Abstract
Prior studies suggest Staphylococcus aureus exotoxins are not produced when the organism is cultured in human blood. Human blood was fractionated into plasma and water-lysed red blood cells and demonstrated that mixtures of α and β globins of hemoglobin (as low as 1 ug/ml) inhibited S. aureus exotoxin production while increasing production of protein A and not affecting bacterial growth. Pepsin but not trypsin digestion destroyed the ability of α and β globin to inhibit exotoxin production. Exotoxin production by both methicillin-resistant and susceptible organisms was inhibited. Production of streptococcal pyrogenic exotoxin A by Streptococcus pyogenes was unaffected by α and β globin chains, but was inhibited when produced in S. aureus. Use of isogenic S. aureus strains suggested the targets of α and β globin chains, leading to inhibition of staphylococcal exotoxins, included the two component system SrrA-SrrB. Delta hemolysin production was also inhibited, suggesting the two component (and quorum sensing) system AgrA-AgrC was targeted. The α and β globin chains represent promising molecules to interfere with the pathogenesis of serious staphylococcal diseases.
Staphylococcus aureus causes large numbers of human diseases, primarily initiated by colonization of mucosal surfaces (1, 2). At any particular time, as many as 40% of humans may be colonized by culturable S. aureus strains on either nasal or vaginal mucosal surfaces (1, 3). The organism may cause relatively benign infections, such as boils, and life threatening infections such as toxic shock syndrome (TSS) (4), scalded skin syndrome (5), necrotizing pneumonia (6–8), and the recently described staphylococcal purpura fulminans (9, 10). Antibiotic resistance in S. aureus strains is an ever increasing problem, with recognition of both community- and hospital-associated methicillin-resistant strains (MRSA) (1, 2, 11). Recently, two methicillin-resistant, vancomycin-resistant strains were isolated in association with human infections (12, 13).
The ability of S. aureus to cause serious human diseases depends on production of both cell-surface and secreted virulence factors by the organism (1, 4). The cell-surface virulence factors, often referred to as microbial surface components recognizing adhesive matrix molecules (MSCRAMMs) (14), allow the organism to attach to host tissues and in some instances evade the host immune system (15). MSCRAMMs include staphylococcal protein A. The secreted virulence factors, including a large array of exotoxins, allow the organism to gain access to nutrients and avoid the host immune system (4, 5, 16). For example, the hemolysin family of exotoxins allows the organism both to resist destruction by leukocytes and at the same time gain access to host tissue nutrients though cell lysis. In addition, the staphylococcal superantigens (17), including toxic shock syndrome toxin-1 (TSST-1) and enterotoxins, divert the host immune system from antibody-based immunity, and allow organisms to cause TSS, purpura fulminans, and related serious illnesses (4).
In the course of our studies of TSS, we observed that staphylococcal exotoxins were not produced when the organism was cultured in human blood and menses, despite the presence of environmental conditions that favor exotoxin production; these include temperatures near 37°C, pH near neutrality, media containing protein, and at least 2% oxygen balanced with 7% CO2 (18, 19) This led to the hypothesis that one or more components of human blood may interfere with their production. The present study was undertaken to characterize the factor(s) in human blood that reduce S. aureus exotoxin production and to evaluate their mechanism of action.
EXPERIMENTAL PROCEDURES (MATERIALS AND METHODS)
Bacterial strains
For the majority of studies, S. aureus strain MN8, a methicillin susceptible (MSSA), clinical isolate from a menstrual TSS patient and representative of 75% of menstrual TSS isolates, was used as the test organism. Strain MN8 secretes TSST-1, hemolysins (including α and δ hemolysins) and glycerol ester hydrolase (lipase). The organism produces cell-surface protein A. S. aureus strains CDC587 (MSSA) and MN128 (MRSA) were used in some studies; both of these strains are clinical isolates that also secrete TSST-1. S. aureus strain RN4220 (pJMY10) contains the chromosomally-encoded two component system SrrA-SrrB that functions as a repressor of exotoxin production under low oxygen conditions (20), and the organism contains the gene for TSST-1 production on a plasmid. Strain MN4010 is an isogenic organism that lacks a functional SrrB gene; the organism contains the gene for TSST-1 on the same plasmid. These latter two organisms were used in studies to assess whether or not two component systems were a target of α and β globin chains. S. aureus MW2 is a clinical isolate from a patient with necrotizing pneumonia; the organism produces the superantigen enterotoxin C (11). Group A streptococcal strain T253cured(T12) produces the superantigen streptococcal pyrogenic exotoxin A (SPE A) (21). S. aureus strain RN4220 (pMIN165) produces SPE A from a plasmid in S. aureus (22). Escherichia coli strain DH5 α was used to evaluate the effect of α and β globin chains on E. coli growth.
Bacterial culture conditions
Multiple culture conditions were used to assess the effects of human blood components on production of S. aureus exotoxins. Initial studies compared the production of exotoxins in S. aureus MN8 cultured in 125 ml Erlenmeyer flasks, containing either 25 ml beef-heart medium (23) (similar in composition to Todd Hewitt broth used in subsequent assays) or 25 ml heparinized (50 units/ml, Baxter, Deerfield, IL) human blood. Culture conditions included inoculation with 107 CFU/ml bacteria, and incubation in ambient air with shaking (200 revolutions per min) at 37°C for up to 7 days. Samples were removed daily for use in plate counts on blood agar for colony forming unit (CFU)/ml determination and TSST-1 assays. Subsequent studies evaluated S. aureus MN8 growth and TSST-1 production after culture in tampon sacs, in the presence and absence of human blood (24). Briefly, commercially available cotton/rayon blend tampons were placed within dialysis tubing (Spectra/Por, 45 mm, 12,000–14,000 molecular weight cut-off, Spectrum Laboratories, Inc, Rancho Dominguez, CA) that had been inoculated with 1×107 S. aureus MN8 CFU/ml in 0.1 ml volumes. Human blood (5 ml) was carefully added to one side of the tampons inside the dialysis tubing, sufficient to cause visible reddening of approximately one-fourth of the tampons. Other areas remained free of blood. The dialysis membranes containing tampons, referred to as tampon sacs, were submerged under Todd Hewitt medium (Becton, Dickinson, and Company, Sparks, MD) containing 0.8% melted agar (Becton, Dickinson, and Company) at 50°C in large test tubes (35 mm internal diameter). The bottom ends of the dialysis tubing were tied off, with the tops remaining open and extending above the agar; this permitted partial extrusion of air during the submersion process. The agar was allowed to solidify by placing the test tubes in cold water (4°C) for 3–5 min, and then the tubes were incubated stationary at 37°C for 18 h in the presence of 7% CO2. Subsequently, the tampon sacs were removed from the test tubes, the sacs were opened with scalpel blades, and various sections of both the dialysis tubing and tampons were evaluated for CFU/ml of S. aureus and concentration of TSST-1 present. In one study, pieces of the tampons were placed in glutaraldehyde fixative with and without 0.1% methylene blue to detect biofilm formation (25) and examined by scanning electron microscopy for growth characteristics. Finally, for assays to assess the ability of α and β globin chains to inhibit exotoxin production, the following protocol was used. Bacterial strains were cultured in the presence and absence of exotoxin-inhibitory fractions in 1:2 diluted (final dilution) of Todd Hewitt broth for various time periods; in most experiments, this was 8 h. The initial bacterial inoculum was 1×105 CFUs/ml, two logs lower than the above experiments to increase assay sensitivity. Culture conditions included incubation in ambient air with shaking (200 RPM) at 37°C. At designated time points, samples were removed for plate counting and exotoxin determination.
Exotoxin measurements
In tampon sac experiments, a quantitative double immunodiffusion assay was employed to measure TSST-1 concentrations (26). We later developed a quantitative Western immunoblot assay (27), which was used in all other experiments for estimation of TSST-1 concentrations in the presence of blood components, including in the presence of antibodies to TSST-1. This assay was verified as acceptable with use of 11 blood samples from women who were positive for antibodies to TSST-1 (average serum antibody titer was 160 by enzyme-linked immunosorbent assay (ELISA) (26) compared to pooled human intravenous immunoglobulin, Immuno AG, Vienna, Austria, with a titer of 320). We spiked each of the 11 blood samples with known concentrations of TSST-1 (ranging from 10 ug/ml to 0.0001 ug/ml), performed Western immunoblotting (compared to control TSST-1 samples), and determined the band density with use of a computer program provided by NIH (ImageJ 1.34S) at http://rsb.info.nih.gov/ij/. Primary antibody against TSST-1 was rabbit hyperimmune antiserum raised against purified TSST-1. Secondary conjugate antibody was goat anti-rabbit IgG conjugated with alkaline phosphatase (Sigma-Aldrich, St. Louis, MO). Results indicated that comparable amounts of TSST-1 were detectable in the spiked human blood samples as in the standard TSST-1 samples in water. The lower limit of TSST-1 detection by this assay varied between 0.01 and 0.001 ug/ml, depending on day of assay. To increase the sensitivity of the assay, samples to be tested for TSST-1 were first treated with 4 volumes of absolute ethanol to precipitate TSST-1, followed by resolubilization in distilled water to 1/10th original volume; we have shown the precipitation/resolubilization procedure allows recovery of nearly 100% of TSST-1 (data not shown). SPE A production was measured comparably. We also tested the reproducibility of the Western assay in triplicate tests of human blood components positive for ability to inhibit TSST-1 production; the assay was highly reproducible.
Hemolysins and lipase were measure by bioassays (28) compared to control staphylococcal a hemolysin and lipase. Briefly, hemolysin concentrations were estimated by ability to lyse rabbit erythrocytes suspended in phosphate buffered saline (0.005 M NaPO4, pH 7.2, 0.15 M NaCl) in 0.85% agarose on microscope slides (taken as a measure of α and δ hemolysins), and lipase concentrations were determined by ability to clear tributyrin suspensions in 0.85% agarose on microscope slides.
Human blood
Human blood was drawn from healthy volunteers with Institutional Review Board (University of Minnesota) approval. Blood was drawn into syringes with heparin (Baxter Healthcare Corporation, Deerfield, IL, 50 units/ml blood).
Purification of human blood substances that inhibit production of staphylococcal exotoxins (inhibitory substances)
First, a definition of one unit of inhibitory activity was established as 75% inhibition of TSST-1 production after 8 h of S. aureus growth in 1:2 Todd Hewitt broth (we used 1:2 diluted Todd Hewitt broth as the standard growth medium because up to 50% volumes of inhibitory substances were added to the cultures), beginning with 2 ml of 105 CFU/ml S. aureus. This allowed determination of specific activity during purification.
The first purification step was to assess whether the inhibitory substance activity was in the plasma fraction or the red blood cell fraction. Plasma was separated from red blood cells by centrifugation from a sample of heparinized (50 units/ml) whole human blood (50 ml). The red blood cells (25 ml packed volume) were washed 3 times with 250 ml room temperature phosphate buffered saline (400 × g, 10 min), then lysed with distilled water, and diluted with water to the original 50 ml volume. The inhibitory substance resided primarily in the red blood cell fraction (see Results section). One unit of activity corresponded to approximately 100 ug of protein (Bio-Rad Protein Assay, Hercules, CA) (specific activity = approximately 10 unit/mg protein). In the red blood cells from 50 ml of blood, there were 5000 mg protein or nearly 50,000 total units of activity.
Subsequently, 5 sets of samples of the red blood cells (each sample from 10 ml of human blood) were subjected to preparative thin-layer isoelectric focusing (23) in pH gradients of 3.5 to 10 (Amersham Pharmacia Biotech AB, Uppsala, Sweden). The fractions with isoelectric points of 7.5 were determined to contain nearly all of the TSST-1 inhibitory activity and were pooled; these fractions were bright red. The pH of individual fractions from isoelectric focusing was determined by scraping fractions from the thin-layer plates, adding 10 ml of distilled water, allowing the matrix gel to settle, and determining the pH with a pH meter.
Finally, samples of the pooled isoelectric point 7.5 isoelectric focusing fractions were subjected to reversed-phase HPLC (rHPLC) (Protein C4 column from Vydac (Hesperia, CA) 0 to 60% gradient of acetonitrile with 0.1% trifluoroacetic acid over a 30 min time period) where 5 sequential eluted fractions containing protein were collected. All five fractions obtained from rHPLC were lyophilized, reconstituted with distilled water, and adjusted to protein concentrations of 5 mg/ml. Each fraction was tested for ability to reduce production of TSST-1 by S. aureus MN8. Fractions purified by this procedure were most often utilized in the present studies since the fractions contained the greatest activity.
The following purification procedure is a modification of the method by Rahbar and Asmerom (29) and was used to provide more complete separation of α and β globin chains, and separation of the chains from heme. The fractions were mixed with an equal volume of 0.1% trifluoroacetic acid in water and then analyzed by rHPLC (Waters 625 LC System with Water 490E Programmable Multiwavelength Detector) on a Vydac 214TP54 (C4, 5 um, 300 Å, 4.6 mm internal diameter × 250 mm length) analytical column. Separation of the hemoglobin chains was carried out with a linear gradient of acetonitrile in 0.1% trifluoroacetic acid; 39 to 40% in 10 min, and then from 40 to 43% of acetonitrile in 60 min with a flow rate of 1 ml/min at ambient temperature. The protein and heme elution was monitored at 225 nm and 410 nm wavelength. Fractions were manually collected, dried under vacuum, and identified using mass spectrometry for protein identification. Briefly, proteins isolated from HPLC were analyzed by either CapLC-MS on an electrospray hybrid quadruple time-of-flight mass spectrometer (Q-TOF, Waters, Milford, MA) or matrix-assisted laser desorption ionization time-of-flight mass spectrometer (MALDI-TOF, Voyager-DE STR, Applied Biosystems, Foster City, CA) for molecular weight determination. Identification of proteins isolated from gel electrophoresis of isoelectric focusing fractions was accomplished by using NanoLC-MS/MS on the CapLC-MS system. Tryptic digests of protein gel bands were first separated with a gradient of 5–80% acetonitrile containing 0.02% trifluoroacetic acid at 0.5 ul/min over 60 min with a capillary column (75 um × 10 cm, C18, New Objective, Woburn, MA). Peptides eluted from the column were detected automatically in data-dependent MS/MS scan mode. Mass spectrometric data were then used to search public NCBI protein database using Mascot software (Matrix Science, London, UK) for protein identification.
Biochemical analysis of rHPLC inhibitory substances
The first two fractions to elute from the initial rHPLC columns reduced production of TSST-1 by S. aureus MN8 relative to the control. Thus, rHPLC fractions 1 and 2 were evaluated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) for homogeneity (30), and N terminal sequencing for identification (Mayo Clinic Microchemical Facility, Rochester, MN).
Fractions 1 and 2 were treated with pepsin (25 ug/10 ul in 0.1M sodium acetate buffer, pH 4.5) for 1 h in attempt to destroy the ability of fractions to inhibit TSST-1 production. After treatment with pepsin, both fractions individually, plus control fractions not treated with pepsin, were incubated with S. aureus MN8 for 8 h in 1:2 diluted Todd Hewitt and then CFUs determined and TSST-1 production assessed by Western immunoblotting. Trypsin digestion was performed comparably except the buffer used was phosphate buffered saline at pH 8.0.
RESULTS
Human blood inhibits production of TSST-1
The tampon sac method (24) was used to evaluate the effect of blood on production of TSST-1 by menstrual TSS S. aureus strain MN8 (31). The tampon was inoculated with S. aureus and along one side with 5 ml human blood, and then after culture sectioned into white pieces (five sections of approximately 0.4 gm each) versus one blood-containing piece (approximately 6 gm); the adjacent regions of the dialysis tubing were also tested separately. S. aureus was evenly distributed throughout the tampon sac, including within the white (range 3.7 to 6.1 × 109 CFU/section) and red (8.5 × 109 CFU/section) tampon sections and along the walls of the dialysis tubing (1.8 × 1010 total CFU). S. aureus grew as a biofilm along the walls of the dialysis tubing and throughout the tampon as demonstrated by staining with methylene blue for exopolysacchaaride (data not shown). In contrast to the wide distribution of S. aureus within all regions of the tampon sac, TSST-1, as measured by quantitative double immunodiffusion, was detected only in regions of the tampon sac that lacked human blood (2.5 ug/section). Incidentally, S. aureus grew as more pronounced biofilms (with more exopolysaccharide visible) along the cellulose acetate dialysis sacs than in the tampons.
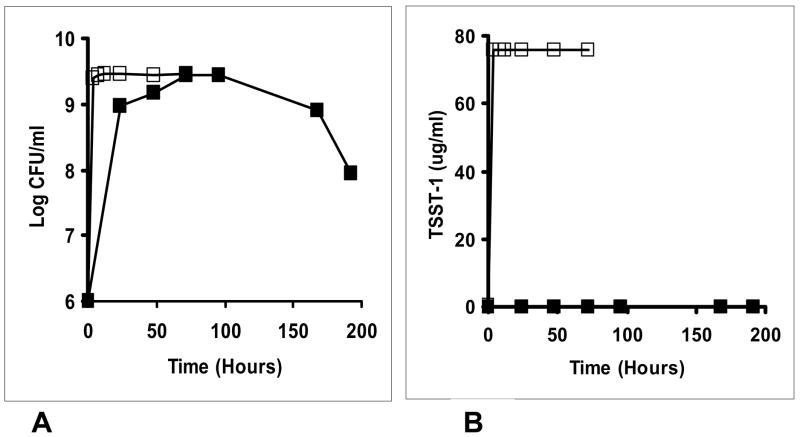
Trivial reasons for the lack of detection of TSST-1 in human blood were the possibilities that human blood would not support the growth of S. aureus or that the organism grew and produced exotoxins but that TSST-1 was being bound by antibodies present in the blood, and not detected by the quantitative double immunodiffusion assay (26). We therefore developed a quantitative Western immunoblot assay for estimation of TSST-1 concentrations that could be used in the presence of antibodies to the toxin (see experimental procedures section). Subsequently, we evaluated S. aureus MN8 growth (initial inoculum size 1 × 107 CFU/ml) and TSST-1 production by Western immunoblot in two menses samples (one from an individual with detectable antibody to TSST-1 [titer = 160 by ELISA] and one from a woman lacking antibodies to TSST-1 [titer <10]) obtained with use of cervical menstrual cups to collect menses. Samples were incubated with shaking in ambient air (200 RPM) for 48 h in a 37°C incubator. S. aureus growth was observed in both menses samples (stationary phase was 2–3 × 109 CFU/ml by 48 h), but no TSST-1 was detected in either sample (data not shown). In contrast, both S. aureus growth (stationary phase 4.3 × 109CFU/ml) and TSST-1 production (76 ug/ml) were obtained after comparable incubation in beef heart medium (23). It should also be noted that we have demonstrated previously that TSST-1 is stable in S. aureus culture media for more than 7 days at 37°C (18). In addition, with the same methods, we evaluated an additional sample of human blood (antibody titer to TSST-1 = 160 by ELISA) over a period of 8 days (Figure 1) for both S. aureus growth (initial inoculum size 1.1 × 107 CFU/ml) and TSST-1 production compared to growth and TSST-1 production in the dialyzable beef heart medium (23) (for 4 days). In this test, only very low concentrations of TSST-1 were detected in the human blood sample, only on days 4 and 7, compared to high production in dialyzable beef heart medium (more than 700 times more than in blood) at all times after 4 h. S. aureus MN8 grew to comparable cell numbers in both media, but was modestly delayed in human blood, thus indicating blood contains sufficient nutrients to support S. aureus growth.
Figure 1.
A. S. aureus MN8 grew well in both a dialyzable beef heart medium (□) and human blood (■). B. production of TSST-1 in human blood (■) was inhibited compared to TSST-1 production in dialyzable beef heart medium (□).
The above findings of reduction of TSST-1 production in human blood were unexpected since it has been assumed previously that TSST-1 production occurs in menses, thus explaining the menstrual association of TSS. However, the data indicate menses and human blood contain one or more factors that prevent TSST-1 production. These data are also consistent with the observation that TSS S. aureus mainly remain localized on mucosal surfaces in causation of menstrual TSS (32, 33), producing TSST-1 in regions without menses.
Purification of the substances from human blood that inhibit TSST-1 production indicates the factors include α and β chains of hemoglobin
Having established that menses and human blood contain one or more substances that inhibit TSST-1 production, while not interfering with growth of TSS S. aureus, we initiated purification of the compound(s) from human blood. The first purification step assessed whether the exotoxin-inhibitory activity was in the plasma fraction or in red blood cells. The inhibitory activity resided primarily in the red blood cell fraction (Figure 2). One unit of activity corresponded to approximately 100 ug of protein (specific activity = approximately 10 unit/mg protein). In the red blood cell fraction from 50 ml of blood, there were 5000 mg protein corresponding to nearly 50,000 total units of activity.
Figure 2.
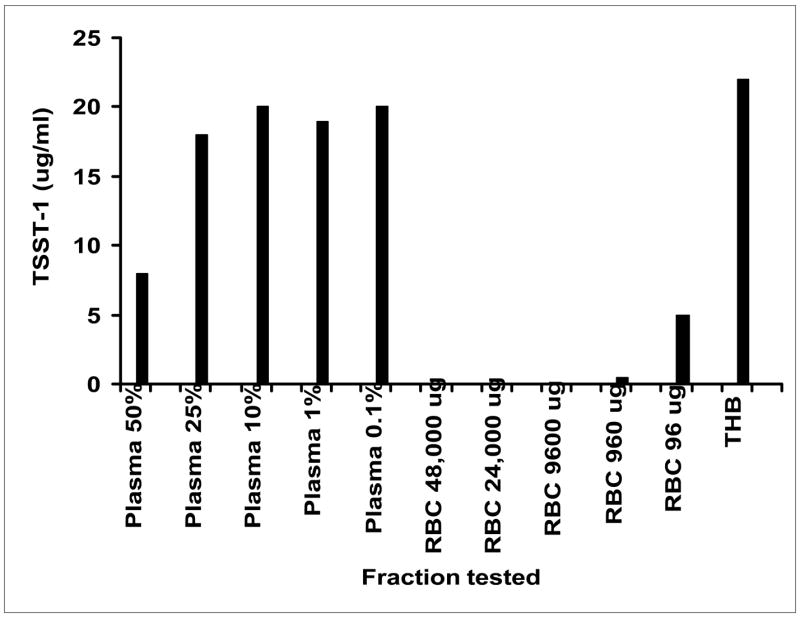
Lysed human blood cells (RBCs) inhibits production of TSST-1 by S. aureus MN8. Fractions (either % plasma or ug RBC protein) were added to Todd Hewitt broth (THB) such that the final Todd Hewitt broth concentration was 1:2 diluted. For those fractions where the added volume was less than 1 ml added, the remainder was made up with phosphate buffered saline. Samples were incubated with shaking at 37°C for 8 h, and then toxin assayed by Western immunoblot.
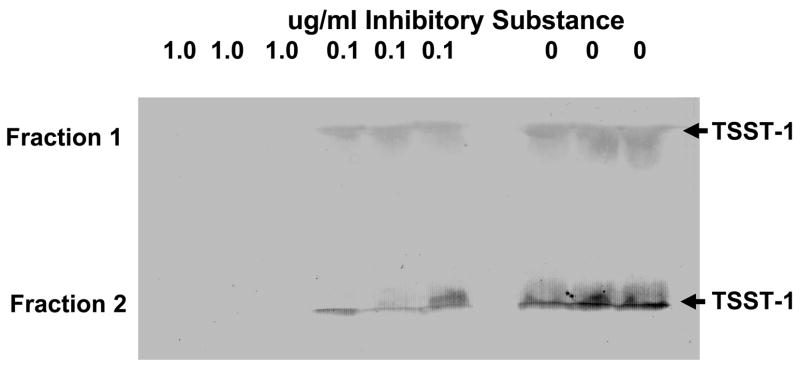
Subsequently, samples of the red blood cells (each sample from 10 ml of human blood) were subjected to preparative thin-layer isoelectric focusing in pH gradients of 3 to 10. The fractions with isoelectric points of 7.5 were determined to contain nearly all of the TSST-1 inhibitory activity (data not shown) and were pooled; these fractions were bright red (Figure 3). Finally, samples of the pooled isoelectric point 7.5 fractions were subjected to rHPLC, where 5 sequentially eluting fractions containing protein were collected. All five fractions obtained from rHPLC were lyophilized, reconstituted with distilled water, and adjusted to protein concentrations of 5 mg/ml. Each fraction was tested for ability to prevent production of TSST-1 by S. aureus MN8. Only fractions 1 and 2 contained the major activity, with fraction 2 being somewhat more active than fraction 1. By N-terminal protein sequencing, fraction 1 contained β globin chains of hemoglobin, and fraction 2 contained α globin chains of hemoglobin. By more extensive rHPLC purification, fraction 1 contained approximately 80% β globin chains and 20% α globin chains. In contrast, fraction 2 contained approximately 85% α globin chains and 15% β globin chains; thus both fractions were mixtures, and both fractions contained heme. One unit of activity in both fractions corresponded to approximately 1 ug protein (Figure 4 shows representative Western immunoblots in triplicate that were used to calculate activity), and there were thus approximately 1000 units/mg of protein (a 100-fold increase in activity per milligram of protein compared to unseparated inhibitory components in the lysed red blood cells). We recovered a combined total of 36 mg protein in the pooled fractions 1 and 2 from the 50 ml blood sample, for a recovery of 36,000 total units of activity (72% recovery of activity compared to total activity present in the red blood cells from 50 ml of blood).
Figure 3.
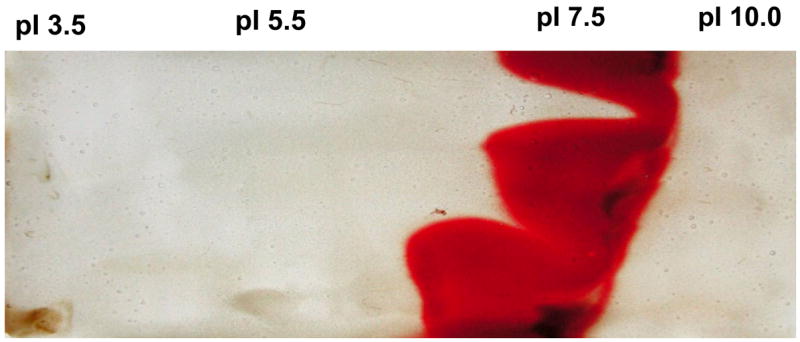
Preparative thin layer isoelectric focusing of human red blood cells (from 10 ml of blood) lysed with distilled water. The red blood cells had been washed 3 times with 50 ml of phosphate buffered saline prior to lysis with water. The fraction with an isoelectric point of 7.5 was determined to inhibit production of TSST-1 by S. aureus MN8.
Figure 4.
Separate Western immunoblot assays demonstrate the ability of Fractions 1 and 2 from rHPLC column to inhibit production of TSST-1 by S. aureus MN8. The isoelectric point 7.5 fractions from preparative thin-layer isoelectric focusing were pooled and then subjected to rHPLC, where 5 protein peaks were collected. Only Fractions 1 and 2 contained activity. Arrows indicate the location of TSST-1. Image J density scans of Fraction 1: 1.0 ug/ml 0; 0.1 ug/ml 96.5±2.4 (standard error); 0 ug/ml 104±3.1. Image J density scans of Fraction 2: 1.0 ug/ml 0; 0.1 ug/ml 109±12.3; 0 ug/ml 150±8.2.
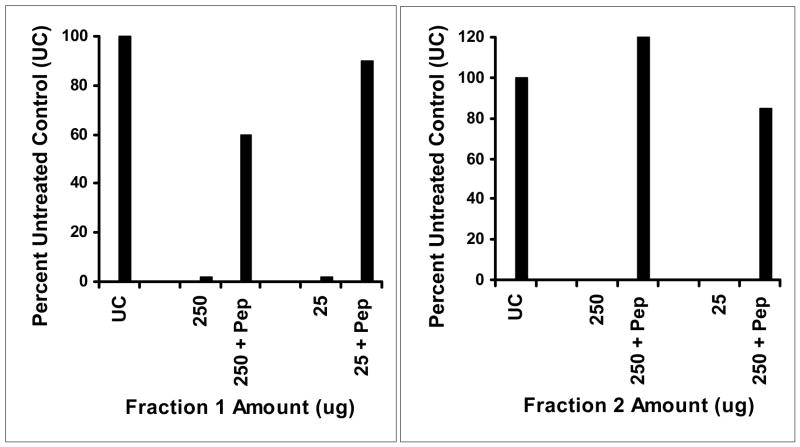
Although the inhibitory substance tracked with protein during purification, it was possible the active fraction was not protein. For example, the fractions were pale pink in color and therefore contained residual heme molecules. Thus, to assess the requirement for protein, rHPLC fractions 1 and 2 were treated with pepsin for 1 h and then tested for activity against S. aureus MN8 (Figure 5). Pepsin treatment of both 250 and 25 ug fractions 1 and 2 eliminated the activity of both fractions, demonstrating the requirement of protein for activity. In addition, the data suggest the heme groups alone present in the fractions were not responsible for the activity. Interestingly, treatment of the fractions with trypsin (data not shown) failed to reduce inhibitory activity but did result in formation of reduced molecular weight proteolysis products.
Figure 5.
Pepsin (Pep) treatment of inhibitory substance Fractions 1 and 2 eliminates their abilities to inhibit TSST-1 production by S. aureus MN8. Fractions 1 and 2 were incubated with 25 ug of pepsin at 37°C for 1 h and then assayed directly for ability to inhibit TSST-1 production.
We also assessed the role of heme by purchasing hemin and evaluating its ability to inhibit TSST-1 production by S. aureus MN8. Hemin, although highly insoluble, lacked any demonstrable activity in inhibition of exotoxin production (data not shown). These data collectively suggest that the inhibitory activity requires α and β globins of hemoglobin, possibly as mixtures, and that heme alone is insufficient for the activity.
The α and β globin chains inhibit production of TSST-1 by other S. aureus strains and inhibit production of other exotoxins
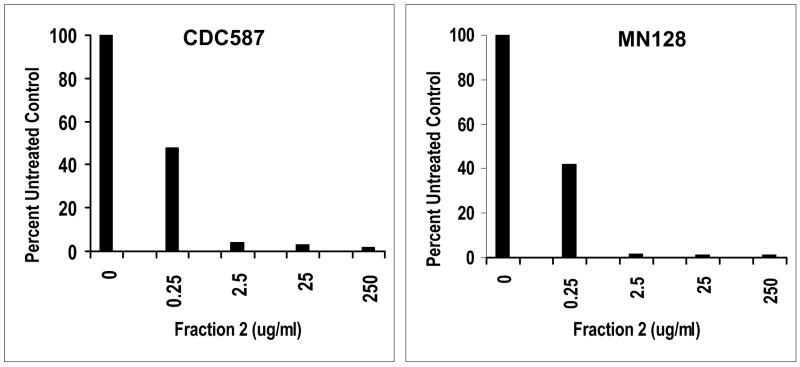
We also tested the hemoglobin chains for activity against two other menstrual TSS isolates to prevent TSST-1 production. S. aureus CDC587 was originally obtained in 1980, is representative of 75% of menstrual TSS isolates, and is MSSA. Strain MN128 was obtained from the Minnesota Department of Health in 2004, likewise represents the major class of menstrual TSS isolates, and is MRSA due to the presence of the mec type II element. Both S. aureus strains tested gave results comparable to those obtained through study of S. aureus MN8 in that approximately 1 ug/ml of fractions 1 and 2 globins was sufficient to inhibit production of TSST-1 by 75% (Figure 6).
Figure 6.
Western immunoblot test demonstrates the ability of the rHPLC Fraction 2 inhibitory substance to inhibit production of TSST-1 by 2 S. aureus strains. Strain CDC587 came originally from the CDC in 1980 and is methicillin sensitive; strain MN128 was kindly provided by the Minnesota Department of Health and is methicillin resistant and was isolated in 2004.
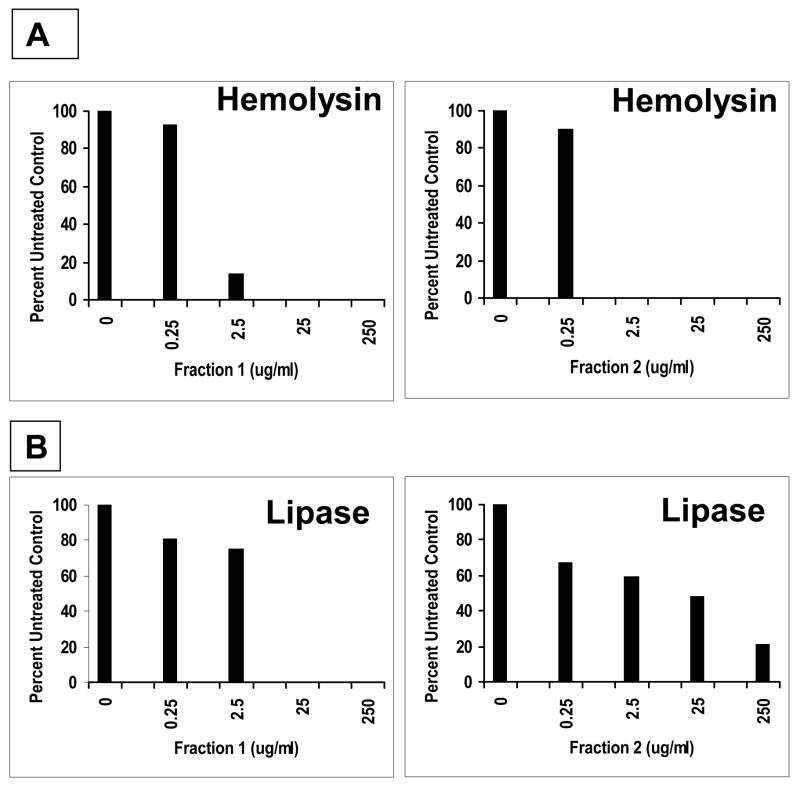
The ability of S. aureus MN8 to produce other virulence factors in the presence of fractions 1 and 2 globin chains (Figure 7) was tested. Production of both hemolysins and lipase were inhibited by the hemoglobin chains (this experiment has been reproduced three times with comparable results). Staphylococcal enterotoxin C production by S. aureus MW2 (methicillin resistant) was reproducibly inhibited by both fractions 1 and 2 (data not shown). However, neither fraction 1 nor 2 inhibited production of SPE A by Streptococcus pyogenes strain T253cured(T12) (data not shown). Interestingly, production of the streptococcal pyrogenic exotoxin A was reproducibly inhibited when produced in S. aureus RN4220 (data not shown). Lastly, we evaluated the fraction 1 and 2 peptides for ability to inhibit the growth of the gram negative organism, E. coli, based on a prior observation that menses hemoglobin peptides were able to inhibit the growth of gram negative bacteria (34, 35). In this representative experiment, growth of the organism was unaffected by concentrations of α and β globin fractions as high as 500 ug/ml (Table 1).
Figure 7.
rHPLC Fractions 1 and 2 inhibit the production of hemolysins (A) and lipase (B) by S. aureus MN8. S. aureus was grown in the presence of the indicated concentration of fractions, the supernates clarified by filtration (0.2 um pore size), and 20 ul of supernates added to wells. The hemolysin slides contained washed rabbit red blood cells in 0.85% agarose made up in phosphate buffered saline; the lipase slides contained tributyrin vortexed for 1 min with 0.85% agarose made up in phosphate buffered saline.
Table 1.
Escherichia coli growth was not affected by Fractions 1 and 2 globins.
| Amount Tested (ug/ml) | Fraction 1 CFU/ml × 109 | Fraction 2 CFU/ml × 109 |
|---|---|---|
| 500 | 8.7 | 8.3 |
| 250 | 9.2 | 10.0 |
| 25 | 8.8 | 8.0 |
| 2.5 | 9.0 | 9.7 |
| 0 | 8.5 | 7.8 |
Final purification of fractions 1 and 2
Both fractions 1 and 2 were subjected to additional purification by rHPLC, until the fractions were homogeneous and free of demonstrable heme. Both fractions retained activity (Figure 8), but the fractions were not quite as active as the mixtures tested above, possibly due to denaturation as a part of the additional purification steps.
Figure 8.
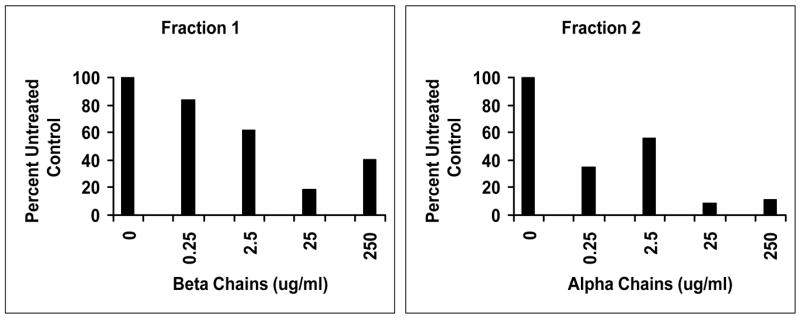
rHPLC final purification of Fractions 1 (β chains) and 2 (α chains) globin retained capacity to inhibit TSST-1 production by S. aureus MN8. Fractions 1 and 2 used to generate figure 6 were subjected to additional rHPLC until the globin chains were homogeneous and lacked detectable heme.
Mechanism of inhibition of exotoxin production
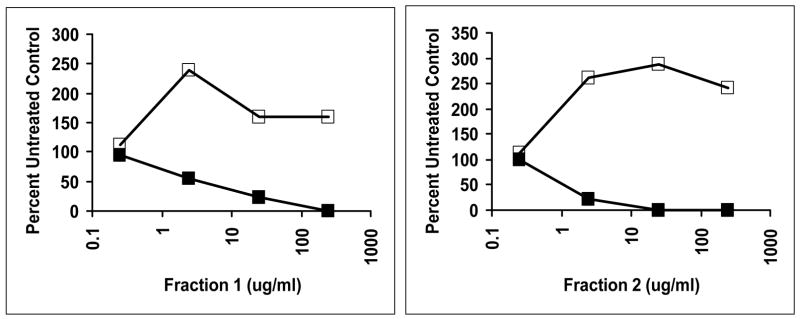
We hypothesized that the action of fractions 1 and 2 was to alter bacterial signal transduction, through the globin chains interacting with membranes or membrane proteins. Studies above in this manuscript showed that both fractions inhibited staphylococcal production of δ hemolysin (as well as α hemolysin) (Figure 7), the translation product of RNA III, which is the effector molecule of the Agr signal transduction system (36). Agr causes up-regulation of genes for exotoxins in post-exponential growth phase, leading to exotoxin production. In addition, Agr simultaneously causes down-regulation of production of the cell surface molecule, protein A. Thus, if fractions 1 and 2 containing α and β globins functioned to inhibit exotoxin production through Agr, it might be expected that production of protein A would be simultaneously increased. This was reproducibly observed (Figure 9).
Figure 9.
Treatment S. aureus MN8 with mixtures of Fractions 1 and 2 globins causes increased production of the cell surface factor protein A, while decreasing amounts of TSST-1. ■ TSST-1; □ Protein A.
The Agr system depends on activation of the AgrA-AgrC two component (and quorum sensing) signal transduction system. The Agr system is regulated by another two component system SrrA-SrrB (20). One effect of α and β globin chains could be inhibition of signal transduction through the two component system SrrA-SrrB. In order to test more rigorously whether or not the fraction 1 and 2 α and β globin chains interfere with two component system signal transduction, we examined the effect of fraction 2 on SrrA-SrrB. Isogenic strains of S. aureus that differed in function of the transmembrane histidine kinase SrrB, were tested for inhibition of TSST-1 production in the presence of fraction 2. The previously reported effect of the knockout strain (for SrrB) is overproduction of TSST-1. This overproduction of TSST-1 was not affected by fraction 2 (Figure 10). In contrast, TSST-1 production by the strain containing wild-type SrrB was inhibited as expected by fraction 2. This experiment was reproduced one additional time.
Figure 10.
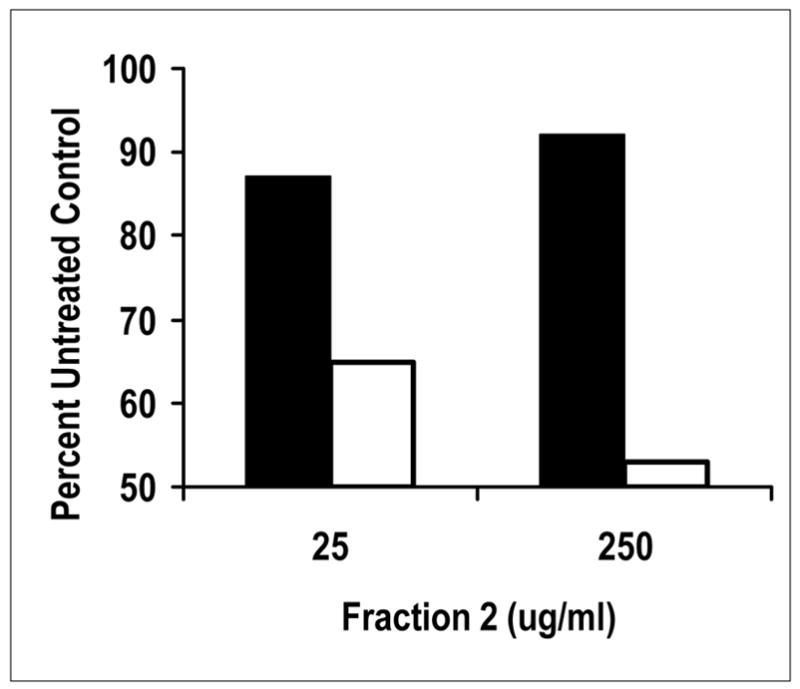
Treatment of isogenic strains of S. aureus with a mixture of Fraction 2 globins indicates the two component system SrrA-SrrB is targeted by the proteins. Data compared production of TSST-1 (production regulated by SrrA-SrrB) by the tested strains. RN4220 (pJMY10) contains the chromosomally-encoded two component system SrrA-SrrB that functions as a repressor of exotoxin production under low oxygen conditions (20), and the organism contains the gene for TSST-1 production on a plasmid (open boxes). Strain MN4010 is an isogenic organism that lacks a functional SrrB gene; the organism contains the gene for TSST-1 on the same plasmid (filled boxes).
DISCUSSION
Staphylococcus aureus causes more infections possibly than nearly any other microorganism. The organism has an array of virulence factors that allow a diverse array of strains to cause disease. Among these virulence factors are large families of secreted exotoxins that facilitate disease production, with many having known requirements in serious diseases. S. aureus infections pose serious treatment problems with the emergence of methicillin-resistant organisms. For many years, it was thought methicillin-resistance was a problem in hospitals, but this has changed such that multiple varieties of methicillin-resistant S. aureus clones have emerged in the community, only to begin spreading into hospitals. In the past the majority of hospital-associated methicillin-resistant strains were negative for production of superantigens capable of causing TSS and related illnesses. With the movement of community-associated methicillin-resistant strains into hospital settings, they carry with them superantigen production (such as staphylococcal enterotoxin C) (11). Very recently, two strains of S. aureus have been described that are resistant to vancomycin—in addition to being methicillin-resistant (13, 37). These occurrences portend the need to develop novel strategies to modulate the ability of S. aureus to cause diseases. Two approaches have been considered by the medical and scientific communities: 1) development of vaccines which have met with very limited success; and 2) development of novel treatment strategies, such as those studied in this report.
Our studies have shown that the α and β globin chains of hemoglobin inhibit production of superantigens (TSST-1 and enterotoxin C) and other exotoxins (α and δ hemolysins and lipase) by several S. aureus strains, while at the same time having minimal effects on staphylococcal growth. Thus, it is possible that these chains or active fragments of the chains may be useful as additives to tampons, wound dressings, or catheters as agents to prevent exotoxin production, with failure of the microbe to initiate disease production. Our studies have provided evidence that heme groups are not required for the activity. Highly purified α and β globin chains retained capacity to inhibit TSST-1 production. Treatment of fractions 1 and 2 with pepsin did not remove heme groups from the mixtures, but the treatment resulted in the complete loss of ability to inhibit TSST-1 production. Finally, hemin added to S. aureus cultures did not result in reduction of exotoxin production. Collectively, these data indicate the agents may be useful as non-pigmented peptides. In preliminary studies with the α globin chain, we have localized the ability to inhibit TSST-1 production to the N-terminal half. We hypothesize that humans may not generate immune responses against α and β globin fractions or peptides fragments, if such fragments can be made and retain inhibitory activity, since they are of human origin; however, future studies are required to test this hypothesis.
There have been prior studies of antimicrobial properties of α and β globin chains of hemoglobin (34, 35). In those studies, it was shown that heme interfered with activity, a property not seen in our studies. In addition, the prior studies observed growth inhibitory activity, particularly against gram negative bacteria such as E. coli. With use of 10-fold high quantities of both hemoglobin chains in the study, we observed no effect on an isolate of E coli. Finally, we observed no inhibition of S. aureus growth at any α or β globin chain concentration tested, also differing from the prior work. A recent study has shown that hemoglobin peptides mediated the antimicrobial activity of the hemoglobin chains, the activity was amplified by defensins and lysozyme, and the peptides were relatively inactive against gram positive bacteria (38)
We initiated studies to clarify the mechanism of action of α and β globin chain inhibition of exotoxin production. δ hemolysin production was inhibited as evidenced by reduction and ultimately complete loss of lysis of rabbit erythrocytes by 8 h cultures of S. aureus MN8. At the same time, production of the cell surface virulence factor protein A was increased. These data are suggestive that the globin chains must have directly or indirectly affected the accessory gene regulator (Agr) global regulatory system (36). δ hemolysin is the translation product of RNA III, the direct activator of exotoxin production in post exponential phase of growth. Simultaneous with inhibition of exotoxin production, a direct or indirect effect on Agr would be expected to increase protein A. Because α and β globin chains have regions of high positive charge, we hypothesize, that like antimicrobial defensin peptides from humans, the hemoglobin chains may have interacted with negatively charged phospholipids in the bacterial membrane or negatively charged amino acids in the histidine kinase components of two components systems. Thus, the globin chains may have interacted directly or indirectly with AgrC, the histidine kinase component of the Agr system to interfere with exotoxin production. A recent study (38) has shown that a β globin peptide (amino acids 115–146) synergizes with defensin peptides and lysozyme to kill Escherichia coli. Interestingly, through an unknown mechanism, the same peptide also enhances the ability of lysozyme to degrade the peptidoglycan of certain Gram positive bacteria.
We have recently identified another highly important staphylococcal two component system SrrA-SrrB (20), an oxygen sensing system that regulates exotoxin production. SrrA-SrrB functions as a repressor of exotoxins under anaerobic conditions, and SrrA-SrrB also regulates the Agr system. Experiments in this study demonstrated that α and β globin chains directly affected SrrB to alter TSST-1 production. In the presence of SrrA-SrrB, TSST-1 production was reduced by the fraction 2 mixture tested, whereas in the absence of a functional SrrA-SrrB two component system, production of TSST-1 was unaffected by the fraction 2 globin chains. It is also interesting that SPE A was not affected by the fraction 1 or 2 mixtures of globin chains when the toxin was produced in the native host Streptococcus pyogenes, but was inhibited when produced in S. aureus. Streptococcus pyogenes is an aerotolerant anaerobe that lacks a SrrA-SrrB homologue. If this two component system is the primary target of the globin chains, then it would be expected that Streptococcus pyogenes would be resistant to the effects.
As a final point of potential importance, it is well recognized that S. aureus initiates nearly all of its infections from mucosal and skin infections, but the organism is also able to cause bloodstream infections. Our studies suggest that development of menstrual TSS is more complicated than originally thought. It has been presumed that TSST-1 is produced by S. aureus strains growing vaginally on menses, leading to development of TSS in women without neutralizing antibodies to the toxin. The studies presented in this manuscript suggest that, whereas TSS S. aureus may grow in menses, TSST-1 production is unlikely to occur. This in turn suggests that TSST-1 production must be occurring in vaginal secretion and tampon regions that lack menses.
It would be expected that S. aureus strains that gain access to the bloodstream would “turn off” exotoxin production due to high exposure to hemoglobin components. However, the organism appears to have adapted a lifestyle in which the organism makes coagulases and quickly is walled off from the host, including blood components, through formation of abscesses. This may allow the organism both a site protected from the human immune system, but at the same time also allow the organism to make exotoxins needed for spread and disease production.
Acknowledgments
Dr. Joanne Bartkus, Minnesota Department of Health is acknowleged for providing S. aureus strain MN128. The late Dr. Stanley Erlandsen is gratefully acknowleged for performing the scanning electron microscopy. Tanuja Chaudhary, Bill Begley, and Jessica Gore are acknowledged for technical assistance.
Footnotes
This work was supported by a research grant from Procter & Gamble, Cincinnati, Ohio and USPHS research grant HL36611 from the National Heart, Lung, and Blood Institute.
Abbreviations: TSS, toxic shock syndrome; MRSA, methicillin resistant Staphylococcus aureus; MSCRAMMS, microbial surface components recognizing adhesive matrix molecules; TSST-1, toxic shock syndrome toxin-1; MSSA, methicillin susceptible Staphylococcus aureus; SPE A, streptococcal pyrogenic exotoxin A; CFU, colony forming units; ELISA, enzyme-linked immunosorbent assay; rHPLC, reversed-phase high pressure liquid chromatography; SDS-PAGE, sodium dodecyl sulfate polyacrylamide gel electrophoresis.
References
- 1.Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998;339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 2.Sheagren JN, Schaberg DR. Gram positive cocci. Staphylococci. In: Gorbach SL, Bartlett JG, Blacklow NR, editors. Infectious Disease. 3. Lippincott Williams and Wilkins; Philadelphia: 2004. pp. 1585–1591. [Google Scholar]
- 3.Kuehnert MJ, Kruszon-Moran D, Hill HA, McQuillan G, McAllister SK, Fosheim G, McDougal LK, Chaitram J, Jensen B, Fridkin SK, Killgore G, Tenover FC. Prevalence of Staphylococcus aureus nasal colonization in the United States, 2001–2002. J Infect Dis. 2006;193:172–179. doi: 10.1086/499632. [DOI] [PubMed] [Google Scholar]
- 4.McCormick JK, Yarwood JM, Schlievert PM. Toxic shock syndrome and bacterial superantigens: an update. Annu Rev Microbiol. 2001;55:77–104. doi: 10.1146/annurev.micro.55.1.77. [DOI] [PubMed] [Google Scholar]
- 5.Ladhani S. Recent developments in staphylococcal scalded skin syndrome. Clin Microbiol Infect. 2001;7:301–307. doi: 10.1046/j.1198-743x.2001.00258.x. [DOI] [PubMed] [Google Scholar]
- 6.From the Centers for Disease Control and Prevention. Four pediatric deaths from community-acquired methicillin-resistant Staphylococcus aureus--Minnesota and North Dakota, 1997–1999. Jama. 1999;282:1123–1125. [PubMed] [Google Scholar]
- 7.Methicillin-resistant Staphylococcus aureus infections in correctional facilities---Georgia, California, and Texas, 2001–2003. MMWR Morb Mortal Wkly Rep. 2003;52:992–996. [PubMed] [Google Scholar]
- 8.Daum RS, Ito T, Hiramatsu K, Hussain F, Mongkolrattanothai K, Jamklang M, Boyle-Vavra S. A novel methicillin-resistance cassette in community-acquired methicillin-resistant Staphylococcus aureus isolates of diverse genetic backgrounds. J Infect Dis. 2002;186:1344–1347. doi: 10.1086/344326. [DOI] [PubMed] [Google Scholar]
- 9.Adem PV, Montgomery CP, Husain AN, Koogler TK, Arangelovich V, Humilier M, Boyle-Vavra S, Daum RS. Staphylococcus aureus sepsis and the Waterhouse-Friderichsen syndrome in children. N Engl J Med. 2005;353:1245–1251. doi: 10.1056/NEJMoa044194. [DOI] [PubMed] [Google Scholar]
- 10.Kravitz G, Dries DJ, Peterson ML, Schlievert PM. Purpura fulminans due to Staphylococcus aureus. Clin Infect Dis. 2005;40:941–947. doi: 10.1086/428573. [DOI] [PubMed] [Google Scholar]
- 11.Fey PD, Said-Salim B, Rupp ME, Hinrichs SH, Boxrud DJ, Davis CC, Kreiswirth BN, Schlievert PM. Comparative molecular analysis of community- or hospital-acquired methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2003;47:196–203. doi: 10.1128/AAC.47.1.196-203.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Summary of Notifiable Diseases, United States, 1998. MMWR Morb Mortal Wkly Rep. 1999;47:ii-92. [PubMed] [Google Scholar]
- 13.Chang S, Sievert DM, Hageman JC, Boulton ML, Tenover FC, Downes FP, Shah S, Rudrik JT, Pupp GR, Brown WJ, Cardo D, Fridkin SK. Infection with vancomycin-resistant Staphylococcus aureus containing the vanA resistance gene. N Engl J Med. 2003;348:1342–1347. doi: 10.1056/NEJMoa025025. [DOI] [PubMed] [Google Scholar]
- 14.Foster TJ, Hook M. Surface protein adhesins of Staphylococcus aureus. Trends Microbiol. 1998;6:484–488. doi: 10.1016/s0966-842x(98)01400-0. [DOI] [PubMed] [Google Scholar]
- 15.Lofdahl S, Guss B, Uhlen M, Philipson L, Lindberg M. Gene for staphylococcal protein A. Proc Natl Acad Sci U S A. 1983;80:697–701. doi: 10.1073/pnas.80.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dinges MM, Orwin PM, Schlievert PM. Exotoxins of Staphylococcus aureus. Clin Microbiol Rev. 2000;13:16–34. doi: 10.1128/cmr.13.1.16-34.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marrack P, Kappler J. The staphylococcal enterotoxins and their relatives. Science. 1990;248:705–711. doi: 10.1126/science.2185544. [DOI] [PubMed] [Google Scholar]
- 18.Schlievert PM, Blomster DA. Production of staphylococcal pyrogenic exotoxin type C: influence of physical and chemical factors. J Infect Dis. 1983;147:236–242. doi: 10.1093/infdis/147.2.236. [DOI] [PubMed] [Google Scholar]
- 19.Yarwood JM, Schlievert PM. Oxygen and carbon dioxide regulation of toxic shock syndrome toxin 1 production by Staphylococcus aureus MN8. J Clin Microbiol. 2000;38:1797–1803. doi: 10.1128/jcm.38.5.1797-1803.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yarwood JM, McCormick JK, Schlievert PM. Identification of a novel two-component regulatory system that acts in global regulation of virulence factors of Staphylococcus aureus. J Bacteriol. 2001;183:1113–1123. doi: 10.1128/JB.183.4.1113-1123.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson LP, Schlievert PM. Group A streptococcal phage T12 carries the structural gene for pyrogenic exotoxin type A. Mol Gen Genet. 1984;194:52–56. doi: 10.1007/BF00383496. [DOI] [PubMed] [Google Scholar]
- 22.Roggiani M, Stoehr JA, Leonard BA, Schlievert PM. Analysis of toxicity of streptococcal pyrogenic exotoxin A mutants. Infect Immun. 1997;65:2868–2875. doi: 10.1128/iai.65.7.2868-2875.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blomster-Hautamaa DA, Schlievert PM. Preparation of toxic shock syndrome toxin-1. Methods Enzymol. 1988;165:37–43. doi: 10.1016/s0076-6879(88)65009-9. [DOI] [PubMed] [Google Scholar]
- 24.Schlievert PM. Effect of Merocel vaginal sponge on growth of Staphylococcus aureus and production of toxic shock syndrome-associated toxins. J Am Coll Surg. 1996;183:19–24. [PubMed] [Google Scholar]
- 25.Vuong C, Kocianova S, Voyich JM, Yao Y, Fischer ER, DeLeo FR, Otto M. A crucial role for exopolysaccharide modification in bacterial biofilm formation, immune evasion, and virulence. J Biol Chem. 2004;279:54881–54886. doi: 10.1074/jbc.M411374200. [DOI] [PubMed] [Google Scholar]
- 26.Schlievert PM. Immunochemical assays for toxic shock syndrome toxin-1. Methods Enzymol. 1988;165:339–344. doi: 10.1016/s0076-6879(88)65050-6. [DOI] [PubMed] [Google Scholar]
- 27.Blake MS, Johnston KH, Russell-Jones GJ, Gotschlich EC. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984;136:175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- 28.Schlievert PM, Osterholm MT, Kelly JA, Nishimura RD. Toxin and enzyme characterization of Staphylococcus aureus isolates from patients with and without toxic shock syndrome. Ann Intern Med. 1982;96:937–940. doi: 10.7326/0003-4819-96-6-937. [DOI] [PubMed] [Google Scholar]
- 29.Rahbar S, Asmerom Y. Rapid HPLC techniques for globin chain synthesis studies. Hemoglobin. 1989;13:475–487. doi: 10.3109/03630268908998086. [DOI] [PubMed] [Google Scholar]
- 30.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 31.Schlievert PM, Kelly JA. Clindamycin-induced suppression of toxic-shock syndrome--associated exotoxin production. J Infect Dis. 1984;149:471. doi: 10.1093/infdis/149.3.471. [DOI] [PubMed] [Google Scholar]
- 32.Davis JP, Chesney PJ, Wand PJ, LaVenture M. Toxic-shock syndrome: epidemiologic features, recurrence, risk factors, and prevention. N Engl J Med. 1980;303:1429–1435. doi: 10.1056/NEJM198012183032501. [DOI] [PubMed] [Google Scholar]
- 33.Shands KN, Schmid GP, Dan BB, Blum D, Guidotti RJ, Hargrett NT, Anderson RL, Hill DL, Broome CV, Band JD, Fraser DW. Toxic-shock syndrome in menstruating women: association with tampon use and Staphylococcus aureus and clinical features in 52 cases. N Engl J Med. 1980;303:1436–1442. doi: 10.1056/NEJM198012183032502. [DOI] [PubMed] [Google Scholar]
- 34.Lipeke C, Baxmann S, Heine C, Breithaupt N, Standker L, Forsmann WG. Human hemoglobin-derived peptides exhibit antimicrobial activity: a class of host defense peptides. J Chromatog b. 2003;791:334–356. doi: 10.1016/s1570-0232(03)00245-9. [DOI] [PubMed] [Google Scholar]
- 35.Mak P, Wojcik K, Wicherek L, Suder P, Dubin A. Antibacterial hemoglobin peptides in human menstrual blood. Peptides. 2004;25:1839–1847. doi: 10.1016/j.peptides.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 36.Novick RP. Autoinduction and signal transduction in the regulation of staphylococcal virulence. Mol Microbiol. 2003;48:1429–1449. doi: 10.1046/j.1365-2958.2003.03526.x. [DOI] [PubMed] [Google Scholar]
- 37.Vancomycin-resistant Staphylococcus aureus--Pennsylvania, 2002. MMWR Morb Mortal Wkly Rep. 2002;51:902. [PubMed] [Google Scholar]
- 38.Mak P, Siwek M, Pohl J, Dubin A. Menstrual hemocidin HbB115-146 is an acidophilic antibacterial peptide potentiating the activity of human defensins, cathelicidin and lysozyme. Am J Reprod Immunol. 2007;57:81–91. doi: 10.1111/j.1600-0897.2006.00456.x. [DOI] [PubMed] [Google Scholar]