Abstract
The pseudoautosomal regions (PAR1 and PAR2) of the human X and Y chromosomes pair and recombine during meiosis. Thus genes in this region are not inherited in a strictly sex-linked fashion. PAR1 is located at the terminal region of the short arms and PAR2 at the tips of the long arms of these chromosomes. To date, 24 genes have been assigned to the PAR1 region. Half of these have a known function. In contrast, so far only 4 genes have been discovered in the PAR2 region. Deletion of the PAR1 region results in failure of pairing and male sterility. The gene SHOX (short stature homeobox-containing) resides in PAR1. SHOX haploinsufficiency contributes to certain features in Turner syndrome as well as the characteristics of Leri-Weill dyschondrosteosis. Only two of the human PAR1 genes have mouse homologues. These do not, however, reside in the mouse PAR1 region but are autosomal. The PAR regions seem to be relics of differential additions, losses, rearrangements and degradation of the X and Y chromosome in different mammalian lineages. Marsupials have three homologues of human PAR1 genes in their autosomes, although, in contrast to mouse, do not have a PAR region at all. The disappearance of PAR from other species seems likely and this region will only be rescued by the addition of genes to both X and Y, as has occurred already in lemmings. The present review summarizes the current understanding of the evolution of PAR and provides up-to-date information about individual genes residing in this region.
Key Words: Pseudoautosomal region, PAR, sex chromosomes, XE7, SHOX
THE X AND Y CHROMOSOMES
The human sex chromosomes (X and Y) originate from an ancestral homologous chromosome pair, which during mammalian evolution lost homology due to progressive degradation of the Y chromosome [1]. The X-chromosome in placental mammals represents approximately 5% of the haploid genome and the gene content is almost completely conserved amongst species. To ensure dosage compensation, most genes on the X are subject to X inactivation in females. The Y chromosome is much smaller than the X, being only 2–3% of the haploid genome, and is largely composed of repeated sequences. Most genes on the Y have relatives on the X chromosome and these are not subject to X inactivation. The degeneration of the Y chromosome has been researched and reviewed extensively [2–6].
THE PSEUDOAUTOSOMAL REGIONS
The pseudoautosomal regions (PAR1 and PAR2) are short regions of homology between the mammalian X and Y chromosomes. The PAR behave like an autosome and recombine during meiosis. Thus genes in this region are inherited in an autosomal rather than a strictly sex-linked fashion.
PAR1 comprises 2.6 Mb of the short-arm tips of both X and Y chromosomes in humans and other great apes [7, 8] and is required for pairing of the X and Y chromosomes during male meiosis. All characterized genes within PAR1 escape X inactivation. X-Y pairing in the PAR is thought to serve a critical function in spermatogenesis, at least in humans and mouse [9–11]. PAR2 is located at the tips of the long arms and is a much shorter region, spanning only 320 kb [12]. PAR2 exhibits a much lower frequency of pairing and recombination than PAR1 and is not necessary for fertility [13–15].
GENES IN HUMAN PAR1 AND PAR2
The sequence of the human X chromosome is nearly complete [16]. This has shown that PAR1 contains at least 24 genes. About half were identified almost a decade ago, while some, like PLCXD1, P2RY8 and DHRSX, have been identified more recently. As well, many novel transcripts were recently assigned to the PAR1 region [16]. The function of known genes in PAR1 is summarized in Table 1. One, designated XE7 when it was described initially [17, 18], but which is now termed CXYorf3, had at that time no function ascribed to it. CXYorf3 is located 1760 kb from the te-lomere in PAR1 and generates two protein isoforms [18]. The shorter one arises from the insertion of an alternative exon containing a stop codon that results in a truncated protein [17, 18]. The longer isoform has a C-terminal region rich in arginine and serine residues, reminiscent of the RS (arginine/serine) domain present in RNA binding/spliceo- somal proteins. The protein, XE7, also termed 721P/B-lymphocyte surface antigen [19], had been identified initially in a spliceosomal screen [20]. Only recently have functional studies been carried out. In these we found that XE7 is an alternative splicing regulator which binds to two important splicing proteins, ASF/SF2 and ZNF265 [21]. Fig. (1) shows the localization of XE7/CXYorf3 amongst other PAR1 genes. Interestingly, exon 3 of XE7/CXYorf3 is identical to exon 1A of another pseudoautosomal gene, ASMTL (acetylserotonin methyltransferase-like) [22]. ASMTL represents a unique fusion product of two distinct genes of different evolutionary origin and function [22]. The N-terminal part is homologous to the bacterial mafl orfE genes and the rest shows 60% homology to the ASMT gene and its encoded protein. Taken together the data suggest that exon duplication and shuffling, as well as gene fusion, may represent common features in the origin of the pseudoautosomal region. Indeed, gene duplications have been shown for other genes in this region.
Table 1.
PAR1 Genes and Protein Function. Only Genes that have been Cloned or Otherwise Characterized are Shown
| Gene symbol | Alternative name/symbol | Protein | Ref. |
|---|---|---|---|
| PLCXD1: phospatidylinositol-specific phospholipase C, X domain containing 1 | FLJ11323 | Function not known. | [70, 71] |
| GTPBP6: GTP binding protein 6 (putative) | PGPL | Function not known. | [72] |
| PPP2R3B: Protein phosphatase 2, regulatory subunit B | PPP2R3L, PR48 protein | Exerts regulatory control over the initiation of DNA replication. Over-expression of PR48 causes G1 cell cycle arrest. | [73] |
| SHOX: short stature homeobox | PHOG, GCFX, SS, SHOXY | Homeobox-containing gene, thought to be a transcription factor related to short stature syndromes. | [34, 36] |
| CRLF2: cytokine receptor-like factor 2 | CRL2, TSLPR | The receptor for TSLP, a cytokine that enhances the maturation process of dendritic cells and promotes the proliferation of CD4+ T cells. | [74–77] |
| CSF2RA: colony-stimulating factor 2 receptor, alpha | CD116, GMCSFR | The alpha subunit of the receptor for the granulocyte-macrophage colony-stimulating factor (GM-CSF). GM-CSF is important for the growth and differentiation of eosinophils and macrophages in the bone marrow, and also regulates cell viability in human embryos. | [55–57, 78–80] |
| IL3RA: interleukin 3 receptor, alpha | CD123 | The alpha subunit of the receptors for interleukin 3. | [81, 82] |
| SLC25A6: solute carrier family 25, member A6 | ANT3, ANT3Y, MGC17525 | A member of the ADP/ATP translocase family, which has a potential role in Th cell survival and immune cell homeostasis. | [83–85] |
| ASMTL: acetylserotonin O-methyltransferase-like | ASMTLX | Function not known. | [22] |
| P2RY8: purinergic receptor P2Y, G-protein coupled, 8 | P2Y8 | A member of the purine nucleotide G-protein coupled receptor gene family. | [86] |
| CXYorf3 | XE7, XE7Y, DXYS155E, MGC39904, B lymphocyte surface antigen 721P, X-escapee, CCDC133 | Alternative splicing regulator. | [17, 18, 21] |
| ASMT: acetylserotonin O-methyltransferase | HIOMT, ASMTY, HIOMTY | Catalyzes the final reaction in the synthesis of melatonin. | [84, 87] |
| DHRSXY: dehydrogenase/reductase (SDR family) X-linked | DHRS5X, DHRS5XY, DHRSY, DHRS5Y | Encodes an oxidoreductase of the short-chain dehydrogenase/reductase family. | [88] |
| ZBED1: zinc finger, BED-type containing 1 | TRAMP, ALTE, KIAA0785 | Has been suggested to be involved in the transposition of other transposable elements. | [89] |
| CD99: CD99 molecule | MIC2, CD99 antigen, “antigen identified by monoclonal antibodies 12E7, F21 and O13” | Is a cell surface molecule involved in T-cell adhesion processes. Activation of a distinct domain of CD99 activates a caspase-independent death pathway in T-cells. | [90–92] |
| XG: XG blood group | PBDX, “XG blood group, pseudoautosomal boundary-divided on the X-chromosome” | The blood group gene XG generates a cell-surface antigen 48 % homologues to CD99. | [93, 94] |
Fig. (1).
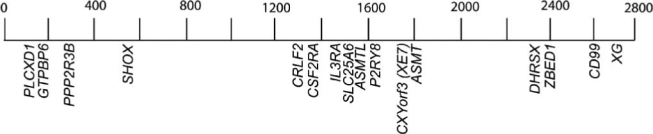
Localization of genes in PAR1. Only characterized genes that are discussed in the text are shown. Their relative position and distance from the telomere is shown in Kb.
CD99 (MIC2) is 73% homologous to the pseudogene MIC2R, and its protein shows 48% homology with XG, while CSF2RA and IL3RA are 54% homologous at the amino acid level. The exon structure in both MIC2/MIC2R and ASMT/ASMTL are also similar [22]. Due to the crossing-over event in each male meiosis between X and Y in the PAR region [23], the recombination rate is 20-fold higher compared with the rest of the genome. This does not, however, fully explain the high rate of gene duplication in PAR.
In the case of the PAR2 region, 4 genes have been identified to date: SPRY3, SYBL1, IL9R and CXYorf1. Of these, only SYBL1 and IL9R have a known function, and these, together with SHOX from the PAR1, are discussed below.
PAR1/PAR2 AND DISEASE
SYBL1 is located in PAR2 but differs from most other PAR genes in that it undergoes both X and Y inactivation. It is a highly conserved gene [24] that codes for a member of the synaptobrevins implicated in cellular exocytosis. In a subset of families with bipolar affective disorder (BPAD), the absence of father-to-son transmission suggested that a susceptibility gene existed on the sex-linked portion of the X chromosome. Saito et al. [25] screened SYBL1 and found a polymorphism (G to C transversion at the intron 7/exon 8 junction) with a statistical trend toward an association with BPAD in males. In addition, Muller et al. [26] observed a significantly increased frequency of genotypes homozygous for the C allele in females with BPAD in comparison with controls, thus strengthening the role of the SYBL1 gene as a candidate gene for BPAD. IL9R (also known as CD129) belongs to the hematopoietin receptor subfamily and PAR2 expresses this gene in both membrane-bound and soluble forms [27]. A role for IL9R in the development of asthma has been suggested [28, 29]. The sDF2∗10 allele of IL9R is more frequently transmitted than untransmitted to asthmatic offspring and the allele was found to be homozygous more often than expected in asthma patients [29]. Also, a specific X-chromosome haplotype (sDF2∗10-sDF1∗6) was found to be associated with asthma [29]. In support of the involvement of IL9R in allergic diseases, a specific IL9R haplotype appears to protect against wheezing in boys [30]. In addition, it has been shown that IL9R is expressed in samples from asthmatic airways but not those from normal subjects [31–33].
The SHOX (Short stature HOmeoboX-containing) gene resides in PAR1 and was first suggested to be involved in the short stature of Turner syndrome by Ellison et al. [34], although they named the gene PHOG for “pseudoautosomal homeobox-containing osteogenic gene”. Turner syndrome is one of the most common chromosomal abnormalities in humans with an incidence of at least 1 in 1850 live female births [35]. It is characterized by features such as short stature, cubitus valgus, short metacarpals, Madelung deformity, high arched palate and short neck. Further data supported the involvement of SHOX in the growth failure of Turner patients and identified a mutation in the SHOX gene in patients with idiopathic growth retardation [36]. It has also been shown that SHOX haploinsufficiency can cause not only short stature but also Turner skeletal anomalities such as short fourth metacarpals, cubitus valgus and characteristics of Leri-Weill dyschondrosteosis (LWD) [37]. LWD is an inherited skeletal dysplasia characterized by disproportionate short stature, mesomelic limb shortening and Madelung deformity of the arm. Later studies have found submicroscopic deletions in the SHOX gene in 34% to 81% of affected families and point mutations in the SHOX gene in 19% to 39% of LWD families studied [38–46]. Patients with SHOX haploin-sufficiency could benefit from early growth hormone treatment, so early screening of children with unexplained short stature has been suggested [47, 48]. A second PAR1 region has been implicated lately in the pathogenesis of LWD. This involved identification of a novel class of PAR1 deletions which did not include SHOX [49]. The finding indicated the presence of distal regulatory elements of SHOX transcription in PAR1 or the existence of an additional locus involved in the control of skeletal development [49]. More recently PAR1 deletions downstream of SHOX have been reported to represent a higher proportion of mutations than SHOX deletions and mutations implicated in LWD [50].
PRE-mRNA SPLICING AND PAR
Alternative splicing generates several mRNA products and thus protein isoforms from a single gene. This is one of the most important mechanisms regulating gene expression. Alternative splicing can lead to the production of protein isoforms with changed binding properties, intracellular localization, enzymatic activity, protein stability, or posttransla-tional modification (such as phosphorylation) and cell type/tissue-specific expression. Alternative splicing can also introduce a stop codon, which, if a pre-mature stop codon, can lead to nonsense-mediated decay (NMD) of the mRNA. Changes in splice site selection can also cause disease, or might be a consequence of disease. Several genes in PAR1 and PAR2 generate multiple protein isoforms as a result of alternative splicing.
SHOX can produce two protein isoforms, SHOXa and SHOXb, of 292 and 225 amino acids, respectively. SHOX consists of 6 exons. The two isoforms diverge after exon IV. Both SHOXa and SHOXb are expressed in skeletal muscle and bone marrow fibroblasts, while SHOXa is also expressed in placenta, pancreas and heart. SHOXb, on the other hand, is also expressed in fetal kidney, but the highest expression has been found in bone marrow fibroblasts [36]. The significance of the two isoforms is at present not known. An insertion in exon 6a in a man with Langer mesomelia dysplasia has led to the conclusion that the SHOXa isoform is essential for normal skeletal development [51].
XE7/CXYorf3 also generates two isoforms. This is a result of the insertion of an additional exon (exon 5), which, as described above, leads to a truncated protein [18]. As mentioned earlier, we have shown that the longer isoform of XE7 is an alternative splicing regulator that affects the splicing of CD44, Tra2β 1 and SRp20 [21]. The significance of the shorter isoform is at present not known, but it has been speculated that this isoform undergoes NMD. This could be a way of regulating the expression of XE7 in different cell types or developmental stages according to need.
CD99 serves as a marker for the Ewing sarcoma family of tumors and has been found recently in primary cutaneous melanoma [52]. CD99 exists in two isoforms, type I and II. An 18 bp insertion between exons 8 and 9 introduces a premature stop codon, generating a truncated protein, as in the case of XE7. The longer isoform of CD99 has been shown to regulate the adhesion of lymphocytes via the LFA-1/ICAM-1 pathway. In contrast, overexpression of the shorter isoform reduces the level of LFA-1 expression and regulates CD99-mediated and spontaneous aggregation of lymphocytes [53]. Type I is expressed in most tissues studied, while type II has been detected at lower levels in a cell-specific manner [53], suggesting that the alternative splicing of CD99 serves a biological functional role.
IL9R exists in two distinct isoforms and this too is due to alternative splicing. Isoform 1 [27] is the longer isoform and is 76% homologous to isoform 2 reported by Chang et al. [54]. Isoform 2 contains an insertion of 125 base pairs in the N-terminal region, and this results in a frameshift. Other base pair changes in the coding region, compared to isoform 1, generate an isoform with distinct N and C-termini and other internal differences. It is at present unknown if a difference in expression of each isoform is related to an association with asthma.
CSF2RA encodes the alpha subunit of the heterodimeric receptor for colony stimulating factor 2, a cytokine controlling the production, differentiation, and function of granulocytes and macrophages. Alternative splicing produces at least 5 isoforms, some being membrane-bound and others being soluble [55–59].
ORIGIN OF HUMAN PAR1 AND PAR2
Marsupials and eutherian mammals diverged about 130 million years ago (Mya), and monotremes and eutherians 170 Mya. The sex chromosomes of marsupials and monotremes differ quite substantially. Marsupials have a small X and an even smaller Y and these do not undergo homologous pairing, while the monotremes have a large X and Y which pair over the entire short arm of the X and the long arm of the Y.
The PAR of placental mammals varies greatly. The mouse and human PAR region are completely non-homologous and even within primates the gene content of the PAR deviates. Cloning and mapping dog and sheep homologues of human Xp22.3 genes PRKX and STS, as well as PAR1 genes ANT3 and CSF2RA, showed that they are all pseudoautosomal in these mammals and must have been part of the sex chromosomes for at least 80 million years [60]. This means that the ancestral eutherian PAR was larger than the present human PAR. Mapping of STS, ANT3 and CSF2RA genes in marsupials showed that these are autoso-mal in marsupials and colocalized with 7 other human Xp genes within a single autosomal cluster in marsupials (61). This implies that the eutherian PAR was part of a larger autosomal addition to the X and Y 130–80 Mya [61]. ANT3 mapped separately on another wallaby autosome, so it may represent a region added independently to the eutherian PAR or a region that has been rearranged in marsupials [61]. The mouse sex chromosomes have a 2 Mb PAR region, but contain only one active gene, Sts [62, 63]. One other gene, Fxy, spans the pseudoautosomal boundary on the mouse X and has a truncated partner at the boundary of the Y PAR [64, 65]. The human homologue resides near the PAR on the X but does not exist on the Y. A recent revelation is that PAR1 resides within a 9 Mb block that has been removed from the X chromosome of a common murine ancestor of mouse and rat [16]. It thus seems that independent additions to PAR1 by gene translocation from autosomes seem to have occurred in eutherians, macropodid marsupials and monotremes, while loss of PAR1 genes is evident in mouse. By comparing human genes in or near PAR1 with those of other mammals it is evident that mutation and loss of genes on the differentiating Y chromosome reduced the homologous region to a different extent in different lineages [60].
Of the four PAR2 genes, only SYBL1 is located on the X chromosome in all species, including marsupials, so it must have been part of the ancient X chromosome. SPRY3 is localized to the X chromosome in all eutherians, but not marsupials, consistent with it having been added to the X after the divergence of eutherians and marsupials 130 Mya, but before the eutherian radiation 80 Mya [66]. Neither SPRY3 nor SYBL1 map to the Y chromosome in primates and mouse. Each are inactive on the Y and subject to X inactivation in humans [67]. CXYorf1 on the other hand is on the X and autosomes in both primate and mouse [68], but is auto-somal in the wallaby [66], so it must have been added 70–130 Mya. IL9R is located on the X only in primates [66], so it seems to be the latest addition to PAR2, occurring 60–70 Mya. Human CXYorf1 and IL9R are expressed from the Y chromosome and are not subject to X inactivation [67]. There are multiple copies of IL9R and CXYorf1 on the auto-somes, so gene duplication has been suggested as playing a role in the evolution of these two genes [66]. Since the order of the genes on human PAR2 is SPRY3, SYBL1, IL9R, followed by CXYorf1, the evolution of this region must have required two inversion events on top of the three independent additions of genes.
FUTURE OF PAR
As mentioned earlier, X-Y pairing in PAR serves a critical function for spermatogenesis in humans and mice [9–11]. PAR is, however, absent in marsupials and the absence of homologous pairing of the X-Y chromosomes in this species causes no disruption to segregation at meiosis [8]. It is at present unclear what has replaced homologous pairing and recombination in marsupials. The mouse PAR also seems to be at the last stage of degradation. The PAR region will only be saved if further additions of genes take place to both X and Y. This has already happened in the case of one mammal, the lemming [69]. The fact that the gene content of PAR in different species is so inconsistent argues for PAR not playing a sequence-dependent role in fertility. It does, however, seems to be an excellent genetic playground.
ABBREVIATIONS
- PAR
Pseudoautosomal region
- RS
Arginine/serine
- BPAD
Bipolar affective disorder
- LWD
Leri-Weill dyschondrosteosis
- NMD
Nonsense-mediated decay
- Mya
Million years ago
REFERENCES
- 1.Charlesworth B. The evolution of sex chromosomes. Science. 1991;4997:1030–3. doi: 10.1126/science.1998119. [DOI] [PubMed] [Google Scholar]
- 2.Charlesworth B, Charlesworth D. The degeneration of Y chromosomes. Philos Trans R Soc Lond B Biol Sci. 2000;1403:1563–72. doi: 10.1098/rstb.2000.0717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Charlesworth B. The evolution of chromosomal sex determination. Novartis Found Symp. 2002:207–19. discussion 20-4, 53-7. [PubMed] [Google Scholar]
- 4.Charlesworth D, Charlesworth B. Sex chromosomes: evolution of the weird and wonderful. Curr Biol. 2005;4:R129–31. doi: 10.1016/j.cub.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 5.Gvozdev VA, Kogan GL, Usakin LA. The Y chromosome as a target for acquired and amplified genetic material in evolution. Bioessays. 2005;12:1256–62. doi: 10.1002/bies.20321. [DOI] [PubMed] [Google Scholar]
- 6.Graves JA. Sex chromosome specialization and degeneration in mammals. Cell. 2006;5:901–14. doi: 10.1016/j.cell.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 7.Rappold GA. The pseudoautosomal regions of the human sex chromosomes. Hum Genet. 1993;4:315–24. doi: 10.1007/BF01247327. [DOI] [PubMed] [Google Scholar]
- 8.Graves JA, Wakefield MJ, Toder R. The origin and evolution of the pseudoautosomal regions of human sex chromosomes. Hum Mol Genet. 1998;13:1991–6. doi: 10.1093/hmg/7.13.1991. [DOI] [PubMed] [Google Scholar]
- 9.Mohandas TK, Speed RM, Passage MB, Yen PH, Chandley AC, Shapiro LJ. Role of the pseudoautosomal region in sex-chromosome pairing during male meiosis: meiotic studies in a man with a deletion of distal Xp. Am J Hum Genet. 1992;3:526–33. [PMC free article] [PubMed] [Google Scholar]
- 10.Burgoyne PS, Mahadevaiah SK, Sutcliffe MJ, Palmer SJ. Fertility in mice requires X-Y pairing and a Y-chromosomal “spermiogenesis” gene mapping to the long arm. Cell. 1992;3:391–8. doi: 10.1016/0092-8674(92)90509-b. [DOI] [PubMed] [Google Scholar]
- 11.Matsuda Y, Moens PB, Chapman VM. Deficiency of X and Y chromosomal pairing at meiotic prophase in spermatocytes of sterile interspecific hybrids between laboratory mice (Mus domesticus) and Mus spretus. Chromosoma. 1992;8:483–92. doi: 10.1007/BF00352471. [DOI] [PubMed] [Google Scholar]
- 12.Freije D, Helms C, Watson MS, Donis-Keller H. Identification of a second pseudoautosomal region near the Xq and Yq telomeres. Science. 1992;5089:1784–7. doi: 10.1126/science.1465614. [DOI] [PubMed] [Google Scholar]
- 13.Kvaloy K, Galvagni F, Brown WR. The sequence organization of the long arm pseudoautosomal region of the human sex chromosomes. Hum Mol Genet. 1994;5:771–8. doi: 10.1093/hmg/3.5.771. [DOI] [PubMed] [Google Scholar]
- 14.Li L, Hamer DH. Recombination and allelic association in the Xq/Yq homology region. Hum Mol Genet. 1995;11:2013–6. doi: 10.1093/hmg/4.11.2013. [DOI] [PubMed] [Google Scholar]
- 15.Kuhl H, Rottger S, Heilbronner H, Enders H, Schempp W. Loss of the Y chromosomal PAR2-region in four familial cases of satellited Y chromosomes (Yqs) Chromosome Res. 2001;3:215–22. doi: 10.1023/a:1012219820317. [DOI] [PubMed] [Google Scholar]
- 16.Ross MT, Grafham DV, Coffey AJ, Scherer S, McLay K, Muzny D, Platzer M, Howell GR, Burrows C, Bird CP, Frankish A, Lovell FL, Howe KL, Ashurst JL, Fulton RS, Sudbrak R, Wen G, Jones MC, Hurles ME, Andrews TD, Scott CE, Searle S, Ramser J, Whittaker A, Deadman R, Carter NP, Hunt SE, Chen R, Cree A, Gunaratne P, Havlak P, Hodgson A, Metzker ML, Richards S, Scott G, Steffen D, Sodergren E, Wheeler DA, Worley KC, Ainscough R, Ambrose KD, Ansari-Lari MA, Aradhya S, Ashwell RI, Babbage AK, Bagguley CL, Ballabio A, Banerjee R, Barker GE, Barlow KF, Barrett IP, Bates KN, Beare DM, Beasley H, Beasley O, Beck A, Bethel G, Blechschmidt K, Brady N, Bray-Allen S, Bridgeman AM, Brown AJ, Brown MJ, Bonnin D, Bruford EA, Buhay C, Burch P, Burford D, Burgess J, Burrill W, Burton J, Bye JM, Carder C, Carrel L, Chako J, Chapman JC, Chavez D, Chen E, Chen G, Chen Y, Chen Z, Chinault C, Ciccodicola A, Clark SY, Clarke G, Clee CM, Clegg S, Clerc-Blankenburg K, Clifford K, Cobley V, Cole CG, Conquer JS, Corby N, Connor RE, David R, Davies J, Davis C, Davis J, Delgado O, Deshazo D, Dhami P, Ding Y, Dinh H, Dodsworth S, Draper H, Dugan-Rocha S, Dunham A, Dunn M, Durbin KJ, Dutta I, Eades T, Ellwood M, Emery-Cohen A, Errington H, Evans KL, Faulkner L, Francis F, Frankland J, Fraser AE, Galgoczy P, Gilbert J, Gill R, Glockner G, Gregory SG, Gribble S, Griffiths C, Grocock R, Gu Y, Gwilliam R, Hamilton C, Hart EA, Hawes A, Heath PD, Heitmann K, Hennig S, Hernandez J, Hinzmann B, Ho S, Hoffs M, Howden PJ, Huckle EJ, Hume J, Hunt PJ, Hunt AR, Isherwood J, Jacob L, Johnson D, Jones S, de, Jong PJ, Joseph SS, Keenan S, Kelly S, Kershaw JK, Khan Z, Kioschis P, Klages S, Knights AJ, Kosiura A, Kovar-Smith C, Laird GK, Langford C, Lawlor S, Leversha M, Lewis L, Liu W, Lloyd C, Lloyd DM, Loulseged H, Loveland JE, Lovell JD, Lozado R, Lu J, Lyne R, Ma J, Maheshwari M, Matthews LH, McDowall J, McLaren S, McMurray A, Meidl P, Meitinger T, Milne S, Miner G, Mistry SL, Morgan M, Morris S, Muller I, Mullikin JC, Nguyen N, Nordsiek G, Nyakatura G, O’Dell CN, Okwuonu G, Palmer S, Pandian R, Parker D, Parrish J, Pasternak S, Patel D, Pearce AV, Pearson DM, Pelan SE, Perez L, Porter KM, Ramsey Y, Reichwald K, Rhodes S, Ridler KA, Schlessinger D, Schueler MG, Sehra HK, Shaw-Smith C, Shen H, Sheridan EM, Shownkeen R, Skuce CD, Smith ML, Sotheran EC, Steingruber HE, Steward CA, Storey R, Swann RM, Swarbreck D, Tabor PE, Taudien S, Taylor T, Teague B, Thomas K, Thorpe A, Timms K, Tracey A, Trevanion S, Tromans AC, d’Urso M, Verduzco D, Villasana D, Waldron L, Wall M, Wang Q, Warren J, Warry GL, Wei X, West A, Whitehead SL, Whiteley MN, Wilkinson JE, Willey DL, Williams G, Williams L, Williamson A, Williamson H, Wilming L, Woodmansey RL, Wray PW, Yen J, Zhang J, Zhou J, Zoghbi H, Zorilla S, Buck D, Reinhardt R, Poustka A, Rosenthal A, Lehrach H, Meindl A, Minx PJ, Hillier LW, Willard HF, Wilson RK, Waterston RH, Rice CM, Vaudin M, Coulson A, Nelson DL, Weinstock G, Sulston JE, Durbin R, Hubbard T, Gibbs RA, Beck S, Rogers J, Bentley DR. The DNA sequence of the human X chromosome. Nature. 2005;7031:325–37. doi: 10.1038/nature03440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ellison J, Passage M, Yu LC, Yen P, Mohandas TK, Shapiro L. Directed isolation of human genes that escape X inactivation. Somat Cell Mol Genet. 1992;3:259–68. doi: 10.1007/BF01233862. [DOI] [PubMed] [Google Scholar]
- 18.Ellison JW, Ramos C, Yen PH, Shapiro LJ. Structure and expression of the human pseudoautosomal gene XE7. Hum Mol Genet. 1992;9:691–6. [PubMed] [Google Scholar]
- 19.Voland JR, Wyzykowski RJ, Huang M, Dutton RW. Cloning and sequencing of a trophoblast-endothelial-activated lymphocyte surface protein: cDNA sequence and genomic structure. Proc Natl Acad Sci USA. 1992;21:10425–9. doi: 10.1073/pnas.89.21.10425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rappsilber J, Ryder U, Lamond AI, Mann M. Large-scale proteomic analysis of the human spliceosome. Genome Res. 2002;8:1231–45. doi: 10.1101/gr.473902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mangs AH, Speirs HJ, Goy C, Adams DJ, Markus MA, Morris BJ. XE7: a novel splicing factor that interacts with ASF/SF2 and ZNF265. Nucleic Acids Res. 2006;17:4976–86. doi: 10.1093/nar/gkl660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ried K, Rao E, Schiebel K, Rappold GA. Gene duplications as a recurrent theme in the evolution of the human pseudoautosomal region 1: isolation of the gene ASMTL. Hum Mol Genet. 1998;11:1771–8. doi: 10.1093/hmg/7.11.1771. [DOI] [PubMed] [Google Scholar]
- 23.Rouyer F, Simmler MC, Johnsson C, Vergnaud G, Cooke HJ, Weissenbach J. A gradient of sex linkage in the pseudoautosomal region of the human sex chromosomes. Nature. 1986;6051:291–5. doi: 10.1038/319291a0. [DOI] [PubMed] [Google Scholar]
- 24.Filippini F, Rossi V, Galli T, Budillon A, D’Urso M, D’Esposito M. Longins: a new evolutionary conserved VAMP family sharing a novel SNARE domain. Trends Biochem Sci. 2001;7:407–9. doi: 10.1016/s0968-0004(01)01861-8. [DOI] [PubMed] [Google Scholar]
- 25.Saito T, Parsia S, Papolos DF, Lachman HM. Analysis of the pseudoautosomal X-linked gene SYBL1in bipolar affective disorder: description of a new candidate allele for psychiatric dis-orders. Am J Med Genet. 2000;3:317–23. doi: 10.1002/1096-8628(20000612)96:3<317::aid-ajmg17>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 26.Muller DJ, Schulze TG, Jahnes E, Cichon S, Krauss H, Kesper K, Held T, Maier W, Propping P, Nothen MM, Rietschel M. Association between a polymorphism in the pseudoautosomal X-linked gene SYBL1 and bipolar affective disorder. Am J Med Genet. 2002;1:74–8. doi: 10.1002/ajmg.10115. [DOI] [PubMed] [Google Scholar]
- 27.Renauld JC, Druez C, Kermouni A, Houssiau F, Uyttenhove C, Van Roost E, Van Snick J. Expression cloning of the murine and human interleukin 9 receptor cDNAs. Proc Natl Acad Sci USA. 1992;12:5690–4. doi: 10.1073/pnas.89.12.5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holroyd KJ, Martinati LC, Trabetti E, Scherpbier T, Eleff SM, Boner AL, Pignatti PF, Kiser MB, Dragwa CR, Hubbard F, Sullivan CD, Grasso L, Messler CJ, Huang M, Hu Y, Nicolaides NC, Buetow KH, Levitt RC. Asthma and bronchial hyperresponsiveness linked to the XY long arm pseudoautosomal region. Genomics. 1998;2:233–5. doi: 10.1006/geno.1998.5445. [DOI] [PubMed] [Google Scholar]
- 29.Kauppi P, Laitinen T, Ollikainen V, Mannila H, Laitinen LA, Kere J. The IL9R region contribution in asthma is supported by genetic association in an isolated population. Eur J Hum Genet. 2000;10:788–92. doi: 10.1038/sj.ejhg.5200541. [DOI] [PubMed] [Google Scholar]
- 30.Melen E, Gullsten H, Zucchelli M, Lindstedt A, Nyberg F, Wickman M, Pershagen G, Kere J. Sex specific protective effects of interleukin-9 receptor haplotypes on childhood wheezing and sensitisation. J Med Genet. 2004;12:e123. doi: 10.1136/jmg.2004.023135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bhathena PR, Comhair SA, Holroyd KJ, Erzurum SC. Interleukin-9 receptor expression in asthmatic airways In vivo. Lung. 2000;3:149–60. doi: 10.1007/s004080000018. [DOI] [PubMed] [Google Scholar]
- 32.Abdelilah S, Latifa K, Esra N, Cameron L, Bouchaib L, Nicolaides N, Levitt R, Hamid Q. Functional expression of IL-9 receptor by human neutrophils from asthmatic donors: role in IL-8 release. J Immunol. 2001;4:2768–74. doi: 10.4049/jimmunol.166.4.2768. [DOI] [PubMed] [Google Scholar]
- 33.Gounni AS, Hamid Q, Rahman SM, Hoeck J, Yang J, Shan L. IL-9-mediated induction of eotaxin1/CCL11 in human airway smooth muscle cells. J Immunol. 2004;4:2771–9. doi: 10.4049/jimmunol.173.4.2771. [DOI] [PubMed] [Google Scholar]
- 34.Ellison JW, Wardak Z, Young MF, Gehron Robey P, Laig-Webster M, Chiong W. PHOG, a candidate gene for involvement in the short stature of Turner syndrome. Hum Mol Genet. 1997;8:1341–7. doi: 10.1093/hmg/6.8.1341. [DOI] [PubMed] [Google Scholar]
- 35.Nielsen J, Wohlert M. Sex chromosome abnormalities found among 34,910 newborn children: results from a 13-year incidence study in Arhus, Denmark. Birth Defects Orig Artic Ser. 1990;4:209–23. [PubMed] [Google Scholar]
- 36.Rao E, Weiss B, Fukami M, Rump A, Niesler B, Mertz A, Muroya K, Binder G, Kirsch S, Winkelmann M, Nordsiek G, Heinrich U, Breuning MH, Ranke MB, Rosenthal A, Ogata T, Rappold GA. Pseudoautosomal deletions encompassing a novel homeobox gene cause growth failure in idiopathic short stature and Turner syndrome. Nat Genet. 1997;1:54–63. doi: 10.1038/ng0597-54. [DOI] [PubMed] [Google Scholar]
- 37.Kosho T, Muroya K, Nagai T, Fujimoto M, Yokoya S, Sakamoto H, Hirano T, Terasaki H, Ohashi H, Nishimura G, Sato S, Matsuo N, Ogata T. Skeletal features and growth patterns in 14 patients with haploinsufficiency of SHOX: implications for the development of Turner syndrome. J Clin Endocrinol Metab. 1999;12:4613–21. doi: 10.1210/jcem.84.12.6289. [DOI] [PubMed] [Google Scholar]
- 38.Schiller S, Spranger S, Schechinger B, Fukami M, Merker S, Drop SL, Troger J, Knoblauch H, Kunze J, Seidel J, Rappold GA. Phenotypic variation and genetic heterogeneity in Leri-Weill syndrome. Eur J Hum Genet. 2000;1:54–62. doi: 10.1038/sj.ejhg.5200402. [DOI] [PubMed] [Google Scholar]
- 39.Belin V, Cusin V, Viot G, Girlich D, Toutain A, Moncla A, Vekemans M, Le Merrer M, Munnich A, Cormier-Daire V. SHOX mutations in dyschondrosteosis (Leri-Weill syndrome) Nat Genet. 1998;1:67–9. doi: 10.1038/ng0198-67. [DOI] [PubMed] [Google Scholar]
- 40.Huber C, Cusin V, Le Merrer M, Mathieu M, Sulmont V, Dagoneau N, Munnich A, Cormier-Daire V. SHOX point mutations in dyschondrosteosis. J Med Genet. 2001;5:323. doi: 10.1136/jmg.38.5.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huber C, Rosilio M, Munnich A, Cormier-Daire V. High incidence of SHOX anomalies in individuals with short stature. J Med Genet. 2006;9:735–9. doi: 10.1136/jmg.2006.040998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ross JL, Scott C, Jr, Marttila P, Kowal K, Nass A, Papenhausen P, Abboudi J, Osterman L, Kushner H, Carter P, Ezaki M, Elder F, Wei F, Chen H, Zinn AR. Phenotypes Associated with SHOX Deficiency. J Clin Endocrinol Metab. 2001;12:5674–80. doi: 10.1210/jcem.86.12.8125. [DOI] [PubMed] [Google Scholar]
- 43.Binder G, Renz A, Martinez A, Keselman A, Hesse V, Riedl SW, Hausler G, Fricke-Otto S, Frisch H, Heinrich JJ, Ranke MB. SHOX haploinsufficiency and Leri-Weill dyschon-drosteosis: prevalence and growth failure in relation to mutation, sex, and degree of wrist deformity. J Clin Endocrinol Metab. 2004;9:4403–8. doi: 10.1210/jc.2004-0591. [DOI] [PubMed] [Google Scholar]
- 44.Schneider KU, Sabherwal N, Jantz K, Roth R, Muncke N, Blum WF, Cutler GB, Jr, Rappold G. Identification of a major recombination hotspot in patients with short stature and SHOX deficiency. Am J Hum Genet. 2005;1:89–96. doi: 10.1086/431655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rappold G, Blum WF, Shavrikova EP, Crowe BJ, Roeth R, Quigley CA, Ross JL, Niesler B. Genotypes and pheno-types in children with short stature: clinical indicators of SHOX haploinsufficiency. J Med Genet. 2006 doi: 10.1136/jmg.2006.046581. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gatta V, Antonucci I, Morizio E, Palka C, Fischetto R, Mokini V, Tumini S, Calabrese G, Stuppia L. Identification and characterization of different SHOX gene deletions in patients with Leri-Weill dyschondrosteosys by MLPA assay. J Hum Genet. 2007;1:21–27. doi: 10.1007/s10038-006-0074-5. Epub 2006 Nov 8. [DOI] [PubMed] [Google Scholar]
- 47.Binder G, Schwarze CP, Ranke MB. Identification of short stature caused by SHOX defects and therapeutic effect of recombinant human growth hormone. J Clin Endocrinol Metab. 2000;1:245–9. doi: 10.1210/jcem.85.1.6375. [DOI] [PubMed] [Google Scholar]
- 48.Blum WF, Crowe BJ, Quigley CA, Jung H, Cao D, Ross JL, Braun L, Rappold For The Shox Study Group, G Growth Hormone is Effective in Treatment of Short Stature Associated with SHOX Deficiency: Two-year Results of a Randomized, Controlled, Multi-Center Trial. J Clin Endocrinol Metab. 2007;92:219–28. doi: 10.1210/jc.2006-1409. [DOI] [PubMed] [Google Scholar]
- 49.Benito-Sanz S, Thomas NS, Huber C, Gorbenko del Blanco D, Aza-Carmona M, Crolla JA, Maloney V, Rappold G, Argente J, Campos-Barros A, Cormier-Daire V, Heath KE. A novel class of Pseudoautosomal region 1 deletions downstream of SHOX is associated with Leri-Weill dyschondrosteosis. Am J Hum Genet. 2005;4:533–44. doi: 10.1086/449313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Benito-Sanz S, del Blanco DG, Aza-Carmona M, Magano LF, Lapunzina P, Argente J, Campos-Barros A, Heath KE. PAR1 deletions downstream of SHOX are the most frequent defect in a Spanish cohort of Leri-Weill dyschondrosteosis (LWD) probands. Hum Mutat. 2006;10:1062. doi: 10.1002/humu.9456. [DOI] [PubMed] [Google Scholar]
- 51.Zinn AR, Wei F, Zhang L, Elder FF, Scott CI, Jr, Marttila P, Ross JL. Complete SHOX deficiency causes Langer mesomelic dysplasia. Am J Med Genet. 2002;2:158–63. doi: 10.1002/ajmg.10422. [DOI] [PubMed] [Google Scholar]
- 52.Wilkerson AE, Glasgow MA, Hiatt KM. Immunoreactivity of CD99 in invasive malignant melanoma. J Cutan Pathol. 2006;10:663–6. doi: 10.1111/j.1600-0560.2006.00524.x. [DOI] [PubMed] [Google Scholar]
- 53.Hahn JH, Kim MK, Choi EY, Kim SH, Sohn HW, Ham DI, Chung DH, Kim TJ, Lee WJ, Park CK, Ree HJ, Park SH. CD99 (MIC2) regulates the LFA-1/ICAM-1-mediated adhesion of lymphocytes, and its gene encodes both positive and negative regulators of cellular adhesion. J Immunol. 1997;5:2250–8. [PubMed] [Google Scholar]
- 54.Chang MS, Engel G, Benedict C, Basu R, McNinch J. Isolation and characterization of the human interleukin-9 receptor gene. Blood. 1994;11:3199–205. [PubMed] [Google Scholar]
- 55.Hayashida K, Kitamura T, Gorman DM, Arai K, Yokota T, Miyajima A. Molecular cloning of a second subunit of the receptor for human granulocyte-macrophage colony-stimulating factor (GM-CSF): reconstitution of a high-affinity GM-CSF receptor. Proc Natl Acad Sci USA. 1990;24:9655–9. doi: 10.1073/pnas.87.24.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Raines MA, Liu L, Quan SG, Joe V, DiPersio JF, Golde DW. Identification and molecular cloning of a soluble human granulocyte-macrophage colony-stimulating factor receptor. Proc Natl Acad Sci USA. 1991;18:8203–7. doi: 10.1073/pnas.88.18.8203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Crosier KE, Wong GG, Mathey-Prevot B, Nathan DG, Sieff CA. A functional isoform of the human granulocyte/macrophage colony-stimulating factor receptor has an unusual cytoplasmic domain. Proc Natl Acad Sci USA. 1991;17:7744–8. doi: 10.1073/pnas.88.17.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brown AL, Peters M, D’Andrea RJ, Gonda TJ. Constitutive mutants of the GM-CSF receptor reveal multiple pathways leading to myeloid cell survival, proliferation, and granulocyte-macrophage differentiation. Blood. 2004;2:507–16. doi: 10.1182/blood-2003-05-1435. [DOI] [PubMed] [Google Scholar]
- 59.Chen J, Carcamo JM, Golde DW. The alpha subunit of the granulocyte-macrophage colony-stimulating factor receptor interacts with c-Kit and inhibits c-Kit signaling. J Biol Chem. 2006;31:22421–6. doi: 10.1074/jbc.M604644200. [DOI] [PubMed] [Google Scholar]
- 60.Toder R, Glaser B, Schiebel K, Wilcox SA, Rappold G, Graves JA, Schempp W. Genes located in and near the human pseudoautosomal region are located in the X-Y pairing region in dog and sheep. Chromosome Res. 1997;5:301–6. doi: 10.1023/B:CHRO.0000038760.84605.0d. [DOI] [PubMed] [Google Scholar]
- 61.Toder R, Graves JA. CSF2RA, ANT3, and STS are autosomal in marsupials: implications for the origin of the pseudoautosomal region of mammalian sex chromosomes. Mamm Genome. 1998;5:373–6. doi: 10.1007/s003359900772. [DOI] [PubMed] [Google Scholar]
- 62.Keitges E, Rivest M, Siniscalco M, Gartler SM. X-linkage of steroid sulphatase in the mouse is evidence for a functional Y-linked allele. Nature. 1985;6016:226–7. doi: 10.1038/315226a0. [DOI] [PubMed] [Google Scholar]
- 63.Salido EC, Li XM, Yen PH, Martin N, Mohandas TK, Shapiro LJ. Cloning and expression of the mouse pseudoautosomal steroid sulphatase gene (Sts) Nat Genet. 1996;1:83–6. doi: 10.1038/ng0596-83. [DOI] [PubMed] [Google Scholar]
- 64.Palmer S, Perry J, Kipling D, Ashworth A. A gene spans the pseudoautosomal boundary in mice. Proc Natl Acad Sci USA. 1997;22:12030–5. doi: 10.1073/pnas.94.22.12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Perry J, Feather S, Smith A, Palmer S, Ashworth A. The human FXY gene is located within Xp22.3: implications for evolution of the mammalian X chromosome. Hum Mol Genet. 1998;2:299–305. doi: 10.1093/hmg/7.2.299. [DOI] [PubMed] [Google Scholar]
- 66.Charchar FJ, Svartman M, El-Mogharbel N, Ventura M, Kirby P, Matarazzo MR, Ciccodicola A, Rocchi M, D’Esposito M, Graves JA. Complex events in the evolution of the human pseudoautosomal region 2 (PAR2) Genome Res. 2003;2:281–6. doi: 10.1101/gr.390503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ciccodicola A, D’Esposito M, Esposito T, Gianfrancesco F, Migliaccio C, Miano MG, Matarazzo MR, Vacca M, Franze A, Cuccurese M, Cocchia M, Curci A, Terracciano A, Torino A, Cocchia S, Mercadante G, Pannone E, Archidiacono N, Rocchi M, Schlessinger D, D’Urso M. Differentially regulated and evolved genes in the fully sequenced Xq/Yq pseudoautosomal region. Hum Mol Genet. 2000;3:395–401. doi: 10.1093/hmg/9.3.395. [DOI] [PubMed] [Google Scholar]
- 68.Gianfrancesco F, Falco G, Esposito T, Rocchi M, D’Urso M. Characterization of the murine orthologue of a novel human subtelomeric multigene family. Cytogenet Cell Genet. 2001;1-2:98–100. doi: 10.1159/000048796. [DOI] [PubMed] [Google Scholar]
- 69.Berend SA, Hale DW, Engstrom MD, Greenbaum IF. Cytogenetics of collared lemmings (Dicrostonyx groenlandicus). I. Meiotic behavior and evolution of the neo-XY sex-chromosome system. Cytogenet Cell Genet. 1997;3-4:288–92. doi: 10.1159/000134746. [DOI] [PubMed] [Google Scholar]
- 70.Kimura K, Wakamatsu A, Suzuki Y, Ota T, Nishikawa T, Yamashita R, Yamamoto J, Sekine M, Tsuritani K, Wakaguri H, Ishii S, Sugiyama T, Saito K, Isono Y, Irie R, Kushida N, Yoneyama T, Otsuka R, Kanda K, Yokoi T, Kondo H, Wagatsuma M, Murakawa K, Ishida S, Ishibashi T, Takahashi-Fujii A, Tanase T, Nagai K, Kikuchi H, Nakai K, Isogai T, Sugano S. Diversification of transcriptional modulation: large-scale identification and characterization of putative alternative promoters of human genes. Genome Res. 2006;1:55–65. doi: 10.1101/gr.4039406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Strausberg RL, Feingold EA, Grouse LH, Derge JG, Klausner RD, Collins FS, Wagner L, Shenmen CM, Schuler GD, Altschul SF, Zeeberg B, Buetow KH, Schaefer CF, Bhat NK, Hopkins RF, Jordan H, Moore T, Max SI, Wang J, Hsieh F, Diatchenko L, Marusina K, Farmer AA, Rubin GM, Hong L, Stapleton M, Soares MB, Bonaldo MF, Casavant TL, Scheetz TE, Brownstein MJ, Usdin TB, Toshiyuki S, Carninci P, Prange C, Raha SS, Loquellano NA, Peters GJ, Abramson RD, Mullahy SJ, Bosak SA, McEwan PJ, McKernan KJ, Malek JA, Gunaratne PH, Richards S, Worley KC, Hale S, Garcia AM, Gay LJ, Hulyk SW, Villalon DK, Muzny DM, Sodergren EJ, Lu X, Gibbs RA, Fahey J, Helton E, Ketteman M, Madan A, Rodrigues S, Sanchez A, Whiting M, Madan A, Young AC, Shevchenko Y, Bouffard GG, Blakesley RW, Touchman JW, Green ED, Dickson MC, Rodriguez AC, Grimwood J, Schmutz J, Myers RM, Butterfield YS, Krzywinski MI, Skalska U, Smailus DE, Schnerch A, Schein JE, Jones SJ, Marra MA. Mammalian Gene Collection Program Team. Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences. Proc Natl Acad Sci USA. 2002;26:16899–903. doi: 10.1073/pnas.242603899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gianfrancesco F, Esposito T, Montanini L, Ciccodicola A, Mumm S, Mazzarella R, Rao E, Giglio S, Rappold G, Forabosco A. A novel pseudoautosomal gene encoding a putative GTP-binding protein resides in the vicinity of the Xp/Yp telomere. Hum Mol Genet. 1998;3:407–14. doi: 10.1093/hmg/7.3.407. [DOI] [PubMed] [Google Scholar]
- 73.Yan Z, Fedorov SA, Mumby MC, Williams RS. PR48, a novel regulatory subunit of protein phosphatase 2A, interacts with Cdc6 and modulates DNA replication in human cells. Mol Cell Biol. 2000;3:1021–9. doi: 10.1128/mcb.20.3.1021-1029.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pandey A, Ozaki K, Baumann H, Levin SD, Puel A, Farr AG, Ziegler SF, Leonard WJ, Lodish HF. Cloning of a receptor subunit required for signaling by thymic stromal lym-phopoietin. Nat Immunol. 2000;1:59–64. doi: 10.1038/76923. [DOI] [PubMed] [Google Scholar]
- 75.Reche PA, Soumelis V, Gorman DM, Clifford T, Liu M, Travis M, Zurawski SM, Johnston J, Liu YJ, Spits H, de Waal Malefyt R, Kastelein RA, Bazan JF. Human thymic stromal lymphopoietin preferentially stimulates myeloid cells. J Immunol. 2001;1:336–43. doi: 10.4049/jimmunol.167.1.336. [DOI] [PubMed] [Google Scholar]
- 76.Zhang W, Wang J, Wang Q, Chen G, Zhang J, Chen T, Wan T, Zhang Y, Cao X. Identification of a novel type I cytokine receptor CRL2 preferentially expressed by human dendritic cells and activated monocytes. Biochem Biophys Res Commun. 2001;4:878–83. doi: 10.1006/bbrc.2001.4432. [DOI] [PubMed] [Google Scholar]
- 77.Al-Shami A, Spolski R, Kelly J, Keane-Myers A, Leonard WJ. A role for TSLP in the development of inflammation in an asthma model. J Exp Med. 2005;6:829–39. doi: 10.1084/jem.20050199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gearing DP, King JA, Gough NM, Nicola NA. Expression cloning of a receptor for human granulocyte-macrophage colony-stimulating factor. EMBO J. 1989;12:3667–76. doi: 10.1002/j.1460-2075.1989.tb08541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Metcalf D, Robb L, Dunn AR, Mifsud S, Di Rago L. Role of granulocyte-macrophage colony-stimulating factor and granulocyte colony-stimulating factor in the development of an acute neutrophil inflammatory response in mice. Blood. 1996;10:3755–64. [PubMed] [Google Scholar]
- 80.Sjoblom C, Wikland M, Robertson SA. Granulocyte-macrophage colony-stimulating factor (GM-CSF) acts independently of the beta common subunit of the GM-CSF receptor to prevent inner cell mass apoptosis in human embryos. Biol Reprod. 2002;6:1817–23. doi: 10.1095/biolreprod.101.001503. [DOI] [PubMed] [Google Scholar]
- 81.Kremer E, Baker E, D’Andrea RJ, Slim R, Phillips H, Moretti PA, Lopez AF, Petit C, Vadas MA, Sutherland GR, et al. A cytokine receptor gene cluster in the X-Y pseudoautosomal region? Blood. 1993;1:22–8. [PubMed] [Google Scholar]
- 82.Kitamura T, Sato N, Arai K, Miyajima A. Expression cloning of the human IL-3 receptor cDNA reveals a shared beta subunit for the human IL-3 and GM-CSF receptors. Cell. 1991;6:1165–74. doi: 10.1016/0092-8674(91)90039-2. [DOI] [PubMed] [Google Scholar]
- 83.Schiebel K, Weiss B, Wohrle D, Rappold G. A human pseudoautosomal gene, ADP/ATP translocase, escapes X-inactivation whereas a homologue on Xq is subject to X-inactivation. Nat Genet. 1993;1:82–7. doi: 10.1038/ng0193-82. [DOI] [PubMed] [Google Scholar]
- 84.Slim R, Levilliers J, Ludecke HJ, Claussen U, Nguyen VC, Gough NM, Horsthemke B, Petit C. A human pseudoautosomal gene encodes the ANT3 ADP/ATP translocase and escapes X-inactivation. Genomics. 1993;1:26–33. doi: 10.1006/geno.1993.1135. [DOI] [PubMed] [Google Scholar]
- 85.Jang JY, Lee CE. IL-4-induced upregulation of adenine nucleotide translocase 3 and its role in Th cell survival from apoptosis. Cell Immunol. 2006;1:14–25. doi: 10.1016/j.cellimm.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 86.Cantagrel V, Lossi AM, Boulanger S, Depetris D, Mattei MG, Gecz J, Schwartz CE, Van Maldergem L, Villard L. Disruption of a new X linked gene highly expressed in brain in a family with two mentally retarded males. J Med Genet. 2004;10:736–42. doi: 10.1136/jmg.2004.021626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yi H, Donohue SJ, Klein DC, McBride OW. Localization of the hydroxyindole-O-methyltransferase gene to the pseudoautosomal region: implications for mapping of psychiatric disorders. Hum Mol Genet. 1993;2:127–31. doi: 10.1093/hmg/2.2.127. [DOI] [PubMed] [Google Scholar]
- 88.Gianfrancesco F, Sanges R, Esposito T, Tempesta S, Rao E, Rappold G, Archidiacono N, Graves JA, Forabosco A, D’Urso M. Differential divergence of three human pseudoautosomal genes and their mouse homologs: implications for sex chromosome evolution. Genome Res. 2001;12:2095–100. doi: 10.1101/gr.197001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Esposito T, Gianfrancesco F, Ciccodicola A, Montanini L, Mumm S, D’Urso M, Forabosco A. A novel pseudoautosomal human gene encodes a putative protein similar to Ac-like transposases. Hum Mol Genet. 1999;1:61–7. doi: 10.1093/hmg/8.1.61. [DOI] [PubMed] [Google Scholar]
- 90.Gelin C, Aubrit F, Phalipon A, Raynal B, Cole S, Kaczorek M, Bernard A. The E2 antigen, a 32 kd glycoprotein involved in T-cell adhesion processes, is the MIC2 gene product. EMBO J. 1989;11:3253–9. doi: 10.1002/j.1460-2075.1989.tb08485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pettersen RD, Bernard G, Olafsen MK, Pourtein M, Lie SO. CD99 signals caspase-independent T cell death. J Immunol. 2001;8:4931–42. doi: 10.4049/jimmunol.166.8.4931. [DOI] [PubMed] [Google Scholar]
- 92.Goodfellow PN, Pym B, Pritchard C, Ellis N, Palmer M, Smith M, Goodfellow PJ. MIC2: a human pseudoautosomal gene. Philos Trans R Soc Lond B. Biol. Sci. 1988;1208:145–54. doi: 10.1098/rstb.1988.0122. [DOI] [PubMed] [Google Scholar]
- 93.Ellis NA, Ye TZ, Patton S, German J, Goodfellow PN, Weller P. Cloning of PBDX, an MIC2-related gene that spans the pseudoautosomal boundary on chromosome Xp. Nat Genet. 1994;4:394–400. doi: 10.1038/ng0494-394. [DOI] [PubMed] [Google Scholar]
- 94.Ellis NA, Tippett P, Petty A, Reid M, Weller PA, Ye TZ, German J, Goodfellow PN, Thomas S, Banting G. PBDX is the XG blood group gene. Nat Genet. 1994;3:285–90. doi: 10.1038/ng1194-285. [DOI] [PubMed] [Google Scholar]


