholar]
- 59.Luo B, Cheu JW, Siegel A. Cholecystokinin B receptors in the periaqueductal gray potentiate defensive rage behavior elicited from the medial hypothalamus of the cat. Brain Res. 1998;796:27–37. doi: 10.1016/s0006-8993(98)00310-2. [DOI] [PubMed] [Google Scholar]
- 60.Lyons WE, Mamounas LA, Ricaurte GA, Coppola V, Reid SW, Bora SH, Wihler C, Koliatsos VE, Tessarollo L. Brain-derived neurotrophic factor-deficient mice develop aggressiveness and hyperphagia in conjunction with brain serotonergic abnormalities. PNAS. 1999;96:15239–15244. doi: 10.1073/pnas.96.26.15239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Macdonald RL, Kelly KM. Antiepileptic drug mechanisms of action. Epilepsia. 1995;36((Suppl 2)):S2–S12. doi: 10.1111/j.1528-1157.1995.tb05996.x. [DOI] [PubMed] [Google Scholar]
- 62.Maier SF, Watkins LR. Cytokines for psychologists: implications of bidirectional immune-brain-communication for understanding behavior, mood, and cognition. Psychol Rev. 1998;105:83–107. doi: 10.1037/0033-295x.105.1.83. [DOI] [PubMed] [Google Scholar]
- 63.Maier SF, Watkins LR, Nance DM. Multiple routes of action of interleukin-1 on the nervous system. In: Ader R, Cohen N, Felten DL, editors. Psychoneuroimmunology. San Diego: Academic Press; 2001. pp. 563–583. [Google Scholar]
- 64.Malone RP, Luebbert J, Pena-Ariet M, Biesecker K, Delaney MA. The Overt Aggression Scale in a study of lithium in aggressive conduct disorder. Psychopharmacol Bull. 1994;30:215–218. [PubMed] [Google Scholar]
- 65.Malone RP, Delaney MA, Luebbert JF, Cater J, Campbell M. A double-blind placebo-controlled study of lithium in hospitalized aggressive children and adolescents with conduct disorder. Arch Gen Psychiatry. 2000;57:649–654. doi: 10.1001/archpsyc.57.7.649. [DOI] [PubMed] [Google Scholar]
- 66.Marsh DM, Dougherty DM, Moeller FG, Swann AC, Spiga R. Laboratory-measured aggressive behavior of women: acute tryptophan depletion and augmentation. Neuropsychopharmacology. 2002;26:660–671. doi: 10.1016/S0893-133X(01)00369-4. [DOI] [PubMed] [Google Scholar]
- 67.McHutchison JG, Gordon SC, Schiff ER, Shiffman ML, Lee WM, Rustgi VK, Goodman ZD, Ling MH, Cort S, Albrecht JK. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. Hepatitis Interventional Therapy Group. N Engl J Med. 1998;339:1485–1492. doi: 10.1056/NEJM199811193392101. [DOI] [PubMed] [Google Scholar]
- 68.McQuade DV. Pharmacological Intervention in Aggression. In: Mattsonm M, editor. Neurobiology of Aggression: Understanding and Preventing Violence. Totawa NJ: Humana Press Inc; 2003. pp. 289–313. [Google Scholar]
- 69.Meloy JR. The Psychopathic Mind: Origins Dynamics and Treatment. Northvale NJ: Jason Aronson Inc; 1988. [Google Scholar]
- 70.Moyer KE. The Psychology of Aggression. New York NY: Harper Row; 1976. . [Google Scholar]
- 71.New AS, Buchsbaum MS, Hazlett RA, Goodman M, Koenigsberg HW, Lo J, Iskander L, Newmark R, Brand J, Flynn KO, Siever LJ. Fluoxetine increases relative metabolic rate in prefrontal cortex in impulsive aggression. Psychopharmacology. 2004;V176:451–458. doi: 10.1007/s00213-004-1913-8. [DOI] [PubMed] [Google Scholar]
- 72.Nickel MK, Nickel C, Mitterlehner FO, Tritt K, Lahmann C, Leiberich PK, Rother WK, Loew TH. Topiramate treatment of aggression in female borderline personality disorder patients: a double-blind, placebo-controlled study. J Clin Psychiatry. 2004;65:1515–1519. doi: 10.4088/jcp.v65n1112. [DOI] [PubMed] [Google Scholar]
- 73.Panksepp J. Aggression elicited by electrical stimulation of the hypothalamus in albino rats. Physiol Behav. 1971;6:321–329. doi: 10.1016/0031-9384(71)90163-6. [DOI] [PubMed] [Google Scholar]
- 74.Petitto JM, Lysle DT, Gariepy JL, Lewis MH. Association of genetic differences in social behavior and cellular immune responsiveness: effects of social experience. Brain Behav Immun. 1994;8:111–122. doi: 10.1006/brbi.1994.1011. [DOI] [PubMed] [Google Scholar]
- 75.Pinner E, Rich CL. Effects of trazodone on aggressive behavior in seven patients with organic mental disorders. Am J Psychiatry. 1988;145:1295–1296. doi: 10.1176/ajp.145.10.1295. [DOI] [PubMed] [Google Scholar]
- 76.Pradhan SN. Aggression and central neurotransmitters. Int Rev Neurobiol. 1973;18:213–262. doi: 10.1016/s0074-7742(08)60036-7. [DOI] [PubMed] [Google Scholar]
- 77.Pucilowski O, Eichelman B, Overstreet DH, Rezvani AH, Janowsky DS. Enhanced affective aggression in genetically bred hypercholinergic rats. Neuropsychobiology. 1991;24:37–41. doi: 10.1159/000119040. [DOI] [PubMed] [Google Scholar]
- 78.Shaikh MB, De Lanerolle NC, Siegel A. Serotonin 5-HT1A and 5-HT2/1C receptors in the midbrain periaqueductal gray differentially modulate defensive rage behavior elicited from the medial hypothalamus of the cat. Brain Res. 1997;765:198–207. doi: 10.1016/s0006-8993(97)00433-2. [DOI] [PubMed] [Google Scholar]
- 79.Shaikh MB, Lu CL, Siegel A. An enkephalinergic mechanism involved in amygdaloid suppression of affective defense behavior elicited from the midbrain periaqueductal gray in the cat. Brain Res. 1991;559:109–117. doi: 10.1016/0006-8993(91)90293-5. [DOI] [PubMed] [Google Scholar]
- 80.Shaikh MB, Schubert K, Siegel A. Basal amygdaloid facilitation of midbrain periaqueductal gray elicited defensive rage behavior in the cat is mediated through NMDA receptors. Brain Res. 1994;635:187–195. doi: 10.1016/0006-8993(94)91438-9. [DOI] [PubMed] [Google Scholar]
- 81.Shaikh MB, Siegel A. GABA-mediated regulation of feline aggression elicited from midbrain periaqueductal gray upon affective defense behavior in the cat. Brain Res. 1990;507:51–56. doi: 10.1016/0006-8993(90)90521-c. [DOI] [PubMed] [Google Scholar]
- 82.Shaikh MB, Steinberg A, Siegel A. Evidence that substance P is utilized in medial amygdaloid facilitation of defensive rage behavior in the cat. Brain Res. 1993;625:283–294. doi: 10.1016/0006-8993(93)91070-9. [DOI] [PubMed] [Google Scholar]
- 83.Siegel A. The Neurobiology of Aggression and Rage. Boca Raton FL: CRC press; 2005. ; pp. 1–293. [Google Scholar]
- 84.Siegel A, Brutus M, Shaikh MB, Edinger H, Blaustein P, Ritter A, Siegel HE. Effects of amygdaloid seizures upon aggressive behavior elicited from the hypothalamus of the cat. In: Oomura Y, editor. Emotions: Neuronal and Chemical Control. Tokyo: Japan Scientific Societies Press; 1986. pp. 219–230. [Google Scholar]
- 85.Siegel A, Pott CB. Neural substrate of aggression and flight in the cat. Prog Neurobiol. 1988;31:261–283. doi: 10.1016/0301-0082(88)90015-9. [DOI] [PubMed] [Google Scholar]
- 86.Siegel A, Roeling TAP, Gregg TR, Kruk MR. Neuropharmacology of brain-stimulation-evoked aggression. Neurosci Biobehav Rev. 1999;23:359–389. doi: 10.1016/s0149-7634(98)00040-2. [DOI] [PubMed] [Google Scholar]
- 87.Siegel A, Schubert KL, Shaikh MB. Neurotransmitters regulating defensive rage behavior in the cat. Neurosci Biobehav Rev. 1997;21:733–742. doi: 10.1016/s0149-7634(96)00056-5. [DOI] [PubMed] [Google Scholar]
- 88.Spreat S, Behar D, Reneski B, Miazzo P. Lithium carbonate for aggression in mentally retarded persons. Compr Psychiatry. 1989;30:505–511. doi: 10.1016/0010-440x(89)90080-1. [DOI] [PubMed] [Google Scholar]
- 89.Stelt M, Broersen M, Berend O, Westenberg GM. Effects of dietary tryptophan variations on extracellular serotonin in the dorsal hippocampus of rats. Psychopharmacology. 2004;V172:137–144. doi: 10.1007/s00213-003-1632-6. [DOI] [PubMed] [Google Scholar]
- 90.Stephens RJ, Bassel C, Sandor P. Olanzapine in the treatment of aggression and tics in children with Tourette’s syndrome--a pilot study. J Child Adolesc Psychopharmacol. 2004;14:255–266. doi: 10.1089/1044546041648959. [DOI] [PubMed] [Google Scholar]
- 91.Strous RD, Nolan KA, Lapidus R, Diaz L, Saito T, Lachman HM. Aggressive Behavior in schizophrenia is associated with the low enzyme activity COMT polymorphism: A replication study. Am J Med Genet Part B. 2003;120B:29–34. doi: 10.1002/ajmg.b.20021. [DOI] [PubMed] [Google Scholar]
- 92.Suarez EC, Lewis JG, Krishnan RR, Young KH. Enhanced expression of cytokines and chemokines by blood monocytes to in vitro lipopolysaccharide stimulation are associated with hostility and severity of depressive symptoms in healthy women. Psychoneuroendocrinology. 2004;29:1119–1128. doi: 10.1016/j.psyneuen.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 93.Tcheremissine OV, Lane SD, Lieving LM, Rhoades HM, Nouvion S, Cherek DR. Individual differences in aggressive responding to intravenous flumazenil administration in adult male parolees. J Psychopharmacol. 2005;19:640–646. doi: 10.1177/0269881105056532. [DOI] [PubMed] [Google Scholar]
- 94.Toot J, Dunphy G, Turner M, Ely D. The SHR Y-chromosome increases testosterone and aggression, but decreases serotonin as compared to the WKY Y-chromosome in the rat model. Behav Gen. 2004;V34:515–524. doi: 10.1023/B:BEGE.0000038489.82589.6f. [DOI] [PubMed] [Google Scholar]
- 95.Volavka J. The effects of clozapine on aggression and substance abuse in schizophrenic patients. J Clin Psychiatry. 1999;60((Suppl 12)):43–46. [PubMed] [Google Scholar]
- 96.Volavka J, Citrome L, Huertas D. Update on the biological treatment of aggression. Actas Esp Psiquiatr. 2006;34:123–135. [PubMed] [Google Scholar]
- 97.Volavka J, Czobor P, Nolan K, Sheitman B, Lindenmayer JP, Citrome L, McEvoy JP, Cooper TB, Lieberman JA. Overt aggression and psychotic symptoms in patients with schizophrenia treated with clozapine, olanzapine, risperidone, or haloperidol. J Clin Psychopharmacol. 2004;24:225–228. doi: 10.1097/01.jcp.0000117424.05703.29. [DOI] [PubMed] [Google Scholar]
- 98.Wasman M, Flynn JP. Directed attack elicited from hypothalamus. Arch Neurol. 1962;6:220–227. doi: 10.1001/archneur.1962.00450210048005. [DOI] [PubMed] [Google Scholar]
- 99.Watson RE, Troiano R, Poulakos JJ, Weiner S, Block CH, Siegel A. A [14C]2-deoxyglucose analysis of the functional neural pathways of the limbic forebrain in the rat. I. The amygdala. Brain Res Rev. 1983;5:1–44. doi: 10.1016/0165-0173(83)90020-6. [DOI] [PubMed] [Google Scholar]
- 100.Wersinger SR, Ginns EI, O’Connell AM, Lolait SJ, Young WS. Vasopressin V1b receptor knockout reduces aggressive behavior in male mice. Mol Psychiatry. 2002;7:975–984. doi: 10.1038/sj.mp.4001195. [DOI] [PubMed] [Google Scholar]
- 101.Woodworth CH. Attack elicited in rats by electrical stimulation of the lateral hypothalamus. Physiol Behav. 1971;6:345–353. doi: 10.1016/0031-9384(71)90166-1. [DOI] [PubMed] [Google Scholar]
- 102.Wroblewski BA, Joseph AB, Kupfer J, Kalliel K. Effectiveness of valproic acid on destructive and aggressive behaviours in patients with acquired brain injury. Brain Inj. 1997;11:37–47. doi: 10.1080/026990597123791. [DOI] [PubMed] [Google Scholar]
- 103.Zalcman SS, Siegel A. The neurobiology of aggression and rage: Role of cytokines. Brain Behav Immun. 2006;20:507–514. doi: 10.1016/j.bbi.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 104.Zanarini MC, Frankenburg FR, Parachini EA. A preliminary, randomized trial of fluoxetine, olanzapine, and the olanzapine-fluoxetine combination in women with borderline personality disorder. J Clin Psychiatry. 2004;65:903–907. doi: 10.4088/jcp.v65n0704. [DOI] [PubMed] [Google Scholar]