Abstract
Intrauterine nutrition status is reported to correlate with risk of cardiovascular diseases in adulthood. Either under- or over-nutrition during early to mid gestation contributes to altered fetal growth and ventricular geometry. This study was designed to examine myocardial expression of ciliary neurotrophic factor receptor α (CTNFRα) and its down-stream mediator signal transducer and activator of transcription 3 (STAT3) on maternal under- or over-nutrition-induced changes in fetal heart weight. Multiparous ewes were fed with 50% (nutrient-restricted, NR), 100% (control) or 150% (overfed, OF) of NRC requirements from 28 to 78 days of gestation (dG; Term 148 dG). Ewes were euthanized on day 78, and the gravid uteri and fetuses recovered. Ventricular protein expression of CTNFRα, STAT3, phosphorylated STAT3, insulin-like growth factor I receptor (IGF-1R) and IGF binding protein 3 (IGFBP3) were quantitated using western blot. Plasma cortisol levels were higher in both NR and OF fetuses whereas plasma IGF-1 levels were lower and higher, in NR and OF fetuses. Fetal weights were reduced by 29.9% in NR ewes and were increased by 22.2% in fetuses from OF ewes compared to control group. Nutrient restriction did not affect fetal heart or ventricular weights whereas overfeeding increased heart and ventricular weights. Protein expression of CTNFRα in fetal ventricular tissue was reduced in OF group whereas STAT3 and pSTAT3 levels were reduced in both NR and OF groups. Expression of IGF-1R and IGFBP3 was unaffected in either NR or OF group. These data suggested that compared with maternal undernutrition, intrauterine overfeeding during early to mid gestation is associated with increases fetal blood concentrations of cortisol and IGF-1 in association with ventricular hypertrophy where reduced expression of CNTFRα and STAT3 may play a role.
Keywords: fetal, under-nutrition, over-nutrition, heart, CNTFR, STAT3, IGF-1
Introduction
Epidemiological studies have described a close association between nutrition status during development and risk for long-term disease. Together with controlled animal studies these data have led to the biological concept of developmental programming (DP). Developmental programming is defined as a response to a specific challenge to the mammalian organism during a critical developmental time window that alters the trajectory of development qualitatively and/or quantitatively with persistent effects on offspring phenotype. Many studies have focused on the effects of exposure of the fetus to either under- or over-nourishment in order to determine the impact on the health of the offspring during adulthood (1-5). Understanding of how maternal nutrition may program fetal as well as postnatal organ geometry and function is still inadequate.
Altered fetal nutritional status, especially during the first two trimesters of pregnancy, has been shown to enhance susceptibility to cardiovascular, metabolic and endocrine diseases during postnatal life (2;6;7). Under-nutrition during early to mid pregnancy leads to birth of offspring with health problems in adulthood, even if adequate nutrition is provided later in gestation (6;8). In addition, an elevated level of prenatal nutrient supply significantly increases the propensity to obesity in adult life (9;10). These gestational nutrition-associated fetal or postnatal defects seem to be consistent with the critical role of early life for fetal growth and development (11). Despite these clinical and animal experimental observations, the precise cellular mechanism(s) responsible for abnormal fetal growth and physiological function as a result of altered maternal nutrient supply remains poorly elucidated.
The objective of this study was to examine the impact of the early life nutrient restriction or overfeeding on fetal cardiac growth and expression of the receptor for ciliary neurotrophic factor (CNTF), which is essential to tissue growth and metabolism (12). The CNTF receptor (CNTFR) complex closely resembles the leptin receptor (ObR) structurally and has a similar distribution in the hypothalamic nuclei associated with regulation of feeding and body weight (13). The CNTF receptor is a trimeric receptor complex with a CNTF binding component (CNTFRα), a leukemia inhibitory factor β subunit, and the signal transducer of IL-6 (gp130) (13;14). Evidence has demonstrated that leptin and CNTF may share similar cellular signaling components including the structural homology between ObR and the gp130 subunit of the CNTFR as well as activation of signaling pathway involving the Janus kinase-signal transducer and activator of transcription (STAT) pathway (15;16). Given the role of insulin-like growth factor I (IGF-1) system in fetal cardiac growth (17;18), levels of circulating IGF-1, protein expression of IGF-1 receptor (IGF-1R) and IGF-1 binding protein 3 (IGFBP3) were also evaluated.
Materials and Methods
Experimental animals
All animal procedures were approved by the University of Wyoming Animal Care and Use Committee. On day 20 of pregnancy, multiparous ewes of mixed breeding were weighed so that individual diets could be calculated on a metabolic body weight basis (weight0.75) as previously described (19). On day 21 of gestation, all ewes were placed in individual pens and fed a highly palatable diet (Table 1) at 100% of National Research Council recommendations (NRC, 1985) which was equivalent to 1.1% of ewe body weight. On day 28, ewes were randomly assigned in equal numbers to be fed the highly palatable diet at 50% (nutrient restricted), 100% (control) or 150% (overfed) of NRC recommendations. All ewes were weighed at weekly intervals and rations adjusted for weight gain or loss. On day 45, ultrasonography was used to determine the number of fetuses present and only twin bearing ewes (7 control, 5 nutrient restricted and 10 overfed fetuses) were entered in the study. Ewes were necropsied on day 78 of gestation (dG) for this study.
Table 1.
Composition of the diet fed to ewes throughout the study
| Ingredients (%) | |
| Ground bromegrass hay1 | 14.02 |
| Ground corn | 63.89 |
| Soybean meal | 13.30 |
| Liquid molasses | 5.60 |
| Limestone | 2.24 |
| Ammonium chloride | 0.50 |
| Mineralized salt2 | 0.24 |
| Magnesium chloride | 0.10 |
| ADE premix3 | 0.10 |
| Rumensin 80 | 0.02 |
| Analyzed composition4 | |
| DM, % | 88.54 |
| NDF, % of DM | 24.09 |
| ADF, % of DM | 9.99 |
| CP, % of DM | 17.39 |
| IVDMD, % | 93.92 |
Mean particle length = 2.54 cm
Contained 13% NaCl, 10% Ca, 10% P, 2% K, 1.5% Mg, 0.28% Fe, 0.27% Zn, 0.12% Mn, 0.01% I, 35 ppm Se, and 20 ppm Co.
Contained 110,000 IU/kg vitamin A, 27,500 IU/kg vitamin D, and 660 IU/kg vitamin E.
DM, dry matter; NDF, neutral detergent fiber; ADF, acid detergent fiber; CP, crude protein; IVDMD, in vitro dry matter digestibility.
Ewes were sedated with ketamine and maintained under isofluorine inhalation anesthesia. Fetal blood was collected from the umbilical vein via puncture with an 18 gauge 1 ½ inch needle and blood was drawn into a 3 cc syringe and subsequently placed in a chilled nonheparinized vacutainer tube (no additives, Sigma, St. Louis, MO). Serum was obtained and frozen at -80°C until assayed for cortisol and insulin-like growth factor (IGF-1). Ewes were then given an overdose of sodium pentobarbital (Abbott Laboratories, Abbott Park, IL) and exanguinated. The gravid uterus was quickly removed. Fetal weight, crown-rump length (CRL), total heart, left and right ventricular weights were determined for each twin. Ventricular samples were snap-frozen in liquid nitrogen for later analysis.
Western analysis of CTNFRα, STAT3, phosphorylated STAT3, IGF-1R and IGFBP3
For western blot analysis, left and right ventricular tissue were homogenized and lysed in RIPA lysis buffer containing 20 mmol/l Tris (pH 7.4), 1 mmol/l M EDTA, 1 mmol/l EGTA, 150 mmol/l NaCl, 1% Triton, 0.1% SDS with 1% protease inhibitor cocktail. Lysates were sonicated and clarified by centrifugation at 13,000 × g for 25 min at 4°C and protein concentrations were determined using the Bio-Rad protein assay reagent (Bio-Rad Laboratories, Inc., Richmond, CA). Equal amounts of protein samples (50 μg/lane) were separated on a 7% SDS-polyacrylamide gels. SeeBlue plus2 Prestained markers (Invitrogen, Carlsbad, CA) were used as standards. Electrophoretic transfer of proteins to nitrocellulose membranes (0.2 μm pore size, Bio-Rad Laboratories, Inc, Hercules, CA) was accomplished in a transfer buffer consisting of 25 mmol/l Tris-HCl, 192 mmol/l glycine and 20% methanol for 60 min. Membranes were blocked for 60 min at room temperature in TBS-T (0.1% Tris-buffered saline Tween-20) with 5% nonfat dry milk. Membranes were incubated overnight with anti-CTNFRα (1:1,000), anti-STAT3 (1:1,000), anti-phosphorylated STAT3 (1:1,000) anti-IGF-1R (1:1,1000) and anti-IGFBP-3 (1:1,1000) antibodies at 4°C (Cell Signaling, Beverly, MA). After incubation with the primary antibody, blots were incubated with the anti-rabbit IgG HRP-linked antibodies (1:5,000) for 60 min at room temperature. After three washes in TBS-T, immunoreactive bands were detected using the Super Signal west Dura Extended Duration Substrate (Pierce, Milwaukee, WI). The intensity of bands was measured with a scanning densitometer (Model GS-800; Bio-Rad, Hercules, CA) coupled with Bio-Rad PC analysis software.
Hormone assay methods
All samples were run in triplicate and sample analysis was completed using a single assay. The intra-assay variability was < 5 %. Cortisol levels were measured by RIA in accordance with the manufacturer recommendation (Coat-A-Count®, Diagnostic Products Corporation, Los Angeles, CA). IGF-1 (single measurement) was run on serum samples using Immulite test kits on an Immulite 1000 (Diagnostics Products Corporation, Los Angeles, CA). The sensitivity of the IGF-1 assay was 20 ng/ml and the intra-assay variability was < 2%.
Statistics
Data are presented as Mean ± SEM. Mean differences between groups were assessed using a 2–way analysis of variance (ANOVA). When an overall significance was determined, a Dunnetts post hoc analysis was incorporated. P value less than 0.05 was considered significant.
Results
General features of ewes and fetuses under intrauterine nutrition restriction and overfeeding
There were no differences in body weight on day 28 of gestation between ewes assigned to nutrient restricted (156 ± 6 kg), control (155 ± 7 kg) and overfed (157 ± 6 kg) groups. By day 78 of gestation, however, nutrient restricted ewes had lost 7.1% body weight (145 ± 6 kg), control ewes had gained 7.7% body weight (167 ± 7 kg) and overfed ewes had gained 29.9 % body weight (204 ± 7kg). There were no fetal losses in any of the dietary groups. On day 78 fetal weight and CRL were significantly reduced or increased in nutrition restricted and overfeeding groups, compared to control fetuses (Table 2). While the whole heart, left and right ventricular weights, as well as the ratio of whole heart, left and right ventricular weights to fetal weights were similar for fetuses between control and nutrition restricted fetuses on day 78, fetal heart, left and right ventricular weights were increased (p < 0.05) in fetuses from overfeeding versus control ewes. However, the ratio of whole heart, left and right ventricular weights to fetal body weight were comparable between overfeeding and control groups (Table 2). Blood cortisol levels were significantly elevated in fetuses from both nutrition-restricted and overfed ewes. However, IGF-1 concentrations were significantly reduced in the blood of fetuses from nutrition restricted ewes and were elevated (p < 0.05) in the blood of fetuses from overfed ewes.
Table 2.
Biometric features, blood concentrations of cortisol and insulin-like growth factor I (IGF-1) in fetus from ewes with control, nutrition restricted and overfed ewes on day 78 of gestation
| Control
(n = 7) |
Nutrition Restricted
(n = 5) |
Overfed
(n = 10) |
|
|---|---|---|---|
|
| |||
| Fetal weight (g) | 308.0 ± 12.2 | 216.0 ± 13.6* | 376.4 ± 12.5*, # |
| CRL (cm) | 21.86 ± 0.26 | 20.20 ± 0.34* | 24.45 ± 0.17*, # |
| Heart weight (g) | 2.47 ± 0.14 | 2.17 ± 0.33 | 3.27 ± 0.23*, # |
| HW/FW (%) | 2.61 ± 0.15 | 4.58 ± 0.49 | 2.30 ± 0.13 |
| Left Ventricle (g) | 1.07 ± 0.05 | 0.89 ± 0.09 | 1.34 ± 0.07*, # |
| Right Ventricle (g) | 0.61 ± 0.03 | 0.50 ± 0.07 | 0.77 ± 0.03*, # |
| LV/FW (%) | 0.35 ± 0.03 | 0.41 ± 0.05 | 0.36 ± 0.03 |
| RV/FW (%) | 0.16 ± 0.02 | 0.23 ± 0.03 | 0.20 ± 0.03 |
| Cortisol (ng/ml) | 7.85 ± 0.76 | 11.51 ± 1.11* | 14.16 ± 0.88*, # |
| IGF-1 (ng/ml) | 44.62 ± 0.90 | 38.78 ± 2.29* | 53.30 ± 1.76*, # |
FW = fetal weight; CRL = crown-rump length; LV = left ventricle; RV = right ventricle, Mean ± SEM,
p < 0.05 compared with control group,
p < 0.05 compared with nutrition restricted group.
Expression of CTNFRα, STAT3, pSTAT3, IGF-1R and IGFBP3 in response to dietary treatments
Intrauterine overfeeding but not nutrient restriction significantly down-regulated (p < 0.05) protein expression of CTNFRα. The protein expression of the CTNFRα downstream signaling molecule STAT3 and its activation measured by phosphorylated STAT3 were increased (p < 0.05) in fetal hearts of both nutrition restricted and overfeeding groups (Fig. 1). Our further study revealed that the expression of both IGF-1R and IGFBP3 was not affected by either nutrient restriction or overfeeding (Fig. 2).
Fig 1.
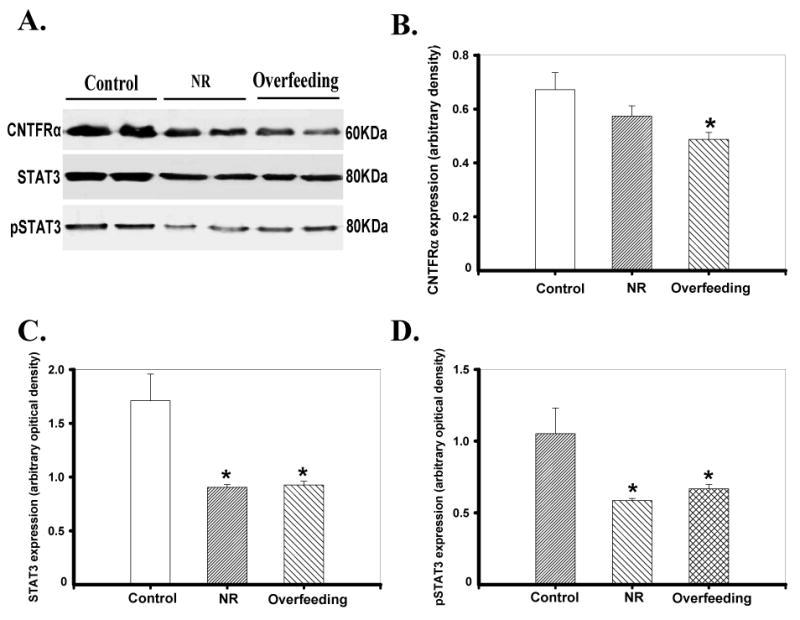
Protein expression of CNTFRα, STAT3 and phosphorylated STAT3 (pSTAT3) in ventricles from fetus of control, nutrient restricted (NR) and overfed ewes. Panel A: Actual gel blotting depicting CNTFRα, STAT3 and pSTAT3 proteins using specific antibodies; Panels B-D: CNTFRα, STAT3 and pSTAT3 protein abundance in the three groups. Mean ± SEM, n = 5-10 per group, * p < 0.05 vs. control.
Fig 2.

Protein expression of IGF-1 receptor (panel A) and IGFBP3 (panel B) in ventricles from fetus of control, nutrient restricted (NR) and overfed ewes. Insets: Actual gel blotting depicting IGF-1R and IGFBP3 proteins using specific antibodies; Mean ± SEM, n = 5-10 per group.
Discussion
As we have previously reported (19), early to mid gestational nutrition restriction significantly reduces fetal growth and CRL in conjunction with minimal effects on absolute weight and size of fetal hearts and both ventricles. In contrast, enhanced gestational nutrient supply by 50% contributes to cardiac (ventricular) hypertrophy, increased fetal growth and CRL. In both under- and over-nutrition ventricular size is increased relative to whole body weight. When exposed to excess nutrients, the fetal heart shows reduced expression of CTNFRα and STAT3 (both non-active and active forms). Interestingly, STAT3 and phosphorylated STAT3 but not CTNFRα were also reduced in the hearts of fetuses exposed to nutrient restriction. Plasma IGF-1 levels were reduced and increased, in fetuses from nutrient restriction and overfeeding groups. Nonetheless, neither IGF-1R nor IGFBP3 was significantly affected by nutrient restriction and overfeeding. Fetal plasma cortisol was increased in both nutrient restriction and overfeeding groups compared to controls. Given that nutrition restriction is associated with retarded cardiac growth (18;19), our data suggest that circulating IGF-1 and CTNFRα-STAT3 signaling cascade may play a role in cardiac hypertrophy in response to early to mid gestational overfeeding.
There are several possible explanations for the observations we have made resulting from gestational overfeeding and nutrient restriction-induced changes in fetal heart growth. First, plasma IGF-1 levels were reduced and elevated, in nutrition restricted and overfed fetuses. This change in plasma IGF-1 levels correlates well with the absolute fetal weights under nutrition restriction (29.9% reduction) and overfeeding (22.2% increase). Earlier studies have indicated that maternal undernutrition during periconceptual and gestational periods may lead to significant falls in fetal IGF and IGF binding protein-3 (IGFBP-3) levels in sheep (17, 20). Nonetheless, our data favored a role of IGF-1 rather than IGF-1R or IGFBP3 in altered fetal heart weights in response to nutrition restriction and overfeeding. On the other hand, plasma levels of cortisol were increased in fetuses from nutrition restricted and overfed ewes, with a much higher degree of rise in the nutrient surplus group. These data suggest that a less likely role of cortisol in cardiac hypertrophy following gestational overfeeding. Our present data suggested a tie between CNTFRα levels and absolute heart weights, which may contribute to gestational overfeeding-induced cardiac hypertrophy. CNTFR is localized to the sarcolemma and plays a role in the determination of cardiac geometry. Activation of CNTFR has been demonstrated to regress established left ventricular hypertrophy in obese mice (15), suggesting a role of CNTF in antagonism of cardiac hypertrophy. This reciprocal relationship between CNTFR and cardiac hypertrophy is supported by our finding of reduced CNTFRα and enhanced heart and ventricular weights in fetuses of overfed mothers. Our data failed to detect any significant change in CNTFRα levels following maternal nutrition restriction, a finding consistent with the unaffected heart and ventricular weight (and size) in nutrient restricted fetus.
Our results revealed reduced STAT3 and STAT3 phosphorylation, downstream signaling molecule of CNTFRα (15), in both nutrient restricted and overfed groups, which does not favor a critical role of STAT3 and its activation in the development of cardiac hypertrophy. Although STAT3 is thought to be important for induction of cell hypertrophy, its activation does not necessarily result in cardiac hypertrophy, at least in response to certain hypertrophic triggers (21). Although our data seem to suggest a correlation between reduced CNTFRα levels and cardiac hypertrophy under gestational overfeeding, we cannot exclude the possible contribution of systemic effects. For example, CNTF-elicited central nervous system effects may directly influence the heart through activation of the central nervous system and thus mediate metabolic actions of CNTF centrally. It has also been speculated that the non-cardiomyocyte distribution of the CNTF receptors such as cardiac ganglia may indirectly affect geometry within the heart (15). In addition, gestational nutrition status may influence placental vascular resistance, peripheral vascular resistance and blood viscosity (22), leading to an alteration in cardiac afterload and compensated cardiac hypertrophy. Further investigation is warranted to determine the possible participation of these mechanism(s) in fetal cardiac hypertrophy following alteration in maternal nutrient supply.
In summary, our findings depicted that early to mid gestational overfeeding promotes fetal cardiac growth whereas maternal nutrient restriction did not affect heart growth despite of retarded fetal growth. A downregulation in the expression of cardiac CTNFRα and its down-stream signaling molecule STAT3 accompanied intrauterine overfeeding-induced cardiac hypertrophy. Given that reduced STAT3 signaling is also seen in nutrition restricted fetuses where cardiac hypertrophy is absent, our data seem to indicate the existence of alternative mechanisms in addition to STAT3 in promoting fetal cardiac growth following alteration in maternal nutrient supply during early to mid gestation. These experimental findings may provide some useful insights for a better understanding of “nutritional programming” of postnatal cardiac hypertrophy.
Acknowledgments
This work was supported in part by a grant from American Heart Association Pacific Mountain Affiliate (0355521Z) and NIH INBRE # P20 RR16474-04.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Langley-Evans SC, Bellinger L, McMullen S. Animal models of programming: early life influences on appetite and feeding behaviour. Matern Child Nutr. 2005;1(3):142–148. doi: 10.1111/j.1740-8709.2005.00015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buckley AJ, Jaquiery AL, Harding JE. Nutritional programming of adult disease. Cell Tissue Res. 2005;322(1):73–79. doi: 10.1007/s00441-005-1095-7. [DOI] [PubMed] [Google Scholar]
- 3.Moore SE. Nutrition, immunity and the fetal and infant origins of disease hypothesis in developing countries. Proc Nutr Soc. 1998;57(2):241–247. doi: 10.1079/pns19980038. [DOI] [PubMed] [Google Scholar]
- 4.Wu G, Bazer FW, Cudd TA, Meininger CJ, Spencer TE. Maternal nutrition and fetal development. J Nutr. 2004;134(9):2169–2172. doi: 10.1093/jn/134.9.2169. [DOI] [PubMed] [Google Scholar]
- 5.Yajnik CS. Obesity epidemic in India: intrauterine origins? Proc Nutr Soc. 2004;63(3):387–396. doi: 10.1079/pns2004365. [DOI] [PubMed] [Google Scholar]
- 6.Godfrey K, Robinson S. Maternal nutrition, placental growth and fetal programming. Proc Nutr Soc. 1998;57(1):105–111. doi: 10.1079/pns19980016. [DOI] [PubMed] [Google Scholar]
- 7.Godfrey KM, Barker DJ. Fetal nutrition and adult disease. Am J Clin Nutr. 2000;71 5:1344S–1352S. doi: 10.1093/ajcn/71.5.1344s. [DOI] [PubMed] [Google Scholar]
- 8.Barker DJ. Intrauterine programming of coronary heart disease and stroke. Acta Paediatr Suppl. 1997;423:178–182. doi: 10.1111/j.1651-2227.1997.tb18408.x. [DOI] [PubMed] [Google Scholar]
- 9.McMillen IC, Muhlhausler BS, Duffield JA, Yuen BS. Prenatal programming of postnatal obesity: fetal nutrition and the regulation of leptin synthesis and secretion before birth. Proc Nutr Soc. 2004;63(3):405–412. doi: 10.1079/pns2004370. [DOI] [PubMed] [Google Scholar]
- 10.Muhlhausler BS. Programming of the appetite-regulating neural network: a link between maternal overnutrition and the programming of obesity? J Neuroendocrinol. 2007;19(1):67–72. doi: 10.1111/j.1365-2826.2006.01505.x. [DOI] [PubMed] [Google Scholar]
- 11.Schneider H. Ontogenic changes in the nutritive function of the placenta. Placenta. 1996;17(1):15–26. doi: 10.1016/s0143-4004(05)80639-3. [DOI] [PubMed] [Google Scholar]
- 12.Sleeman MW, Garcia K, Liu R, Murray JD, Malinova L, Moncrieffe M, et al. Ciliary neurotrophic factor improves diabetic parameters and hepatic steatosis and increases basal metabolic rate in db/db mice. Proc Natl Acad Sci U S A. 2003;100(24):14297–14302. doi: 10.1073/pnas.2335926100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elmquist JK, Elias CF, Saper CB. From lesions to leptin: hypothalamic control of food intake and body weight. Neuron. 1999;22(2):221–232. doi: 10.1016/s0896-6273(00)81084-3. [DOI] [PubMed] [Google Scholar]
- 14.Sleeman MW, Anderson KD, Lambert PD, Yancopoulos GD, Wiegand SJ. The ciliary neurotrophic factor and its receptor, CNTFR alpha. Pharm Acta Helv. 2000;74(23):265–272. doi: 10.1016/s0031-6865(99)00050-3. [DOI] [PubMed] [Google Scholar]
- 15.Raju SV, Zheng M, Schuleri KH, Phan AC, Bedja D, Saraiva RM, et al. Activation of the cardiac ciliary neurotrophic factor receptor reverses left ventricular hypertrophy in leptin-deficient and leptin-resistant obesity. Proc Natl Acad Sci U S A. 2006;103(11):4222–4227. doi: 10.1073/pnas.0510460103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ziotopoulou M, Erani DM, Hileman SM, Bjorbaek C, Mantzoros CS. Unlike leptin, ciliary neurotrophic factor does not reverse the starvation-induced changes of serum corticosterone and hypothalamic neuropeptide levels but induces expression of hypothalamic inhibitors of leptin signaling. Diabetes. 2000;49(11):1890–1896. doi: 10.2337/diabetes.49.11.1890. [DOI] [PubMed] [Google Scholar]
- 17.Gallaher BW, Breier BH, Keven CL, Harding JE, Gluckman PD. Fetal programming of insulin-like growth factor (IGF)-I and IGF-binding protein-3: evidence for an altered response to undernutrition in late gestation following exposure to periconceptual undernutrition in the sheep. J Endocrinol. 1998;159(3):501–508. doi: 10.1677/joe.0.1590501. [DOI] [PubMed] [Google Scholar]
- 18.Dong F, Ford SP, Fang CX, Nijland MJ, Nathanielsz PW, Ren J. Maternal nutrient restriction during early to mid gestation up-regulates cardiac insulin-like growth factor (IGF) receptors associated with enlarged ventricular size in fetal sheep. Growth Horm IGF Res. 2005;15(4):291–299. doi: 10.1016/j.ghir.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 19.Vonnahme KA, Hess BW, Hansen TR, McCormick RJ, Rule DC, Moss GE, et al. Maternal undernutrition from early- to mid-gestation leads to growth retardation, cardiac ventricular hypertrophy, and increased liver weight in the fetal sheep. Biol Reprod. 2003;69(1):133–140. doi: 10.1095/biolreprod.102.012120. [DOI] [PubMed] [Google Scholar]
- 20.Osgerby JC, Wathes DC, Howard D, Gadd TS. The effect of maternal undernutrition on ovine fetal growth. J Endocrinol. 2002;173(1):131–141. doi: 10.1677/joe.0.1730131. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi N, Saito Y, Kuwahara K, Harada M, Tanimoto K, Nakagawa Y, et al. Hypertrophic responses to cardiotrophin-1 are not mediated by STAT3, but via a MEK5-ERK5 pathway in cultured cardiomyocytes. J Mol Cell Cardiol. 2005;38(1):185–192. doi: 10.1016/j.yjmcc.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 22.Ferrazzi E, Bozzo M, Rigano S, Bellotti M, Morabito A, Pardi G, et al. Temporal sequence of abnormal Doppler changes in the peripheral and central circulatory systems of the severely growth-restricted fetus. Ultrasound Obstet Gynecol. 2002;19(2):140–146. doi: 10.1046/j.0960-7692.2002.00627.x. [DOI] [PubMed] [Google Scholar]


