Abstract
Parkinson’s disease (PD) is characterized clinically by resting tremor, rigidity, bradykinesia and postural instability due to progressive and selective loss of dopamine neurons in the ventral substantia nigra, with the presence of ubiquitinated protein deposits called Lewy bodies in the neurons. The pathoetiology of cell death in PD is incompletely understood and evidence implicates impaired mitochondrial complex I function, altered intracellular redox state, activation of proapoptotic factors and dysfunction of ubiquitinproteasome pathway. Now it is believed that genetic aberration, an environmental toxin or combination of both leads to a cascade of events culminating in the destruction of myelinated brainstem catecholaminergic neurons. Also the role of production of significant levels of abnormal proteins, which may misfold, aggregate and interfere with intracellular processes causing cytotoxicity has recently been hypothesized. In this article, the diverse pieces of evidence that have linked the various factors responsible for the pathophysiology of PD are reviewed with special emphasis to various candidate genes and proteins. Furthermore, the present therapeutic strategies and futuristic approaches for the pharmacotherapy of PD are critically discussed.
Key Words: Parkinson’s disease, lewy bodies, protein aggregation, pathophysiology, α-Synuclein, Parkin, Mitochondrial dysfunction
INTRODUCTION
Parkinson’s disease (PD) is one of the most common neurodegenerative motor disorders affecting 1% of the population over age 50. PD was first described by James Parkinson in 1817 and characterized by resting tremor, rigidity, bradykinesia and postural instability (collectively called parkinsonism). The disease is progressive and the pathologic features of classical PD are the selective loss of dopamine neurons in the ventral substantia nigra with the presence of ubiquitinated protein deposits in the cytoplasm of neurons (Lewy bodies), and thread like proteinaceous inclusions within neuritis (Lewy neuritis). The pathoetiology of cell death in PD remains incompletely understood, but evidence implicates impaired mitochondrial complex I function, altered intracellular redox state, activation of proapoptotic factors, and dysfunction of ubiquitinproteasome pathway. The resultant state of dopamine deficiency results in disequilibrium of the extrapyrimidal motor circuits and development of parkinsonism [14, 208, 225].
EPIDEMIOLOGY
The prevalence of PD in industrialized countries is estimated to be 0.3% of the general population [44, 217]. The disease increasingly strikes with age affecting about 1% of 65-year individuals and rising to 5% by 85-years of age [179]. The annual incidence of PD ranges between 16 and 19 individuals per 100, 000 [265]. PD occurs throughout the world in all ethnic groups and affect both sexes roughly equally or with slight predominance among males [289]. The lowest reported incidence is among Asian and African blacks where as the highest is among European and North Americans [124].
PD greatly shortens life-mortality upto 2-5 % [170]. PD along with other neurodegenerative diseases is projected to be the second most common cause of death among elderly by the year 2040 [154]. PD not only shortens the life expectancy but also causes debility during life affecting quality of life [278]. Mean age of onset of PD was estimated to be in the late 50s [111], however now it is thought to be in the early-to-mid 60s [121]. In 5-10% of the patients initial symptoms arise between age 10 and 40 (sometimes 50) years, hence is termed as young onset PD [85]. However the first symptoms of juvenile onset PD occur before the age of 20 years [187]. Young onset and juvenile onset PD are familial PD since family history is found in these patients. Idiopathic PD or typical sporadic PD is the term probably reserved for PD cases without or known etiology or familial connection [225].
CLINICAL FEATURES
PD is characterized clinically by three motor symptoms such as resting tremors, rigidity and bradykinesia. The fourth symptom postural instability is non-specific and is usually absent in early disease especially in the younger patients [224]. The motor abnormalities are many times accompanied by various non-motor symptoms including autonomic dysfunction[50], depression [105], other psychiatric changes such as cognitive deficit, anxiety, psychosis, hallucinations [32, 57, 166], dementia [66], sensory symptoms [141] and disturbed sleep [1, 251].
A resting tremor with a frequency of 3-5 Hz (classically resembling pill-rolling) is the first symptom in 70% of PD patients. Tremor is usually asymptomatic at disease onset and worsens with anxiety, contralateral motor activity and during ambulation. Resting foot tremor is much less common than hand tremor as a presenting sign [24, 122]. Rigidity is the raised resistance noted during passive joint movement that is uniform throughout the range of motion of the joint. Rigidity is enhanced by contralateral motor activity or mental task performance [168].
Bradykinesia is the most disabling symptom of early PD. It initially manifests by difficulties with fine motor tasks such as handwriting and reduced arm swing while walking. Limb bradykinesia can be tested by finger tapping alternating forearm pronation or supination, foot tapping and fist closing and opening [168].
Postural instability refers to the gradual development of poor-balance, leading to an increased risk of falls. Gait becomes slower with shuffling and turning is en bloc. Freezing is characterized by difficulty initiating gait or striking gait hesitation on arriving at a real or perceived obstacle. Postural and gait abnormalities are rarely prominent early in the course of PD [225].
Over the course of PD, more than 90% of patients experience symptoms of autonomic dysfunction [50]. Depression is common, affecting nearly half of the patients [105]. Other psychiatric symptoms associated with PD are cognitive deficit, anxiety, psychosis, hallucinations etc [32, 57].
Sensory symptoms arise in PD in various patterns [216]. Disturbed sleep is common in PD and has many different causes, including nocturnal stiffness, nocturia, depression, restless leg syndrome and REM sleep behavior disorder [1, 251].
DIFFERENTIAL DIAGNOSIS
The resembling clinical features are shown by normal aging, essential tremors, progressive supranuclear palsy, multiple system atrophy, dementia with Lewy bodies, cortico-basal degeneration, basal ganglia vascular disease, normal pressure hydrocephalus and other Parkinson plus syndromes [24, 122]. Less common entities with PD include dopa-responsive dystonia [79], juvenile onset Huntington’s disease, pallidopontonigral degeneration and drug induced PD [130, 167]. Drug induced PD usually arises after exposure to neuroleptics, antiemetics and promotility agents (promethazine, prochlorperazine, metoclopramide), reserpine, tetrabenazine and some calcium channel blockers. The pathologies underlying these diseases are different [244].
Hughes and colleagues as well as many others described differential criteria for diagnosis of PD [80, 116]. The supportive criteria described by Hughes included an asymmetric onset, progression, persistent asymmetry as an excellent response to L-DOPA treatment (dyskinesia). The exclusion criteria include corticospinal tract signs, severe dysautonomia, moderate to severe gait disturbance, and the presence of more than one clinically affected relative history of encephalitis and hydrocephalus and exposure to PD inducing drugs [225]. The exclusion criteria are designed to remove cause of an akinetic rigid syndrome not due to Lewy body nigral degeneration [116].
ETIOLOGY
The bicentenary of James Parkinson’s original description of the disease will be marked in ten years, still the etiology debate of PD being environmental caused or genetically determined continues [167].
Environmental
Numerous studies have presented evidences suggesting the role of well water consumption [88, 89], toxic substances exposure as manganese, copper and other heavy metals [88, 89], 1-methyl-4-phenyl-1,2,3,6 tetrahydropyridine (MPTP) [148], dopamine derivatives as 6-hydroxy dopamine, salsonilol [163, 177], herbicides and pesticides as paraquat, rotenone [201, 230], endotoxins as lipopolysaccharide by Salmonella minnesota infections [183], frontal lobe tumors, defects or variation in metabolic pathways [7, 245] in development of parkinsonism. However, epidemiological studies that evaluated the association between environmental factors and risk of PD have reported inconsistent results for rural residence, drinking well water and exposure to pesticides [6].
Genetic
The role of genetic factor was suggested as early as 1880s, by observations made by Charcott and Gower separately of positive family history in patients of PD. The suggestion is supported by number of studies [207]. Approximately 15% of cases have first and second degree relatives [164]. Yet in the light of modern epidemiology techniques many of these studies appear to have been methodologically flawed, failing to examine affected family member to account for other factors such as family bias [223], hence cannot be considered conclusive.
It was hypothesized that, if PD has strong genetic determinant, concordance in monozygotic twins, who are genetically identical, should be greater than in dizygotic twins. However results of twin study, a frequently used tool to study the role of genetics, showed little difference in concordance in twins when PD develops after age of 50 years, whereas complete concordance was shown in monozygotic twins for PD with onset before 50 years of age. This finding suggests that genetic factors play a more significant part in the young-onset than late-onset or typical sporadic PD [260]. Finding of many other twin studies do not show enhanced concordance in monozygotic twins [132, 277]. Data from cross sectional studies was consistently refuted by longitudinal twin studies using 18FDOPA positron emission tomography [146]. A large difference in concordance rates of monozygotic and dizygotic twins was observed by one of such studies [213]. With the identified gene mutations in familial PD and common genetic risk factors for idiopathic PD, the genetic basis for the pathophysiology of PD has gained its place over the environmental factors, even though no gene has been identified so far as being solely responsible for idiopathic PD (Table 1).
Table 1.
Genes Involved in Parkinson’s Disease
| Gene | Chromosome Locus | Gene Product | Age Onset | Clinical Features | Reference |
|---|---|---|---|---|---|
| PARK1 | 4q21-q23 | α-synuclein | Late | Rapid progression, tremor uncommon | [242] |
| PARK2 | 6q25-q27 | parkin | Early/Juvanile | Slow progression, dystonia or dyskinesia | [156] |
| PARK3 | 2p13 | - | Late | Cognitive decline | [156] |
| PARK4 | 4p15 | α-synuclein | Late | Postural tremor; dementia with weight loss | [242] |
| PARK5 | 4p14 | UCHL1 | Late | Similar to idiopathic PD | [153] |
| PARK6 | 1p35-p36 | PINK1 | Early | Slow progression, tremor at rest | [223] |
| PARK7 | 1p36 | DJ-1 | Early | Focal dystonia, psychiatric symptoms | [45] |
| PARK8 | 12p11.2-q13.1 | LRRK-2 | Late | Similar to idiopathic PD | [222] |
| PARK10 | 1p32(Icelandic) | - | Late | Similar to idiopathic PD | [108] |
| PARK11 | 2q(USA) | - | Late | Similar to idiopathic PD | [200] |
In the patient population of PD there were less than 5% patients with monogenetic inheritance of the disease, 10-15% patient with familial history and vast majority of patients about 80% were essentially sporadic or idiopathic with complex etiology [147]. Current information suggests that PD is likely to result from a combination of both genetic and environmental factors [99].
MOLECULAR PATHOGENESIS
Whichever the etiological factor to initiate PD, be it a genetic aberration, an environmental toxin or combination of both, there follows a cascade of events culminating in the destruction of myelinated brainstem catecholaminergic neurons particularly dopaminergic neurons of SNPc. There is no clear explanation for selective neurodegeneration. One possible answer may lie in the ability of these neurons to take up both endogenous and extrinsic toxic compounds through selective carrier mechanisms such as dopamine transporter. Other possible explanations include increased metabolic stress, high physiologic rates of protein oxidation, selective generation of potential toxins or failure to detoxify or dispose off them (possibly because of the presence of neuromelanin) and specific requirement for neurotrophic support. Irrespective of above factors, the death of dopaminergic neuron is prevalent in PD. The symptoms of PD are caused by a >50% loss of dopaminergic neurons in the SNPc leading to >80% reduction in dopamine levels in the striatum [47]. The underlying mechanisms that provoke cell death in PD are largely unknown. The mechanism that has been implicated in neuronal degeneration and now gaining support is protein mishandling (as aberrant degradation) and subsequent accumulation of proteins [94].
PROTEIN AGGREGATION
The production of significant levels of abnormal proteins (as incomplete, mutant, misfolded, denatured, oxidized or otherwise damaged proteins) is associated with normal cellular functions. These abnormal proteins may misfold, aggregate and interfere with intracellular processes causing cytotoxicity. Hence their production should be limited or their clearance must be rapid for survival of cell [87, 235]. The production of abnormal proteins is prominent in the CNS due to high oxidative metabolism in neuronal cells [8, 97] and may be triggered or promoted by iron toxicity [18], glutathione deficiency [18], oxidative stress [64, 290], proteasomal dysfunction [46], mitochondrial impairment [234] and inflammation [196]. The limited ability of neuronal cells to repair/regenerate and their long life span demands equilibrium between the production and degradation of abnormal proteins for cell survival.
Ubiquitin proteasome system (UPS) is the main pathway that mediates the degradation of abnormal and soluble proteins as well as short lived regulatory functional proteins in cytoplasm, nucleus and endoplasmic reticulum [17]. The other system, autophagy uses cathepsins and cystein proteases to degrade membrane and extracellular components following endocytosis into the lysosome [17]. If the production of abnormal proteins overwhelms, the protein degradation mechanism is inhibited or impaired, there occurs the accumulation and aggregation of these proteins [235]. This adverse state is called proteolytic stress [174]. In PD, where proteolytic stress is a key factor, inclusion bodies are seen in the cytoplasm and axons of neurons at various pathological sites. These inclusion bodies are termed as Lewy bodies (LB) and Lewy neuritis since they were originally described by Fredrich Lewy in 1912 [74].
All forms of familial and idiopathic PD have LBs with the exception of autosomal recessive form [74]. LBs are found in surviving neurons and nigrostriatal pathway as well as extranigral neurons as in dorsal mater nucleus of the vagus, substantia innominata, thalamus, hypothalamus, amy-gdala, piriform cortex and olfactory bulb [74]. LBs are also seen in extracellular space following destruction of the host neurons [134].
LBs are cytoplasmic rounded inclusions of 8-30 μ in diameter with spherical dense core and peripheral halo radiating filaments of diameter 7-20 nm. The LBs in cortical region are diffuse since they frequently lack a distinct peripheral halo. The LBs contain heterogenous mixture of insoluble proteins and lipids [48]. Among proteins α-synuclein (αSN) derivatives are the major proteins [48, 250]. Apart from αSN, LBs contain various proteins [14, 49, 149, 250]. These proteins include 1) proteins related to UPS as ubiquitin, ubiquitinated proteins, ubiquitin carboxyterminal hydrolase (UCHL 1), ubiquitin ligase, elements of proteasomes, ubiquitin binding protein, parkin and DJ-1 2) cytoskeletal proteins as neurofilaments, intermediate filaments, tubulins, microtubule associated proteins, tropomyosin family, gelsolin (actin disassembling protein, septin) 3) protein kinases and phosphatases 4) chaperones as heat shock proteins (HSP70, HSP90) 5) αβ crystalline, torsin A, 14-3-3 6) proteins associated with inflammation as various complements 7) αSN binding proteins as synphillin, MAP1B 8) Alzheimer’s disease related proteins as tau, apoJ, cdk5, β-amyloid precursor proteins 9) proteins associated with apoptosis, Bcl-2 associated death proteins, extracellular regulated kinase 10) other proteins as glutathione peroxidase, transglutaminase, cytochrome C. These proteins may be oxidized, nitrated, phosphorylated and/or ubiquitinated [14, 49, 249, 250].
α-synuclein: Structure
αSN gene spans about 111 kb, contains 6 exons and encodes 140 amino acid protein that has a molecular weight of ~14 KDa. This extremely heat resistant small acidic protein remains uncoiled in solution but assumes α-helical configuration within lipid containing vesicles and β-sheet at high concentration [220]. The sequence of αSN can be subdivided into three distinct domains i) The highly conserved amino terminal domain of residues 1-65, containing six imperfect repeats with KTKEGV consensus motif. This domain is unordered in solution but can shift to an α-helical confirmation of two distinct helices interrupted by a short break [28, 81]. The amino terminus can bind to lipids and play role in association with lipid micelles, synaptic vesicles, lipid bilayer [63, 131] ii) The central portion of αSN known as non Aβ component of plaque (NACP) has residues 61-95 and is responsible for various confirmations of αSN [266] NACP helps to attain random coil, β sheet or single cylindrical β-sheet or protofibrils and fibrils [53, 61, 82] iii) The acidic C-terminal of residues 97-140 appears critical for other protein binding and chaperone like activity [138, 202]. The C-terminal has several phosphorylation sites as Y125, 133, 136 and S129 [77]. αSN in LB is extensively and specifically phosphorylated at S129. Whereas post translational modifications in the C-terminal include glycosylation at S129 [236], oxidation [27] and nitration at Y125, 133, 136 [259]. These modifications affect chaperone-like activity.
αSN Protein Functions
Potential Loci for Pathology: The functions ascribed to αSN include physiological regulation of certain enzymes, transporters, neurotransmitter vesicles as well as roles in neuronal survival. αSN has been demonstrated to inhibit protein kinase C [197], phospholipase D2 [123], tyrosine hydroxylase [208]; to down regulate extracellular signal regulated kinase (ERK) [100], Bcl2 associated death protein (BAD) [151]. Also αSN facilitates an increase in the number of plasma membrane dopamine transporter (DAT) molecule. Phospholipase D2 triggers secretory vesicle production by catalyzing hydrolysis of phosphotidyl choline to phospha-tidic acid [5]. Tyrosine hydroxylase is rate limiting enzyme in dopamine synthesis. DAT regulates dopamine uptake.
αSN, in addition to binding to lipids, binds to variety of ligands and has been reported to interact with more than 200 proteins [5] and functions as a chaperone. The various proteins with which αSN interacts include synphillin, parkin, UCJL1, 14-3-3, tubulin and many others [5, 237]. Parkin, UCHL 1 are involved in UPS whereas 14-3-3 regulates cell growth and neuronal development, antagonizes BAD and increases tyrosine hydroxylase activity [286].
The changes in cellular conditions such as pH, temperature and oxidative stress, mutations and aging could trigger changes in αSN gene expression as well as post translational and/or conformational protein modifications. The changes in the proteins make it more prone to self aggregation hence more difficult to destroy or alternatively the production of misfolded proteins that cannot be degraded [22, 276]. The undegraded αSN accumulates in cytoplasm. This causes molecular crowding or formation of spherical protofibrils, a process that involves β-sheet formations, oligomers and subsequently chains and annular/ring protofibrils [53, 275]. The generated anuular protofibrils would exert toxicity due to their binding and permiabilization of vesicles and formation of amyloid pores [149, 275]. αSN fibrils are thought to act initially as protective sink i.e. protofibrils are consumed for formation of fibrils therefore cell gets temporarily relieved from αSN toxicity. However the accumulation of fibrils eventually results in toxicity. Indeed studies using cultured human dopaminergic neurons suggests that mutant αSN triggers an elevation in cytosolic dopamine, oxidative stress and dopamine-dependent toxicity [281]. The mutant αSN causes an increase in toxicity probably via a reduction in synaptic storage of dopamine [257]. The putative toxic forms of αSN, apart from fibrillar aggregates (Lewy bodies, Lewy neuritis, cytoplasmic glial inclusions), amorphous aggregates, oligomers & protofibrils include soluble forms as partially folded intermediates, S129 phosphorylated αSN, phosphorylated, nitrosylated, glucosylated, ubiquitylated αSN species [53].
Soluble forms of αSN complex with different chaperones as 14-3-3 an anti-apoptotic protein [281] and hasten apoptosis. Protein confirmation dependent neurotoxcity is seen as increased propensity of partially folded intermediates to aggregate and oligomerise and act as chaperone [138, 185]. Various non-genetic etiological factors as pesticides, metal ions, oxidized trimethyl-N-oxide have shown to make αSN acquire increased level of secondary structure [211, 267-270]. Oxidative stress conditions facilitate catecholamines including dopamine to be readily converted in the presence of iron to highly reactive metabolites such as dopamine-quinone [37, 211, 267-270], these can bind to αSN. Adducts of dopamine quinone αSN block αSN fibril formation and stabilizes the potentially most toxic ααSN protofibrils at the expense of fibrils [37]. The toxicity of dopamine quinone adduct have a role in dopamine dependent toxicity that involves αSN proteins complexes such as with 14-3-3 [281], interacting possibly with various functions of 14-3-3 [157]. Other derivatives as Ser/Tyr phosphorylated αSN, Tyrnitrosylated αSN, Tyr-phosphorylated αSN perhaps affect vesicle movement and potentiate fibril formation [157, 158]. In addition to αSN overexpression in cultured cells, particularly of mutant forms has been linked to mitochondrial deficit [114], defective cellular trafficking [91], impaired chaperone-mediatd autophagy [39] and increased sensitivity to oxidative stress [140].
Mutations in αSN
Three missense mutations have been identified with autosomal dominant inheritance pattern as A53T [140], A30P [140, 143], E46K [287]. These missense mutations develop Parkinson’s disease at 40-60 years of age with typical PD symptoms. Genomic duplication [29] and triplication [242] are also identified. Genetic polymorphism in the promoter of αSN gene have been identified as PD susceptibility markers [70, 71, 112] which implicates that variability of αSN levels can predispose individuals to disease [199].
UPS, PARKIN, UCHL 1, PROTEASOMES
Ubiquitin proteasome systems are responsible for rapid degradation control of 30% or more of newly made proteins within the cell [239]. This process plays a crucial role in a number of cellular events such as cell-cycling, protein trafficking, antioxidant defense mechanisms, mitochondrial func-tion, inflammatory response, immune response, apoptotic signaling, synaptic function and neurotransmission, signal transduction, gene transcription, cell development and differentiation [176].
The UPS comprises two sequential processes: marking of target proteins with ubiquitin-ubiquitination and proteolytic action by proteasome, on marked proteins. The ubiquitination is a highly ordered ATP dependant process catalyzed by three enzyme systems viz. E1 (ubiquitin activating enzyme), E2 (ubiquitin conjugating enzyme), E3 (ubiquitin ligase) [107]. For mammalian cells only a single E1 is known to exist at present, whereas E2 family consists of over 20 members as UbcH7, UbcH8 and E3 family consist of hundreds of members, parkin is one of them of importance [214].
In the first step of ubiquitination, molecules of ubiquitin (a 76 amino acid, 8.5 KDa polypeptide) are activated by E1. In the second step, the activated ubiquitin is transferred to E2. In the third step, target protein is recognized by E3 which then catalyses the transfer of ubiquitin from E2 to target protein via a covalent bond between carboxyl group of C-terminal residue and internal Lys residue/s of the target protein [214].
Subsequent cycles of covalent addition of Ub molecules result in addition of many Ub molecules to target proteins. A minimum of 4 Ub moieties are required for efficient marking for the proteasome. The marked proteins are degraded by 20/26S proteasomes to small peptides as of 2-24 amino acid residues that are further degraded by peptides to their constituent amino acids ready to be recycled and then hydrolysed by ubiquitin carboxy terminal hydrolase (UCHL 1) to monomeric ubiquitin [118]. Chaperones like heat shock proteins function significantly with UPS, in several ways, notably in their ability to facilitate the recognition and entry into 20/26S proteasomes [118]. Small ubiquitin modifiers (SUMO) may undergo similar and similarly varied reactions with targeted proteins [212].
The dysfunction/inhibition of the protein/s operating in UPS could cause aggregation of unwanted proteins resulting in toxicity. For example, pathogenic species of αSN are more resistant to proteasomal degradation and can directly bind to 20/26S proteasomal subunit and impair proteolytic activity [13, 155, 246]. Over expression of mutant αSN also sensitizes cultured cells to the toxicity associated with proteasome inhibitors, suggesting a prior levels of proteasome impairment [261].
PARKIN
Structure
Parkin, an E3 ligase, gene spans about 1.5Mbp contains 12 exons and encodes a 465 aminoacid protein that has a molecular weight of ~52KDa. Parkin contains i) An amino acid terminal (residues 1-76) ubiquitin homology domain UHD that shares 62% homology to Ub and may be involved in substrate recognition and proteasome binding ii) a central domain (residues 145-232) with unknown function iii) a carboxy terminal RING (RING-Really Interesting New Gene) box (residues 237-449) involved in substrate and E2 interaction. A RING box is composed of two RING-finger motifs. RING1 and RING2 and a cys rich domain termed in between RING (RING1-IBR-RING2) [49, 171].
Parkin Functions
Potential Loci for Pathology: Parkin, the E3 ubiquitinprotein ligase is found to be localized in mitochondria [145]. Numerous substrates that are identified and recruited by parkin in UPS include CDCrel (cell division control related), Pael R (parkin associated endothelial like receptor), O-glyco-sylated αSN, synphillin, cyclin E, CHIP, CASK, septin, α/β tubulin, aminoacyl tRNA synthetase cofactor, p38/JIV 1 and others [49, 171].
The substrate CDCrel is synaptic vesicle associated protein suggested to regulate synaptic vesicle release of cytokines. Parkin mutations could affect CDCrel-1 modulation of dopamine release which may contribute to PD [42]. The second substrate of parkin Pael R is related to aggregation and cell death via stress response in endoplasmic reticulum [120, 237]. Parkin suppresses Pael R induced toxicity by ubiquitination and degradation of this protein and can protect dopaminergic neurons against various insults [119].
Interaction of parkin with αSN, synphillin suggests parkin may play role in vesicle transport and LB associated autosomal PD [35, 237]. Over expression of parkin can rescue cells from proteolytic stress [119] as with mutant αSN induced toxicity. Parkin also has been suggested to play a protective role in apoptosis cell death by delaying mitochondrial swelling and reducing cytochrome C release [41].
Mutations in Parkin
Mutations in parkin are associated with autosomal recessive juvenile parkinsonism (ARJP) characterized by early onset disease mostly before 40 years of age. Parkin mutations have been found in about 60% familial cases and about 20% of cases without positive family history [184]. More than 40 different mutations have been identified globally in varied ethnic populations as European, Japanese, Indian, German etc. These mutations include missense mutations, nonsense mutations, frame shift mutations, deletions, insertions, duplications etc [34, 75, 159, 171, 209]. Lack of parkin function due to mutation has been suggested to lead to toxic accumulation of substrate proteins or indeed itself. Over expression of mutated forms of parkin cause oxidative stress, excessive nitric oxide production and lead to cell death probably via proteasomal inhibition.
Mutations in parkin can disrupt interaction of parkin with E2s and impair substrate recognition such as Q311stop, T240R abrogate interaction between parkin and UbcH7 [236, 237]. Mutation of R42P does not affect UbcH7 interaction still abolishes ubiquitination [236, 237]. T415N and Q311 stop mutations eliminate UbcH8 binding to parkin and T240 R, W453stop reduces the binding [288]. Wild type parkin can promote the ubiquitination of αSP22 (Oglycosylated αSN) but the R42P and T240R mutants do not display this activity [288]. Exon 3-4 deletion (parkin without aminoacids 58-178) prevents interaction of parkin UbcH7,8 and loss of E3 ligase [288, 119]. The mutations lying in Exon 7 leading to R256C, R275T, C253Y, D280N are especially predisposing to the late onset form of PD due to variable functional reduction in parkin [194]. The Lewy body pathology has been found in a ARJP case with R275 W substitution and exon 3 deletion [70]. As in the case of αSN, the parkin gene promoter has several functional variants and those that of lower transcriptional activity have been reported to be associated with increased risk of PD [279].
DJ-1
Structure
DJ-1 gene spans about 24 kb contain 8 exons and encodes 189 amino acid protein that has a molecular weight of ~20KDa [115]. The crystal structure shows that DJ-1 exists as a homodimer in vitro. Dimerization of protein appears to be critical for its normal cellular function, the failure of dimerization results in increased degradation and hence loss of function [115].
DJ-1 Functions
Potential Loci for Pathology: DJ-1 expression is ubiquitous in the brain with higher levels of the transcript in subcortical regions such as the caudate nucleus, thalamus, substantia nigra and hippocampus which are more often affected in PD [9]. Unlike αSN and parkin, DJ-1 is not an essential component of Lewy bodies. DJ-1, which belongs to DJ-1/Thi J/Pfp 1 super family, participates in multiple protein-protein interactions. The important proteins with which DJ-1 interacts are PIASxα (protein inhibitor of activated STAT (signal transducer and activator of transcription)) and DJBp (DJ-1 binding protein) [190, 259]. Both these proteins bind to DNA binding domain of androgen receptor controlled genes. The exact function of DJBp is unknown, while PIASxα, is a member of PIAs family of proteins known as SUMO 1, ligases and regulates various transcription factors such as p53 and steroid hormone receptors [115].
DJ-1 may have a role in sensing and protecting neurons from oxidative stressors and/or protecting against mitochondrial damage. For example in cell culture studies and under stress conditions, wild type DJ-1 appears to translocate to the outer mitochondrial membrane and has shown to confer protection against some toxins such as MPTP [25].
DJ-1 demonstrates an acidic shift in isoelectric point in cultured cells following oxidative stress owing mainly to oxidation of cystein residues, particularly Cys106, which can be converted to cysteine sulfinic acid (CysSO2OH) [25]. In cultured cells, overexpression of DJ-1 protects against oxidative harm whereas breakdown of DJ-1 by small interfering RNA (siRNA) enhances the susceptibility to oxidative stress [258]. DJ-1 has been found to shift to more acidic isoforms (pH 5.8) after treatment of cells with herbicide paraquat causing αSN upregulation and aggregation by oxidizing proteins [162]. DJ-1 may also confer protection against endoplasmic reticulum stress, proteasomal inhibition and the toxicity induced by over expression of PaelR [284].
Mutations in DJ-1
DJ-1 mutations are rare cause of early onset autosomal recessive parkin-like parkinsonism. The frequency of DJ-1 mutations in early onset familial disease is approximately 1-2 %. To date no mutations have been described in idiopathic PD. The identified DJ1 mutations include L166P, 14kbdel, M26I, D149A [2], Ivs61G→C, c56 del Cc.56G→A, A104T [96], Ex 5-7 del, IV S5+2-12 del [103]. L166P mutant DJ-1 exists as a monomer instead of dimer because of impaired ability to self-interact. As a result the mutant protein is highly unstable and is degraded by 20/26S proteasome [183]. Wild type DJ-1 over expression can rescue cells from H2O2 induced oxidative stress but L166P mutant DJ-1 cannot do this [284]. The effect of other potent mutations remains to be identified. The majority of mutations result in aberrant protein transcription and truncation causing loss of functionality of the protein.
DARDARIN OR LRRK2
Structure
LRRK2 (Leucine Rich Repeat Kinase 2) or dardarin (in Basque dardara means tremor) gene spans about 14 kb contains 51 exons and encodes 2527 amino acid protein that has molecular weight of about 286 KDa [198].
The LRRK2 belong to ROCO protein that contains numerous highly conserved domains as Leucine-rich repeat LRR; Ras of complex proteins (Roc) a Ras-like/small GTPase superfamily domain of mitogen activated protein kinase (MAPK), a tyrosine kinase like domain and WD 40 domain [198].
Very little is known about the normal functions of LRRK2. It functions as kinase and kinase activity most closely resembles the family tyrosine like kinases that phosphorylate serine/threonine residues and lie upstream of MAPK pathways. These receptor interacting protein (RIP) kinases activate downstream pathways by scaffolding function, in addition to and independent of their kinase activity [262].
Mutations in LRRK2
Mutations in LRRK2 tend to be distributed throughout these functional domains and are responsible for ≤7% of familial autosomal dominant late onset PD and account for 0.5-3% of sporadic PD [51, 83, 198].The mutations found include R1441C [198, 291], Y1699C [198, 291], G2019S [83], I1122 V [291], I2020T [78]. Many more putative amino acid substitutions have been identified [15, 137]. At present it is unclear how mutations in LRRK2 cause PD, but it will be of particular interest to determine whether LRRK2 is related to other gene products or molecular pathways associated with PD. It is tempting to speculate that the putative kinase activity may be involved in the phosphorylation of protein implicated in PD pathogenesis such as αSN [182].
In contrast to mutations in parkin and DJ-1 which likely cause PD by loss of function mechanisms the I2020T mutation within kinase domain of LRRK2 has been found to increase the kinase activity of LRRK2 by 40% in vitro suggesting that increased LRRK 2 kinase causes PD [86].
PINK1
Structure
PTEN [(phosphatase and tensin homolog deleted on chromosome ten)] induced putative kinase, PINK1 gene spans about 18 kb contains 8 exons and encodes 581 amino acid protein that has a molecular weight of about ~63KDa. The N-terminus of PINK1 protein has a mitochondrial targeting motif at N-terminus with 34 amino acid and a highly conserved protein kinase domain (amino acid 156-509) homologous to serine/threonine kinases of Ca2+ - calmodulin family [241].
Studies of PINK1 are at an early stage and currently little is known about the physiological functions of PINK1. PINK1 is found to be localized in mitochondria and suggested to have mitochondrial function in phosphorylating mitochondrial proteins in response to cellular stress [241]. Although the kinase activity of PINK1 has been demonstrated and as such no putative mitochondrial substrates or interacting proteins have been found. In vitro studies suggest that PINK1 may afford some protection against mitochondrial dysfunction and apoptosis induced by protesasomal inhibition, although the mechanism for this action is not understood [241].
Mutations in PINK1
Mutations in PINK1 probably account for only 1-2% of early onset cases of Parkinson’s disease [101]. Mutations in PINK1 are thought to cause PD through loss of function with most mutations clustering in and around putative kinase domain. Several mutations reported in PINK1 include W437stop, G309D [234], H271Q, E417G etc [101]. Reports show that loss of Drosophila PINK1 leads to defects in mitochondrial function resulting in male sterility, apoptotic muscle degeneration, and minor loss of dopamine neurons that is rescued by overexpression of the ubiquitin E3 ligase, parkin. Thus, PINK1 and parkin appear to function in a common pathway suggesting a convergence of the two genes most commonly associated with autosomal recessive PD [101].
OTHERS
Several other genes have been implicated in the pathology of PD or have been re-examined. These are genes for UCHL-1, synphillin-1, NR4A2 and glucocerebrosidase. UCHL-1 protein functions in the UPS of protein degradation and is also found in LBs. An I93M mutation in gene encoding UCHL-1 resulting in reduced enzymatic activity is thought to cause autosomal dominant PD in one family of German descent [153]. NR4A2 protein acts as a transcription factor required for the differentiation and maintenance of dopaminergic neurons and heterozygous mutations were identified in multiple PD families [150].
Synphillin-1 has been implicated in the pathogenesis of PD after one group identified heterozygous mutations and because it may interact with both αSN and parkin [169]. For all three genes there has been little or no replication of the original study in independent populations.
Mutations are found in gene for glucocerebrosidase and the findings have been replicated by most studies [4, 160].
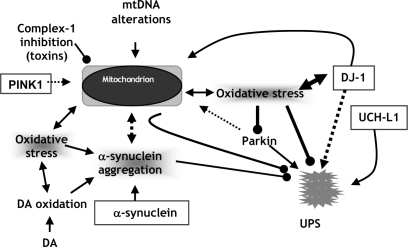
MITOCHONDRIAL DYSFUNCTION
The link between Parkinson’s disease and mitochondria was first established with the identification of a deficiency in the activity of complex I (NADH ubiquinone reductase) in PD substantia nigra and subsequently in the peripheral tissues of patients. Inhibition of complex I results in increased free radical generation and could contribute to the oxidative mediated damage seen in the PD affected substantia nigra. Inhibition of mitochondrial respiration reduces the levels of ATP required for normal UPS function of protein degradation and oxidative stress adds to the substrate load. These changes in turn may induce protein damage and misfolding [26]. The neurotoxic compounds as MPTP, rotenone which selectively damage dopaminergic neurons and cause PD like clinical features are selective inhibitors of complex I of mitochondrial respiratory chain [93]. There is 30-40% decrease in complex I dysfunction in PD which could thus play a role in oxidative stress, free radical mediated protein damage, impaired proteasomal function and protein accumulation [232]. Mitochondria are also intimately involved with both necrotic and apoptotic cell death. Recently identified nuclear gene mutations of mitochondrial proteins include mutations of PINK1, DJ1 causing Parkinson’s disease, and mutations of MtDNA polymerase γ (POLG) causing parkinsonism. The relation between mitochondrial dysfunction PD has been highlighted further by the recent description of mtDNA abnormalities in PD patients. Occasional mtDNA point mutations have been identified in PD but these have not been present in the general PD population. Thus, their association with PD might merely represent part of the wide clinical spectrum of mtDNA mutations and not necessarily imply a more common role in sporadic PD. POLG mutations have been described in patients with PD with ragged red fibres and multiple mtDNA deletions, but mutations of this gene have not been found in sporadic PD [256]. A mutation in the mtDNA 12S RNA was identified in a patient with maternally inherited early onset PD, deafness, and neuropathy. Several studies that have sequenced mtDNA in PD patients have not identified any consistent mutations. Two studies have shown a link between mtDNA haplotypes and the risk for developing PD. The first showed a reduced risk for PD in individuals with haplotypes J and K and the second a 22% decrease in PD risk in those with the UKJT haplotype cluster. In contrast, a smaller study reported an increased risk for PD with haplotypes J and T.
Inhibition of mitochondrial respiration causes the release of reactive oxygen species from the respiratory chain and reduces the levels of ATP required for normal ubiquitin-proteasome system function of protein degradation. These changes in turn may induce protein damage and misfolding [26]. The neurotoxic compounds as MPTP, rotenone which selectively damage dopaminergic neurons and cause PD like clinical features are selective inhibitors of complex I (NADH ubiquinone reductase) of mitochondrial respiratory chain [93]. There is 30-40% decrease in complex I dysfunction in PD which could thus play a role in oxidative stress, free radical mediated protein damage, impaired proteasomal function and protein accumulation [232]. Mitochondria are also intimately involved with both necrotic and apoptotic cell death.
Oxidative Stress
Oxidative stress develops from increased production of highly reactive free radicals, decreased scavenging of these reactive species, increased generation of oxidatively damaged proteins, lipids, DNA and decreased clearance of oxidized products that could be toxic for cells [233].
Signs of oxidative stress are abundant in the substantial nigra of patients of PD [128]. These include reactive oxygen species (ROS) such as hydrogen peroxide, hydroxyl, superoxide and peroxyl radicals. Other oxidants are reactive nitrogen species (RNS) as nitric oxide. These strong oxidants react with DNA, protein and lipid altering their structure and causing cellular damage [127]. The oxidants as ROS are produced by dopamine metabolism, the electron transport chain of mitochondria, iron metabolism and inflammation (Fig. 1).
Fig. (1).
Putative roles of various candiate genes and other factors on abnormal mitochondrial ATP synthesis in PD.
The enzymatic metabolism or auto-oxidation of dopamine generates free radicals [97]. Dopamine metabolism by MAO-A leads to production of dihydroxy phenylacetic acid (DOPAC) and H2O2 with the consumption of O2 and H2O. Intracellular auto-oxidation of dopamine generates dopamine-quinone and H2O2. Dopamine-quinone participates in nucleophilic reaction with sulfhydryl groups of protein leading to structural modification and reduced glutathione levels [253], and is shown to inhibit glutamate and dopamine transporter function in synaptosome [16], inhibit tyrosine hydroxylase [144] in cell free systems and promote H+ leakage from mitochondria resulting in uncoupling of respiration to ATP synthesis [137]. H2O2 produced can be converted to hydroxyl radical by Fenton reaction in presence of ferrous ion. Hydroxyl radical can react with virtually every cellular macromolecule. In the presence of ferrous ion, the superoxide anion and H2O2 two weakly reactive free radical species can combine in the Haben-Weiss reaction to produce the more reactive OH radical [15, 110]. Reactive oxygen species inhibit complex I and provoke mitochondrial dysfunction.
The iron mediated catalysis of hydroxyl radical generation could be a key mechanism in pathogenesis mediated by oxidative stress. Elevation of iron levels are detected in the SNPc in patients of PD [15, 110]. The iron levels are increased by 35% in PD patients compared to age matched control [247]. Glutathione (GSH) is a major cellular antioxidant counteracting oxidative stress. The oxidative stress indeed is reflected by low levels of GSH and its transfer enzyme glutathione transferase (GST). The depletion of GSH has shown to enhance toxicity of dopamine and H2O2. It is worth to note that, the depletion of GSH and reduced GST activity is reported in PD [243].
Excitotoxicity
The concept of excitotoxicity has been applied to a number of neurodegenerative diseases including PD [92]. Glutamate is the principal excitatory neurotransmitter and mediate its action by interacting with receptors such as NMDA (N-Methyl D-Aspartate), AMPA (α-amino-3-hydroxy-5-methyl 4-isoxalopropionate) and kainate receptors. Persistent activation of the glutaminergic NMDA receptor increases intracellular levels of Ca2+ ions potentially leading to activation of proteases, endonucleases, phospholipases and NO synthase with resulting generation of reactive NO. The process, furthermore releases iron from ferritin induces lipid peroxidation and impairs mitochondrial function. Increased intracellular Ca2+concentration and the subsequnt events leading to cell death is supported by the observation that dopaminergic neurons expressing calcium binding protein calbindin may be selectively preserved in PD [109]. Glutamate can inhibit the uptake of cysteine which is required for intracellular synthesis of glutathione [186]. Persistent glutamate activation may occur by overactivity of subthalamic glutamate neurons or impairment of complex I activity in SNPc leading to partial depolarization dopaminergic neurons, relieving the voltage dependant blockade of NMDA receptors by Mg2+, making them susceptible to damage consequent to normal physiological levels of glutamate [12, 43]. Glutamate mediated excitotoxicity may contribute to the protein aggregation via nitration [58].
Inflammation
Gliosis in the substantia nigra and striatum is a prominent feature of sporadic PD [228]. Activation and neuronal homing of microglia occurs prior to the death of dopaminergic neuron [195]. The massive microglial activity is reported in PD [151, 152, 173], which may be a general consequence of neuronal death or may be implication of active participation of microglia in the neurodegeneration. Activated microglia release proinflammatory molecules as interleukin 1β (IL-1β), tumor necrosis factor α (TNFα), interferon γ (IFNγ), which can stimulate the expression of nitric oxide synthase (NOS) and increase nitric oxide production. Cytokines such as TNFα, and IL-1β trigger the microglial activation and increased levels of these cytokines are observed in the substantial nigra of sporadic PD. Also there is an increased expression of glial inducible NOS and nitrites has been observed in substantia nigra of PD [180]. NO readily reacts with superoxide radical, also produced by activated microglia to produce highly reactive peroxy nitrite anions ONOO−. The ONOO− can lead to DNA damage, enzyme dysfunction, disruption of structural protein integrity, the events that trigger cellular apoptosis or necrosis [136, 290].
Lipopolysaccharide activated microglia exhibits increase in cyclooxygenase 2 (COX2) synthesis [113]. COX toxicity is mediated by prostaglandins and reactive oxygen species generated through arachidonic acid cascade and inhibition of glutamate uptake causing excitotoxicity [263].
Deficiency of Neurotropic Factors
Neurotropic factors such as glial-derived neurotropic fac-tors (GDNF) and brain derived neurotropic factors (BDNF) have shown to produce potent protective and regenerative effects on dopaminergic neurons. Delivering GDNF directly into the putamen of PD patients greatly improves their symptoms and significantly increases putaminal dopamine uptake and storage [84]. BDNF appears as an important determinant of dopamine cell survival and D3 receptor expression [95]. The beneficial effects of BDNF and GDNF raises the possibility that the supply of these or other neurotropic substances may be limited or that their down stream signaling path may be dysfunctional in PD leading to degeneration of dopaminergic cells.
CURRENT TREATMENT STRATEGIES
The management of PD can be subdivided into three categories as symptomatic treatment, protective or preventive treatment restorative or regenerative treatment. At present only symptomatic treatments are proven efficacy [225]. The treatments encompass pharmacotherapy with levodopa, dopaminergic agonists, MAO inhibitors, COMT inhibitors, anticholinergics etc to support or restore dopaminergic functions (Table 2).
Table 2.
Therapies for Parkinson’s Disease
| Agent | Pharmacological Class | Adverse Effects |
|---|---|---|
| Levodopa (with carbidopa and benserazide) | Dopamine precursors | Central side effects (motor fluctuations, dyskinesia, psychiatric disturbances) and peripheral side effects (nausea, vomiting, orthostatic hypotension) |
| Seligiline | MAO-B inhibitors | Insomnia, neuropsychiatric side effects. Dopaminergic effects of the other drugs may be accentuated. |
| Tolcapone, Entacapone | COMT inhibitors | Hepatotoxicity, diarrhea, potentiation of levodopa side effects |
| Ergot derivatives (Bromocriptin, pergolide, lisuride, carbergoline) | Dopamine agonists | Peripheral and central dopaminergic side effects, pedal edema, pleuropulmonary reaction retroperitoneal fibrosis, erythromelagia, Raynaud’s phenomenon, postural hypotension |
| Ropinirole, Pramipexole | D2/D3/D4 agonist | Somnolence, nausea, vomiting, dyspepsia, abdominal pain, hypotension, confusion, hallucination and dyskinesia |
| Apomorphine | D1/D2 agonist | Nausea and injection site reactions, neuropsychiatric side effects |
| Benztropine, procyclidine etc | Anticholinergics | Peripheral effects (dry mouth, blurred vision, constipation, difficulties with urination) and central effects (confusion, hallucinations, memory problems) |
| Amantadine | NMDA receptor antagonist | Confusion, hallucination, pedulary edema, livedo reticularis |
Levodopa
The most reliable and effective symptomatic treatment of PD makes use of levodopa in combination with a peripheral dopa-decarboxylase inhibitors such as carbidopa, benserazide. Unfortunately chronic levodopa treatment is accompanied by motor complications as motor fluctuations (end of dose deterioration , on-off phenomenon, dystonia, athetosis). The motor side effects are compounded by treatment induced psychiatric disturbances such as psychosis, mania or delirium [238]. After 5 years of treatment about 50% of the patients taking levodopa develop motor fluctuations and 30-40% develop dyskinesia. The prevalence is higher in young patients. The occurrence of both motor fluctuations and dyskinesia being almost inevitable after 5 years of treatment [264]. Moreover, there have been suggestions about toxicity of levodopa and its potentiating effects on disease progression [67, 68]. However there is no evidence as yet to support these suggestions [3]. The mechanisms of motor response fluctuations are not completely understood and still a matter of debate. These motor side effects may be caused by alterations in dopamine receptor expression due to progression of the disease process and/or adaptive response of the drug [38]. This may arise from pulsatile delivery of levodopa to striatal neurons, since levodopa has a short plasma half-life of about 90-120 minutes [192]. To take care of this probable cause, prodrugs of levodopa as ethyl ester or methyl ester of levodopa are developed [54].
MAO Inhibitors
The dopamine deficiency can be countered by inhibiting metabolism of dopamine by means of inhibitors of metabolizing enzymes as monoamine oxidase (MAO) and catechol-O-methyl transferase (COMT). The specific MAO enzyme MAO-B catalyses the breakdown of dopamine in the basal ganglia. MAO- B inhibitors such as selegiline and rasagiline both improve motor function in early and advanced PD. Although there was some concern regarding the long term safety of selegiline, several studies have allayed these fears and shown this drug to be both effective and safe for PD patients. Selegiline is a clinically used irreversible MAO-B inhibitor. Other MAO-B inhibitors as rasagiline, lazabemide appear to be promising compounds for the management of PD [205, 252]. Besides decreasing the degradation of dopamine the MAO-B inhibitors possibly possesses neuroprotective properties [226] and are used as adjunct therapy.
COMT Inhibitors
The newly developed catechol-O-methyltransferase (COMT) inhibitors increase the availability of levodopa to the brain, and their action is complementary to that of the dopa decarboxylase inhibitors. There are concerns about the hepatotoxicity of tolcapone, whereas entacapone, another catechol-O-methyltransferase inhibitor, seems safer in this respect [192]. These compounds however, accentuate side effects of levodopa.
Dopaminergic Agonists
Dopamine-replacement therapy has dominated the treatment of motor symptoms of PD since the early 1960s. The effects are predictable and so are the side effects. None of the more recently introduced synthetic dopamine agonists has surpassed the clinical benefit derived from levodopa [218]. Dopamine agonists, although are less effective, avoid some of the motor complications associated with chronic use of levodopa. The multiplicity of dopamine receptors such as D1-like (D1 and D5) and D2-like (D2, D3 and D4) in the brain offers a range of potential targets [248]. Most currently used drugs however activate D2 and D3 dopamine receptors only [188]. So far exploitation of drugs acting on specific receptor subtypes has been disappointing and no major advance has been made in producing D1 dopamine agonists, a known target for anti-Parkinsonian agents [129]. Partial D2 dopamine agonists are being developed as they might treat the motor symptoms of Parkinson’s disease while suppressing both psychosis and dyskinesia. The most important DA receptor agonists which are currently approved are ergolinederivatives such as bromocriptine, lisuride, pergolide, cabergoline as well as the non-ergoline derivatives like pramipexole, ropinirole and apomorphine.
Theoretically, the advantage of being both D1 and D2 agonist such as lisuride seems to be of better therapeutic index [271]. The non-ergoline derived dopaminergic agonists have preferential affinity for D2-like receptors except apomorphine which is active at both D1 and D2 receptors [125]. Ropinirole, pramipexole bind preferably to D3 receptors [36, 181]. A novel compound sumanirole with a 200 fold selectivity for D2 receptor over D3 receptor was recently been shown to be effective in reversing motor symptoms in animal models of PD and did not cause dyskinesia [175]. The D1 specific agonists such as SKF-81297, SKF-82958, ABT-431 are under investigation [65]. A novel mixed dopamine D1/D2/D3 agonist roligotine has proven to be effective antiparkinsonisan agent in clinical trials [126]. Recently, non-oral delivery has provided more long-lasting anti-Parkin-sonian activity through the subcutaneous or intravenous infusion of apomorphine and transdermal patch technology with roligotine or lisuride. Valvular Heart Disease is observed with the use of dopamine agonists [135].
Anticholinergic Agents
Dopamine deficiency in PD results in overactivity of acetylcholine. The symptoms of excess cholinergic activity can be overcome by anticholinergic drugs such as benztropine, trihexylphenidyl, procyclidine etc [104].
Others
Amantadine, an antiviral agent was found by chance to be effective in PD and is particularly effective in reducing dyskinesia [20]. This antidyskinetic effect is likely to be mediated by NMDA receptor antagonistic action [254].
EMERGING THERAPIES
Supporting Reduced Dopaminergic Functions
Traditionally, this approach makes use of levodopa, MAO-B inhibitors, COMT inhibitors and dopamine agonists. The novel compounds based on diverse therapeutic strategies are developed as SIB1508Y, an agonist selective for specific nicotinic acetylcholine receptor subtype which enhances release of dopamine from striatum in vivo [274, 280]. Other compounds such as NS-2214, NS-2380 which are tropane derivatives of cocaine block the dopamine transporter (DAT) and increase endogenous dopamine levels in the synaptic cleft [280].
INTERVENTION TO PREVENT DEVELOPMENT OF MOTOR COMPLICATIONS
NMDA Receptor Agonists
Dopamine depletion and dopamine replacement strategies result in substantial alteration in basal ganglia NMDA receptor structure, localization and function [30]. With the help of this knowledge and experience of amantadine, a number of studies have examined the efficacy of antagonists of NMDA receptor such as remacemide, however remacemide showed no clear effects in PD [203]. A nonselective, non-competitive antagonist of NMDA receptor is MK801 which is found to be well tolerated in rodents (but not in primates) can improve akinesia and can have synergistic action with dopaminergic drugs [165]. To avoid the sedative adverse effects of MK-801 selective NMDA receptor antagonists are under development. For example NR2B subunit containing NMDA selective antagonists are ifenprodil, CP101606, Co101244, CI1041 and NRI/ NR2B antagonist MDL100453 have been examined in animal studies [98]. Alternative to traditional antagonists other strategies such as drugs that alter the trafficking and regulation of NMDA receptors are also worth exploring. These might include drugs with actions at the phosphatase and kinase systems that modulate NMDA receptor or perhaps drugs acting at upstream modulatory systems such as metabotropic glutamate receptors [161].
AMPA Receptor Antagonists
An excitatory amino acid glutamate acts on another ionotropic receptor AMPA receptor. Stimulation of NMDA and AMPA receptor seems to considerably contribute to motor complications and levodopa induced dyskinesia [23]. Hence AMPA receptor antagonist could be of use in PD as NMDA receptor antagonists. Many of the homophthalazine derivatives as GYKI52466, GYKI53655, GYKI54015, GYKI53405, and GYKI54065 have been developed as AMPA antagonists. Of these talampanel (GYKI53405) has shown promise in decreasing or abolishing levodopa caused dyskinesia [255].
Serotonergic Agents
Advances in knowledge of 5-HT receptors and its subtypes suggests putative role of these receptors in the control of movement and possible novel intervention strategies for modulating dopaminergic and nondopaminergic systems in PD [19]. Based on the distribution, localization and function in the basal ganglia, the subtypes 5-HT1A, 5-HT1B/1D, 5-HT2A and 5-HT2C receptors are clearly linked with modulation of nigrostriatal pathway [10]. Therefore, receptor subtype specific serotonergic agents can act at several sites to modify dopaminergic activity. Sarizotan (EMD12830), a 5-HT2A inverse agonist has been reported to exhibit encouraging results in phase II trials. Other agents being developed with this strategy include SR46349D (5-HT2A antagonist), ACP103 (5-HT2A inverse agonist), SB206553 (5-HT2C/2B antagonist), SB 242084 (5-HT2C antagonist) and mCPP, MK 212, RO 600175 (5-HT2C antagonists) [11].
A2A Receptor Antagonists
Adenosine, an endogenous purine nucleoside acts as a homeostatic modulator by controlling neurotransmitter release or postsynaptic neuronal response [40]. These actions are mediated by receptors A1, A2A, A2B and A3. Among these adenosine receptors, A2A receptors appear to play the most important role in the control of motor behavior and in the modulation of dopamine mediated responses since A2A receptor is enriched in striatum compared with other brain regions [55, 73]. There exists a functional antagonistic interaction between A2A and D2 receptors as their co-localization in striatopalladial neurons. Stimulation of A2 receptor has shown to decrease the affinity of D2 receptors in striatal membrane preparation [73]. This functional interactions has suggested new therapeutic approach for PD based on the use of selective A2A receptor antagonists. Several A2A receptor antagonists have been developed as xanthine derivatives KF-17837, istradefylline, triazoloquinazoline CGS15943, triazolopyrimidine SCH5826, triazolotriazine ZM241385 and VER 2006 [133]. Among the most advanced molecules, istradefylline has moved successfully to phase III clinical evaluation [102].
Interacting with Crucial Steps in Neuronal Death
Programmed cell death, one of the processes of neuronal death occurs by two ways, apoptosis and autophagy. Apoptosis is catalyzed by aspartate proteases called caspases. About 14 different caspases have been identified in mammalian nervous tissue and separated into apoptotic initiators [62]. Selective caspase inhibition would reduce neuronal apoptosis and is considered as a potential therapeutic strategy to develop drugs [221]. This strategy includes caspase inhibitors, p53 inhibitors, JNK inhibitors and GAPDH ligands.
Caspase Inhibitors
The second generation tetracycline derivatives minocycline is referred as caspase inhibitor. Minocycline inhibits immunologic processes for example inhibition of matrix metalloproteases p38-mitogen activated protein kinase, reactive oxygen species release from neutrophils, inducible nitric oxide synthases and complexes with Ca2+ [52, 276]. It also inhibits caspase 1 and 3 presumably by interacting with upstream activation [276].
p53 Inhibitors
A synthetic inhibitor of p53 induced transcriptional activation, pifithrin α, was originally developed to protect non-cancerous cells in cancer therapy, but has been also found as protective in the mouse MPTP PD model [56]. However, the long term application of p53 inhibitors as in PD could prove problematic due to its anticancer activity.
JNK Inhibitors
The compound presently in clinical trial is CEP-1347 a staurosporin derivative. It inhibits the jun-N-terminal kinase (JNK) pathway by inhibition of mixed-linkage kinase [206].
GAPDH Ligands
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH), an enzyme in glycolytic pathway is implicated in p53 dependant apoptosis. TCH 346 binds to GAPDH stabilizes the dimeric form of the protein and prevents apoptosis related up regulation and prevents nuclear translocation of GAPDH known to be involved in the mediation of p53 dependent apoptosis [56]. TCH346 (previously known as CGP3466p), a tricyclic propagylamine derivative has shown inhibition of apoptosis in various cell lines and its systemic administration prevented motor symptoms and nigrostriatal degeneration induced by MPTP in primates. However in a double-blind, randomised, controlled trial TCH346 did not show evidence of a neuroprotective effect. The discrepancy between the pre-clinical promise of TCH346 and the clinical outcome could have arisen because of the use of laboratory models that do not accurately reflect the pathogenesis of Parkinson’s disease, the doses of study drug used, insensitive clinical endpoints, and the patient population selected for study [196].
HALTING OR SLOWING THE PROGRESSION OF NEURONAL LOSS
Induction of Autophagy
Malfunction of UPS or chaperone mediated autophagy (CMA) results in toxic accumulation of proteins leading to cell death. This toxic accumulation of proteins can be circumvented by inducing autophagy. Rapamycin, one such autophagy inducer, suppresses the inhibitory activity of the mammalian target of rapamycin (mTOR) an autophagy protein. Use of rapamycin has demonstrated to reverse pathology in a model of Huntington’s disease [219]. Although this is a promising approach, it should be remembered that autophagy also constitutes a non-apoptotic, caspase-independent form of programmed cell death, a significant site of Alzheimers Aβ peptide generation and inducing autophagy might increase the production of this neurotoxic peptide [285].
Addressing Mitochondrial Dysfunction
The positively confirmed links of mitochondrial dysfunction and protein aggregation causing PD provides a rationale for the use of antioxidants such as vitamin C and E as conjunctive therapies. The result of studies carried to check the validity of the rational gave ambiguous results. One study including vitamin C and E delayed the use of levodopa or dopamine agonists for 2 years. However, the results were not confirmed by further studies [205,227]. Coenzyme Q10 is an intrinsic component of mitochondrial respiratory chain and use of this might be beneficial as suggested by reduced rate of deterioration in motor functions found in double blind placebo controlled pilot studies with longer follow-up [240].
Reducing Oxidative Stress
Oxidative stress caused by increased free radical generation as observed in PD can be reduced by use of spin-trapping compounds such as nitrones. One such compound developed is stibazulenyl nitrone [283]. These developments of new antioxidants will broaden the therapeutic strategies for PD. Since iron plays a pivotal role in the process of oxidative stress, inflammatory process and cell death, it is apparent that reducing iron-induced oxidation is protective to the neurons and renders iron-chelators as an approach to combat neurodegeneration in PD. VK28, a quinoline compound and its derivatives are developed as iron-chelators and tested in rats [231]. Depletion of an endogenous antioxidant glutathione is one of the important pathogenic factors of PD. Hence it is plausible that one of the ways to counteract this problem is to replenish glutathione pool either by increasing synthesis of glutathione or by slowing its degradation or by administering glutathione or administering precursors of glutathione or its analogues [229, 282]. A GSH analogue YM737 has been tested in animal PD model [282].
Inhibiting αSN/Protein Aggregation
The association of αSN aggregation with PD has led to efforts to develop inhibitors of αSN aggregation, both as peptide based and dopachrome based and proven effective in vitro [60, 191]. Geldanamycin, a naturally occurring benzoquinone ansamycin, has been shown to inhibit αSN aggregation and toxicity in cultured cells and in Drosophila [174]. However, it should be taken into account that inhibiting αSN aggregation is not without risk. If protein aggregation is protective mechanism as suggested by protofibril formation, then aggregation inhibitors might liberate toxic oligomeric species and aggravate rather than inhibit the disease process.
Immunotherapy
Therapeutic antibodies can be used to target αSN, however αSN is primarily an intracellular cytosolic protein and as a result might be less accessible to antibodies. An intrabody approach has been reported in which the expression of single-chain Fv antibody inhibits the formation of αSN aggregates [178]. More recently immunization of αSN transgenic mice with αSN has been reported to inhibit the aggregation of αSN in neuronal cell bodies and synapses and increased levels of synaptophysin suggesting recovery from a neurodegenerative process [170]. The use of antibodies might cause immune mediated cellular response also.
Inhibiting Inflammatory Process
Microglia mediated deleterious inflammatory reactions controlled by anti-inflammatory therapy. The anti-inflam-matory drugs may inhibit COX2 and COX2 mediated dopamine oxidation and may scavenge hydroxyl radical and NO [31, 72, 263]. Agonists of peroxisome proliferator activated receptor γ (PPARγ), which has been reported to possesses anti-inflammatory qualities is found to attenuate glial activation and rescue dopaminergic cells of substantia nigra in MPTP treated mice [21].
Inducing Recovery of Surviving Dysfunctional Neurons: Neurotrophic Factors
A neuroprotective approach to treat PD is the use of neurotrophic factors to nourish and enhance the survival of dopaminergic cells in the substantia nigra. Several of these factors including neurotrophin4/5, BDNF, GDNF have been shown to be promising in animal models [117, 142]. Unfortunately, results from a recent controlled clinical study delivering GDNF directly into brain did not demonstrate efficacy and safety of such a treatment. A critical review of available data suggests that there are questions that need to be answered before the future of GDNF as a therapy for PD can be determined. Exhaustive human trials should be undertaken with proper route of administration.
Gene Therapy
Gene therapy is considered as one of the most promising approaches to develop a novel effective treatment for PD. Among the candidate genes that have been tested include genes for enzymes of dopamine synthesis such as tyrosine hydroxylase, guanosine triphosphate cyclohydrolase 1, aromatic-L-amino acid decarboxylase and genes for neurotrophic factors [33]. The genes encoding vesicular monoamine transporter-2 and glutamic acid decarboxylase have also produced therapeutic effects in animal models of PD [59]. The important factor to be addressed in gene therapy is regulation of gene expression for its clinical use.
Replacing Lost Neurons by Transplantation
The idea of replacing the dead dopaminergic neurons of the substantial nigra has emerged more than 20 years ago and is tried now for the treatment of PD [106, 210]. The cells used for transplantation are human primary cells, porcine primary cells, expanded stem cells and neural progenitor cells [76]. Promising results have been achieved in clinical trials but much remains to be done before cell based therapies can be practiced extensively such as addressing adverse effects of dyskinesia; improvements in reproducibility of the transplantation; robust solutions to various safety issues, regulatory and ethical consideration.
CONCLUDING REMARKS
Though our knowledge and understanding of the pathophysiology of disease process has been improved with the advent of molecular pharmacological tools, the precise etiology of PD is still a matter of debate. However, now it is generally agreed that, the genetic factors lead to various protein aggregation and subsequent loss of dopamine neurons. The role of candidate genes and the plausible therapies to target them are the exciting current interest of researchers worldwide. The unmet needs of PD therapy still exist as it is a disorder with variety of complex etiologies. It remains the hope of research scientists that in the not-too-distant future we shall see a new class of drugs arising logically from the understanding of molecular pathophysiology of PD.
ACKNOWLEDGEMENTS
We thank Dr. A. Veeranjaneyulu and Mrs. A. Uma Maheswari for critical comments on the manuscript. We acknowledge Drs. S. S. Kadam & K. R. Mahadik (Bharati Vidyapeeth Deemed University, Poona College of Pharmacy, Pune, India) and the management of Allana College of Pharmacy (Pune, India) for their constant encouragement in the work.
REFERENCES
- 1.Abad VC, Guilleminault C. Review of rapid eye movement behavior sleep disorders. Curr Neurol Neurosci Rep. 2004;4:157–63. doi: 10.1007/s11910-004-0031-7. [DOI] [PubMed] [Google Scholar]
- 2.Abou-Sleiman PM, Healy DG, Quinn N, Lees AJ, Wood NW. The role of pathogenic DJ-s1 mutations in Parkinson’s disease. Ann Neurol. 2003;54:283–286. doi: 10.1002/ana.10675. [DOI] [PubMed] [Google Scholar]
- 3.Agid Y. Levodopa: is toxicity a myth? Neurology. 1998;50:858–863. doi: 10.1212/wnl.50.4.858. [DOI] [PubMed] [Google Scholar]
- 4.Aharan-Peretz J, Rosnbaum H, Gershani-Baruch R. Mutations in the glucocerebrosidase gene and Parkinson’s disease in Ashkenazi Jews. N Eng J Med. 2004;351:1972–1977. doi: 10.1056/NEJMoa033277. [DOI] [PubMed] [Google Scholar]
- 5.Ahn BH, Rhim H, Kim SY, Sung YM, Lee MY, Choi JY. α-synuclein interacts with phospholipase D isozymes and inhibits pervandate induced phospholipase D activation in human embroyo in kidney-293 cells. J Biol Chem. 2002;277:12334–12342. doi: 10.1074/jbc.M110414200. [DOI] [PubMed] [Google Scholar]
- 6.Allam MF, Del Castillo AS, Navajas RF. Parkinson’s disease risk factors: genetic, environmental or both? Neurol Res. 2005;27:206–208. doi: 10.1179/016164105X22057. [DOI] [PubMed] [Google Scholar]
- 7.Armstrong M, Daly AK, Cholerten S, Bateman DN, Idle JR. Mutant debrisoquine hydroxylation genes in Parkinson’s disease. Lancet. 1992;339:1017–1018. doi: 10.1016/0140-6736(92)90537-d. [DOI] [PubMed] [Google Scholar]
- 8.Asanuma M, Miyazaki I, Diaz-Corlaes FJ, Ogawa N. Quinone formation as dopaminergic neuron-specific oxidative stress in the pathogenesis of sporadic PD and neurotoxin induced parkinsonism. Acta Med Okayama. 2004;58:221–233. doi: 10.18926/AMO/32105. [DOI] [PubMed] [Google Scholar]
- 9.Bandopadhay R, Kingsbury AE, Cookson MR, Reid AR, Evans IM, Hope AD, Pittman AM, Lashley T. The expression of DJ-1 (PARK7) in normal human CNS and idiopathic Parkinson’s disease. Brain. 2004;127:420–430. doi: 10.1093/brain/awh054. [DOI] [PubMed] [Google Scholar]
- 10.Barnes NM, Sharp T. A review of central 5-HT receptors and their functions. Neuropharmacology. 1999;38:10083–11152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- 11.Bartoszyk GD, Van Amsterdam C, Greiner HE, Rauternberg W, Russ H, Seyfried CA. Sarizotan, a serotonin 5-HT1A receptor agonist and dopamine receptor ligand 1.Neurochemical Profile. J Neural Trans. 2004;111:113–126. doi: 10.1007/s00702-003-0094-7. [DOI] [PubMed] [Google Scholar]
- 12.Beal MF. Excitotoxicity and nitric oxide in Parkinson’s disease pathogenesis. Ann Neurol. 1998;44:110–114. doi: 10.1002/ana.410440716. [DOI] [PubMed] [Google Scholar]
- 13.Bennett MC, Bishop JF, Leng Y, Chock PB, Chase TN, Mouradian MM. Degradation of α-synuclein by proteasome. J Biol Chem. 1999;274:33855–33858. doi: 10.1074/jbc.274.48.33855. [DOI] [PubMed] [Google Scholar]
- 14.Bennett MC. The role of α-synuclein in neurodegerative diseases. Pharmacol Ther. 2005;105:311–331. doi: 10.1016/j.pharmthera.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 15.Berg D, Gerlach M, Youdin MB, Double KL. Brain iron pathways and their relevance to Parkinson’s disease. J Neurochem. 2001;79:225–236. doi: 10.1046/j.1471-4159.2001.00608.x. [DOI] [PubMed] [Google Scholar]
- 16.Berman SB, Hastings TG. Inhibition of glutamate transport in synaptosomes by dopamine oxidation and reactive oxygen species. J Neurochem. 1997;69:1185–1195. doi: 10.1046/j.1471-4159.1997.69031185.x. [DOI] [PubMed] [Google Scholar]
- 17.Betarbet R, Sherer TB, Greenamyre JT. Ubiquitin-proteasome system and Parkinson’s disease. Expt Neurol. 2005;191:17–27. doi: 10.1016/j.expneurol.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 18.Bharath S, Hsu M, Kaur D, Rajgopalan S, Anderson JK. Glutathione iron and Parkinson’s disease. Biochem Pharm. 2002;64:1037–1048. doi: 10.1016/s0006-2952(02)01174-7. [DOI] [PubMed] [Google Scholar]
- 19.Blackburn T. Serotonergic agents and Parkinson’s disease. Drug Discov Today. 2004;1:35–41. [Google Scholar]
- 20.Blanchet PJ, Metman LV, Chase TN. Renaissance of amantadine in the treatment of Parkinson’s disease. Adv Neurol. 2003;91:251–257. [PubMed] [Google Scholar]
- 21.Breidert T, Calebert J, Heneka MT, Landreth G, Launay JM, Hirsch EC. Protective action of the peroxisome proliferator activated receptor γ agonist pioglitazone in mouse models of Parkinson’s disease. J Neurochem. 2002;82:615–624. doi: 10.1046/j.1471-4159.2002.00990.x. [DOI] [PubMed] [Google Scholar]
- 22.Bucciantini M, Giannoni E, Chiti F, Baroni F, Formigli L, Zurolo J. Inherent toxicity of aggregates implies a common mechanism of protein misfolding diseases. Nature. 2002;416:507–511. doi: 10.1038/416507a. [DOI] [PubMed] [Google Scholar]
- 23.Calan F, Rajput AH, Homkiewiez O, Bedard PJ, Dipalo T. Levodopa- induced motor complications are associated with alterations of glutamate receptors in Parkinson’s disease. Neurobiol Disord. 2003;14:404–416. doi: 10.1016/j.nbd.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 24.Calne DB, Snow BJ, Lee C. Criteria for Parkinson’s disease. Adv Neurol. 1992;32:121–127. doi: 10.1002/ana.410320721. [DOI] [PubMed] [Google Scholar]
- 25.Canet-Aviles RM, Wilson MA, Miller DW. The PD protein DJ-1 is neuroprotective due to cystein-sulfinic acid driven mitochondrial localization. Proc Natl Acad Sci USA. 2004;101:9103–9108. doi: 10.1073/pnas.0402959101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cassarino DS, Benneltt JP., Jr An evaluation of the role of mitochondrial mutations and oxidative pathology protective nuclear responses and cell death in neurodegeneration. Brain Res Rev. 1999;29:1–25. doi: 10.1016/s0165-0173(98)00046-0. [DOI] [PubMed] [Google Scholar]
- 27.Castellani RJ, Perry G, Siedlak SL, Nunomura A, Shimohama S, Zhang J. Hydroxnoneal adducts indicate a role for lipid peroxidation in neocortical and brainstem Lewy bodies in humans. Neurosci Lett. 2002;319:25–28. doi: 10.1016/s0304-3940(01)02514-9. [DOI] [PubMed] [Google Scholar]
- 28.Chandra S, Chen X, Rizo J, Jahn R, Sudho TC. A broken αhelix in folded α-synuclein. J Biol Chem. 2003;278:15313–15318. doi: 10.1074/jbc.M213128200. [DOI] [PubMed] [Google Scholar]
- 29.Chartier-Harlim MC. α-synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet. 2004;364:1167–1169. doi: 10.1016/S0140-6736(04)17103-1. [DOI] [PubMed] [Google Scholar]
- 30.Chase TN. Levodopa therapy: consequences of nonphysiologic replacement of dopamine. Neurology. 1998;50:17–25. doi: 10.1212/wnl.50.5_suppl_5.s17. [DOI] [PubMed] [Google Scholar]
- 31.Chen H, Zang SM, Herman MA, Schwarzschild MA, Willet WC, Colditz GA, Speizer FE, Ascherio A. Nonsteroidal antiinflammatory drugs and the risk of Parkinson’s disease. Arch Neurol. 2003;60:1059–1064. doi: 10.1001/archneur.60.8.1059. [DOI] [PubMed] [Google Scholar]
- 32.Chen JJ. Anxiety, depression and psychosis in Parkinson’s disease. Unmet needs and treatment challenges. Neurol Clin. 2004;41:15–23. doi: 10.1016/j.ncl.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 33.Chen Q, He Y, Keyi Y. Gene therapy for Parkinson’s disease: progress and challenges. Curr Gene Ther. 2005;5:71–80. doi: 10.2174/1566523052997505. [DOI] [PubMed] [Google Scholar]
- 34.Choudhary S, Behari M, Dihana M, Swaminathann PJ, Govindappa ST. Parkin mutations in familial and sporadic Parkinson’s disease among Indians. Parkinsonism Relat Disord. 2006;12:239–45. doi: 10.1016/j.parkreldis.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 35.Chung KK, Zhang Y, Lim KL, Tanaka Y, Huang H, Gao J, Ross CA, Dawson TM. Parkin ubiquitinates the α-synuclein interacting protein, synphilin-1: implications for Lewy body formation in Parkinson’s disease. Nat Med. 2001;10:1144–1150. doi: 10.1038/nm1001-1144. [DOI] [PubMed] [Google Scholar]
- 36.Coldwell MC, Haga J, Middlemiss D, Telloch I, Boyfield I. Ropinirole is a D3-selective agonist at cloned human D2 analog D3 and D4 receptors in functional studies using microphysiometry. Br J Pharmacol. 1997;119:346–346. (abstract) [Google Scholar]
- 37.Conway K, Rochet JC, Bieganski RM, Lanbury PTJ. Kinetic stabilization of α-synuclein protofibril by a dopamine-α-synuclein adduct. Science. 2001;294:1346–1349. doi: 10.1126/science.1063522. [DOI] [PubMed] [Google Scholar]
- 38.Crossman AR. A hypothesis on the pathophysiological mechanisms that underlie levodopa or dopamine agonist-induced dyskinesia in Parkinson’s disease: implications for the future strategies in treatment. Mov Disord. 1990;5:100–108. doi: 10.1002/mds.870050203. [DOI] [PubMed] [Google Scholar]
- 39.Cuervo AM, Stefani L, Fredenburg R, Lansburry PT, Sulzer D. Impaired degradation of mutant α-synuclein by chaperone-mediated autophagy. Science. 2004;305:1292–95. doi: 10.1126/science.1101738. [DOI] [PubMed] [Google Scholar]
- 40.Cunha PA. Adenosine as a neuromodulator and as a homeostatic regulator in the nervous system: different roles, different sources and different receptors. Neurochem Int. 2001;38:107–125. doi: 10.1016/s0197-0186(00)00034-6. [DOI] [PubMed] [Google Scholar]
- 41.Darios F, Corti O, Lucking CB, Hampe C, Muriel MP, Abbas N, Gu WJ, Hirsch EC, Rooney T, Ruberg M, Brice A. Parkin prevents mitochondrial swelling and cytochrome C release in mitochondria dependant cell death. Hum Mol Genet. 2003;12:517–526. doi: 10.1093/hmg/ddg044. [DOI] [PubMed] [Google Scholar]
- 42.Dawson TM, Dawson VL. Rare genetics mutations shed light on the pathogenesis of Parkinson’s disease. J Clin Invest. 2003;111:145–151. doi: 10.1172/JCI17575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dawson VL, Dawson TM. Nitric oxide in neurodegeneration. Prog Brain Res. 1998;118:215–29. doi: 10.1016/s0079-6123(08)63210-0. [DOI] [PubMed] [Google Scholar]
- 44.deRijk MC, Launer LJ, Berger K . Prevalence of Parkinson’s disease in Europe: a collaborative study of population based cohorts. Neurology. 2000;55:1358–63. [PubMed] [Google Scholar]
- 45.Dekker M, Bonifati V, van Swieten J, Leenders N, Galjaard RJ. Clinical features and neuroimaging of PARK7-linked parkinsonism. Mov Disord. 2003;18:51–757. doi: 10.1002/mds.10422. [DOI] [PubMed] [Google Scholar]
- 46.Demasi M, Davies KJ. Proteasome inhibitors induce intracellular protein aggregation and cell death by an oxygen dependant mechanism. FEBS Lett. 2003;542:89–94. doi: 10.1016/s0014-5793(03)00353-3. [DOI] [PubMed] [Google Scholar]
- 47.Deumens R, Blokland A, Prickaerts J. Modeling RD in rats: an evolution of 6- OH DA lesions of the nigrostriatal pathway. Exp Neurol. 2002;175:303–317. doi: 10.1006/exnr.2002.7891. [DOI] [PubMed] [Google Scholar]
- 48.Dev KK, Hofele K, Barbieri S, Buchman VL, van der Putten H. Part II α- synuclein and its molecular pathophysiological role in neurodegenerative disease. Neuropharmacology. 2003;45:14–44. doi: 10.1016/s0028-3908(03)00140-0. [DOI] [PubMed] [Google Scholar]
- 49.Dev KK, van der Putten H, Sommer B, Rovelli G. Part I Parkin associated proteins and Parkinson’s disease. Neuropharmacology. 2003;45:1–13. doi: 10.1016/s0028-3908(02)00337-4. [DOI] [PubMed] [Google Scholar]
- 50.Dewy RB., Jr Autonomic dysfunction in Parkinson’s disease. Neurol Clin. 2004;22:127–139. doi: 10.1016/j.ncl.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 51.Di Fonzo A, Rohe CF, Ferreira J. A frequent LRRK2 gene mutation associated with autosomal dominant Parkinson’s disease. Lancet. 2005;365:412–415. doi: 10.1016/S0140-6736(05)17829-5. [DOI] [PubMed] [Google Scholar]
- 52.Diguet E, Fernagut PO, Wei X, Du Rouland R, Gross C, Bezard E, Tison F. Deleterous effects of minocycline in animal models of Parkinson’s disease and Huntington’s disease. Eur J Neurosci. 2004;19:3266–3276. doi: 10.1111/j.0953-816X.2004.03372.x. [DOI] [PubMed] [Google Scholar]
- 53.Ding TT, Lee SJ, Rochet JC, Lansbury PTJ. Annular α-synuclein protofibrils are produced when spherical protofibrils are incubated in solution or bound to brain derived membranes. Biochemistry. 2002;41:10209–10217. doi: 10.1021/bi020139h. [DOI] [PubMed] [Google Scholar]
- 54.Djaldetti R, Melamed E. Levodopa fluctuations in Parkinson’s disease. Ann Neurol. 1996;390:400–404. doi: 10.1002/ana.410390321. [DOI] [PubMed] [Google Scholar]
- 55.Druncan MJ, Morgan PF. Evidence for adenosine A2b receptor involvement in the hypermobility effects of adenosine analogues in mice. Eur J Pharmacol. 1989;168:285–290. doi: 10.1016/0014-2999(89)90789-9. [DOI] [PubMed] [Google Scholar]
- 56.Duan W, Zhi X, Landenheim B, Yu QS, Guo Z, Oyler J. p53 inhibitors preserve dopamine neurons and motor function in experimental Parkinsonism. Ann Neurol. 2002;52:597–606. doi: 10.1002/ana.10350. [DOI] [PubMed] [Google Scholar]
- 57.Dubois B, Pillon B. Cognitive deficit in Parkinson’s disease. J Neurol. 1997;244:2–8. doi: 10.1007/pl00007725. [DOI] [PubMed] [Google Scholar]
- 58.Duda JE, Giasson BI, Chen Q, Gur TL, Hartig HI, Stern MB. Widespread nitration pathological inclusions in neurodegenerative synucleinopathies. Am J Pathol. 2000;157:1439–45. doi: 10.1016/S0002-9440(10)64781-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.During MJ, Kaplitt MG, Stern MP, Eidelberg D. Subthalamic GAD gene transfer in Parkinson’s disease patients who are candidates for deep brain stimulation. Hum Gene Ther. 2001;12:1589–1591. [PubMed] [Google Scholar]
- 60.El- Agnaf OM, Paleologou KE, Greer B, Abogrein AM, King JE, Salem SA, Fullwood NJ. A strategy for designing inhibitors of αSN aggregation and toxicity as a novel treatment for PD and related disorders. FASEB J. 2004;18:1315–1317. doi: 10.1096/fj.03-1346fje. [DOI] [PubMed] [Google Scholar]
- 61.El- Agnaf OM, Irvine GB. Aggregation and neurotoxicity of α-synuclein and related peptides. Biochem Soc Trans. 2002;30:559–565. doi: 10.1042/bst0300559. [DOI] [PubMed] [Google Scholar]
- 62.Eldadah BA, Faden AT. Caspase pathways, neuronal apoptosis and CNS injury. J Neurotrauma. 2000;17:811–829. doi: 10.1089/neu.2000.17.811. [DOI] [PubMed] [Google Scholar]
- 63.Eliezer D, Kutluay E, Bussell R, Brown G. Conformational properties of α- synuclein in its free and lipid-associated states. J Mol Biol. 2001;307:1061–1073. doi: 10.1006/jmbi.2001.4538. [DOI] [PubMed] [Google Scholar]
- 64.Emerit J, Edeas M, Bricaire F. Neurodegenerative diseases and oxidative stress. Biomed Pharmacother. 2004;58:39–46. doi: 10.1016/j.biopha.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 65.Emilien G, Maloteaux J, Geurts M, Hoogenberg K, Cragg S. Dopamine receptors-physiological understanding to therapeutic intervention potential. Pharmacol Ther. 1999;84:133–156. doi: 10.1016/s0163-7258(99)00029-7. [DOI] [PubMed] [Google Scholar]
- 66.Emre M. Dementia associated with Parkinson’s disease. Lancet Neurol. 2003;2:229–37. doi: 10.1016/s1474-4422(03)00351-x. [DOI] [PubMed] [Google Scholar]
- 67.Fahn S. Is levodopa toxic? Neurology. 1996;47:184–195. doi: 10.1212/wnl.47.6_suppl_3.184s. [DOI] [PubMed] [Google Scholar]
- 68.Fahn S. Welcome news about levodopa, but uncertainty remains. Ann Neurol. 1998;43:551–554. doi: 10.1002/ana.410430502. [DOI] [PubMed] [Google Scholar]
- 69.Fahn SA. A pilot trial of high dose alpha-tocopherol and ascorbate in early Parkinson’s disease. Ann Neurol. 1992;32:128–132. doi: 10.1002/ana.410320722. [DOI] [PubMed] [Google Scholar]
- 70.Farrer M, Maraganore DM, Lockhart P, Singleton A, Lesnick TG, de Andrade M, West A, de Silva R, Hardy J, Hernandez D. α-synuclein gene haplotypes are associated with Parkinson’s disease. Hum Mol Genet. 2001;10:1847–1851. doi: 10.1093/hmg/10.17.1847. [DOI] [PubMed] [Google Scholar]
- 71.Farrer M, Chan P, Chen R, Tan L, Lincoln S, Hernandez D. Lewy bodies and Parkinsonism in families with Parkin mutations. Ann Neurol. 2001;50:293–300. doi: 10.1002/ana.1132. [DOI] [PubMed] [Google Scholar]
- 72.Feng ZH, Wang TG, Li DD, Fung P, Wilson BC, Liu B, Ali SF, Langenbach R, Hong JS. Cyclooxygenase-2 deficient mice are resistant to 1-methyl-4-phenyl-1,2,3,6 tetrahydropyridine induced damage of dopaminergic neurons in the substantia nigra. Neurosci Lett. 2002;329:354–358. doi: 10.1016/s0304-3940(02)00704-8. [DOI] [PubMed] [Google Scholar]
- 73.Ferre S, Von Euler G, Johansson B, Fredholm BB, Fuxe K. Stimulation of high affinity adenosine A2 receptors decrease the affinity of dopamine D2 receptors in rat striatal membranes. Proc Natl Acad Sci USA. 88:7238–7241. doi: 10.1073/pnas.88.16.7238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Forono LS. Neuropathology of Parkinson’s disease. J Neuropathol Expt Neurol. 1996;55:259–272. doi: 10.1097/00005072-199603000-00001. [DOI] [PubMed] [Google Scholar]
- 75.Foroud T, Uniacke SK, Liu L, Parkartz N, Rudolph A, Halter C, Shults C, Marder K, Conneally PM, Nicholas WC. Heterozygosity for a mutation in the gene leads to later onset Parkinson’s disease. Neurology. 2003;60:796–801. doi: 10.1212/01.wnl.0000049470.00180.07. [DOI] [PubMed] [Google Scholar]
- 76.Foster GA, Stringer BMJ. Cell based therapies for PD. Drug Discov Today. 2004;1:43–49. [Google Scholar]
- 77.Fujiwara H, Hasegawa M, Dohmae N, Kawashima A, Masliah E, Goldberg MS, Shen J, Takio K, Iwatsubo T. α-synuclein is phosphorylated in synuclepathy lesions. Nat Cell Biol. 2002;4:160–164. doi: 10.1038/ncb748. [DOI] [PubMed] [Google Scholar]
- 78.Funayama M, Hasegawa K, Ohta E, Kawashima N, Komiyama M, Kowa H, Tsuji S, Obata F. A LRRK 2 mutation as a cause factor for the parkinsonism in the original PARK 8 family. Ann Neurol. 2005;57:918–921. doi: 10.1002/ana.20484. [DOI] [PubMed] [Google Scholar]
- 79.Furukawa Y, Kish SJ. Dopa-responsive dystonia: recent advances and remaining issues to be addressed. Mov Disord. 1999;14:709–15. doi: 10.1002/1531-8257(199909)14:5<709::aid-mds1001>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 80.Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson’s disease. Arch Neurol. 1999;56:33–39. doi: 10.1001/archneur.56.1.33. [DOI] [PubMed] [Google Scholar]
- 81.George JM. The synucleins. Genome Biol. 2002;3:3002–3006. doi: 10.1186/gb-2001-3-1-reviews3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Giasson BI, Muray JVJ, Trojanowski JQ, Lee VM. A hydrophobic stretch of 12 amino acid residues in the middle of α-synuclein is essential for filament stability. J Biol Chem. 2001;276:2380–2386. doi: 10.1074/jbc.M008919200. [DOI] [PubMed] [Google Scholar]
- 83.Gilks WP, Abou- Sleiman PM, Gandhi S, Jain S, Singleton A, Lees AJ, Shaw K, Bhatia KP, Bonifati V. A common LRRK2 mutation in idiopathic Parkinson’s disease. Lancet. 2005;365:415–416. doi: 10.1016/S0140-6736(05)17830-1. [DOI] [PubMed] [Google Scholar]
- 84.Gill SS, Patel NK, Hotton GR, O’ Sullivan K. Direct brain infusion of GDNF in Parkinson’s disease. Nat Med. 2003;9:589–595. doi: 10.1038/nm850. [DOI] [PubMed] [Google Scholar]
- 85.Globe LI. Young onset Parkinson’s disease: a clinical review. Neurology. 1991;41:168–73. doi: 10.1212/wnl.41.2_part_1.168. [DOI] [PubMed] [Google Scholar]
- 86.Gloeckner CJ, Kink N, Schumacher A, Braun RJ, O’ Neil E, Meitinger T, Kolch W, Prokishch H, Ueffing M. The Parkinson’s disease causing LRRK2 mutations I2020T is associated with increased kinase activity. Hum Mol Genet. 2006;15:223–232. doi: 10.1093/hmg/ddi439. [DOI] [PubMed] [Google Scholar]
- 87.Goldberg AL. Protein degradation and protection against misfolded or damaged proteins. Nature. 2003;426:895–9. doi: 10.1038/nature02263. [DOI] [PubMed] [Google Scholar]
- 88.Gorell JM, Jhonson CC, Rybicki BA. Occupational exposure to manganese, copper, lead, iron, mercury and zinc and risk of Parkinson’s disease. Neurotoxicology. 1990;20:239–48. [PubMed] [Google Scholar]
- 89.Gorell JM, Johnson CC, Rybicki BA, Peterson EL, Richardson RJ. The risk of Parkinson’s disease with exposure to pesticides, farming, well water and rural living. Neurology. 1998;50:1346–50. doi: 10.1212/wnl.50.5.1346. [DOI] [PubMed] [Google Scholar]
- 90.Gorell JM, Peterson EL, Rybcki BA, Johnson CC. Multiple risk factors for Parkinson’s disease. J Neurol Sci. 2004;217:169–174. doi: 10.1016/j.jns.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 91.Gosavi N, Lee HJ, Lee JS, Patel S, Lee SJ. Golgi fragmentation occurs in the cells with protofibrillar α-synuclein aggregates and proceeds the formation of fibrillar inclusion. J Biol Chem. 2002;277:48984–92. doi: 10.1074/jbc.M208194200. [DOI] [PubMed] [Google Scholar]
- 92.Green JG, Greenamyre JT. Bioenergetics and glutamate excitotoxicity. Prog Neurobiol. 1996;48:613–34. doi: 10.1016/0301-0082(96)00006-8. [DOI] [PubMed] [Google Scholar]
- 93.Greenamyre JT, Betarbet R, Sherer TB. The rotenone model of Parkinson’s disease: genes environment and mitochondria. Parkinsonism Relat Disord. 2003;9(Suppl 2):59–64. doi: 10.1016/s1353-8020(03)00023-3. [DOI] [PubMed] [Google Scholar]
- 94.Grune T, Jung T, Merker K, Davies KJ. Decreased proteolysis caused by protein aggregates, inclusion bodies, plaques, lipofuscin, ceroid and aggreosomes during oxidative stress, aging and disease. Int J Biochem Cell Biol. 2004;36:2579–2630. doi: 10.1016/j.biocel.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 95.Guillin O, Diaz J, Carrol P, Griffen N. BDNF controls D3 receptor expression and triggers behavioral sensitization. Nature. 2001;411:86–89. doi: 10.1038/35075076. [DOI] [PubMed] [Google Scholar]
- 96.Hague R, Rogaeva E, Hernandez D, Gulick C, Singleton A, Hanson M, Johnson J, Weiser R, Gallardo M, Ravina B, Gwinn- Hardy K, Crawley A, George- Hyslop PH, Lang AE. Early onset Parkinson’s disease caused by compound heterozygous DJ 1 mutations. Ann Neurol. 2003;54:271–274. doi: 10.1002/ana.10663. [DOI] [PubMed] [Google Scholar]
- 97.Hald A, Lotharius J. Oxidative stress and inflammation in Parkinson’s disease: is there a causal link? Exp Neurol. 2005;193:279–290. doi: 10.1016/j.expneurol.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 98.Hallelt P, Standaert DG. Rationale for the use of NMDA receptor antagonists in Parkinson’s disease. Pharmacol Ther. 2004;102:155–174. doi: 10.1016/j.pharmthera.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 99.Hardy J, Cookson MR, Singleton A. Genes and Parkinson’s disease. Lancet Neurol. 2003;2:221–28. doi: 10.1016/s1474-4422(03)00350-8. [DOI] [PubMed] [Google Scholar]
- 100.Hashimoto M, Takenouchi T, Rockenstein E, Masliah E. α-synuclein upregulates expression of caveolin-1 and down regulates extracellular signal regulated kinase activity in B1O3 neuroblastoma cells: role in the pathogenesis of PD. J Neurochem. 2003;85:1468–1479. doi: 10.1046/j.1471-4159.2003.01791.x. [DOI] [PubMed] [Google Scholar]
- 101.Hatano Y, Li Y, Sato K, Asakawa S, Yamamura Y, Tomiyama H, Yoshino H. Novel PINK1 mutations in early-onset parkinsonism. Ann Neurol. 2004;56:424–427. doi: 10.1002/ana.20251. [DOI] [PubMed] [Google Scholar]
- 102.Hauser RA, Hubble JP. Istradefylline US001 study group. Randomized trial of adenosine A2A receptor antagonist istradefylline in advanced Parkinson’s disease. Neurology. 2003;61:297–303. doi: 10.1212/01.wnl.0000081227.84197.0b. [DOI] [PubMed] [Google Scholar]
- 103.Heidrich K, Djarmati A, Schater N, Hering R, Wellenbrock C, Weiss PH, Bonifati V, Schwinger E. DJ 1 (PARK 7) mutations are less frequent that parkin (PARK 2) mutations in early onset Parkinson’s disease. Neurology. 2004;62:389–394. doi: 10.1212/01.wnl.0000113022.51739.88. [DOI] [PubMed] [Google Scholar]
- 104.Hely MA, Fung VSC, Morris JG. Treatment of PD. J Clin Neurosci. 2000;7:484–494. doi: 10.1054/jocn.2000.0766. [DOI] [PubMed] [Google Scholar]
- 105.Henderson R, Kurlan R, Kersyn JM, Como P. Preliminary examination of comorbidity of anxiety and depression in Parkinson’s disease. J Neuropsychiatry Clin Neurosci. 1992;4:257–64. doi: 10.1176/jnp.4.3.257. [DOI] [PubMed] [Google Scholar]
- 106.Hermann A, Gerlach M, Scharz J, Starch A. Neurorestoration in Parkinson’s disease by cell replacement and endogenous regeneratioin. Exp Opin Biol Ther. 2004;4:131–143. doi: 10.1517/14712598.4.2.131. [DOI] [PubMed] [Google Scholar]
- 107.Hershko A, Ciechanover A. The ubiquitin system. Ann Rev Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- 108.Hicks AA, Petursson H, Jonsson T, Stefansson H, Johannsdotrir HS, Sainz J. A suceptibility gene for late-onset idopathic Parkinson’s disease. Ann Neurol. 2002;52:549–555. doi: 10.1002/ana.10324. [DOI] [PubMed] [Google Scholar]
- 109.Hirsch EC, Mouatt A, Thomassa M. Expression of calbindin D 28Klike immunoreactivity in catecholaminergic cell groups of human midbrain normal distribution in Parkinson’s disease. Neurodegeneration. 1992;1:83–93. [Google Scholar]
- 110.Hirsch EC, Faucheux BA. Iron metabolism and Parkinson’s disease. Mov Disord. 1998;13:39–45. [PubMed] [Google Scholar]
- 111.Hoehn MM, Yahr MD. Parkinson’s disease: onset, progression and mortality. Neurology. 1967;17:427–42. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 112.Holzmann C, Kruger R, Saecker AM, Schmitt E, Schols L, Berger K, Reiss O. Polymorphism of α-synuclein promoter: expression analysis and association studies in Parkinson’s disease. J Neurol Trans. 2003;110:67–76. doi: 10.1007/s00702-002-0769-5. [DOI] [PubMed] [Google Scholar]
- 113.Hoozemans JJ, Veerhuis R, Janssen I, Janvan EIKE, Rozemuller AJM, Eikelenboom P. The role of cyclooxygenase 1 and 2 activity in prostaglandin E2 secretion by cultured human adult microglia: implication for Alzheimer’s disease. Brain Res. 2002;951:218–226. doi: 10.1016/s0006-8993(02)03164-5. [DOI] [PubMed] [Google Scholar]
- 114.Hsu LJ, Sagara Y, Arroyo A, Rockenstein E, Sisk A. α-synuclein promotes mitochondrial deficit and oxidative stress. Am J Pathol. 2000;157:401–10. doi: 10.1016/s0002-9440(10)64553-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Huai Q, Sun Y, Wang H, Clain L, Li L, Rabinson H, Ke H. Crystal structure of DJ-1/RS and implication on familial Parkinson’s disease. FEBS Lett. 2003;549:171–175. doi: 10.1016/s0014-5793(03)00764-6. [DOI] [PubMed] [Google Scholar]
- 116.Hughes AJ, Daniel SE, Ben- Shlomo Y, Lees AJ. The accuracy of diagnosis of parkinsonian syndromes in a specialist movement disorder service. Brain. 2002;125:861–870. doi: 10.1093/brain/awf080. [DOI] [PubMed] [Google Scholar]
- 117.Hyman C, Hofer MB, Arde YA, Juhar M, Yancopoulos GD, Squinto SP, Lindsay RM. A BDNF is a neuropathic factor for dopaminergic neurons of the substantia nigra. Nature. 1991;350:230–232. doi: 10.1038/350230a0. [DOI] [PubMed] [Google Scholar]
- 118.Imai J, Yashiroda H, Maruya M, Yahara I, Tanaka K. Proteasomes and molecular chaperones: cellular machinery responsible for folding and destruction of unfolded proteins. Cell Cycle. 2003;2:585–590. [PubMed] [Google Scholar]
- 119.Imai Y, Soda M, Takahashi R. Parkin suppresses unfolded protein stress- induced cell death through E3 ubiquitin-protein ligase activity. J Biol Chem. 2000;275:35661–35664. doi: 10.1074/jbc.C000447200. [DOI] [PubMed] [Google Scholar]
- 120.Imai Y, Soda M, Inoue H, Hattori N, Mizuono Y, Takahashi R. An unfolded putative transmembrane polypeptide which can lead to endoplasmic reticulum stress, a substrate for parkin. Cell. 2001;105:891–902. doi: 10.1016/s0092-8674(01)00407-x. [DOI] [PubMed] [Google Scholar]
- 121.Inzelberg R, Schechtman E, Paleacu D. Onset age of Parkinson’s disease. Am J Med Genet. 2002;111:459–60. doi: 10.1002/ajmg.10586. [DOI] [PubMed] [Google Scholar]
- 122.Jankovic J. Essential tremor and Parkinson’s disease. Ann Neurol. 1989;25:211–12. doi: 10.1002/ana.410250226. [DOI] [PubMed] [Google Scholar]
- 123.Jenco JM, Rawlingson A, Daniels B, Morris AJ. Regulation of phospholipase D2: selective inhibition of mammalian phospholipase D isoenzymes by α- and β-synucleins. Biochemistry. 1998;37:4901–4909. doi: 10.1021/bi972776r. [DOI] [PubMed] [Google Scholar]
- 124.Jendroska K, Olasode DJ. Incidental Lewy body disease in black Africans. Lancet. 1994;344:882–3. doi: 10.1016/s0140-6736(94)92854-1. [DOI] [PubMed] [Google Scholar]
- 125.Jenner P, Marsden CD. Motor activity following the administration of selective D1 and D2 dopaminergic drugs to MPTP treated common marmosets. Psychopharmacol (Berlin) 1992;109:49–56. doi: 10.1007/BF02245479. [DOI] [PubMed] [Google Scholar]
- 126.Jenner P. A novel dopamine agonist for the transdermal treatment of PD cases. Neurology. 2005;65:3–5. doi: 10.1212/wnl.65.2_suppl_1.s3. [DOI] [PubMed] [Google Scholar]
- 127.Jenner P. Oxidative mechanism in nigral cell death in Parkinson’s disease. Mov Disord. 1998;13:24–34. [PubMed] [Google Scholar]
- 128.Jenner P. Oxidative stress in Parkinson’s disease. Ann Neurol. 2003;53:26–36. doi: 10.1002/ana.10483. [DOI] [PubMed] [Google Scholar]
- 129.Jenner P. The rational use of dopamine agonist in PD. Neurology. 1995;45:6–12. doi: 10.1212/wnl.45.3_suppl_3.s6. [DOI] [PubMed] [Google Scholar]
- 130.Jimenz FJ, Garcia- Ruiz PJ, Molina JA. Drug induced movement disorders. Drug Saf. 1997;16:180–204. doi: 10.2165/00002018-199716030-00004. [DOI] [PubMed] [Google Scholar]
- 131.Jo E, McLaurin J, Yip CM, St Geroge-Hyslop P, Fraser PE. α-synuclein membrane interaction and lipid specificity. J Biol Chem. 2000;275:34328–34334. doi: 10.1074/jbc.M004345200. [DOI] [PubMed] [Google Scholar]
- 132.Johnson WG, Hodge SE, Duvoisin R. Twin studies and the genetics of Parkinson’s disease: a reappraisal. Mov Disord. 1990;5:187–94. doi: 10.1002/mds.870050302. [DOI] [PubMed] [Google Scholar]
- 133.Kase H, Mori A, Jenner P. Adenosine A2A receptor antagonists beyond dopaminergic therapies for Parkinson’s disease. Drug Discov Today. 2004;1:51–57. [Google Scholar]
- 134.Katsuse O, Iseki E, Marui W, Kosaka Y. Developmental stages of cortical Lewy bodies and their reduction to axonal transport blockage in brains of patients with demential with Lewy bodies. J Neurol Sci. 2003;211:29–35. doi: 10.1016/s0022-510x(03)00037-6. [DOI] [PubMed] [Google Scholar]
- 135.Katzenschlager R, Hughes A, Evans A, Manson AJ, Hoffman M, Swinn L, Watt H, Bhatia K, Quinn N, Lees AJ. Continuous subcutaneous apomorphine therapy improves dyskinesias in Parkinson’s disease: a prospective study using single- dose challenges. Mov Disord. 2005;20:151–157. doi: 10.1002/mds.20276. [DOI] [PubMed] [Google Scholar]
- 136.Kennedy LJ, Moore K, Jr, Caulfield JL, Tannenbaun SR, Dedon PC. Quantitation of 8-oxoguanine and strand breaks produced by four oxidising agents. Chem Res Toxicol. 1997;10:386–392. doi: 10.1021/tx960102w. [DOI] [PubMed] [Google Scholar]
- 137.Khan FM, Saha M, Chakrabarti S. Dopamine induced protein damage in mitochondrial- synaptosomal fraction in rat brain. Brain Res. 2001;895:245–249. doi: 10.1016/s0006-8993(00)03284-4. [DOI] [PubMed] [Google Scholar]
- 138.Kim TD, Paik SR, Yang CH, Kim J. Structural changes in α-synuclein affect its chaperone-like activity in vitro. Protein Sci. 2000;9:2489–2496. doi: 10.1110/ps.9.12.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kim TD, Paik SR, Yang CH. Structural and functional implication of C- terminal regions of α-synuclein. Biochemistry. 2002;41:13782–13790. doi: 10.1021/bi026284c. [DOI] [PubMed] [Google Scholar]
- 140.Ko L, Mehta ND, Farrer M, Eassen C, Hussey J. Sensitization of neuronal cells to oxidative stress with mutated human α-synuclein. J Neurochem. 2000;75:2546–54. doi: 10.1046/j.1471-4159.2000.0752546.x. [DOI] [PubMed] [Google Scholar]
- 141.Koller WC. Sensory symptoms in Parkinson’s disease. Neurology. 1984;34:1957–59. doi: 10.1212/wnl.34.7.957. [DOI] [PubMed] [Google Scholar]
- 142.Kordower JH, Emborg ME, Bloch J, Ma SY, Chu Y, Leventhal L, McBride J, Chen EY, Palfi S. Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson’s disease. Science. 2000;290:767–73. doi: 10.1126/science.290.5492.767. [DOI] [PubMed] [Google Scholar]
- 143.Kruger R, Kuhn W, Muller T, Woitalla D, Graber M, Kosel S, Przuntek H, Epplen JT, Schols L, Reiss O. Ala30 Pro mutation in the gene encoding α- synuclein in Parkinson’s disease. Nat Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- 144.Kuhn DM, Arthur AE, Jr, Thomas DM, Elferink LA. Tyrosine hydroxylase is inactivated by catechol-quinones and converted to redox-cycling quinoprotein: possible relevance to Parkinson’s disease. J Neurochem. 1999;279:48384–96. doi: 10.1046/j.1471-4159.1999.0731309.x. [DOI] [PubMed] [Google Scholar]
- 145.Kuroda Y, Mitsui T, Kunishige M, Shono M, Akaike M, Azuma H, Matsumoto T. Parkin enhances mitochondrial biogenesis in proliferating cells. Hum Mol Genet. 2006;1:883–95. doi: 10.1093/hmg/ddl006. [DOI] [PubMed] [Google Scholar]
- 146.Laihinen A, Ruottien H, Rinne JO, Haaparanta M, Bergman J, Salin O. Risk of Parkinson’s disease: twin studies for the detection of asymptomic subject using 6 fluro dopa PET. J Neurol. 2000;247:110–3. doi: 10.1007/pl00022911. [DOI] [PubMed] [Google Scholar]
- 147.Lang AE, Lozano AM. Parkinson’s disease. N Eng J Med. 1998;339:1044–53. doi: 10.1056/NEJM199810083391506. [DOI] [PubMed] [Google Scholar]
- 148.Langston JW, Ballard PA, Tetrud JW, Irwin I. Chronic Parkinsonism in humans due to a product of mepridine analog synthesis. Science. 1983;219:979–80. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- 149.Lashuel HA, Hartley D, Petre BM, Waltz T, Lansbury PTJ. Neurodegenerative disease: amyloid pores from pathogenic mutation. Nature. 2002;418:291–291. doi: 10.1038/418291a. [DOI] [PubMed] [Google Scholar]
- 150.Le WD, Xu P, Jankovic J, Jiang H, Appel SH, Smith RG, Vassilatis DK. Mutations in NR4A2 associated with familial Parkinson’s disease. Nat Genet. 2003;33:85–89. doi: 10.1038/ng1066. [DOI] [PubMed] [Google Scholar]
- 151.Lee M, Hyuan D, Halliwell B, Jenner P. Effect of the over expression of wild type mutant α-synuclein on cell susceptibility to insult. J Neurochem. 2001;76:998–1009. doi: 10.1046/j.1471-4159.2001.00149.x. [DOI] [PubMed] [Google Scholar]
- 152.Lee W, Rowe D, Yie W, Ortiz I, He Y, Apple SH. Microglial activation of dopaminergic cell injury: an in vitro model relevant to Parkinson’s disease. J Neurosci. 2001;21:8447–8455. doi: 10.1523/JNEUROSCI.21-21-08447.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Leroy E, Baer R, Auburgen G, Leube B, Ulm G . The ubiquitin pathway in Parkinson’s disease. Nature. 1998;395:451–452. doi: 10.1038/26652. [DOI] [PubMed] [Google Scholar]
- 154.Lilienfield ED, MarderCote L, Tang M, Mayeux R. Projected neurodegenerative disease mortality in US, 1990-2040. Neuroepidemiology. 1993;12:219–28. doi: 10.1159/000110320. [DOI] [PubMed] [Google Scholar]
- 155.Lindersson E, Beedholm R, Hojrup P, Moos T, Gai W. Proteasomal inhibition of α-synuclein filaments and oligomers. J Biol Chem. 2004;279:12924–34. doi: 10.1074/jbc.M306390200. [DOI] [PubMed] [Google Scholar]
- 156.Lohmann E, Periquet M, Bonifati V, Wood NW, De Michele G, Bonnet AM. How much phenotypic variation can be attributed parkin genetype? Ann Neurol. 2004;56:336–341. doi: 10.1002/ana.10613. [DOI] [PubMed] [Google Scholar]
- 157.Lotharius J, Brundin B. Pathogenesis of Parkinson’s disease: dopamine, vesicles and α- synuclein. Nat Rev Neurosci. 2002;3:1–11. doi: 10.1038/nrn983. [DOI] [PubMed] [Google Scholar]
- 158.Lotharius J, Brundin P. Impaired dopamine storage resulting from α-synuclein mutations may contribute to the pathogenesis of Parkinson’s disease. Hum Mol Genet. 2002;11:2395–2407. doi: 10.1093/hmg/11.20.2395. [DOI] [PubMed] [Google Scholar]
- 170.Louis ED, Mareter K, Cote L, Tang M, Mayeux R. Mortality from Parkinson’s disease. Arch Neurol. 1997;54:260–264. doi: 10.1001/archneur.1997.00550150024011. [DOI] [PubMed] [Google Scholar]
- 159.Lucking CB, Durr A, Bonifati V, Vaughan J, De Michele G. Association between early onset Parkinson’s disease and mutations in the parkin gene. N Eng J Med. 2000;342:1560–67. doi: 10.1056/NEJM200005253422103. [DOI] [PubMed] [Google Scholar]
- 160.Lwin A, Orvisky E, Gokr- Alpan O, LaMarca ME, Sidransky E. Glucocerebroside mutations in the subjects with parkinsonism. Mol Genet Metab. 2004;81:70–73. doi: 10.1016/j.ymgme.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 161.Maino MJ, Valenti O, Cann PJ. Glutamate receptors and Parkinson’s disease: opportunities for intervention. Drugs Aging. 2003;20:377–397. doi: 10.2165/00002512-200320050-00006. [DOI] [PubMed] [Google Scholar]
- 162.Manning- Bog AB, McComack AL, Li J, Uversky VN, Fink AL, Di Monte DA. The herbicide paraquat causes upregulation and aggregation of α-synuclein in mice:paraquat and α-synuclein. J Biol Chem. 2002;277:1641–1644. doi: 10.1074/jbc.C100560200. [DOI] [PubMed] [Google Scholar]
- 163.Maoi M, Marugam W. Dopamine derived salsolinol derivatives as exogenous MAO inhibitor: occurrence, metabolism and function in human brains. Neurotoxicology. 2004;25:193–204. doi: 10.1016/S0161-813X(03)00099-8. [DOI] [PubMed] [Google Scholar]
- 164.Marder K, Tang MX, Mejia H, Alfaro B, Cote L. Risk of Parkinson’s disease among fist degree relatives: a community based study. Neurology. 1996;47:155–60. doi: 10.1212/wnl.47.1.155. [DOI] [PubMed] [Google Scholar]
- 165.Marin C, Papa S, Engber TM, Bonastre M. Levodopa induced motor response alterations in Parkinsonian rats. Brain Res. 1996;736:202–205. doi: 10.1016/0006-8993(96)00693-2. [DOI] [PubMed] [Google Scholar]
- 166.Marsh L. Neuropsychiatric aspects of Parkinson’s disease. Psychosomatics. 2000;41:15–23. doi: 10.1016/S0033-3182(00)71169-8. [DOI] [PubMed] [Google Scholar]
- 167.Marti- Masso JF, Poza JJ, Lopez de Munain A. Drugs inducing or aggrevating Parkinsonism: a review. Therapie. 1996;5:568–577. [PubMed] [Google Scholar]
- 168.Martinez- Martin P, Gil- Nagel A, Gracia LM, Germez JB, Martinez- Sarries J, Bermejo F. Unified PD rating scale characteristics and structure. Mov Disord. 1994;9:76–83. doi: 10.1002/mds.870090112. [DOI] [PubMed] [Google Scholar]
- 169.Marx FP, Holzmann C, Strauss KM. Identification and functional characterization of novel R621C mutations in the synphilin-1 gene in PD. Hum Mol Genet. 2003;12:1223–1231. doi: 10.1093/hmg/ddg134. [DOI] [PubMed] [Google Scholar]
- 170.Masliah E, Rockenstein A, Adame E, Alferd M, Crews L, Alashimoto M, Secubent P, Lee M. Effects of αSN immunization in mouse model of PD. Neuron. 2005;46:857–868. doi: 10.1016/j.neuron.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 171.Mata IF, Lockhart PJ, Farrer M. Parkin genetics: one model for Parkinson’s disease. Hum Mol Genet. 2004;13:27–33. doi: 10.1093/hmg/ddh089. [DOI] [PubMed] [Google Scholar]
- 172.McCall RB, Lookingland KJ, Bedard PJ, Huff RM. Sumanirole a highly dopamine D2 selective receptor agonist: in vitro and in vivo pharmacological characterization and efficacy in animal model of Parkinson’s disease. J Pharmacol Exp Ther. 2005;314:1248–1256. doi: 10.1124/jpet.105.084202. [DOI] [PubMed] [Google Scholar]
- 173.McGeer PL, Schwab C, Parent A, Doudet D. Presence of reactive microglia in monkey substantia nigra after MPTP administration. Ann Neurol. 2001;169:219–230. doi: 10.1002/ana.10728. [DOI] [PubMed] [Google Scholar]
- 174.McLean PJ, Klucken J, Shin Y, Hyman BT. Geldanamycin induces Hsp 70 and prevents αSN aggregation and toxicity in vitro. Biochem Biophys Res Commun. 2004;321:665–669. doi: 10.1016/j.bbrc.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 175.McNaught KS, Olanow CW. Proteolytic stress: a unifying concept in the etiopathogenesis of familial and sporadic Parkinson’s disease. Ann Neurol. 2003;53:73–86. doi: 10.1002/ana.10512. [DOI] [PubMed] [Google Scholar]
- 176.McNaught KS, Olanow CW. Protein aggregation in the pathogenesis of familial and sporadic Parkinson’s disease. Neurobiol Aging. 2006;27:530–545. doi: 10.1016/j.neurobiolaging.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 177.Michel PP, Hefti F. Toxicity of 6-hydroxy dopamine and dopamine for dopaminergic neurons in culture. J Neurosci Res. 2004;26:428–35. doi: 10.1002/jnr.490260405. [DOI] [PubMed] [Google Scholar]
- 178.Miller TW, Messer A. Intrabody applications in neurological disorders: progress and future prospects. Mol Ther. 2005;12:394–401. doi: 10.1016/j.ymthe.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 179.Moghal S, Rajput AH, D’ Arcy C, Rajput R. Prevalence of movement disorder in elderly community residents. Neuroepidemiology. 1994;13:175–178. doi: 10.1159/000110376. [DOI] [PubMed] [Google Scholar]
- 180.Mogi M, Harada M, Kondo T, Riederer P, Inayaki H, Minami M. Interleukin 1-beta, interleukin 6, epidermal growth factor and transforming growth factor-alpha are activated in ventricular cerebrospinal fluid in juvanile parkinsonism and Parkinson’s disease. Neurosci Lett. 1996;211:13–16. [Google Scholar]
- 181.Molho ES, Facter SA, Weiner WT, Sanchez- Ramas JR, Singer C, Shul- man L, Brown D, Sheldon C. The use of pramipexole a novel dopamine agonist in advanced Parkinson’s disease. J Neurol Transm. 1995;45:225–230. [PubMed] [Google Scholar]
- 182.Moore DJ, West AB, Dawson VL, Dawson TM. Molecular pathophysiology of Parkinson’s disease. Ann Rev Neurosci. 2005;28:57–87. doi: 10.1146/annurev.neuro.28.061604.135718. [DOI] [PubMed] [Google Scholar]
- 183.Moore DJ, Zhang L, Dawson TM, Dawson VL. A missense mutation (L166P) is DJ-1 linked to familial Parkinson’s disease confers reduced protein stability and impairs homo-oligomerization. J Neurochem. 2003;87:1558–67. doi: 10.1111/j.1471-4159.2003.02265.x. [DOI] [PubMed] [Google Scholar]
- 184.Morris HR. Genetics of Parkinson’s disease. Ann Med. 2005;37:86–96. doi: 10.1080/07853890510007269. [DOI] [PubMed] [Google Scholar]
- 185.Muchowski PJ. Protein misfolding, amyloid formation and neurodegeneration: a critical role for molecular chaperones? Neuron. 2002;35:9–12. doi: 10.1016/s0896-6273(02)00761-4. [DOI] [PubMed] [Google Scholar]
- 186.Murphy TH, Miyamoto M, Sastre A, Achnaar RL, Coyle JT. Glutamate toxicity in neuronal cell line involves inhibition of cysteine transport leading to oxidative stress. Neuron. 1989;2:1547–58. doi: 10.1016/0896-6273(89)90043-3. [DOI] [PubMed] [Google Scholar]
- 187.Muthane UB, Swamy HS, Satischandra P, Subhash MN, Rao S, Subbakrishna D. Early onset Parkinson’s disease: are juvenile and young onset different? Mov Disord. 1994;9:539–544. doi: 10.1002/mds.870090506. [DOI] [PubMed] [Google Scholar]
- 188.Newman- Tancredi A, Cussac D, Audinot V, Nicolas J, De Ceuninck F, Boutin JA, Millan MJ. Differential actions of antiparkinson agents at multiple classes of monoaminergic receptor. II. Agonist and antagonist properties at subtypes of dopamine D(2)-like receptor and α(1)/α(2)-adrenoceptor. J Pharmacol Exp Ther. 2002;303:805–814. doi: 10.1124/jpet.102.039875. [DOI] [PubMed] [Google Scholar]
- 189.Niehaus I, Lange JH. Endotoxins: is it an environmental factor in the cause of Parkinson’s disease? Occup Environ Med. 2003;60:378. doi: 10.1136/oem.60.5.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Niki T, Takahashi- Nik A, Taira T, Iguchi- Agira SM, Agira H. DJBP : a novel DJ 1 binding protein, negatively regulates the androgen receptor by recruiting histome deacetylase complex and DJ 1 antagonizes this inhibition by abrogation of this complex. Mol Cancer Res. 2003;1:247–261. [PubMed] [Google Scholar]
- 191.Norris FH, Giasson BI, Hodara R, Xu S, Trojanowski JQ. Reversible inhibition of αSN fibrillization by dopaminochrome mediated conformational alterations. J Biol Chem. 2005;280:21212–21219. doi: 10.1074/jbc.M412621200. [DOI] [PubMed] [Google Scholar]
- 192.Olanow CW. Tolcapane and hepatic effects. Arch Neurol. 2000;57:263–267. doi: 10.1001/archneur.57.2.263. [DOI] [PubMed] [Google Scholar]
- 193.Olanow CW, Schapira AH, LeWitt PE, Kieburtz K, Sauer D, Olivieri G, Pohlmann H, Hubble J. TCH346 as a neuroprotective drug in Parkinson’s disease: a double-blind, randomised, controlled trial . Lancet Neurol. 2006;5:1013–1020. doi: 10.1016/S1474-4422(06)70602-0. [DOI] [PubMed] [Google Scholar]
- 194.Oliveira SA, Scott WK, Martin ER, Nance MA, Watt RL, Hubble JP, Koller WC, Pahwa R. Parkin mutations and susceptibility alleles in late onset Parkinson’s disease. Ann Neurol. 2003;53:624–629. doi: 10.1002/ana.10524. [DOI] [PubMed] [Google Scholar]
- 195.Orr CF, Rowe DB, Halliday GM. An inflammatory review of Parkinson’s disease. Prog Neurobiol. 2002;68:325–340. doi: 10.1016/s0301-0082(02)00127-2. [DOI] [PubMed] [Google Scholar]
- 196.Orth M, Schapira AH. Mitochondrial involvement in Parkinson’s disease. Neurochem Int. 2002;40:53–41. doi: 10.1016/s0197-0186(01)00124-3. [DOI] [PubMed] [Google Scholar]
- 197.Ostrerova V, Petrucelli L, Farrer M, Mehta N, Choi P, Hardy J, Wolozin B. α-synuclein shares physical and functional homology with 14-3-3 proteins. J Neurosci. 1996;19:5782–5791. doi: 10.1523/JNEUROSCI.19-14-05782.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Paisan- Ruiz C, Jain S, Evans EW, Gilks WP, Simon J. Cloning of the gene containing mutations that cause PARK 8 linked Parkinson’s disease. Neuron. 2004;44:595–600. doi: 10.1016/j.neuron.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 199.Pals P, Lincoln S, Manning J, Heckman M, Skipper L. α-synuclein promoter confers susceptibility to Parkinson’s disease. Ann Neurol. 2004;56:591–95. doi: 10.1002/ana.20268. [DOI] [PubMed] [Google Scholar]
- 200.Pankratz N, Nichols WC, Uniacke SK, Halter C, Rudolph A, Shults C. Significant linkage of Parkinson’s disease to chromosome 2q-36-37. Am J Hum Genet. 2003;72:1053–1057. doi: 10.1086/374383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Paolini M, Sapone A, Gonzalez EJ. Parkinson’s disease, pesticides and individual vulnerability. Trends Pharmacol Sci. 2004;25:124–129. doi: 10.1016/j.tips.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 202.Park SM, Jung HY, Kim TD, Park JH, Yang CH, Kim J. Distinct roles of N-terminal binding domain and C-terminal solubilizing domain of α-synuclein, a molecular chaperone. J Biol Chem. 2002;277:28512–28520. doi: 10.1074/jbc.M111971200. [DOI] [PubMed] [Google Scholar]
- 203.Parkinson’s Study Group. Evaluation of dyskinesias in a pilot, randomized, placebo-controlled trial of the remacemide in advanced Parkinson’s disease. Arch Neurol. 2001;58:1660–1668. doi: 10.1001/archneur.58.10.1660. [DOI] [PubMed] [Google Scholar]
- 204.Parkinson’s Study Group. Impact of deprenyl and tocopherol treatment of Parkinson’s disease in DATATOP patients requiring levodopa. Ann Neurol. 1996;39:37–45. doi: 10.1002/ana.410390107. [DOI] [PubMed] [Google Scholar]
- 205.Parkinson’s Study Group. Lazabenide. Ann Neurol. 1996;39:37–45. doi: 10.1002/ana.410390107. [DOI] [PubMed] [Google Scholar]
- 206.Parkinson’s Study Group. The safety and tolerability of a mixed linkage kinase inhibitor (CEP-1347) in PD. Neurology. 2004;62:330–332. doi: 10.1212/01.wnl.0000103882.56507.20. [DOI] [PubMed] [Google Scholar]
- 207.Payami H, Larsen K, Bernard S, Nutt J. Increased risk of Parkinson’s disease in parents and siblings of patients. Ann Neurol. 1994;36:659–69. doi: 10.1002/ana.410360417. [DOI] [PubMed] [Google Scholar]
- 208.Perez RG, Waymire JC, Lin E, Liu JJ, Guo F, Zigmond MJ. A role for α- synuclein in the regulation of dopamine biosynthesis. J Neurosci. 2002;22:3090–3099. doi: 10.1523/JNEUROSCI.22-08-03090.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Periquet M, Lucking C, Vaughan J, Bonifat V, Durr A, De Michele G, Horstink, M, Farrer M. Origin of the mutations in the parkin gene in Europe: exon rearrangements are independent recurrent events, where as point mutations may result from founder effects. Am J Hum Genet. 2001;68:617–627. doi: 10.1086/318791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Perlow MJ, Fred WJ, Hoffer BJ, Seiger A, Olsen L, Wyatt RJ. Brain grafts reduce motor abnormalities produced destruction of nigrostriatal dopamine system. Science. 1979;204:643–647. doi: 10.1126/science.571147. [DOI] [PubMed] [Google Scholar]
- 211.Perrin RJ, Woods WS, Clayton DF, George JM. Interaction of human α- synuclein and Parkinson’s disease variants with phospholipids. Structural analysis using site-directed mutagenesis. J Biol Chem. 2000;275:34393–34398. doi: 10.1074/jbc.M004851200. [DOI] [PubMed] [Google Scholar]
- 212.Petrucelli L, O’ Farrel L, Lockhart PJ, Baptista M, Kehoe K, Vink L, Choi P, Wolozinn B, Farrer M, Hardy J, Cookson MR. Parkin protects against the toxicity associated with mutant α-synuclein: proteasome dysfunction selectively affects catecholaminergic neuron. Neuron. 2002;36:1007–1019. doi: 10.1016/s0896-6273(02)01125-x. [DOI] [PubMed] [Google Scholar]
- 213.Piccini P, Brook DJ. Etiology of Parkinson’s disease: contribution from 18F- DOPA PET. Adv Neurol. 1999;80:227–31. [PubMed] [Google Scholar]
- 214.Pickart CM. Mechanisms of underlying ubiquitination. Ann Rev Biochem. 2001;70:503–533. doi: 10.1146/annurev.biochem.70.1.503. [DOI] [PubMed] [Google Scholar]
- 215.Pyle A, Foltynie T, Tiangyou W. Mitochondrial DNA haplogroup cluster UKJT reduces the risk of PD. Ann Neurol. 2005;57:564–67. doi: 10.1002/ana.20417. [DOI] [PubMed] [Google Scholar]
- 216.Quinn NP, Koller WC, Lang AE, Marsden CD. Painful Parkinson’s disease. Lancet. 1986;1:1366–69. doi: 10.1016/s0140-6736(86)91674-0. [DOI] [PubMed] [Google Scholar]
- 217.Rajput AH. Frequency and cause of Parkinson’s disease. Can J Neurol Sci. 1992;19(Suppl 1):103–107. [PubMed] [Google Scholar]
- 218.Rascol O, Brooks DJ, Korczyn AD, De Deyn PP, Clarke CE, Lang AE. A five-year study of the incidence of dyskinesia in patients with early Parkinson’s disease who were treated with ropinirole or levodopa. N Engl J Med. 2000;342:1484–1491. doi: 10.1056/NEJM200005183422004. [DOI] [PubMed] [Google Scholar]
- 219.Ravikumar B, Vacher C, Berer Z, Davies JE, Luo S. Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington’s disease. Nat Genet. 2004;36:589–595. doi: 10.1038/ng1362. [DOI] [PubMed] [Google Scholar]
- 220.Recchia A, Debetto P, Negro A, Guidolin D, Skaper S, Giusti P. α- synuclein and Parkinson’s disease. FASEB J. 2004;18:617–626. doi: 10.1096/fj.03-0338rev. [DOI] [PubMed] [Google Scholar]
- 221.Rideout HJ, Stefanis L. Caspase inhibitors: a potential therapeutic strategy in neurological diseases. Histo Histopathol. 2001;16:895–908. doi: 10.14670/HH-16.895. [DOI] [PubMed] [Google Scholar]
- 222.Ross OA, McCormack R, Maxwell LD. mt4216C variant in linkage with the mtDNA TJ cluster may confer a susceptibility to mitochondrial dysfunction resulting in an increased risk of Parkinson’s disease in the Irish. Exp Gerontol. 2003;38:397–405. doi: 10.1016/s0531-5565(02)00266-8. [DOI] [PubMed] [Google Scholar]
- 223.Sackett DL. Bias in analytical research. J Chronic Dis. 1979;32:51–63. doi: 10.1016/0021-9681(79)90012-2. [DOI] [PubMed] [Google Scholar]
- 224.Samii A, Letwin SR, Calne DB. Prospects for new drug treatment in idiopathic Parkinsonism. Drug Discov Today. 1998;3:131–140. [Google Scholar]
- 225.Samii A, Nutt JG, Ransom BR. Parkinson’s disease. Lancet. 2004;363:1783–93. doi: 10.1016/S0140-6736(04)16305-8. [DOI] [PubMed] [Google Scholar]
- 226.Schapira AH, Olanow CW. Neuroprotection in Parkinson’s disease mysteries, myths and misconception. JAMA. 2004;291:358–364. doi: 10.1001/jama.291.3.358. [DOI] [PubMed] [Google Scholar]
- 227.Scheiden WL, Hershey LA, Vena JE, Holmulund T, Marshall JR, Freudenhein T. Dietary antioxidants and other dietary factors in the etiology of PD. Mov Disord. 1997;12:190–196. doi: 10.1002/mds.870120209. [DOI] [PubMed] [Google Scholar]
- 228.Schneider JS, Denaro FJ. Astrocytic responses to the dopaminergic neurotoxin MPTP in cat and mouse brain. J Neuropathol Exp Neurol. 1988;47:452–458. doi: 10.1097/00005072-198807000-00006. [DOI] [PubMed] [Google Scholar]
- 229.Schulz JB, Lindenau J, Seyfried J, Dichgans J. Glutathione, oxidative stress and neurodegeneration. Eur J Biochem. 2000;267:4904–4911. doi: 10.1046/j.1432-1327.2000.01595.x. [DOI] [PubMed] [Google Scholar]
- 230.Semchuk KM, Love EJ, Lee RC. Parkinson’s disease and exposure to agricultural work and pesticide chemicals. Neurology. 1992;42:1328–1335. doi: 10.1212/wnl.42.7.1328. [DOI] [PubMed] [Google Scholar]
- 231.Shachar DB, Kahana N, Kampel V, Warshawsky A, Youdim MB. Neuroprotection by a novel brain permeable iron chelator, VK-28, against 6- hydroxydopamine lesion in rats. Neuropharmacology. 2004;46:254–263. doi: 10.1016/j.neuropharm.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 232.Shamato- Nagai M, Maruyama W, Kato Y, Isobe K, Tanaka M, Naoi M. An inhibitor of complex I rotenone inactivates proteasome by oxidative modification and induces aggregation of oxidized protein in SH-SY5Y cells. J Neurosci Res. 2003;74:589–97. doi: 10.1002/jnr.10777. [DOI] [PubMed] [Google Scholar]
- 233.Shang F, Lu M, Dudek E, Reddan J, Taylor A. Vitamin C and vitamin E restore the resistance of GSH-depleted lens cell to H2O2. Free Radic Biol Med. 2003;34:521–530. doi: 10.1016/s0891-5849(02)01304-7. [DOI] [PubMed] [Google Scholar]
- 234.Shen J, Cookson MR. Mitochondria and dopamine - new insights into recessive parkinsonism. Neuron. 2004;43:301–304. doi: 10.1016/j.neuron.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 235.Sherman MY, Goldberg AL. Cellular defenses against unfolded proteins: a cell biologist thinks about neurodegenerative diseases. Neuron. 2001;29:15–32. doi: 10.1016/s0896-6273(01)00177-5. [DOI] [PubMed] [Google Scholar]
- 236.Shimura H, Hattori N, Kubo S, Mizuno Y, Asakwa S, Minoshima S, Shimuzu N, Iwai K, Chiba T. Familial PD gene product parkin is an ubiquitin-protein ligase. Nat Genet. 2000;25:302–305. doi: 10.1038/77060. [DOI] [PubMed] [Google Scholar]
- 237.Shimura H, Schlossmacher MG, Hattori N, Frosch MP, Trockenbacher A, Schneider R. Ubiquitination of a new form of α-synuclein by parkin from human brain: implications for Parkinson’s disease. Science. 2001;293:263–269. doi: 10.1126/science.1060627. [DOI] [PubMed] [Google Scholar]
- 238.Shrag A. Psychiatric aspects of Parkinson’s disease - an update. J Neurol. 2004;251:795–804. doi: 10.1007/s00415-004-0483-3. [DOI] [PubMed] [Google Scholar]
- 239.Shubert V, Anton LC, Gibbs J, Norbury CC, Yewdell JW, Bennink JR. Rapid degradation of a large fraction of newly synthesized proteins by proteasomes. Nature. 2000;404:770–774. doi: 10.1038/35008096. [DOI] [PubMed] [Google Scholar]
- 240.Shutts CW, Oakes D, Kieburtz K, Beal ME, Hao SR. Effects of coenzyme Q10 in early Parkinson’s disease: evidence of slowing the functional decline. Arch Neurol. 2002;59:1541–1550. doi: 10.1001/archneur.59.10.1541. [DOI] [PubMed] [Google Scholar]
- 241.Silvestri L, Caputo V, Bellacchi E, Atorino L, Dallapiccola B, Valente EM, Casai G. Mitochondrial import and enzymatic activity of PINK1 mutants associated to recessive parkinsonism. Hum Mol Genet. 2005;14:3477–3492. doi: 10.1093/hmg/ddi377. [DOI] [PubMed] [Google Scholar]
- 242.Singleton AB, Farrer M, Jhonson J. α-synuclein locus triplication causes Parkinson’s disease. Science. 2003;302:481. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- 243.Sion J, Dexter DT, Lee AJ, Daniel S, Agid Y. Alternation in glutathione levels in Parkinson’s disease and other neurodegenerative disorders affecting basal ganglia. Ann Neurol. 1994;36:348–355. doi: 10.1002/ana.410360305. [DOI] [PubMed] [Google Scholar]
- 244.Skipper L, Farrer M. Parkinson’s genetics: molecular insights for the new millennium. Neurotoxicology. 2002;23:503–514. doi: 10.1016/s0161-813x(02)00038-4. [DOI] [PubMed] [Google Scholar]
- 245.Smith CA, Gough AC, Leigh PN. Debrisoquin hydroxylase gene polymorphism and susceptibility to Parkinson’s disease. Lancet. 1992;339:1375–1377. doi: 10.1016/0140-6736(92)91196-f. [DOI] [PubMed] [Google Scholar]
- 246.Snyder H, Mensah K, Theisler C, Lee J, Matouschek A, Wolozin B. Aggregated and monomeric α-synuclein bind S6 proteasomal protein and inhibit proteasomal function. J Biol Chem. 2003;278:11753–59. doi: 10.1074/jbc.M208641200. [DOI] [PubMed] [Google Scholar]
- 247.Sofic E, Riderer P, Heinsen H, Beckmann H, Reynolds GP. Increased Iron (III) and total iron content in postmortem SN of Parkinsonian brain. J Neurol Transm. 1988;74:199–205. doi: 10.1007/BF01244786. [DOI] [PubMed] [Google Scholar]
- 248.Sokoloff P, Schwartz JC. Novel dopamine receptors: half a decade later. Trends Pharmacol Sci. 16:270–275. doi: 10.1016/s0165-6147(00)89044-6. [DOI] [PubMed] [Google Scholar]
- 249.Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M. α- synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with Lewy bodies. Proc Natl Acad Sci USA. 1998;95:6469–6473. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Spillantini MG, Schmidt ML, Lee VM, Trojanwoski JQ, Jakes R, Goedert M. α-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 251.Stacy M. Sleep disorders in Parkinson’s disease: epidemiology and management. Drugs Aging. 2002;19:733–39. doi: 10.2165/00002512-200219100-00002. [DOI] [PubMed] [Google Scholar]
- 252.Stern MB, Marck KL, Friedman J, Hauser RA, Lewitt PA, Tarsy D. Double blind randomized controlled trials of rasagilin as monotherapy in early Parkinson’s patients. Mov Disord. 2004;19:916–923. doi: 10.1002/mds.20145. [DOI] [PubMed] [Google Scholar]
- 253.Stokes AH, Hastings TG, Vrana KE. Cytotoxic and genotoxic potential of dopamine. J Neurosci Res. 1999;55:659–665. doi: 10.1002/(SICI)1097-4547(19990315)55:6<659::AID-JNR1>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 254.Stoof JC, Booij J, Drukcarch B. Amantadine as N-methyl-Daspartic acid receptor antagonist: new possibilities for therapeutic applications? Clin Neurol Neurosurg. 1992;94:4–6. doi: 10.1016/0303-8467(92)90006-o. [DOI] [PubMed] [Google Scholar]
- 255.Szenasi G, Harsing LG., Jr Pharmacology and perspective therapeutic usefulness of negative allosteric modulators of AMPA receptors. Drug Discov Today. 2004;1:69–76. [Google Scholar]
- 256.Taanman JW, Schapira AH. Analysis of the trinucleotide CAG repeat from the DNA polymerase gamma gene (POLG) in patients with Parkinson’s disease. Neurosci Lett. 2005;376:56–59. doi: 10.1016/j.neulet.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 257.Tabrizi SJ, Orth M, Wilkinson JM, Taanman JW, Warner TT, Cooper JM, Schapira AH. Expression of mutant alpha-synuclein causes increased susceptibility to dopamine toxicity. Hum Mol Genet. 2000;9:2683–9. doi: 10.1093/hmg/9.18.2683. [DOI] [PubMed] [Google Scholar]
- 258.Taira T, Saito Y, Niki T, Iguchi- Ariga SM, Takahashi K, Ariga H. DJ-1 has a role in antioxidative stress to prevent cell death. EMBO Rep. 2004;5:213–218. doi: 10.1038/sj.embor.7400074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Takahashi K, Taira T, Niki T, Seino C, Iguchi- Ariga SMM, Ariga H. DJ-1 positively regulates androgen receptor by imparing the binding of PISAXα to the receptor. J Biol Chem. 2001;276:37556–37563. doi: 10.1074/jbc.M101730200. [DOI] [PubMed] [Google Scholar]
- 260.Tanner CM, Ottman R, Goldman SM, Ellenberg J, Chan P, Mayeux R, Langsten JW. Parkinson’s disease in twin: an etiologic study. JAMA. 1999;281:341–346. doi: 10.1001/jama.281.4.341. [DOI] [PubMed] [Google Scholar]
- 261.Tanuka Y, Engelender S, Igarashi S, Rao RK, Wanner T. Inducible expression of mutant α-synuclein decreases proteasome activity and increases sensitivity to mitochondria-dependant apoptosis. Hum Mol Genet. 2001;10:919–26. doi: 10.1093/hmg/10.9.919. [DOI] [PubMed] [Google Scholar]
- 262.Taylor JP, Mata IF, Farrer MJ. LRRK 2: a common pathway for parkinsonism pathogenesis and prevention. Trends Mol Med. 2006;12:76–82. doi: 10.1016/j.molmed.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 263.Teismann P, Tieu K, Choi DK, Wu DC, Naini S, Hynot M, Jackson- Lewis VV, Przedborski S. Cyclooxygenase 2 is instrumental in Parkinson’s disease neurodegeneration. Proc Natl Acad Sci USA. 2003;100:5473–5478. doi: 10.1073/pnas.0837397100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Thobios S, Delamarre- Damier F, Derkindern P. The treatment of motor dysfunction in PD: an overview. Clin Neurol Neurosurg. 2005;107:269–281. doi: 10.1016/j.clineuro.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 265.Twelves D, Perkins KS, Counsell C. Systematic review of incidence of studies of Parkinson’s disease. Mov Disord. 2003;18:19–31. doi: 10.1002/mds.10305. [DOI] [PubMed] [Google Scholar]
- 266.Ueda K, Fukushima H, Masliah E, Xia Y, Twai A, Yoshimoto M, Otero DA, Kondo J, Ihara Y, Saitoh T. Molecular cloning of cDNA encoding an unrecognized component of amyloid in Alzheimer’s disease. Proc Natl Acad Sci USA. 1993;90:11282–11286. doi: 10.1073/pnas.90.23.11282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Uversky VN, Lee HJ, Li J, Fink AL, Lee SJ. Stabilization of partially folded conformation during α-synuclein oligomerization in both purified and cytosolic preparations. J Biol Chem. 2001;276:43495–43498. doi: 10.1074/jbc.C100551200. [DOI] [PubMed] [Google Scholar]
- 268.Uversky VN, Li J, Fink AL. Metal triggered structural transformation, aggregation and fibrillation of human α-synuclein: A molecular link between Parkinson’s disease and heavy metal exposure. J Biol Chem. 2001;276:44284–44296. doi: 10.1074/jbc.M105343200. [DOI] [PubMed] [Google Scholar]
- 269.Uversky VN, Li J, Fink AL. Pesticides directly accelerate the rate of α- synuclein fibril formation: a possible factor in Parkinson’s disease. FEBS Lett. 2001;500:105–108. doi: 10.1016/s0014-5793(01)02597-2. [DOI] [PubMed] [Google Scholar]
- 270.Uversky VN, Li J, Fink AL. Trimethylamine-N-oxide induced folding of α- synuclein. FEBS Lett. 2001;509:31–35. doi: 10.1016/s0014-5793(01)03121-0. [DOI] [PubMed] [Google Scholar]
- 271.Vaamonde J, Luquin MR, Obeso JA. Subcutaneous lisuride infusion in Parkinson’s disease, response to chronic administration in 34 patients. Brain. 1991;114:601–614. doi: 10.1093/brain/114.1.601. [DOI] [PubMed] [Google Scholar]
- 272.Valente EM, Salvi S, Ialango T, Marogiu R, Elia A, Caputo V, Romito L. PINK1 mutations are associated with sporadic early onset parkinsonism. Ann Neurol. 2004;56:336–341. doi: 10.1002/ana.20256. [DOI] [PubMed] [Google Scholar]
- 273.Valente MJ, Lansbury PTJ. Vesicle permiabilization by protofibrillar α- synuclein is sensitive to Parkinson’s disease linked mutations and occurs by a pore-like mechanism. Biochemistry. 2002;41:4595–4602. doi: 10.1021/bi0121353. [DOI] [PubMed] [Google Scholar]
- 274.Waldmeir PC, Tatton WG. Interrupting apoptosis in neurodegenerative disease potential for effective therapy? Drug Discov Today. 2004;9:210–218. doi: 10.1016/S1359-6446(03)03000-9. [DOI] [PubMed] [Google Scholar]
- 275.Walsh DM, Hartley DM, Kusumoto Y, Fezoui Y, Condron MM, Lomakin A. Amyloid beta-protein fibrillogenesis structure and biological activity of protofibrillar intermediates. J Biol Chem. 1999;274:25945–25952. doi: 10.1074/jbc.274.36.25945. [DOI] [PubMed] [Google Scholar]
- 276.Wang X, Zhu S, Drozda M, Zhang W, Stavrovskaya IG, Caltaneo E, Ferrante RJ, Kristol BS, Friedlanda RM. Minocycline inhibits caspase-independent and dependant mitochondrial cell death pathways in models of Huntington’s disease. Proc Natl Acad Sci USA. 2003;100:10483–10487. doi: 10.1073/pnas.1832501100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Ward CD, Duvoisin RC, Ince SE, Nutt JD, Eldridge R, Calne DP. Parkinson’s disease in 65 pairs of twins and in a set of quadreuplets. Neurology. 1983;33:815–24. doi: 10.1212/wnl.33.7.815. [DOI] [PubMed] [Google Scholar]
- 278.Welsh M. Parkinson’s disease and quality of life: issues and challenges beyond motor symptoms. Neurol Clin. 2004;22:141–148. doi: 10.1016/j.ncl.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 279.West AB, Maraganore D, Crook J. Functional dissociation of parkin gene promoter with idiopathic Parkinson’s disease. Hum Mol Genet. 2002;11:2787–2792. doi: 10.1093/hmg/11.22.2787. [DOI] [PubMed] [Google Scholar]
- 280.Williams M, Wright S, Lloyd GK. Improved therapies for Parkinson’s disease: life beyond dopamine D2/D3 receptor agonist. Trends Pharmacol Sci. 1997;18:307–310. [PubMed] [Google Scholar]
- 281.Xu J, Kao SY, Lee FJ, Song LW, Yanker BA. Dopamine dependent neurotoxicity of α-synuclein: a mechanism for selective neurodegeneration in Parkinson’s disease. Nat Med. 2002;8:600–606. doi: 10.1038/nm0602-600. [DOI] [PubMed] [Google Scholar]
- 282.Yamamoto M, Sakamoto N, Iwai A, Yatsugi S, Hidaka K, Naguchhi K, Yuasa T. Protective actions of YM737, a new glutathione analogue against cerebral ischemia in rats. Res Commun Chem Pathol Pharmacol. 1993;81:221–232. [PubMed] [Google Scholar]
- 283.Yang L, Calingasan NY, Chen J, Ley JJ, Becker DA. A novel azulenyl nitrone antioxidant protects against MPTP and 3-nitropropionic acid neurotoxicities. Exp Neurol. 2005;191:86–93. doi: 10.1016/j.expneurol.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 284.Yokata T, Sugawara K, Ito K, Takahashi R, Ariga H, Mizusawa H. Downregulation of DJ-1 enhances cell death by oxidative stress, ER stress and proteasome inhibition. Biochem Biophy Res Commun. 2003;312:1342–1348. doi: 10.1016/j.bbrc.2003.11.056. [DOI] [PubMed] [Google Scholar]
- 285.Yu WH, Kumar A, Peterhoff C, Kulnane LS, Vachiyama Y. Autophagic vacuoles are enriched in amyloid precursor protein secretase activities: implications for beta-amyloid peptides over production and localization in Alzheimer’s disease. Int J Biochem Cell Biol. 2004;36:2531–2540. doi: 10.1016/j.biocel.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 286.Yuan J, Yanker BA. Apoptosis in the nervous system. Nature. 2000;407:802–809. doi: 10.1038/35037739. [DOI] [PubMed] [Google Scholar]
- 287.Zarranz JJ, Alegre J, Gimez- Esteban JC, Lezeano E, Ross R, Ampuero I, Vidal L, Hoenicka J, Roriguez O. The new mutation E46K of α-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004;55:164–173. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- 288.Zhang Y, Gao J, Chung KK, Huang H, Dawson VL, Dawson TM. Parkin function as an E2 dependent ubiquitin protein ligase and promotes the degradation of the synaptic vesicle associated protein CDCrel 1. Proc Natl Acad Sci USA. 2000;97:13354–59. doi: 10.1073/pnas.240347797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Zhang ZX, Roman GC. Worldwide occurrence of Parkinson’s disease: an updated review. Neuroepidemiology. 1993;12:195–208. doi: 10.1159/000110318. [DOI] [PubMed] [Google Scholar]
- 290.Zhang L, Dawson VL, Bricaire F. Role of nitric oxide in Parkinson’s disease. Pharmacol Ther. 2006;109:33–41. doi: 10.1016/j.pharmthera.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 291.Zimprich A, Biskup S, Leitner P, Lichtner P, Farrer M, Lincoln S, Kachergus J, Hulihan M, Uitti RJ. Mutations in LRRK2 cause autosomal dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44:601–607. doi: 10.1016/j.neuron.2004.11.005. [DOI] [PubMed] [Google Scholar]