Abstract
The yeast TRPY1 (Yvc1) channel is activated by membrane stretch to release vacuolar Ca2+ into the cytoplasm upon osmotic upshock. Exogenously added indole greatly enhances the upshock-induced Ca2+ release in vivo. Indole also reversibly activates the channels under patch clamp. A minimum of 10-6 M Ca2+ is needed for membrane stretch force to open TPRY1, but indole activation appears to be Ca2+ independent. A deletion of 30 residues at the predicted cytoplasmic domain, 570-600Δ, renders TRPY1 insensitive to stretch force up to 10-3M Ca2+. Nonetheless, indole readily activates this mutant channel. Several other aromatic compounds, e.g. the antimicrobial parabens, also activate TRPY1. These compounds likely alter the innate forces in the lipid bilayer received by the channel.
Keywords: TRP channels, aromatic compounds, mechanosensitivity, lipid bilayer, YVC1
1. Introduction
Aromatic compounds favor the two membrane-water interface regions of a lipid bilayer. For example, indole has this disposition, whether in its free form, or in the form of tryptophan as either a free amino acid [1], a peptide [2] or a protein [3]. Belts of aromatic residues [4], are found at the level of the interfaces [5] in many membrane proteins including ion channels KcsA [6], KirBac1.1 [7] etc.
TRPY1 (Yvc1), in Saccharomyces cerevisiae, is a member of the “transient receptor potential” superfamily [8]. This channel corresponds to a 320-pS cation conductance in the yeast vacuolar membrane that can be activated by membrane stretch force under patch clamp [9]. It releases Ca2+ from the vacuole into the cytoplasm when live yeast cells are confronted with a sudden osmotic upshock [10]. TRPY1 activated by cytoplasmic Ca2+ provides a positive feedback in a Ca2+-induced Ca2+ release (CICR). We also isolated “gain-of-function” mutants that give larger Ca2+ signals at mild upshocks [11] [9,12]. These mutant channels show kinetic defects, indicative of destabilization of various functional states (conformations) [11] [12]. Seven of the ten gain-of-function mutations are in the predicted transmembrane domains of TRPY1. Surprisingly, they all involve aromatic amino-acid residues, leading to the conclusion that at least some of these aromatics are anchors for state stabilization [12]. In one case, the wild-type tyrosine was replaced with all 19 possible amino acids. Only replacements to the other two aromatic residues (trp and phe) retain the wild-type like channel kinetics [11]. We therefore decided to investigate whether exogenously added aromatic compounds can affect TRPY1 behavior.
2. Materials and methods
2.1. Yeast Strains and media
The wild-type parental strain, BY4742 (MATα his3Δ, leu2Δ, lys2Δ, ura3Δ), and BY4742 yvc∷kanmx4 (the yvc1Δ mutant) were used. Cells were cultured in either YPD (yeast extract peptone with glucose) for patch-clamp experiments cells or in CMD leu-minus (complete minimal medium with glucose, leucine dropout) for luminometry.
2.2. Luminometry
The transgenic-aequorin-based luminometric assay of Ca2+ release through TRPY1 was as described [11] [12].
2.3. Patch clamp
Vacuolar preparation, patch clamp, and data acquisition and analyses were as described [8].
3. Results
3.1. Indole enhances upshock-induced Ca2+ release in vivo
Free Ca2+ in the cytoplasm of live yeast cells can be measured by the luminescence of transgenic aequorin [13]. A jump in the osmolarity of the BAPTA-containing medium causes an immediate rise of cytoplasmic Ca2+ in yeast cells, peaking in tens of second. This wave of Ca2+ is not observed in yvc1Δ, showing that it is the result of a release from the vacuole through the TRPY1 channel [10] [11] [12]. We found exogenously added indole to greatly enhance this response to osmotic upshock. This enhancement is robust and consistently observed (n > 60 experiments). Fig. 1, left, shows that 1 mM indole increases the peak response by more than 10 fold. There is no response to the upshock, with or without indole, when the YVC1 gene is deleted (Fig. 1, right).
Fig. 1.
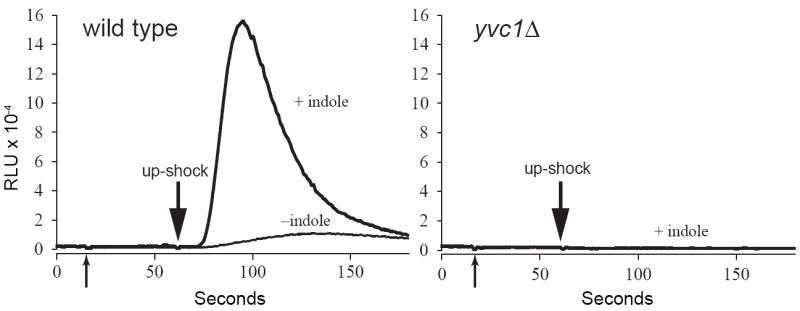
Indole potentiates up-shock-induced Ca2+ release through TRPY1 (Yvc1) in vivo. The luminescence of wild-type (left) or yvc1Δ (right) yeast cells expressing aequorin were examined. At the first arrow, nine volumes of either 0.5% DMSO (- indole curve) or 1 mM indole in 0.5% DMSO were added to the cells. Sorbitol was added at the second arrow at a final concentration of 1 M. Indole’s ability to potentiate the wild-type luminometric signal (in relative luminescence units, RLU) is evident (left). No response was seen in yvc1Δ cells (right) with the same indole treatment, indicating that the Ca2+ release and the indole effect are entirely due to the TRPY1 channel.
3.2. Indole activates TRPY1 channel under patch clamp
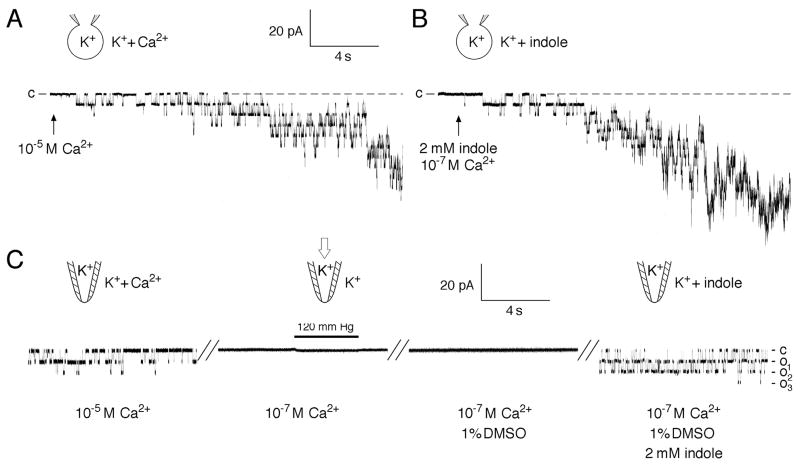
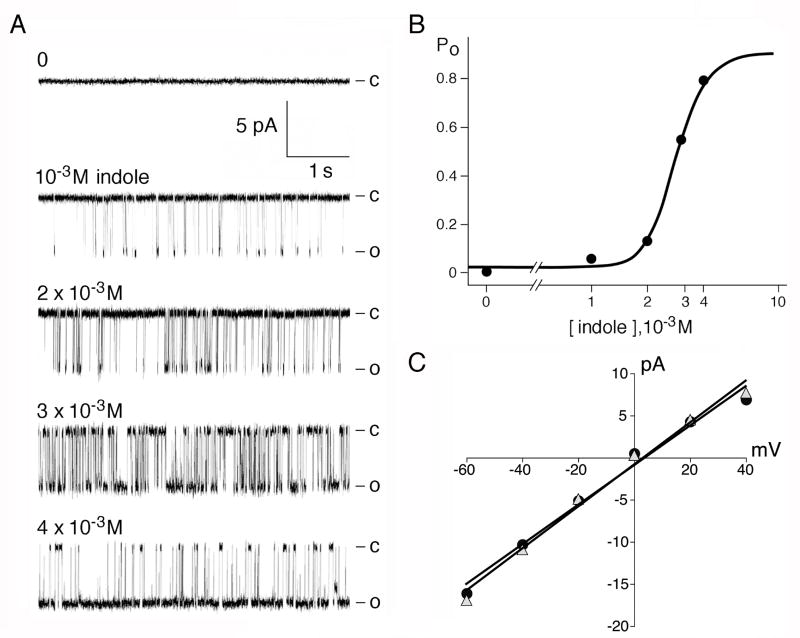
Channel activation by indole was readily observed in either whole-vacuole mode (Fig. 2B) or in excised cytoplasmic-side-out mode of recording (Fig.2C) (n >30, activation observed in every preparation). Stretching the membrane with applied pressures up to lysis failed to activate the channel at or below 10-7 M Ca2+ in repeated independent experiments (n > 10) (Fig. 2C, left two segments). Indole bypassed this requirement and readily activated channels at 10-7 M Ca2+ (Fig. 2C, rightmost segment) or even 10-9 M Ca2+ (not shown). Once activated at 10-7 M Ca2+ the channels were responsive to applied pressure. Submillimolar indole is rarely effective. Within the millimolar range, however, open probability rises steeply with concentration (Fig. 3a,b). Calculating directly from the concentration of indole in water and DMSO, the activation has a Hill coefficient of ~ 6, suggesting cooperativity. Unitary conductance when activated by indole is indistinguishable from that activated by Ca2+ (Fig 3c). Activated with Ca2+ or stretch force, TRPY1 rectifies inwardly, shows a nonspecific, cation selectivity and ~ 300-pS unitary conductance when recorded in symmetric 150 mM K+ [8] [9]. All these properties are the same when channels are activated by indoles.
Fig. 2.
Indole activates the wild-type TRPY1. In whole-vacuole recording mode (A, B) and in excised cytoplasmic-side-out patch mode (C); currents flowing into the cytoplasm are shown downward by convention. A. Ca2+ activation. Increasing [Ca2+] from 10-7 M to 10-5 M Ca2+ (arrow) activated an ensemble of the ~300-pS unitary conductances (in 150 mM KCl). B. Indole activation. Addition of indole in 1% DMSO to the final concentration of 2 mM indole activated the ensemble current as well. C. Unitary conductances were activated in an excised patch with 10-5 M Ca2+ (left segment). Activities subsided after perfusion of 10-7 M Ca2+. At this [Ca2+], the application of 120 mm Hg pressure failed to activate the channel (second segment). The same patch bathed with 1% DMSO control solution did not activate the channel (third segment), but the addition of 2 mM indole did (final segment).
Fig. 3.
Dose response of the Indole effect. (A) The left five traces show activities of a single Yvc1 channel in an excised cytoplasmic-side-out patch bathed in 10-7 M Ca2+ and different concentrations of indole. (B) Open probability (Po) is plotted against concentration at the bottom and fitted with the Hill-Langmuir equation, showing a steep rise in millimolar range. (C) Unitary conductances activated by 10-5 M Ca2+ (triangle) or with 10-7 M Ca2+ and 2 mM indole (circle).
3.3. Indole activates 570-600Δ
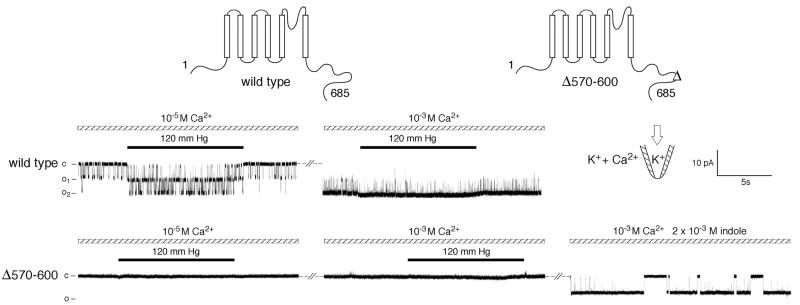
We have carried out extensive scanning and deletion analyses of the TPRY1 protein and the findings will be detailed elsewhere. Of interest here is a 30 amino acid deletion in the predicted cytoplasmic domain of TRPY1 (between position 570 and 600, diagramed at the top of Fig. 4). This produced a channel that can no longer be activated by high concentration of Ca2+ (10-3 M), membrane stretch force (120 mm Hg), or the combination of the two (Fig. 4, bottom trace, center segment). Nevertheless, indole readily activates the 570-600Δ channel (Fig. 4, last segment) in patches without BAPTA. Like the wild type, 570-600Δ channels are not active at 10-7 M, but the addition of indole at this low [Ca2+] readily activates them. Once activated these channels can be further activated by pressure (data not shown).
Fig. 4.
570-600Δ TRPY1 fails to respond to Ca2+ and applied stretch force but can be activated by indole (Top trace) In a patch excised from a wild-type vacuole, two conducting units were inactive activated by 10-5 M Ca2+, and further activated by the exertion of 120 mm Hg pressure (left). Addition of 10-3 M Ca2+ activated the two units nearly completely, such that the additional effect of applied pressure was not evident (right). (Bottom trace) In a comparable experiment with a patch excised from a 570-600Δ Yvc1 vacuole, no channel activities could be elicited with 10-5 M Ca2+ and pressure (left), or even with 10-3 M Ca2+ and pressure (right). Addition of 2 mM indole clearly activated a unitary conductance in this patch.
3.4. Several other aromatics also activate
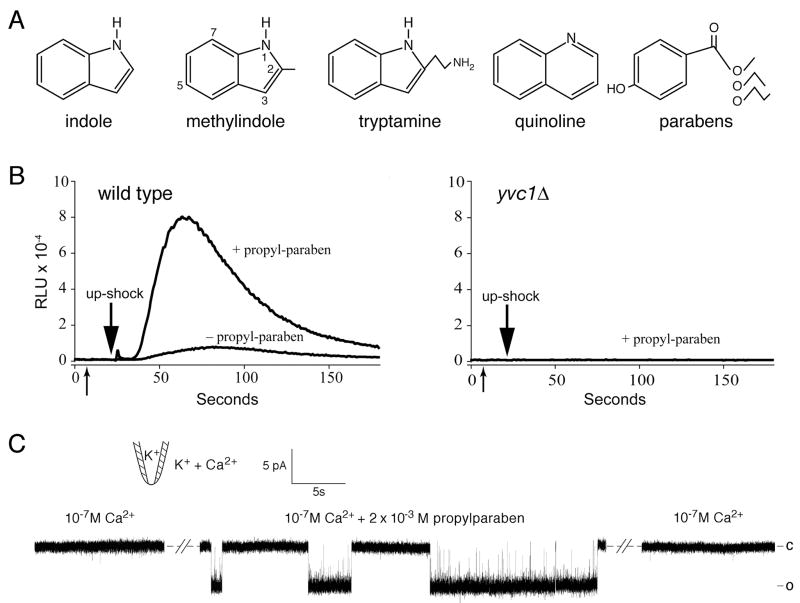
To see whether the effects are unique to indole, we tested several other aromatic compounds. In repeated trials, activation was observed in both patch clamp and luminometry when indole was methylated in position 1, 2, 3, 5, or 7 of the indole ring (diagrammed in Fig. 5A). Quinoline, with two six-member rings, also activates. We also tested para-hydroxybenzoates, commonly called parabens. At millimolar concentrations we found that while para-hydroxybenzoic acid (paraben) did not activate, propylparaben activates TRPY1 in vivo and in vitro as effectively as indole. As shown in Fig. 5, 1 mM propylparaben enhances the upshock-induced release of Ca2+ into the cytoplasm of live yeast cells in a yvc1-dependent manner (Fig. 5B) similar to the effect of indole (Fig. 1). Under patch clamp, channel activation by propylparaben, even at 10-7 M Ca2+, is evident (Fig. 5C) (n = 4). Tryptamine, the amine equivalent of tryptophan, weakly activates. On the other hand, the comparable derivative of tyrosine, tyromine, activates very poorly; and that of phenylalanine, phenylamine, does not activate at all. In short, several of the very different aromatic compounds tested activate TRPY1, while others do not. This suggests that aromatic character maybe a key property but that there are additional contributing factors.
Fig. 5.
Other aromatic compounds also activate TRPY1 channel. (A) Structures of the various aromatics tested. (B) Para-propylbenzoate (propylparaben) (first arrow) potentiated the Ca2+ release induced by osmotic upshock (second arrow) in wild-type cells (left). This release was not observed in the yvc1Δ mutant (right). Same procedure as in Fig. 1. (C) Para-propylbenzoate reversibly activated a wild-type TRPY1 conductance in an excised patch bathed in 10-7 M Ca2+.
4. Discussion
How do these aromatic compounds activate TRPY1? One possibility is that the compound enters the interior of the channel protein to affect gating. The millimolar concentration requirement and steep dependency indicates that multiple low affinity binding sites are involved. Therefore the mechanism is not likely to be as specific as those found with aromatic agonists and antagonists of dihydropyridine (DHP)-sensitive calcium channels [14]. Furthermore, the lack of geometrical specificity suggests that the site is not as restrictive as seen with indole inhibition of Shaker K+ but not IRK currents [15]. In both these cases experimental evidence indicates that specific aromatic residues in the protein are important for the effect on channel activity. Our recent mutagenesis studies of TRPY1 show that more than one aromatic side chain plays a role in maintaining the closed and open form of the channel [11] [12]. However, some aromatic compounds did not activate, implying that there are additional requirements that have yet to be clarified. One possible factor might be that the network of aromatic side chains in TRPY1 require compounds to penetrate to a certain depth into the interface in order to influence the side chains that play a role in maintaining the protein’s dynamic structure. We found that the compounds which activated tended to have greater calculated positive partioning coefficients (logP or logD) into hydrophobic environments. Paraben has been shown to activate other channels including TRPA1 [16], a K+ efflux in bacteria involving OmpF [17] and the mechano-sensitive bacterial channels MscL and MscS [18]. In the latter case paraben was proposed to work from within the channel protein [18].
A second possibility is that the aromatic compounds act at the channel-lipid boundary. The high concentrations of aromatics needed to activate TRPY1 make this unlikely. L- and D-form of tarantula venom toxin GsMTx4 inhibits the stretch-activated channel (SACs) of vertebrate cells [19] by acting at this boundary. However, GsMx4 works at micromolar concentration to inhibit SACs [19], while aromatics work at millimolar to activate TRPY1 (present study) and TRPA1 [16]. If the exogenous aromatics compete with the channel protein’s aromatic residue Y458 for lipid anchors, the un-anchored channel might be expected to show the “gain-of-function” phenotype, rapidly flickering between the closed and the open state as previously described [11] [12]. However, as shown in Fig. 2B, “gain-of-function” flickers were not observed upon opening with aromatic compounds. Flickers were observed in prolonged incubations of some of patches, such that a boundary effect of indole cannot be completely ruled out at the later stage of activation.
It also seems likely that, at these concentrations, the compounds significantly affect the properties of the entire lipid bilayer, which then influences TRPY1 gating. Agents that alter bilayer elasticity are known to regulate the activities of gramicidin A and the voltage-dependent Na+ channel [20]. Calculations [21] and molecular dynamic simulations [22] show that there is a large tension in the bilayer at the two water-membrane interfaces. Amphipathic compounds activate mechanosensitive channels such as MscS, MscL [23], and TREK [24]. The general model is that entry of such amphipathic compounds induces curvature stress in the monolayers and differential insertion into the two monolayers causes bilayer bending, both perturbing the bilayer’s internal forces to favor conformational changes of the embedded channels. We observed that certain amphipaths such as chlorpromazine can activate TRPY1 but others such as trinitrophenol and lysophosphotidylcholine do not (unpublished results). The blockage of the natural activations by deletion 570-600Δ may be due to a defect in the cytoplasmic domain that prevents Ca2+ binding and consequently the resultant reconfiguration needed for stretch-force activation. That indole activates the wild-type channel below critical [Ca2+] (Fig. 2C) and that even the apparently “dead” 570-600Δ can be activated by indole (Fig. 4) may indicate that aromatic compounds that infiltrate deep into the water-lipid interface generates a force profile in the bilayer that is far more favorable for the open conformation. This mechanosensitive channel now offers a unique opportunity to explore the relationship between mechanical forces and the effects caused by aromatic and other organic compounds.
Acknowledgments
We thank Dr. Andriy Anishkin for discussion. This work was supported by National Institutes of Health Grants GM054867 (to Y.S.) and GM047856 (to CK) and the Vilas Trust of the University of Wisconsin - Madison.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yau WM, Wimley WC, Gawrisch K, White SH. The preference of tryptophan for membrane interfaces. Biochemistry. 1998;37:14713–8. doi: 10.1021/bi980809c. [DOI] [PubMed] [Google Scholar]
- 2.de Planque MR, Kruijtzer JA, Liskamp RM, Marsh D, Greathouse DV, Koeppe RE, 2nd, de Kruijff B, Killian JA. Different membrane anchoring positions of tryptophan and lysine in synthetic transmembrane alpha-helical peptides. J Biol Chem. 1999;274:20839–46. doi: 10.1074/jbc.274.30.20839. [DOI] [PubMed] [Google Scholar]
- 3.Nyholm TK, Ozdirekcan S, Killian JA. How protein transmembrane segments sense the lipid environment. Biochemistry. 2007;46:1457–65. doi: 10.1021/bi061941c. [DOI] [PubMed] [Google Scholar]
- 4.Ulmschneider MB, Sansom MS, Di Nola A. Properties of integral membrane protein structures: derivation of an implicit membrane potential. Proteins. 2005;59:252–65. doi: 10.1002/prot.20334. [DOI] [PubMed] [Google Scholar]
- 5.White SH, Wimley WC. Membrane protein folding and stability: physical principles. Annu Rev Biophys Biomol Struct. 1999;28:319–65. doi: 10.1146/annurev.biophys.28.1.319. [DOI] [PubMed] [Google Scholar]
- 6.Doyle DA, Cabral JM, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, MacKinnon R. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- 7.Domene C, Vemparala S, Klein ML, Venien-Bryan C, Doyle DA. Role of aromatic localization in the gating process of a potassium channel. Biophys J. 2006;90:L01–3. doi: 10.1529/biophysj.105.072116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palmer CP, Zhou XL, Lin J, Loukin SH, Kung C, Saimi Y. A TRP homolog in Saccharomyces cerevisiae forms an intracellular Ca(2+)-permeable channel in the yeast vacuolar membrane. Proc Natl Acad Sci U S A. 2001;98:7801–5. doi: 10.1073/pnas.141036198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou XL, Batiza AF, Loukin SH, Palmer CP, Kung C, Saimi Y. The transient receptor potential channel on the yeast vacuole is mechanosensitive. Proc Natl Acad Sci U S A. 2003;100:7105–10. doi: 10.1073/pnas.1230540100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denis V, Cyert MS. Internal Ca(2+) release in yeast is triggered by hypertonic shock and mediated by a TRP channel homologue. J Cell Biol. 2002;156:29–34. doi: 10.1083/jcb.200111004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou X, Su Z, Anishkin A, Haynes WJ, Friske EM, Loukin SH, Kung C, Saimi Y. Yeast screen show aromatic residues at the end of the sixth helix anchor TRP-channel gate. Proc Natl Acad Sci. 2007;104:15555–15559. doi: 10.1073/pnas.0704039104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Su Z, Zhou X, Haynes WJ, Loukin SH, Anishkin A, Saimi Y, Kung C. Yeast gain-of-function mutations reveal structure function relationships conserved among different subfamilies of transient receptor potential channels. Proc Natl Acad Sci U S A. 2007 doi: 10.1073/pnas.0708584104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Batiza AF, Schulz T, Masson PH. Yeast respond to hypotonic shock with a calcium pulse. J Biol Chem. 1996;271:23357–62. doi: 10.1074/jbc.271.38.23357. [DOI] [PubMed] [Google Scholar]
- 14.Peterson BZ, Tanada TN, Catterall WA. Molecular determinants of high affinity dihydropyridine binding in L-type calcium channels. J Biol Chem. 1996;271:5293–6. doi: 10.1074/jbc.271.10.5293. [DOI] [PubMed] [Google Scholar]
- 15.Avdonin V, Hoshi T. Modification of voltage-dependent gating of potassium channels by free form of tryptophan side chain. Biophys J. 2001;81:97–106. doi: 10.1016/S0006-3495(01)75683-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujita F, Moriyama T, Higashi T, Shima A, Tominaga M. Methyl p-hydroxybenzoate causes pain sensation through activation of TRPA1 channels. Br J Pharmacol. 2007;151:153–60. doi: 10.1038/sj.bjp.0707219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bredin J, Davin-Regli A, Pages JM. Propyl paraben induces potassium efflux in Escherichia coli. J Antimicrob Chemother. 2005;55:1013–5. doi: 10.1093/jac/dki110. [DOI] [PubMed] [Google Scholar]
- 18.Nguyen T, Clare B, Guo W, Martinac B. The effects of parabens on the mechanosensitive channels of E. coli. Eur Biophys J. 2005;34:389–95. doi: 10.1007/s00249-005-0468-x. [DOI] [PubMed] [Google Scholar]
- 19.Suchyna TM, Tape SE, Koeppe RE, 2nd, Andersen OS, Sachs F, Gottlieb PA. Bilayer-dependent inhibition of mechanosensitive channels by neuroactive peptide enantiomers. Nature. 2004;430:235–40. doi: 10.1038/nature02743. see comment. [DOI] [PubMed] [Google Scholar]
- 20.Lundbaek JA, et al. Capsaicin regulates voltage-dependent sodium channels by altering lipid bilayer elasticity. Mol Pharmacol. 2005;68:680–9. doi: 10.1124/mol.105.013573. [DOI] [PubMed] [Google Scholar]
- 21.Cantor RS. The influence of membrane lateral pressures on simple geometric models of protein conformational equilibria. Chemistry & Physics of Lipids. 1999;101:45–56. doi: 10.1016/s0009-3084(99)00054-7. [DOI] [PubMed] [Google Scholar]
- 22.Gullingsrud J, Schulten K. Lipid bilayer pressure profiles and mechanosensitive channel gating. Biophys J. 2004;86:3496–509. doi: 10.1529/biophysj.103.034322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martinac B, Adler J, Kung C. Mechanosensitive ion channels of E. coli activated by amphipaths. Nature. 1990;348:261–3. doi: 10.1038/348261a0. [DOI] [PubMed] [Google Scholar]
- 24.Patel AJ, Lazdunski M, Honore E. Lipid and mechano-gated 2P domain K(+) channels. Curr Opin Cell Biol. 2001;13:422–8. doi: 10.1016/s0955-0674(00)00231-3. [DOI] [PubMed] [Google Scholar]