Abstract
Motivationally significant agendas guide perception, thought and behaviour, helping one to define a ‘self’ and to regulate interactions with the environment. To investigate neural correlates of thinking about such agendas, we asked participants to think about their hopes and aspirations (promotion focus) or their duties and obligations (prevention focus) during functional magnetic resonance imaging and compared these self-reflection conditions with a distraction condition in which participants thought about non-self-relevant items. Self-reflection resulted in greater activity than distraction in dorsomedial frontal/anterior cingulate cortex and posterior cingulate cortex/precuneus, consistent with previous findings of activity in these areas during self-relevant thought. For additional medial areas, we report new evidence of a double dissociation of function between medial prefrontal/anterior cingulate cortex, which showed relatively greater activity to thinking about hopes and aspirations, and posterior cingulate cortex/precuneus, which showed relatively greater activity to thinking about duties and obligations. One possibility is that activity in medial prefrontal cortex is associated with instrumental or agentic self-reflection, whereas posterior medial cortex is associated with experiential self-reflection. Another, not necessarily mutually exclusive, possibility is that medial prefrontal cortex is associated with a more inward-directed focus, while posterior cingulate is associated with a more outward-directed, social or contextual focus.
Keywords: self-reflection, medial frontal cortex, posterior cingulate cortex, precuneus, regulatory focus, rumination
There has been considerable interest recently in the neural correlates of the ‘self ’, and results across a number of studies indicate that both anterior medial cortex (medial frontal gyrus and/or anterior cingulate cortex) and posterior medial cortex (posterior cingulate cortex and/or precuneus) are involved in self-referential processing (e.g. Ochsner et al., 2005; Vogt and Laureys, 2005). For example, Johnson et al. (2002) reported greater activity in these areas when participants answered yes/no questions about whether traits, attitudes and abilities were characteristic of them (e.g. I often forget things) than when they answered general semantic knowledge questions (e.g. You need water to live). Fossati et al. (2003) reported medial prefrontal and posterior cingulate activity associated with evaluating positive and negative traits for self-relevance vs for social desirability. Macrae et al. (2004) showed that activity in medial prefrontal cortex during evaluation of the self-relevance of traits was greater for those traits judged to be self-relevant than for those judged not to be self-relevant and was greater for those subsequently recognized compared with those subsequently forgotten. Collectively, these and other findings (e.g. Craik et al., 1999) suggest that anterior and posterior medial cortical areas are recruited during self-reflective thought. Furthermore, these areas often appear to ‘deactivate’ during cognitive tasks, which has led to the suggestion that self-reflective thought may be a common ‘default mode’ when individuals are not otherwise engaged (e.g. Gusnard et al., 2001). Activity in medial prefrontal cortex is also observed in tasks in which participants make judgments about others, especially close ‘others’ (Ochsner et al., 2005), or people they see as similar to themselves (Mitchell et al., 2005); these findings are consistent with the idea that people draw on self-reflection in understanding others (e.g. Frith and Frith, 2001; Mitchell et al., 2005).
In spite of this growing evidence that both anterior and posterior medial areas are involved in self-referential thought (see Macrae et al., 2004; Ochsner et al., 2005 for reviews of findings focusing on medial frontal areas, and Vogt and Laureys, 2005; Cavanna and Trimble, 2006, for reviews focusing on medial posterior areas), the relative roles and specific functions of anterior and posterior medial regions during self-reflection remain to be clarified. One approach to clarifying functions of these brain regions is to contrast judgments of the self with the same judgments about others (Ochsner et al., 2004, 2005). Another approach is to contrast different types of judgments about the same targets (e.g. mentalistic vs perceptual, Mitchell et al., 2005). A third approach, which we have taken here, is to contrast different types of self-relevant thoughts, in this case, about one's agendas (i.e. goals, e.g. Johnson and Reeder, 1997).
Motivationally significant agendas guide our perception, thought and behaviour, helping to define a ‘self’ and to regulate our interactions with others and the general environment. As discussed above, studies investigating neural correlates of self-relevant processing often ask participants for judgments about the self-relevance of traits. However, in characterizing a self, an individual's agendas (e.g. to have a family, become an actor, keep healthy, pay the bills) are perhaps as equally important as their traits and are a frequent target of self-reflection in everyday life. Furthermore, just as there are a multitude of traits that can describe a self, there are a multitude of potential agendas, from the immediate (e.g. finding a restaurant) to the longer term [e.g. making progress on a life task (Cantor and Kihlstrom, 1987) or achieving a possible future self (Markus and Nurius, 1986)].
Nevertheless, at a relatively abstract level of categorization, agendas may be divided into two broad classes based on the two self-regulatory, motivational systems proposed by Higgins (1997, 1998): promotion and prevention. A promotion orientation is associated with seeking advancement and accomplishment, whereas a prevention orientation is associated with concerns of safety and responsibility. This distinction is based on the idea that while individuals generally approach pleasure and avoid pain, the nature and relative importance of seeking pleasure and avoiding pain differs across individuals and within individuals across situations (see also Carver and White, 1994).
The idea of variation in individuals’ regulatory focus highlights the difference between agendas and traits; two people could both be described by the trait ‘planful’, but planful about what? A person with a predominantly promotion focus would be more likely to be planful about attaining positive rewards or outcomes, while a person with a predominantly prevention focus would be more likely to be planful about avoiding negative events or outcomes. Although a promotion or prevention focus may dominate, the aspects of the self that are active change dynamically across situations (e.g. Markus and Wurf, 1987), thus most individuals have both promotion and prevention agendas. For example, the same person can hold both the hope of becoming rich (a promotion agenda) and the duty to support an aging parent (a prevention agenda), or the aspiration to be a good citizen and the obligation to be a well-informed voter. As individuals, hopes and aspirations and duties and obligations make up a large part of our mental life and constitute the motivational scaffolding for much of our behaviour.
Higgins and colleagues have reported a number of findings from behavioural studies consistent with their self-regulatory framework (e.g. Higgins et al., 1997; Shah and Higgins, 2001; Freitas and Higgins, 2002). For example, Freitas and Higgins (2002) found that individuals rated a search task as more enjoyable when the goal was framed to be consistent with their orientation (e.g. finding helpful elements for promotion-focused individuals and finding harmful elements for prevention-focused individuals). Rating the emotionality of words was faster when ratings were along orientation-consistent emotional dimensions (Shah and Higgins, 2001).
A recent neuroimaging study provides converging evidence that self-regulatory focus influences attentional processes and affective responses. Cunningham et al. (2005) found that during an evaluative task in which participants made judgments about the valence of concepts (e.g. recycling, terrorism, love, affirmative action), activity in the amygdala, anterior cingulate cortex and extra-striate cortex was correlated with the participants’ scores on a later promotion/prevention questionnaire. Specifically, a promotion focus was associated with sensitivity to positive stimuli in these regions and a prevention focus was associated with sensitivity to negative stimuli in these same regions, suggesting that regulatory focus may tune a single neural system to detect motivationally significant stimuli. Although the Cunningham et al. (2005) study provides support for the idea that self-regulatory orientation modulates processing of externally presented stimuli, to our knowledge, the neural activity associated with self-reflection in the context of a promotion focus vs a prevention focus has not been investigated.
The present studies investigated neural activity when participants were asked to think about self-relevant agendas related to either a promotion (think about your hopes and aspirations) or prevention (think about your duties and obligations) focus. We compared neural activity associated with thinking about these two different types of self-relevant agendas and with thinking about non-self-relevant topics (distraction). We expected greater activity in anterior and/or posterior medial regions associated with these two self-reflection conditions compared with the distraction control condition because thinking about one's agendas, like thinking about one's traits, is self-referential. Such a finding would also be consistent, for example, with Luu and Tucker's (2004) proposal that both anterior cingulate and posterior cingulate cortex contribute to action regulation by representing goals and expectancies.
Clinical findings also suggest that there should be differences in neural activity associated with self-reflection vs distraction. Behavioural data show that the distraction manipulation used here provides a short-term reduction of negative self-relevant thought in people prone to depression, with a concomitant reduction in depressed mood (Nolen-Hoeksema, 2004). If distraction reduces self-focused rumination, we might expect to find less activity in brain areas associated with self-focus in distraction compared with self-reflection conditions.
In Experiment 1, self-reflection was induced prior to scanning using a between subjects essay task that focused some participants on their hopes and aspirations (promotion focus) and others on their duties and obligations (prevention focus) (Freitas and Higgins, 2002). These promotion and prevention inductions, like the rumination inductions used in behavioural studies of depression (e.g. Nolen-Hoeksema, 2004), encouraged attention to the self but were more goal-directed than those typically used in rumination studies (e.g. where participants might be asked to think about how they are feeling). During scanning, short periods of self-reflection about the pre-scan essay agenda were intermixed with distraction. The distraction condition used items (e.g. think about polar bears fishing) from the standard distraction condition in previous behavioural rumination studies (Nolen-Hoeksema, 2004). Experiment 2 was a completely within-subjects design in which there was no pre-scan essay induction phase. Rather, focus was induced ‘online’ with randomly intermixed promotion, prevention and distraction prompts. Experiment 2 thus provided information about whether the same regions were identified in promotion and prevention self-focus when participants were asked to flexibly consider both promotion and prevention agendas on different trials as when a single type of self-focus was repeatedly engaged over an extended period, as in Experiment 1. In both experiments, marked differences in patterns of neural activity in anterior and posterior medial cortex during self-reflection compared with distraction were observed, as well as a double dissociation between anterior and posterior medial regions in the relative activity levels during promotion and prevention focused self-reflection.
RESULTS
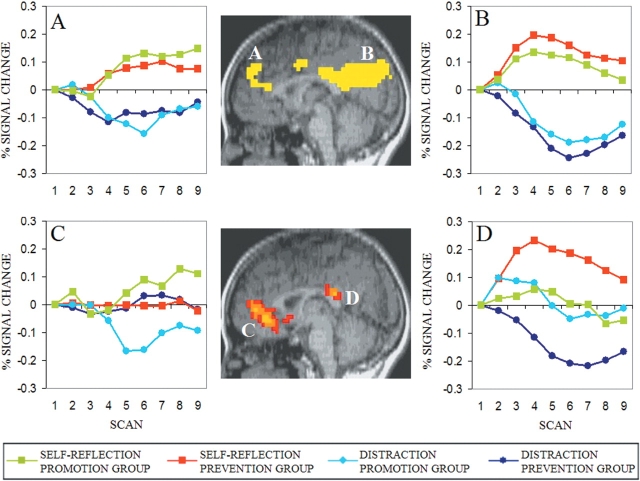
In Experiment 1, there was an area of dorsomedial PFC (Figure 1A) and an area of precuneus/posterior cingulate cortex (Figure 1B) that each showed greater activity in the self-reflection conditions than the distraction condition, and no difference between the two self-reflection conditions. In contrast, in other medial areas, a dissociation was found between self-reflection conditions: an area of anterior cingulate, medial frontal cortex (Figure 1C) showed greater activity during self-reflection than during distraction in the promotion focus group but not the prevention focus group, and an area of posterior cingulate (Figure 1D) and an area of precuneus (Table 1) showed greater activity during self-reflection than distraction in the prevention focus group but not the promotion focus group.
Fig. 1.
Experiment 1. (A) Area of medial frontal gyrus extending into anterior cingulate gyrus (0, 45, 32) identified as showing a condition × time interaction (P < 0.00001) with greater activation in self-reflection than distraction conditions. (B) Area of precuneus/posterior cingulate gyrus (−8, −66, 28) identified as showing a condition × time interaction (P < 0.00001) with greater activation in self-reflection than distraction conditions. (C) Area of anterior cingulate gyrus and medial frontal gyrus (1, 45, 4) identified as showing a focus × condition × time interaction (P < 0.01); subsequent comparisons showed self-reflection > distraction within promotion (P < 0.004) and self-reflection = distraction within prevention. (D) Area of posterior cingulate gyrus (−1, −34, 20) identified as showing a focus × condition × time interaction (P < 0.01); subsequent comparisons showed self-reflection > distraction within prevention (P < 0.001) and self-reflection = distraction within promotion.
Table 1.
Brain areas identified in Experiment 1
| Anatomical area | H | BA | x | y | z | max F |
|---|---|---|---|---|---|---|
| Promotion > distraction; prevention = distraction | ||||||
| 1CAnterior cingulate, medial frontal gyrus | M | 32, 10 (24, 25/11) | 1 | 45 | 4 | 4.71 |
| Middle temporal gyrus (superior occipital gyrus) | L | 39, 19 | −42 | −74 | 24 | 4.71 |
| Promotion = distraction; prevention > distraction | ||||||
| 1DPosterior cingulate | M | 23 (29, 30) | −1 | −34 | 20 | 4.46 |
| Precuneus | L | 31, 7 (19) | −20 | −70 | 28 | 3.65 |
| Middle (inferior) occipital gyrus | L | 19 (18) | −42 | −82 | 4 | 4.33 |
| Promotion < distraction; prevention = distraction | ||||||
| Fusiform gyrus | R | 37 | 34 | −66 | −19 | 4.08 |
| Fusiform gyrus (lingual gyrus) | L | 37, 19 | −27 | −46 | −15 | 3.56 |
| Promotion = distraction; prevention < distraction | ||||||
| Middle frontal gyrus | L | 10/46 | −42 | 45 | 8 | 4.34 |
| Inferior frontal gyrus | R | 45/46 | 49 | 24 | 20 | 2.94 |
| Self-reflection > distraction | ||||||
| 1ADorsomedial frontal gyrus (anterior cingulate) | M | 9 (8/10, 24, 32) | 0 | 45 | 32 | 8.11 |
| 1BPrecuneus/posterior cingulate, cuneus | M | 31 (7/23/18) | −8 | −66 | 28 | 34.74 |
| Superior temporal gyrus | L | 38 | −35 | 24 | −27 | 6.88 |
| Middle/inferior temporal gyrus | L | 21/20 | −54 | −8 | −23 | 7.14 |
| Middle temporal gyrus | L | 21 | −50 | −30 | −4 | 7.91 |
| Middle frontal gyrus | L | 10, 46 | −27 | 49 | 16 | 8.10 |
| Middle temporal gyrus (angular gyrus, superior temporal gyrus) | L | 39(22) | −46 | −66 | 32 | 15.73 |
| Self-reflection < distraction | ||||||
| Temporal pole | L | 20, 36, 38, 28 | −31 | −8 | −34 | 7.79 |
| Fusiform gyrus/parahippocampal gyrus, middle temporal gyrus | R | 36, 37, 21 | 49 | −50 | −8 | 17.34 |
| Fusiform gyrus, middle temporal gyrus, inferior temporal gyrus, middle occipital gyrus | L | 37, 19, 21 (20, 36) | −50 | −58 | −11 | 31.11 |
| Amygdala, parahippocampal gyrus, lentiform nucleus, superior temporal gyrus | L | amyg, 35, 38 | −31 | −1 | −11 | 7.30 |
| Precentral gyrus, inferior frontal gyrus (middle frontal gyrus/superior temporal gyrus) | R | 6, 44 (9, 38/42) | 45 | 2 | 36 | 16.18 |
| Middle (inferior) frontal gyrus | R | 46 (9, 10) | 45 | 35 | 12 | 10.53 |
| Middle (inferior) frontal gyrus | L | 46 (9, 45, 8) | −42 | 35 | 16 | 10.56 |
| Precentral gyrus (inferior frontal gyrus) | L | 6 (44) | −46 | 2 | 24 | 18.53 |
| Inferior parietal lobule (supramarginal gyrus, postcentral gyrus) | L | 40 (2) | −54 | −38 | 40 | 15.61 |
| Inferior parietal lobule (supramarginal gyrus, postcentral gyrus) | R | 40 (2) | 53 | −34 | 44 | 13.17 |
| Cingulate gyrus | M | 24 | −4 | 2 | 36 | 8.19 |
| Intraparietal sulcus | L | 7 | −27 | −70 | 40 | 6.34 |
Superscripts in column 1 indicate corresponding figure. Areas showed a focus × condition × time interaction with a minimum of six contiguous voxels each significant at P < 0.01, or a condition × time interaction with a minimum of six contiguous voxels each significant at P < 0.00001 (Forman et al., 1995). For identified areas, contrasts between conditions were performed on percent signal change at times 5, 6, 7 from time 1, P < 0.05. H, hemisphere; L, left; M, medial; R, right; BA, Brodmann area. For each area of activation, the major anatomical regions and BA numbers are listed in descending order of approximate size, with areas of approximately equal size indicated by a slash (parentheses indicate a small extent relative to other areas listed). Talairach coordinates (x, y, z) are shown for the voxel with the maximum F-value (BA area in bold) in each area of activation. In keeping with SCAN-recommended nomenclature, z > 27 (dorsomedial PFC), −10 < z < 27 (medial PFC), and z < −10 (ventromedial PFC).
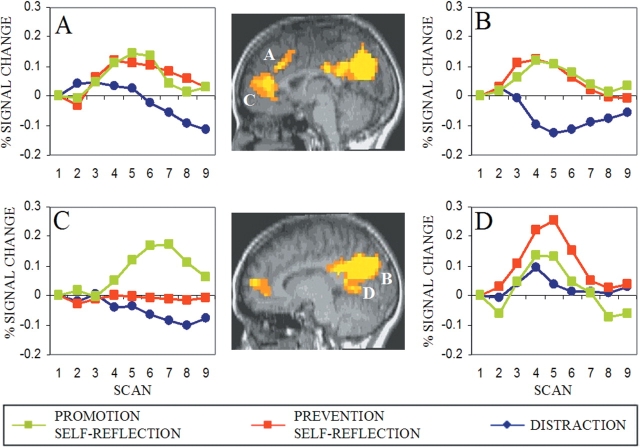
Figure 2 shows data from medial cortical regions identified in Experiment 2. Again, an area of anterior cingulate/dorsomedial PFC (Figure 2A) and an area of posterior cingulate, precuneus (Figure 2B) showed greater activity during self-reflection than distraction, and no difference between promotion and prevention conditions. In contrast, in other medial areas, we found greater activity for the promotion than prevention condition (anterior cingulate, Figure 2C) and greater activity for the prevention than promotion condition (lingual gyrus, posterior cingulate, Figure 2D). The local maximum of the medial PFC region showing the largest promotion > prevention difference in Experiment 2 (Talairach coordinates: x = 2, y = 39, z = −1) was within two voxels of the local maximum in Experiment 1 (1, 45, 4). The local maximum of the posterior medial region showing the largest prevention > promotion difference in Experiment 2 (−10, −53, 2) was more inferior and posterior than the local maximum found in Experiment 1 (−1, −34, 20). Both regions (Figures 1D and 2D) were, however, anterior to those showing equal activation in promotion and prevention conditions (Figures 1B and 2B).1
Fig. 2.
Experiment 2. (A, C) Areas of anterior cingulate and medial frontal gyrus identified as showing a condition × time interaction (P < 0.001) in which the dorsal area (A: −2, 24, 31) showed promotion = prevention > distraction and the medial area (C: 2, 39, −1) showed promotion > prevention > distraction (P < 0.04). (B, D) Areas including precuneus, posterior cingulate/cuneus identified in condition × time interaction (P < 0.001) in which the more superior/posterior area (B: −6, −68, 23) showed prevention = promotion > distraction (P < 0.001) and a more inferior/anterior area (D: −10, −53, 2) showed prevention > promotion = distraction (P < 0.03).
A double dissociation was found when participants were cued to think about promotion and prevention agendas on different trials for the first time during scanning (Experiment 2) and when they spent several minutes thinking about either promotion or prevention agendas before scanning (Experiment 1), indicating that it results from what participants are thinking about during the scan and not from some general effect (e.g. mood) carried over from the pre-scan period of self-reflection. However, the fact that the anterior medial region showing greater activity when participants thought about hopes and aspirations was more similar across experiments than the posterior medial region showing greater activity when participants thought about duties and obligations suggests that perhaps posterior medial cortex is more sensitive to a within vs between subjects manipulation of self-reflection than is anterior medial cortex.
We also obtained participants’ prevention and promotion scores on a brief questionnaire assessing more chronic promotion and prevention orientation (Cunningham et al., 2004). For each participant for each region in Figure 1 or Figure 2, we found the maximum percent change in each trial and averaged across trials. These mean peak percent changes were correlated with the promotion and prevention orientation scores. In Experiment 1, promotion orientation scores were positively correlated with activity in medial PFC (area shown in Figure 1C) during promotion trials, r = 0.69, P < 0.04, suggesting that activity in this area may reflect a combination of state and trait factors. No other correlations approached significance.
Although anterior and posterior medial regions are of primary interest here, other regions identified in the whole brain analyses of Experiments 1 and 2 are shown in Tables 1 and 2, respectively.
Table 2.
Brain areas identified in Experiment 2
| Anatomical area | H | BA | x | y | z | max F |
|---|---|---|---|---|---|---|
| Promotion > prevention = distraction | ||||||
| 2CAnterior cingulate | M | 32 (24) | 2 | 39 | −1 | 4.11 |
| Prevention > promotion = distraction | ||||||
| 2DLingual gyrus (posterior cingulate, parahippocampus) | M | 19, 18/30, 29 | −10 | −53 | 2 | 3.78 |
| Self-reflection > distraction | ||||||
| 2AAnterior cingulate/dorsomedial frontal gyrus | M | 32 (6, 10) | −2 | 24 | 31 | 3.98 |
| 2BPosterior cingulate, cuneus, precuneus | M | 31/18, 23 (30, 29) | −6 | −68 | 23 | 13.24 |
| Middle, superior temporal gyrus | L | 39, 22 | −46 | −58 | 18 | 3.63 |
| Self-reflection < distraction | ||||||
| Fusiform gyrus, inferior temporal gyrus, middle occipital gyrus, middle temporal gyrus | L | 37, 19 | −49 | −51 | −13 | 10.37 |
| Amygdala (hippocampus) | L | amyg, 34 | −24 | −4 | −16 | 3.32 |
| Fusiform gyrus, middle temporal gyrus, inferior temporal gyrus | R | 37, 21 | 55 | −47 | −8 | 4.51 |
| Inferior frontal gyrus | L | 45 | −45 | 26 | 14 | 3.28 |
| Middle temporal gyrus (middle occipital gyrus) | L | 39 (19) | −33 | −69 | 10 | 3.09 |
| Inferior parietal lobule (supramarginal gyrus) | L | 40 | −58 | −43 | 43 | 4.62 |
| Inferior frontal gyrus (precentral gyrus) | R | 44 (6) | 45 | 6 | 27 | 4.26 |
| Precentral gyrus | L | 6 | −44 | −4 | 26 | 3.30 |
| Precentral gyrus/posterior central gyrus | R | 4, 3 | 35 | −22 | 43 | 4.02 |
Superscripts in the first column indicate corresponding figure. Areas showed a condition × time interaction with a minimum of six contiguous voxels each significant at P < 0.001 (Forman et al., 1995). For identified areas, contrasts between conditions were performed on percent signal change at times 5, 6, 7 from time 1, P < 0.05. H, hemisphere; L, left; M, medial; R, right; BA, Brodmann area. For each area of activation, the major anatomical regions and BA numbers are listed in descending order of approximate size, with areas of approximately equal size indicated by a slash (parentheses indicate a small extent relative to other areas listed). Talairach coordinates (x, y, z) are shown for the voxel with the maximum F-value (BA area in bold) in each area of activation. In keeping with SCAN-recommended nomenclature, z > 27 (dorsomedial PFC), −10 < z < 27 (medial PFC), and z < −10 (ventromedial PFC).
Pre-scan essays (Experiment 1)
The pre-scan essays of the promotion and prevention groups were coded for content. They did not differ in the total number of codes assigned (Ms = 112.67 and 120.80, for promotion and prevention, respectively, t [17] = −0.48). Participants asked to write about their hopes and aspirations referred more often to mental activities or emotional responses (‘think’, ‘worry’, etc.) than those asked to write about their duties and responsibilities (Ms 6.33 and 3.00, respectively, t [17] = 3.27, P < 0.005). In contrast, participants asked to write about duties and obligations tended to refer more often to others (Ms = 4.20 and 2.33, respectively, t [17] = −1.76, P < 0.09). No other coding categories approached significance.
DISCUSSION
As noted in the introduction, activity in various regions of medial anterior and posterior cortex has been associated with self-referential processing (e.g. Macrae et al., 2004; Ochsner et al., 2005; Vogt and Laureys, 2005; Cavanna and Trimble, 2006). Consistent with these previous findings, in Experiments 1 and 2, we found that two types of self-reflection, thinking about hopes and aspirations (promotion focus) and thinking about duties and obligations (prevention focus), showed greater activity than a non-self-referential distraction condition in dorsomedial PFC and posterior cingulate/precuneus.
A novel finding in both experiments was that, relative to distraction, a more inferior area of medial PFC was more active during promotion oriented self-reflection and an area of posterior cingulate was more active during prevention oriented self-reflection. Given the procedural differences between Experiments 1 and 2 (self-reflection focus was manipulated between participants in Experiment 1 and within participants in Experiment 2; participants wrote a self-reflective essay in advance of scanning in Experiment 1 and did not engage in self-reflection prior to scanning in Experiment 2), the replication of a double dissociation between the type of self-reflection (promotion vs prevention) and activity in medial PFC vs posterior cingulate regions is particularly striking.
Ochsner et al. (2005) and Mitchell et al. (2005) recently speculated that different regions of medial PFC may have somewhat different functions. Ochsner et al. (2005) subdivided medial PFC into ventral (z < 10) and dorsal (z > 10) regions and proposed that they may be involved in maintaining different types of representations, with ventromedial PFC maintaining representations of affective and motivational states and dorsomedial PFC maintaining more symbolic representations involved in describing and reasoning about these states. Mitchell et al. (2005), using what appears to be a division along the z-dimension of about 17 [see Table 4 in Mitchell et al. (2005)], suggested that ventromedial PFC activity may be associated with self-referential processing and dorsomedial PFC activity may ‘instantiate more universally applicable social-cognitive processes’ (p. 1311). In Tables 1 and 2, we have followed the suggested nomenclature for Social Cognitive and Affective Neuroscience that divides anterior medial PFC into three regions: ventromedial PFC (z < −10), medial PFC (−10 < z < 27) and dorsomedial PFC (z > 27). Thus the area of medial PFC for which we found greater sensitivity to thinking about hopes and aspirations than to thinking about duties and obligations (Figures 1C and 2C), falls within the region labelled ventromedial by Ochsner et al. (2005) and Mitchell et al. (2005). If, compared with dorsomedial PFC, a more inferior medial (or ventromedial) region is more specifically self-referential or more associated with motivation, then our findings would suggest that for our young adult participants, hopes and aspirations may be more closely related to the sense of self than duties and obligations, or that they are more motivated or emotionally engaged by thinking about hopes and aspirations than duties and obligations (Figures 1C and 2C).
In addition to providing evidence that further specifies the dissociation of function between different regions of anterior medial frontal cortex, our findings provide new evidence that different areas of posterior medial cortex may also subserve different functions with respect to self-reflective processing. In both Experiments 1 and 2, the posterior medial regions (Figures 1D and 2D) in which thinking about duties and obligations resulted in relatively greater activity than thinking about hopes and aspirations were somewhat anterior and inferior to those regions that were equally active for both types of self-reflection (Figures 1B and 2B). There is emerging evidence of differences in cytological organization and circuitry associated with the ventral and dorsal posterior cingulate (Vogt and Laureys, 2005). Interestingly, Vogt and Laureys suggest that ventral posterior cingulate may play a greater role in emotional processing than does dorsal posterior cingulate, through connections with anterior cingulate cortex. With respect to the precuneus, Cavanna and Trimble (2006) make an anterior (y closer to −60) vs posterior (y closer to −70) distinction, and their review suggests that more anterior regions tend to be found in studies of self-processing [see Table 4 in Cavanna and Trimble (2006)] and more posterior regions are more likely to be found in studies of episodic retrieval [see Table 3 in Cavanna and Trimble (2006)]. These reviews indicate that both dorsal/ventral and anterior/posterior differences will be of interest in future investigations of the role of medial posterior cortex.
In short, the double dissociation between medial PFC and anterior/inferior medial posterior areas and our two self-reflection conditions indicates that these brain areas serve somewhat different functions during self-focus. There are a number of interesting possibilities that remain to be sorted out. Differential activity in these anterior medial and posterior medial regions as a function of the types of agendas participants were asked to think about could reflect: (i) differences in the representational content in the specific features of agendas, schemas, possible selves and so forth that constitute hopes and aspirations on the one hand and duties and obligations on the other (cf. Luu and Tucker, 2004); (ii) differences in the type(s) of component processes these agendas are likely to engage and/or the representational content they are likely to activate, for example, discovering new possibilities (hopes) vs retrieving episodic memories (e.g. Maddock et al., 2001) of past commitments (duties); (iii) differences in affective significance of hopes and aspirations (attaining the positive) and duties and obligations (avoiding the negative, Higgins, 1997; 1998); (iv) different aspects of the subjective experience of self, such as the subjective experience of control (an instrumental self) vs the subjective experience of awareness (an experiential self; Johnson, 1991; Johnson and Reeder, 1997; compare, e.g. Searle, 1992 and Weiskrantz, 1997, vs Shallice, 1978 and Umilta, 1988); (v) differences in the social significance of hopes and aspirations (more individual) and duties and obligations (involving others). This last possibility is suggested by findings linking the posterior cingulate with taking the perspective of another (Jackson et al., 2006). It may be that thinking about duties and obligations (a more outward focus) tends to involve more perspective-taking than does thinking about hopes and aspirations (a more inward focus). The greater number of mental/emotional references from the promotion group on the pre-scan essay and the tendency for a greater number of references to others from the prevention group are consistent with the hypothesis that medial PFC activity is associated with a more inward focus whereas posterior cingulate/precuneus activity is associated with a more outward, social focus. Clarifying the basis of the similarities and differences between neural activation associated with thinking about hopes and aspirations vs duties and obligations would begin to help differentiate the relative roles of brain regions in different types of self-reflective processing.
Finally, the present findings suggest more specific target regions for investigating the impact of various types of self-reflection and distraction in clinical compared with non-clinical populations in future studies. Many psychological disorders appear to involve disruption of the sense of self, for example, a loss of agency or ownership of ideas (schizophrenia), loss of control of emotions (anxiety, depression) or unstable self-image (borderline personality disorder). Exploration of potential differential disruption in dorsomedial, medial and ventromedial PFC and identifiable posterior cingulate/precuneus components of a self-reflection/self-regulation circuit in these clinical populations should yield further information about the relative contributions of these regions to self-reflection and self-regulation, and to cognition and emotion more generally.
Procedure
Experiment 1 was a 2 (condition: self-reflection, distraction) × 2 (focus: prevention, promotion) mixed design, with condition manipulated within subjects and focus manipulated between subjects. Participants were 19 healthy young adults (14 female, M age = 21.3 years, range = 18–27 years). Before entering the scanner, participants spent an average of 7 min (range = 5–9 min) writing an essay on either their hopes and aspirations (promotion focus condition, n = 9) or duties and obligations (prevention focus condition, n = 10). About 10 min later, during a structural scan, participants were asked to think further about their essay (∼4 min) and encouraged to elaborate on their hopes and aspirations (or duties and obligations). Participants next engaged in an unrelated cognitive task (∼25 min), the results of which will be reported in a separate paper. Finally, they engaged in the self-reflection and distraction tasks as functional MRI data were collected. As in rumination induction experiments (e.g. Nolen-Hoeksema, 2004), participants were told that they would see short phrases and that they should ‘focus on the idea expressed by the phrase and use your imagination to visualize or think about the idea … think about the idea for the entire time it is shown’. On each trial, they saw one of the two self-reflection cues (hopes and aspirations or duties and obligations, depending on their group) or a distraction cue, randomly intermixed. Distraction cues were adapted from those of Nolen-Hoeksema (2004; e.g. polar bears fishing, pattern on oriental rug, shape of USA). The same 12 distraction cues were used for all participants.
In Experiment 2, the participants did not write an essay in advance of the scan; they were simply instructed while in the scanner to think about the ideas suggested by the cues. Experiment 2 was a within-subjects design (N = 11 healthy young adults, 8 females, M age = 20.8 years, range = 18–26 years) in which trials of the three cue types—hopes and aspirations (promotion), duties and obligations (prevention), and distraction (e.g. polar bears fishing)—were randomly intermixed.
In both experiments, each trial was 18 s, with the cue shown for 14 s and a crosshair shown throughout a 4 s ITI. In Experiment 1, there were two runs of 12 trials each (six trials each of self-reflection and distraction pseudo-randomly intermixed). In Experiment 2, there were three runs of 12 trials each (four trials each of promotion self-reflection, prevention self-reflection and distraction pseudo-randomly intermixed). Thus, in both experiments there were 12 trials (108 images) per participant per condition. All participants were paid, and informed consent was obtained from each. The Human Investigation Committee of Yale University Medical School approved the protocol.
Anatomical images were acquired for each participant using a 1.5T Siemens Sonata scanner at the Magnetic Resonance Research Center at Yale University. Functional scans were acquired with a single-shot echoplanar gradient-echo pulse sequence (TR = 2000 ms, TE = 35 ms, flip angle = 80°, FOV = 24). The oblique axial slices (26 in Experiment 1, 24 in Experiment 2) were 3.8 mm thick with an in-plane resolution of 3.75 × 3.75 mm; they were aligned with the AC–PC line. Each run began with 12 blank seconds to allow tissue to reach steady state magnetization, and was followed by a 1 min rest interval. One volume was collected every 2 s, or nine full brain scans for each trial (108 images per participant per condition in each experiment).
fMRI analyses
Data were motion-corrected using a six-parameter automated algorithm (AIR; Woods et al., 1992). A 12-parameter AIR algorithm was used to co-register participants’ images to a common reference brain. Data were mean-normalized across time and participants and spatially smoothed (3D, 8 mm FWHM gaussian kernel). The data were analysed using NeuroImaging Software (NIS; Laboratory for Clinical Cognitive Neuroscience, University of Pittsburgh, and the Neuroscience of Cognitive Control Laboratory, Princeton University). We used analysis of variance (ANOVA) with Participant as a random factor and all other factors fixed. Figure 1A and B shows anterior and posterior medial brain regions from Experiment 1 showing Condition (self-reflection, distraction) X Time (within trial, scans 1–9) interactions with a minimum of six contiguous voxels, each significant at P < 0.00001, and Figure 1C and D shows anterior and posterior medial regions identified as showing Focus (prevention, promotion) X Condition X Time interactions, six contiguous voxels, P < 0.01 (Forman et al., 1995). For Experiment 2, anterior and posterior medial brain regions were first identified from Condition (promotion, prevention, distraction) × Time interactions with a minimum of six contiguous voxels, each significant at P < 0.001. (The P-values used reflect the fact that the power to identify regions varies with the number of factors in the design, e.g. two way vs three way interactions, and the number of participants: N = 19 in Experiment 1 and N = 11 in the Experiment 2 replication.) Because the outcome of Experiment 1 suggested that there might be a difference in the pattern of activity across conditions in different regions of mPFC and PCC, in Experiment 2 these two regions were separated into subregions along the z-dimension. Timelines for the most superior and most inferior areas are shown in Figure 2. Subsequent comparisons between conditions were conducted on percent change from time 1 at times 5–7. For both experiments, F-maps were transformed to Talairach space using AFNI (Cox, 1996), and areas of activation were localized using AFNI and Talairach Daemon software (Lancaster et al., 1997) as well as manually checked with the Talairach and Tournoux (1988) or Duvernoy (1999) atlases. Timecourses in figures show percent change in BOLD signal value from Time 1 within trial.
Pre-scan essays (Experiment 1)
Pre-scan essays from Experiment 1 were scored for the following coding categories: mention of mental activities or emotional responses (e.g. think, decided, happy, worry); nouns (growth, school, job, consumer); verbs (buy, avoid, achieve); to be verbs (was, been, am, is); quantities (enough, some, more than, a lot); adjectives (honest, healthy, independent, silly); others (parents, friends, peers, teacher); time (as a child, in the future, now, this summer); explicit reference to the self (I, my, me); miscellaneous (and, but, about, also).
Acknowledgments
This research was supported by NIA grants AG09253 and AG15793. We thank MR technologists Hedy Sarofin and Cheryl McMurray for assistance in collecting the MR data, Kristen Pring-Mill for making figures and scoring data, and Jessica Jacobson for scoring data.
Footnotes
1 Using regions identified in Experiment 1 as a mask for analysing the data from Experiment 2, there was greater activity on promotion than prevention (P < 0.03) and distraction (P < 0.02) and no difference between prevention and distraction for the region shown in Figure 1C, and greater activity on both promotion (P < 0.004) and prevention (P < 0.001) trials than distraction, with no difference between promotion and prevention trials for the region shown in Figure 1D. Differences between experiments in the location of the posterior medial region relatively more active during prevention trials (and differences in shapes of timelines in all conditions) may reflect interesting differences depending on whether participants focus on both hopes and duties during scanning (as in Experiment 2) or only on hopes or duties (as in Experiment 1), and/or on whether or not they have thought in some detail in advance about such agendas (as in Experiment 1) or only think about them during the scanning phase (as in Experiment 2).
REFERENCES
- Cantor N, Kihlstrom J. Personality and Social Intelligence. NJ: Englewood Cliffs, Prentice Hall; 1987. [Google Scholar]
- Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: the BIS/BAS scales. Journal of Personality and Social Psychology. 1994;67:319–33. [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–83. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers & Biomedical Research. 1996;29:162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Craik FIM, Moroz TM, Moscovitch M, et al. In search of the self: a positron emission tomography study. Psychological Science. 1999;10:26–34. [Google Scholar]
- Cunningham WA, Raye CL, Johnson MK. Implicit and explicit evaluation: fMRI correlates of valence, emotional intensity, and control in the processing of attitudes. Journal of Cognitive Neuroscience. 2004;16:1717–29. doi: 10.1162/0898929042947919. [DOI] [PubMed] [Google Scholar]
- Cunningham WA, Raye CL, Johnson MK. Neural correlates of evaluation associated with promotion and prevention regulatory focus. Cognitive, Affective, & Behavioral Neuroscience. 2005;5:202–11. doi: 10.3758/cabn.5.2.202. [DOI] [PubMed] [Google Scholar]
- Duvernoy HM. The Human Brain: Surface, Three-dimensional Sectional Anatomy with MRI, and Blood Supply. 2nd edn. New York: Springer; 1999. [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995;33:636–47. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Fossati P, Hevenor SJ, Graham SJ, et al. In search of the emotional self: an FMRI study using positive and negative emotional words. American Journal of Psychiatry. 2003;160:1938–45. doi: 10.1176/appi.ajp.160.11.1938. [DOI] [PubMed] [Google Scholar]
- Freitas AL, Higgins ET. Enjoying goal-directed action: the role of regulatory fit. Psychological Science. 2002;13:1–6. doi: 10.1111/1467-9280.00401. [DOI] [PubMed] [Google Scholar]
- Frith U, Frith C. The biological basis of social interaction. Current Direction in Psychological Science. 2001;10:151–5. [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:4259–64. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins ET. Beyond pleasure and pain. American Psychologist. 1997;52:1280–300. doi: 10.1037//0003-066x.52.12.1280. [DOI] [PubMed] [Google Scholar]
- Higgins ET. Promotion and prevention: regulatory focus as a motivational principle. Advances in Experimental Social Psychology. 1998;46:1–46. [Google Scholar]
- Higgins ET, Shah J, Friedman R. Emotional responses to goal attainment: strength of regulatory focus as moderator. Journal of Personality & Social Psychology. 1997;72:515–25. doi: 10.1037//0022-3514.72.3.515. [DOI] [PubMed] [Google Scholar]
- Jackson PL, Brunet E, Meltzoff AN, Decety J. Empathy examined through the neural mechanisms involved in imagining how I feel versus how you feel pain. Neuropsychologia. 2006;44:752–61. doi: 10.1016/j.neuropsychologia.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Johnson MK. Reflection, reality monitoring, and the self. In: Kunzendorf RG, editor. Mental Imagery. New York: Plenum Press; 1991. pp. 3–16. [Google Scholar]
- Johnson MK, Reeder JA. Consciousness as meta-processing. In: Cohen JD, Schooler JW, editors. Scientific Approaches to Consciousness. Hillsdale, NJ: Erlbaum; 1997. pp. 261–93. [Google Scholar]
- Johnson SC, Baxter LC, Wilder LS, Pipe JG, Heiserman JE, Prigatano GP. Neural correlates of self-reflection. Brain. 2002;125:1808–14. doi: 10.1093/brain/awf181. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Summerlin JL, Rainey L, Freitas CS, Fox PT. The Talairach Daemon, a database server for Talairach Atlas Labels. Neuroimage. 1997;5:S633. [Google Scholar]
- Luu P, Tucker DM. Self-regulation by the medial frontal cortex: Limbic representation of motive set-points. In: Beauregard M, editor. Consciousness, Emotional Self-regulation and the Brain. Amsterdam: John Benjamins; 2004. pp. 123–61. [Google Scholar]
- Macrae CN, Moran JM, Heatherton TF, Banfield JF, Kelley WM. Medial prefrontal activity predicts memory for self. Cerebral Cortex. 2004;14:647–54. doi: 10.1093/cercor/bhh025. [DOI] [PubMed] [Google Scholar]
- Maddock RJ, Garrett AS, Buonocore MH. Remembering familiar people: the posterior cingulate cortex and autobiographical memory retrieval. Neuroscience. 2001;104:667–76. doi: 10.1016/s0306-4522(01)00108-7. [DOI] [PubMed] [Google Scholar]
- Markus H, Nurius P. Possible selves. American Psychologist. 1986;41:954–69. [Google Scholar]
- Markus H, Wurf E. The dynamic self-concept: a social psychological perspective. Annual Review of Psychology. 1987;38:299–337. [Google Scholar]
- Mitchell JP, Banaji MR, Macrae CN. The link between social cognition and self-referential thought in the medial prefrontal cortex. Journal of Cognitive Neuroscience. 2005;17:1306–15. doi: 10.1162/0898929055002418. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. The response style theory. In: Papageorgiou C, Wells A, editors. Depressive Rumination: Nature, Theory, and Treatment of Negative Thinking in Depression. New York: Wiley; 2004. pp. 107–23. [Google Scholar]
- Ochsner KN, Beer JS, Robertson ER, et al. The neural correlates of direct and reflected self-knowledge. Neuroimage. 2005;28:797–814. doi: 10.1016/j.neuroimage.2005.06.069. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Knierim K, Ludlow DH, et al. Reflecting upon feelings: an fMRI study of neural systems supporting the attribution of emotion to self and other. Journal of Cognitive Neuroscience. 2004;16:1746–72. doi: 10.1162/0898929042947829. [DOI] [PubMed] [Google Scholar]
- Searle JR. The Rediscovery of the Mind. Cambridge, MA: The MIT Press; 1992. [Google Scholar]
- Shah J, Higgins ET. Regulatory concerns and appraisal efficiency: the general impact of promotion and prevention. Journal of Personality & Social Psychology. 2001;80:693–705. [PubMed] [Google Scholar]
- Shallice T. The dominant action system: an information-processing approach to consciousness. In: Pope KS, Singer JL, editors. The Stream of Consciousness: Scientific Investigations into the Flow of Human Experience. New York: Plenum; 1978. pp. 117–57. [Google Scholar]
- Talairach J, Tournoux P. Co-planar Sterotaxic Atlas of the Human Brain-3-dimentional Proportional System: An Approach to Cerebral Imaging. New York: Thieme; 1988. [Google Scholar]
- Umilta C. The control operations of consciousness. In: Marcel AJ, Bisiach E, editors. Consciousness in Contemporary Science. Oxford, England: Clarendon; 1988. pp. 334–56. [Google Scholar]
- Vogt BA, Laureys S. Posterior cingulate, precuneal and retrosplenial cortices: cytology and components of the neural network correlates of consciousness. In: Laureys S, editor. Progress in Brain Research. Vol 150. Elsevier; 2005. pp. 205–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiskrantz L. A Neuropsychological Exploration. Oxford: Oxford University Press; 1997. Consciousness Lost and Found. [Google Scholar]
- Woods RP, Cherry SR, Mazziotta JC. Rapid automated algorithm for aligning and reslicing PET images. Journal of Computer Assisted Tomography. 1992;16:620–33. doi: 10.1097/00004728-199207000-00024. [DOI] [PubMed] [Google Scholar]