Abstract
Unbiased genome-wide studies of longevity in S. cerevisiae and C. elegans have led to the identification of more than one hundred genes that determine life span in one or both organisms. Key pathways have been uncovered linking nutrient and growth factor cues to longevity. Quantitative measures of the degree to which aging is evolutionary conserved are now possible. A major challenge for the future is determining which of these genes play a similar role in human aging and using that information to develop therapies toward age-associated diseases.
INTRODUCTION
The relatively long life span enjoyed by most mammals presents a difficulty in studying the genetic and environmental features that determine longevity. Experiments designed to directly test the hypothesis that a particular gene or treatment influences life span require a few years in a mouse, several decades in monkeys, and are impractical in humans. Thus, a handful of simple eukaryotes have been used to characterize the pathways that regulate aging in short-lived organisms. Of these, the fruit fly Drosophila melanogaster, the nematode Caenorhabditis elegans, and the budding yeast Saccharomyces cerevisiae, have emerged as the most widely used and, hence, best characterized, model organisms in bio-gerontology.
When considering the use of simple eukaryotes to study aging and age-related disease, it is pertinent to ask whether, and to what degree, the aging process is evolutionarily conserved. Does a yeast cell age by the same mechanism(s) as a mouse? Is the longevity of a nematode determined in the same way as that of a person? The complete answers to these questions remain largely unknown; however, discoveries made over the last several years have unequivocally demonstrated that at least some of the factors regulating longevity are shared between yeast, worms, flies, and mice. The degree to which these pathways will be relevant to human longevity and age-associated disease is an important unanswered question.
The most direct method to address how well the features that determine longevity have been conserved is to identify genes or interventions that function similarly to modulate life span in different organisms. Components of insulin/IGF-1-like signaling pathway, the sirtuin family of protein deacety-lases, and the nutrient-responsive TOR kinase, among others, have been found to have this property (Table 1). Until recently, however, the genetic analysis of longevity was largely limited to mutagenesis screens for secondary phenotypes (such as stress resistance) or targeted studies of specific genes, based on prior knowledge. While many important insights were gained from such studies, they, by necessity, self-selected for mutants with specific properties that are (at best) secondarily related to longevity. Thus, it remains unclear to what degree the pathways regulating longevity are evolutionarily conserved and whether the known longevity genes represent most of the important players or only a small fraction.
Table 1.
A selection of Evolutionarily Conserved Longevity Determinants
A small number of genes or interventions are known to increase life span in different model organisms. A selection of these are shown here.
| Antioxidant enzymes — Increased expression of antioxidant enzymes, such as superoxide dismutase and catalase increase life span in yeast, flies, and mice [23, 26–29]. The free radical theory of aging posits that many age-associated phenotypes result from damage caused by oxidative free radicals, which are likely to be reduced by increased expression of antioxidant enzymes [30]. |
| Dietary restriction (calorie/caloric restriction) — Dietary restriction increases life span in yeast, worms, flies, mice, rats, and other organisms [31, 32]. The mechanisms by which dietary restriction promotes longevity and retards disease remain unknown, although several hypotheses have been proposed. |
| Insulin/IGF-1 — Mutations that decrease insulin/IGF-1-like signaling increase life span in worms, flies, and mice [33–35]. |
| Resveratrol — Increases life span in fish, worms, and flies [36, 37]. Resveratrol is a polyphenolic compound found in red wine and grapes that acts on several cellular proteins, including sirtuins, AMP kinase, mitochondrial ATP synthase and complex III, fatty acid synthase, protein kinase C, p53, MEK1, TNF– and NFκB [38]. |
| Ribosomal Proteins — Deletion or RNAi inhibition of individual ribosomal proteins increases life span in yeast and worms [6, 13, 39]. |
| S6 Kinase — Decreased S6 kinase activity increases life span in worms and flies [14, 21]. S6 kinase is a TOR target that promotes ribosome maturation. |
| Sch9/Akt — Deletion of Sch9 increases life span in yeast and RNAi of Akt isoforms increases life span in C. elegans [3, 24, 40, 41]. |
| Sirtuins — Increased activity of Sir2 orthologs increases life span in yeast, worms, and flies [42–44]. |
| TOR — Mutations that decrease activity of the nutrient-responsive TOR kinase increase life span in yeast, worms, and flies [6, 7, 19–21]. |
Recent technological and methodological advances have allowed researchers to consider, for the first time, genome-wide studies directly measuring longevity [1]. To date, four such studies have been reported: two in the nematode Caenorhabditis elegans [2–5] and two in the budding yeast Saccharomyces cerevisiae [6, 7]. In each of these studies, large-scale libraries were used to identify genetic perturbations that increase life span. The emphasis on mutations that increase life span is important, since there are many ways to shorten life span without accelerating aging. Here I summarize some of the implications of these studies and describe efforts now underway to utilize genomic approaches to identify genes that influence human longevity and age-associated disease.
GENOME-WIDE RNAi SCREENS IN C. elegans
The first true genomic longevity screens to be reported were carried out in C. elegans by the Kenyon and Ruvkun/Li labs [2–5]. Both groups took advantage of the relative ease with which gene expression can be knocked down using RNAi in this organism. For essentially any C. elegans ORF, expression can be decreased by feeding animals E. coli expressing double-stranded RNAi corresponding to that gene [8]. Two different libraries have been created that contain E. coli clones corresponding to most of the C. elegans OR-Feome [9, 10]. Both of the published genome-wide RNAi longevity screens used the library created by the Ahringer lab containing more than 15,000 clones [9, 11]. In each case, effort was concentrated on identification of RNAi clones that conferred increased life span [2–5] (Fig. 1).
Fig. (1).
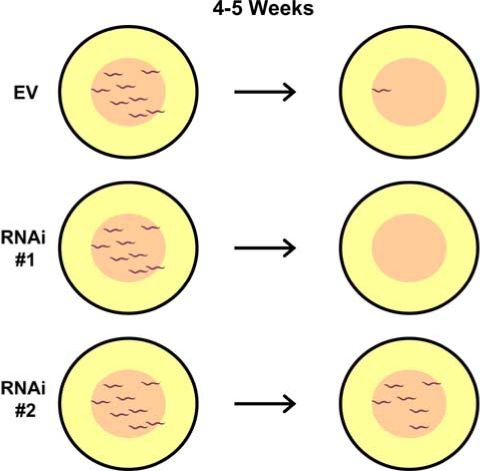
Schematic of worm RNAi screens for increased life span. Individual ORFs can be specifically knocked down in C. elegans by feeding worms E. coli expressing double-stranded RNA corresponding to the ORF (RNAi). A library of ~15,000 different RNAi clones has been screened for long life span by identifying RNAi clones that have a high percentage of live animals on them after a length of time sufficient for most control (empty vector = EV) animals to have died. RNAi #2 in this example corresponds to a gene that limits life span.
Taken together, these two RNAi screens identified 130 ORFs that modulate longevity [12]. Among the life span enhancing RNAi clones, both groups observed enrichment for genes involved in similar functions. For example, genes important for mitochondrial function were enriched in both screens [2–5], demonstrating the important role that this organelle plays in aging of C. elegans. Epistasis analysis was used to putatively place genes into known aging pathways, such as those defined by insulin/IGF-1-like signaling and dietary restriction.
Despite the similarities in functional categories of genes identified from each screen, the overlap among individual ORFs was surprisingly small. Of the 130 ORFs identified between the two studies, only three were identified in both studies. The same RNAi library was used by both groups [9], and although methodology differed somewhat, it seems unlikely that this would account for such a low degree of agreement. One possibility is that both studies contained a high rate of false negatives. Consistent with this idea, more recent reports have identified additional RNAi clones that increase life span, but were not uncovered by either screen (e.g. see refs. [13, 14]). Variability in RNAi efficiency could contribute to a high false negative rate. It seems likely, however, that false positives were also a factor in the relatively small overlap between the two genomic RNAi longevity screens. Although both groups carried out internal validation of their hits, an independent reanalysis of the 130 putative longevity-modulating ORFs has not been reported. Thus, it is difficult to estimate the false positive rates. Regardless, it seems likely that additional longevity-determining ORFs, and perhaps novel aging pathways, remain to be identified in C. elegans.
GENOME-WIDE DELETION SCREENS IN S. cere-visiae
A major advantage of yeast as a model for longevity studies is the ability to separate the aging of mitotic cells from the aging of post-mitotic cells [15]. Mitotic, or “replicative”, life span in yeast is defined as the number of daughter cells produced by a mother cell prior to senescence. Post-mitotic, or “chronological”, life span is defined as the length of time that a cell can survive in a non-dividing, quiescent-like state. Like the case for C. elegans, studies of aging in yeast are also facilitated by the large amount of comparative genomic data and community resources available. For example, the yeast deletion collection is a set of isogenic, single-gene deletion strains covering ~6000 unique ORFs in the yeast genome, which have proven particularly useful for genome-wide studies of a variety of phenotypes, including longevity. Parallel screens of the yeast deletion collection have been initiated with the goal of systematically measuring both replicative and chronological life span for each of the ~5000 non-essential single-gene deletion strains present in the set [16].
Genome-Wide Analysis of Yeast Replicative Life Span
Measuring replicative life span for ~5000 different strains is a daunting task. The replicative life span assay in yeast involves manual micromanipulation of daughter cells away from mother cells, while tallying the number of daughters produced by each mother [15]. In a typical experiment comparing a control versus experimental group, approximately 50 mother cells are assayed per strain in triplicate. Since the average life span of the deletion set parental strain (BY4742) is ~26 generations (daughter cells produced), standard methodology would require microdissection of approximately (50*3*5000*26 = )19.5 million daughter cells.
In order to reduce the effort required to assay replicative life span for each strain in the deletion collection, statistical methods were used to develop an iterative method (Fig. 2) in which 5 cells are initially assayed for each deletion mutant [6]. Based on the average replicative life span of these 5 cells, each mutant is classified as unlikely to be long-lived, likely to be long-lived, or intermediate. In the case of either of the latter two classifications, additional cells are assayed, until a definitive classification can be made. This iterative method was used to characterize the replicative aging properties for 564 single-gene deletion strains in an initial report published in Science. Of the 564 deletion mutants, 13 were verified to have extended replicative life span, relative to the parental strain [6]. The false positive rate is expected to be low, due to validation of long-lived strains by testing of an independently derived deletion allele. The false negative rate is unknown. It is worth noting, however, that the hit rate for the initial 564 strains examined (~2%) is about twice that of the pooled RNAi screens in C. elegans (~1%).
Fig. (2).
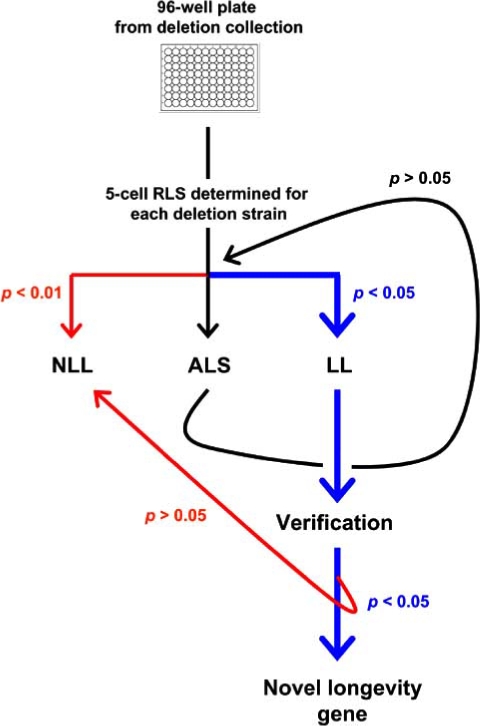
An interative method for measuring yeast replicative life span for ~5000 single gene deletion strains. For each single gene deletion strain, replicative life span is determined initially for 5 mother cells. Each strain is classified as either “not long-lived” (NLL), “long-lived” (LL) or “ambiguous life span” (ALS), based on the 5-cell average. For strains classified as ALS, replicative life span is determined for additional mother cells, until a classification as either NLL or LL can be made. Verification of LL strains involves replicative life span analysis of an independently derived deletion mutant corresponding to the particular ORF.
Among the 13 replicatively long-lived single-gene deletion strains, at least 5 are known to function in a well-characterized TOR (target of rapamycin) signaling pathway [6]. TOR kinases are evolutionarily conserved proteins that function to mediate mRNA translation, cell growth, metabolism, degradation, and stress resistance (among other processes) in response to nutrient and growth factor cues [17, 18]. Mutations that decrease TOR activity have also been reported to increase life span in both C. elegans [19, 20] and Drosophila melanogaster [21], suggesting an evolutionarily conserved link between TOR signaling and aging. Unlike most multicellular eukaryotes, yeast have two TOR paralogs: TOR1 and TOR2. The tor1Δ strain (Tor2 is essential), along with two strains deleted for TOR-regulatory factors (ure2Δ and rom2Δ) were found to have a significantly increased replicative life span. Interestingly, two single-gene deletion strains lacking different genes coding for ribosomal large subunit proteins (rpl31aΔ and rpl6bΔ), which are known to be transcriptionally up-regulated by TOR, were also among the long-lived mutants [6]. As with the TOR genes, most yeast ribosomal protein genes are duplicated, allowing for viable deletion of one, but not both, paralogs. Thus, one possibility is that decreased TOR signaling increases replicative life span by down-regulating mRNA translation, either specifically or generally.
The genome-wide analysis of yeast replicative life span is ongoing, with initial replicative life span data having been obtained for nearly 100% of the strains in the ORF deletion collection (MK and B. Kennedy, unpublished data). Validation of putative long-lived deletion mutants continues, and several dozen novel longevity genes have been identified. As with the initial set of 13, several of these genes have known links to the TOR pathway, both upstream and downstream of TOR, further solidifying the importance of this key nutrientresponsive factor as a primary determinant of replicative longevity in yeast.
Genome-Wide Analysis of Yeast Chronological Life Span
Yeast chronological life span refers to the length of time cells can maintain viability during quiescence [22]. Chronological longevity has typically been assayed by culturing cells into stationary phase in liquid culture, while periodically measuring the percent of cells still alive by dilution and plating onto a nutrient rich agar-based media [15]. Viability is then calculated based on the number of colonies arising (colony forming units) on the nutrient agar. As with the yeast replicative life span assay, this methodology requires a relatively large investment of time and resources, and is not suited for high-throughput study.
A high-throughput method for measuring chronological life span (Fig. 3) was recently described, in which cells are aged in 96-well microtiter plates [7]. Rather than monitoring survival of individual cells based on colony forming units, relative cell viability of the population can be determined by the rate of outgrowth in liquid culture (based on optical density at 600 nm) following dilution into rich media. All cell and liquid transfers are automated using high-density replica pinning robot. while less quantitative than the traditional methodologies, this drawback is offset somewhat by the ability to monitor survival for several thousand strains simultaneously.
Fig. (3).
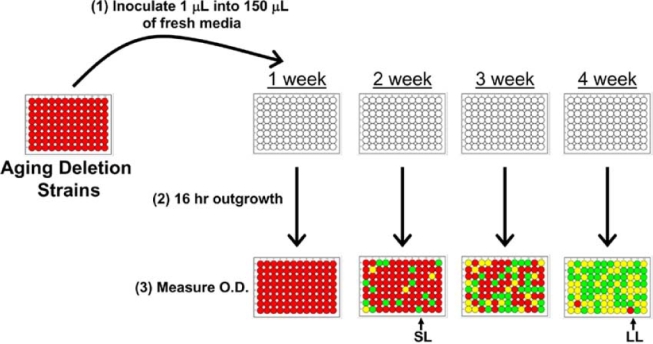
A high-throughput method for measuring yeast chronological life span. Yeast deletion strains are aged in individual wells of 96-well microtiter plates. To assay viability, a small volume from the aging culture is inoculated into a larger volume of fresh media contained in a 96-well microtiter plate. The optical density of the new culture is measured after a fixed period of outgrowth under standard conditions. The relative optical density after outgrowth at different age-points is used to calculate the relative viability of each strain at each age-point. SL = short-lived. LL = long-lived.
This high-throughput chronological life span assay was used to measure the relative chronological longevity for each of the strains in the yeast ORF deletion collection [7]. The entire set of ~5000 deletion mutants was ranked based on relative survival. Among the 90 highest ranked strains, 16 contained deletions in genes implicated in TOR signaling and nutrient uptake, including TOR1, the ammonium per-meases MEP2 and MEP3, the amino acid permease AGP1, and the TOR-regulated transcription factor GLN3 [7].
The two longevity screens of the yeast ORF deletion collection demonstrated that TOR signaling modulates both replicative and chronological aging, with decreased TOR activity correlating with increased life span in both cases [6, 7]. The mechanistic basis for life span extension appears to diverge, however, downstream of TOR. Unlike the case for replicative life span, the genome-wide chronological aging study uncovered no evidence that decreased translation or ribosome function played a role in chronological longevity downstream of TOR. Rather, chronological life span was correlated with increased stress resistance, mediated by the TOR-regulated Msn2/Msn4 transcription factors [7]. Msn2 and Msn4 had been previously implicated in chronological life span extension [23, 24]. Specifically, Fabrizio et al. proposed that Msn2/Msn4-mediated regulation of the gene coding for mitochondrial superoxide dismutase, SOD2, is important for chronological life span extension from deletion of another nutrient-responsive kinase, Sch9 [23]. Thus, it appears that (oxidative) stress resistance is a critical factor determining the longevity of non-dividing yeast cells. Future efforts will likely further clarify the specific TOR target genes involved in regulating replicative and chronological life span in yeast cells, and perhaps in higher organisms as well.
CONSERVATION OF AGING ACROSS SPECIES
Although a handful of genes and interventions are known to modulate life span in different organisms (Table 1), the degree to which mechanisms of aging are conserved remains largely unknown. As the genetic determinants of aging in simple organisms become mapped in finer detail, new opportunities to address this question become available. For example, computational algorithms can identify putative ortholog pairs between yeast and worms [25]. Given a list of potential orthologs, it will be possible to compare the frequency with which ortholog pairs modulate life span similarly in both organisms to the frequency at which a gene randomly picked from the genome modulates longevity in each organism. This type of analysis will begin to provide a quantitative measure of the degree to which longevity determination has been conserved between yeast and worms.
Genomic comparisons of longevity across species also provide an opportunity to identify novel factors that modulate aging and age-associated disease in humans. The evolutionary distance between yeast and worms is approximately equivalent to the evolutionary distance between worm and humans. Therefore, if an ortholog pair has maintained a conserved longevity determining function between yeast and worms, it is reasonable to speculate that the function will also be retained in mammals. At lease one effort is underway to directly test this assumption (http://www.pathology. washington.edu/research/bioage/ellison/). A consortium of laboratories at the University of Washington is utilizing the data from the genome-wide yeast and worm longevity screens described above to identify candidate genes for longevity studies as gene knock-outs in mice [1]. A CRE-based conditional knock-out system is being employed for these studies, to allow either complete knock-out of a particular gene or tissue specific (or post-development) gene deletion. Along with longevity, a select group of potential aging-related biomarkers will be assayed for each of these mouse models. In addition, it should be possible to assay several of these mouse lines for resistance to specific age-associated diseases, such as diabetes and neurological disorders, by crossing them into the appropriate transgenic disease background.
CONCLUSION
Our understanding of the basic mechanisms of aging have benefited greatly from the use of simple model systems such as yeast and worms. The development of technologies that allow direct analysis of longevity on a genome-wide scale in these organisms has provided a wealth of new data regarding the genes and pathways that modulate longevity. Some of these genes and pathways are specific to each organism; however, others appear to be evolutionarily conserved. Future efforts will move toward translating the data from genomic longevity studies in yeast and worms into mammalian models. Any gene that functions similarly to modulate longevity and disease in yeast, worms, and mice will be an outstanding candidate for therapeutic intervention targeting age-associated diseases in people.
ACKNOWLEDGEMENTS
Studies related to this topic in MK’s lab are funded by grants from The Ellison Medical Foundation, The American Federation for Aging Research, and the NIH through the University of Washington Nathan Shock Center of Excellence in the Basic Biology of Aging.
REFERENCES
- 1.Kaeberlein M. Aging-related research in the ‘-omics’ age. Sci Aging Knowledge Environ. 2004;2004:pe39. doi: 10.1126/sageke.2004.42.pe39. [DOI] [PubMed] [Google Scholar]
- 2.Dillin A, Hsu AL, Arantes-Oliveira N, Lehrer-Graiwer J, Hsin H, Fraser AG, Kamath RS, Ahringer J, Kenyon C. Rates of behavior and aging specified by mitochondrial function during development. Science. 2002;298:2398–2401. doi: 10.1126/science.1077780. [DOI] [PubMed] [Google Scholar]
- 3.Hamilton B, Dong Y, Shindo M, Liu W, Odell I, Ruvkun G, Lee SS. A systematic RNAi screen for longevity genes in C. elegans. Genes Dev. 2005;19:1544–1555. doi: 10.1101/gad.1308205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hansen M, Hsu AL, Dillin A, Kenyon C. New genes tied to endocrine, metabolic, and dietary regulation of lifespan from a Caenorhabditis elegans genomic RNAi screen. PLoS Genet. 2005;1:119–128. doi: 10.1371/journal.pgen.0010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee SS, Lee RY, Fraser AG, Kamath RS, Ahringer J, Ruvkun G. A systematic RNAi screen identifies a critical role for mitochondria in C. elegans longevity. Nat Genet. 2003;33:40–48. doi: 10.1038/ng1056. [DOI] [PubMed] [Google Scholar]
- 6.Kaeberlein M, Powers RW, 3rd, Steffen KK, Westman EA, Hu D, Dang N, Kerr EO, Kirkland KT, Fields S, Kennedy BK. Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science. 2005;310:1193–1196. doi: 10.1126/science.1115535. [DOI] [PubMed] [Google Scholar]
- 7.Powers RW, 3rd, Kaeberlein M, Caldwell SD, Kennedy BK, Fields S. Extension of chronological life span in yeast by decreased TOR pathway signaling. Genes Dev. 2006;20:174–184. doi: 10.1101/gad.1381406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Timmons L, Fire A. Specific interference by ingested dsRNA. Nature. 1998;395:854. doi: 10.1038/27579. [DOI] [PubMed] [Google Scholar]
- 9.Kamath RS, Fraser AG, Dong Y, Poulin G, Durbin R, Gotta M, Kanapin A, Le Bot N, Moreno S, Sohrmann M, Welchman DP, Zipperlen P, Ahringer J. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature. 2003;421:231–237. doi: 10.1038/nature01278. [DOI] [PubMed] [Google Scholar]
- 10.Rual JF, Ceron J, Koreth J, Hao T, Nicot AS, Hirozane-Kishikawa T, Vandenhaute J, Orkin SH, Hill DE, van den Heuvel S, Vidal M. Toward improving Caenorhabditis elegans phenome mapping with an ORFeome-based RNAi library. Genome Res. 2004;14:2162–2168. doi: 10.1101/gr.2505604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamath RS, Ahringer J. Genome-wide RNAi screening in Caenorhabditis elegans. Methods. 2003;30:313–321. doi: 10.1016/s1046-2023(03)00050-1. [DOI] [PubMed] [Google Scholar]
- 12.Smith ED, Kennedy BK, Kaeberlein M. Genome-wide identification of conserved longevity genes in yeast and worms. Mech Ageing Dev. 2007;128:106–111. doi: 10.1016/j.mad.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 13.Hansen M, Taubert S, Crawford D, Libina N, Lee SJ, Kenyon C. Lifespan extension by conditions that inhibit translation in Caenorhabditis elegans. Aging Cell. 2007;6:95–110. doi: 10.1111/j.1474-9726.2006.00267.x. [DOI] [PubMed] [Google Scholar]
- 14.Pan KZ, Palter JE, Rogers AN, Olsen A, Chen D, Lithgow GJ, Kapahi P. Inhibition of mRNA translation extends lifespan in Caenorhabditis elegans. Aging Cell. 2007;6:111–119. doi: 10.1111/j.1474-9726.2006.00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaeberlein M. Longevity and aging in the budding yeast. In: Conn PM, editor. Hand-book of models for human aging. Boston: Elsevier Press; 2006. pp. 109–120. [Google Scholar]
- 16.Kaeberlein M, Kennedy BK. Large-scale identification in yeast of conserved ageing genes. Mech Ageing Dev. 2005;126:17–21. doi: 10.1016/j.mad.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 17.Arsham AM, Neufeld TP. Thinking globally and acting locally with TOR. Curr Opin Cell Biol. 2006;18:589–597. doi: 10.1016/j.ceb.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 18.Martin DE, Hall MN. The expanding TOR signaling network. Curr Opin Cell Biol. 2005;17:158–166. doi: 10.1016/j.ceb.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 19.Jia K, Chen D, Riddle DL. The TOR pathway interacts with the insulin signaling pathway to regulate C. elegans larval development, metabolism and life span. Development. 2004;131:3897–3906. doi: 10.1242/dev.01255. [DOI] [PubMed] [Google Scholar]
- 20.Vellai T, Takacs-Vellai K, Zhang Y, Kovacs AL, Orosz L, Muller F. Genetics: influence of TOR kinase on lifespan in C. elegans. Nature. 2003;426:620. doi: 10.1038/426620a. [DOI] [PubMed] [Google Scholar]
- 21.Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, Benzer S. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol. 2004;14:885–890. doi: 10.1016/j.cub.2004.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fabrizio P, Longo VD. The chronological life span of Saccharomyces cerevisiae. Aging Cell. 2003;2:73–81. doi: 10.1046/j.1474-9728.2003.00033.x. [DOI] [PubMed] [Google Scholar]
- 23.Fabrizio P, Liou LL, Moy VN, Diaspro A, SelverstoneValentine J, Gralla EB, Longo VD. SOD2 functions downstream of Sch9 to extend longevity in yeast. Genetics. 2003;163:35–46. doi: 10.1093/genetics/163.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fabrizio P, Pozza F, Pletcher SD, Gendron CM, Longo VD. Regulation of longevity and stress resistance by Sch9 in yeast. Science. 2001;292:288–290. doi: 10.1126/science.1059497. [DOI] [PubMed] [Google Scholar]
- 25.Tatusov RL, Fedorova ND, Jackson JD, Jacobs AR, Kiryutin B, Koonin EV, Krylov DM, Mazumder R, Mekhedov SL, Nikolskaya AN, Rao BS, Smirnov S, Sverdlov AV, Vasudevan S, Wolf YI, Yin JJ, Natale DA. The COG database: an updated version includes eukaryotes. BMC Bioinformatics. 2003;4:41. doi: 10.1186/1471-2105-4-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parkes TL, Elia AJ, Dickinson D, Hilliker AJ, Phillips JP, Boulianne GL. Extension of Drosophila lifespan by overexpression of human SOD1 in motorneurons. Nat Genet. 1998;19:171–174. doi: 10.1038/534. [DOI] [PubMed] [Google Scholar]
- 27.Phillips JP, Parkes TL, Hilliker AJ. Targeted neuronal gene expression and longevity in Drosophila. Exp Gerontol. 2000;35:1157–1164. doi: 10.1016/s0531-5565(00)00117-0. [DOI] [PubMed] [Google Scholar]
- 28.Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, Coskun PE, Ladiges W, Wolf N, Van Remmen H, Wallace DC, Rabinovitch PS. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science. 2005;308:1909–1911. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- 29.Sun J, Tower J. FLP recombinase-mediated induction of Cu/Zn-superoxide dismutase transgene expression can extend the life span of adult Drosophila melanogaster flies. Mol Cell Biol. 1999;19:216–228. doi: 10.1128/mcb.19.1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harman D. Free radical theory of aging: an update: increasing the functional life span. Ann N Y Acad Sci. 2006;1067:10–21. doi: 10.1196/annals.1354.003. [DOI] [PubMed] [Google Scholar]
- 31.Masoro EJ. Overview of caloric restriction and ageing. Mech Ageing Dev. 2005;126:913–922. doi: 10.1016/j.mad.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 32.Weindruch RH, Walford RL. The Retardation of Aging and Disease by Dietary Restriction. Springfield, IL: Thomas; 1988. [Google Scholar]
- 33.Bartke A. Minireview: role of the growth hormone/insulin-like growth factor system in mammalian aging. Endocrinology. 2005;146:3718–3723. doi: 10.1210/en.2005-0411. [DOI] [PubMed] [Google Scholar]
- 34.Bartke A, Chandrashekar V, Dominici F, Turyn D, Kinney B, Steger R, Kopchick JJ. Insulin-like growth factor 1 (IGF-1) and aging: controversies and new insights. Biogerontology. 2003;4:1–8. doi: 10.1023/a:1022448532248. [DOI] [PubMed] [Google Scholar]
- 35.Kenyon C. The plasticity of aging: insights from long-lived mutants. Cell. 2005;120:449–460. doi: 10.1016/j.cell.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 36.Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, Sinclair D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- 37.Valenzano DR, Terzibasi E, Genade T, Cattaneo A, Domenici L, Cellerino A. Resveratrol prolongs lifespan and retards the onset of age-related markers in a short-lived vertebrate. Curr Biol. 2006;16:296–300. doi: 10.1016/j.cub.2005.12.038. [DOI] [PubMed] [Google Scholar]
- 38.Kaeberlein M, Rabinovitch PS. Medicine: grapes versus gluttony. Nature. 2006;444:280–281. doi: 10.1038/nature05308. [DOI] [PubMed] [Google Scholar]
- 39.Chiocchetti A, Zhou J, Zhu H, Karl T, Haubenreisser O, Rinnerthaler M, Heeren G, Oender K, Bauer J, Hintner H, Breitenbach M, Breitenbach-Koller L. Ribosomal proteins Rpl10 and Rps6 are potent regulators of yeast replicative life span. Exp Gerontol. 2006 doi: 10.1016/j.exger.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 40.Hertweck M, Gobel C, Baumeister RC. elegans SGK-1 is the critical component in the Akt/PKB kinase complex to control stress response and life span. Dev Cell. 2004;6:577–588. doi: 10.1016/s1534-5807(04)00095-4. [DOI] [PubMed] [Google Scholar]
- 41.Oh SW, Mukhopadhyay A, Svrzikapa N, Jiang F, Davis RJ, Tissenbaum HA. JNK regulates lifespan in Caenorhabditis elegans by modulating nuclear translocation of forkhead transcription factor/DAF-16. Proc Natl Acad Sci USA. 2005;102:4494–4499. doi: 10.1073/pnas.0500749102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaeberlein M, McVey M, Guarente L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 1999;13:2570–2580. doi: 10.1101/gad.13.19.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rogina B, Helfand SL. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc Natl Acad Sci USA. 2004;101:15998–16003. doi: 10.1073/pnas.0404184101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tissenbaum HA, Guarente L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature. 2001;410:227–230. doi: 10.1038/35065638. [DOI] [PubMed] [Google Scholar]


