Abstract
The cornea is one of the most highly innervated tissues in the mammalian host. We hypothesized changes to cornea innervation through chemical sympathectomy would significantly alter the host response to the neurotropic viral pathogen, herpes simplex virus type 1 (HSV-1) following ocular infection. Mice treated with 6-hydroxydopamine hydrobromide displayed reduced tyrosine hydroxylase-positive fibers residing in the cornea. Sympathectomized mice were also found to show a transient rise in virus recovered in infected tissues and succumbed to infection in greater numbers. Whereas there were no differences in infiltrating leukocyte populations including HSV-1-specific cytotoxic T lymphocytes in the infected tissue, an increase in substance P and a decrease in IFN-γ levels in the trigeminal ganglion but not brain stem of sympathectomized mice were noted. Sympathectomized mice treated with the neurokinin-1 receptor antagonist L703,606 had delayed mortality implicating the involvement of substance P in HSV-1-mediated death.
Keywords: herpes Simplex Virus 1, 6-hydroxydopamine, substance P
1. Introduction
A complex network of specialized cells and soluble factors comprise the mammalian immune system that is broadly divided into innate and adaptive components. Both endogenous and exogenous pathways regulate the integrity and quality of the immune response to various stimuli. For example, it is now recognized organized immune tissue (e.g., spleen, lymph nodes, and thymus) is “hard-wired” with cholinergic, peptidergic, and adrenergic nerve fibers (Felten and Felten, 1991). The importance of innervated immune organs is underscored by findings that suggest disruption of noradrenergic fibers using the neurotoxin, 6-hydroxydopamine (6OHDA) results in changes in antibody production (Livnat et al., 1985; Kohm and Sanders, 1999), delayed-type hypersensitivity responses (Madden et al., 1989), natural killer (NK) cell activity (Reder et al., 1989), and T cell proliferation (Moynihan et al., 2004) which collectively, can modify host susceptibility to infection (Leo et al., 1998; Miura et al., 2001) and severity of autoimmune disease including experimental autoimmune encephalitis (Chelmicka-Schorr et al., 1988) and experimental rheumatoid arthritis (Felton et al., 1992; Härle et al., 2005). 6OHDA treatment results in the activation of the hypothalamic pituitary adrenal axis leading to an elevation in circulating corticosterone (Leo et al., 1998), a potent immunomodulator (McEwen et al., 1997). Likewise, 6OHDA treatment in adult rodents results in the reversible destruction of noradrenergic termini through a loss in membrane integrity elicited by the production of hydrogen peroxide and hydroxyl radicals (Picklo, 1997). As a result, a limited but bolus release of norepinephrine ensues that can bind to adrenergic receptors on lymphocytes and modify immune cell function (Ackerman et al., 1991).
To further explore the relationship between innervation of organized immune organs and the host response to infection, a study was undertaken using the neurotropic DNA virus, herpes simplex virus 1 (HSV-1) in an ocular mouse model of infection. The cornea is one of the most highly innervated tissues of the mammalian host with sensory and autonomic fibers that reside between the basal epithelial cell layer and Bowman's capsule (Whitear, 1960; Ohara et al., 2000). Following the introduction of HSV-1 onto a scarified cornea, the virus replicates locally detected by pattern recognition receptors including toll-like receptor 9 which then elicit the expression of a variety of chemokines and cytokines that act to suppress virus replication and recruit leukocytes including neutrophils, macrophages, NK cells, and CD4+ T cells (Carr and Tomanek, 2006; Wuest et al., 2006; Lepisto et al., 2007). This model is also useful for characterization of HSV-1 latency and subsequent reactivation, the major issue in human morbidity (Shimeld et al., 1995; Turner and Jenkins, 1997). It was anticipated sympathectomy would have dramatic (suppressive) effects on the immune response to ocular HSV-1 infection. While 6OHDA-treated mice were rendered more susceptible to infection, changes in immune status were generally unremarkable with increased susceptibility to infection associated only with a reduction in IFN-γ in the TG of 6OHDA-treated mice.
2. Materials and Methods
2.1 Mice
Male and female C57BL/6 mice were obtained from The Jackson Labs (Bar Harbor, ME). Chemical sympathectomy was initiated by intraperitoneal injections of 80 mg/kg 6-hydroxydopamine hydrobromide (6OHDA) in 0.1% ascorbic acid daily over 3 consecutive days. This treatment regimen has previously been found to sympathectomize animals for 10 days (Härle et al., 2005). Immediately following the last treatment with 6OHDA, mice were anesthetized and the cornea of age (5-8 weeks old) and sex-matched (male and female) mice were scarified using a 25-gauge needle. Next, 1,000 plaque forming units (pfu) of HSV-1 (strain McKrae) was applied in a volume of 3.0 μl in RPMI-1640. At the indicated time post infection (pi), the mice were euthanized and perfused with PBS (pH 7.4). The corneas, trigeminal ganglia (TG), and brain stems (BS) were removed and placed in RPMI-1640 medium for determination of virus quantity by plaque assay. The tissue was homogenized in 500 μl of medium and the supernatant was clarified (10,000 × g, 1 min) and used immediately for the detection of virus (by plaque assay). Alternatively, the corneas, TG, and BS of infected were removed and placed in PBS containing a protease inhibitor cocktail set I (Calbiochem, San Diego, CA). Following homogenization on ice, the supernatant was centrifuged (10,000 × g, 1 min) and subsequently assessed for cytokine/chemokine content by ELISA. To determine if the effect of 6OHDA on survival of HSV-1-infected mice was dependent on substance P, mice were sympathectomized and infected as described. On day 3, 5, and 7 pi, mice were administered intraperitoneally with vehicle (PBS) or 5.0 mg/kg L-703,606 in a volume of 100 μl and monitored for survival over a 9 day pi period. All procedures were approved by The University of Oklahoma Health Sciences Center and Dean A McGee Eye Institute animal use committees.
2.2 Viral plaque assay
Clarified supernatant from homogenized tissue was serially diluted and placed onto Vero cell monolayers in 96-well cultured plates in a 100 μl volume. After a 1-hr incubation at 37° C in 5% CO2, and 95% humidity, the supernatants were removed, and 75 μl of an overlay solution (0.5% methylcellulose in RPMI-1640 supplemented with 10% FBS, gentamicin, and antibiotic/anti-mycotic solution) was added to the well. The cultures were incubated at 37° C in 5% CO2, and 95% humidity for 30-34 h to observe plaque formation, and the amount of infectious virus was reported as pfu/tissue.
2.3 Measurement of cytokines, chemokines and substance P
The detection of CXCL1, CCL2, IFN-γ, TNF-α, and substance P was performed using commercially available kits (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions. The sensitivity for the detection of the chemokines/cytokines ranged from 15.0-25.0 pg/tissue. Each sample was assayed in duplicate along with a standard provided in the kit to generate a standard curve used to determine the unknown amount of targeted cytokine/chemokine. Standard curves did not fall below a correlation coefficient of .9800.
2.4 Flow cytometry
At the designated time, mice were exsanguinated and corneas, TG, BS, spleen and draining (mandibular and cervical) lymph nodes were removed. Tissues were processed to generate single cell suspensions as described (Carr et al., 2006) and were transferred into 5 ml polystyrene round-bottomed tubes (Becton Dickinson, Franklin Lakes, NJ). The cells were incubated with 2 μl of anti-mouse CD16/32 (Fcγ III/II receptor, 2.4G2, BD Biosciences Pharmingen, San Diego, CA) for 20 min on ice. For measuring CD4 T, CD8 T, and NK cell content, cells were labeled with 1-2 μg of FITC-conjugated anti-CD3 (17A2), and PE-conjugated anti-CD4 (RM4-5) or anti-CD8α (53-6.7) or anti-NK1.1 (PK136), and PE-Cy5-anti-CD45 (30-F11) and allowed to incubate on ice in the dark. After a 30 min incubation, the cells were washed (300 × g, 5 min at 4° C) and resuspended in 1% paraformaldehyde for 60 min. Cells were then washed as before and resuspended in 3 ml of 1x PBS containing 1% BSA. A known number of Countbright™ beads (20,800) (Invitrogen, Eugene, OR) was immediately added to the sample which was then briefly vortexed and then analyzed on a Coulter Epics XL flow cytometer (Beckman Coulter, Miami, FL). Cells were gated on CD45high expressing cells, and a second gate was established to capture the number of fluorescent beads that passed through during the sampling time. The absolute number of leukocytes (CD45high) in the tissue was determined by multiplying the ratio of the number of beads collected per sample over the total number of beads added X the number of CD45high events X sample dilution factor. Isotypic control antibodies were included in the analysis to establish background fluorescence levels. Likewise, samples from uninfected mice were also analyzed to determine the degree of contamination from incomplete perfusion. For measuring draining lymph nodes and spleen T and NK cell content, single cell suspensions (1×106 cells) were transferred into 5 ml polystyrene round-bottomed tubes and labeled with antibodies and analyzed by flow cytometry. The absolute number of cells was determined by multiplying the percentage of T or NK cell populations found in each lymphoid organ by the total number of cells recovered.
2.5 Whole-mount preparation
Corneas from vehicle- and 6OHDA-treated, HSV-1-infected mice were removed and fixed for 20 minutes in 4% paraformaldehyde at 4° C. Following fixation, the corneas were washed fives times with 1% Triton-X100 in 1X PBS for 15 minutes each and blocked by soaking each cornea in 100ul of PBS-BGEN(3% BSA, .25% Gelatin, 5mM EDTA, .025% Nonidet-P40 in 1X PBS) with 5ug CD16/32 (BD Biosciences). After a two hour incubation, 5ul of normal goat serum was added to the blocking buffer and the corneas were incubated overnight at 4° C. The corneas were then stained with 2 μl of rabbit anti-tyrosine hydroxylase (Chemicon International, AB152) for 2 hrs on ice. The corneas were washed five times and incubated with a 1:500 dilution of Alex fluor 594-conjugated goat anti-rabbit IgG (Invitrogen Molecular Probes, Gaithersburg, MD) for 2 hrs on ice. Corneas were then incubated over night with 50 μl of 1.5 μg/ml Vectashield Mounting Media with Dapi (Vector Labs, Burlingame, CA) and visualized by confocal miscroscopy.
2.6 Confocal microscopy
Corneas were imaged using an Olympus IX81-FV500 epifluorescence/confocal laser-scanning microscope with a UApo 40X water immersion lens. Samples were excited with 405, 488, and 546 nm wavelength lasers. Scanning images were taken with a step size of 1.2 μm in the z axis and image analysis was performed using Fluoview software (Olympus).
2.7 Tetramer staining
For tetramer staining, single cell suspensions obtained from the draining lymph nodes, TG, and BS of HSV-1-infected vehicle- or 6OHDA-treated mice at day 7 pi were labeled with 1-2 μg of the HSV peptide gB498-505 (SSIEFARL) specific MHC tetramer (MHC Tetramer Lab, Houston, TX) for 60 min. The cells were washed (300 × g, 5 min at 4° C) and labeled with 1-2 μg of FITC-conjugated anti-CD8 and PE-Cy5-conjugated anti-CD45. Following a 30 min incubation, cells were washed (300 × g, 5 min at 4° C) and resuspended in 1% paraformaldehyde. After a 60 min incubation, the cells were resuspended in 1x PBS. Cells were subsequently analyzed by flow cytometry as described above.
2.8 CTL assay
MC57G (CRL-2295™, ATCC, Manassas, VA) were infected with HSV-1 at a multiplicity of infection of 5.0 for 6 hrs at 37°C, 5% CO2, and 95% humidity. Following infection, ten thousand MC57G cells were resuspended in pre-warmed (37°C) PBS containing 0.25 μM carboxyfluorescein diacetate succinimidyl ester (CFSE, Invitrogen) and incubated for 15 min at 37°C. Cells were washed with 1x PBS and resuspended in fresh pre-warmed complete medium. The desired number of isolated leukocytes from the processed draining lymph nodes of HSV-1 infected, vehicle- or 6OHDA-treated mice was added to the CFSE-labeled HSV-1 infected MC57G target cells in 96 well microtiter plate wells in a total volume of 200 μl of complete medium at an effector-to-target cell ratio of 10:1. After a four hr incubation, 0.5 μl of propidium iodide (0.5 μg) was added to cells followed by a 15 min incubation at 37°C, 5% CO2 and 95% humidity. Cells were then washed and resuspended in 1x PBS and immediately analyzed by XL flow cytometry. The gate was set for CFSE-expressing cells. The percent lysis (%) was calculated by dividing the number of propidium iodide labeled CFSE-expressing cells by the total number of CFSE-expressing cells multiplied by 100. The background level was determined by using target cells without effector cells and target cells incubated with spleen cells from uninfected mice.
2.9 Granzyme and perforin expression
Single cell suspensions generated from TG and BS tissue of vehicle- and 6OHDA-treated mice were carefully placed onto a Percoll gradient (20-80% Percoll in RPMI-1640) in a volume of 0.5 ml. The cells were centrifuged at 2,000 × g for 20 min at 20° C. Cells that migrated to the 30% Percoll layer were removed and washed in RPMI-1640 containing 10% FBS. The cells (1 × 105) were then placed in wells of 24-well culture plates and 10 μg of HSV peptide gB498-505 peptide was added. After a 4 hr incubation at 37° C in 95% humidity, the cells were collected, centrifuged (300 × g, 5 min) and subsequently labeled initially with CD16/32 (Fc block) and then FITC-conjugated anti-CD8 and PE-Cy5-conjugated anti-CD45 as described above. After a 30 min incubation period on ice in the dark, the cells were washed (300 × g, 5 min at 4 ° C) in PBS containing 1% BSA. Next, 100 μl of Cytofix/Cytoperm (BD Biosciences Pharmingen) was added and the cells were incubated for 20 min at room temperature. The cells were then washed (300 × g, 5 min at 4 ° C) twice and then labeled with PE-conjugated anti-granzyme B, anti-perforin antibody, or isotypic control (EBiosciences, San Diego, CA). Following a 20 min incubation, the cells were washed twice as before and resuspended in 1.0 ml of PBS containing 1% BSA and analyzed by flow cytometry for the percent granzme B- or perforin-positive CD8+ T cells.
2.10 Measurement of mouse excrement
Vehicle- and 6OHDA-treated mice were housed in single mouse metabolic cages (Nalgene, Rochester, NY) for the measurement of urine and fecal output starting immediately after infection with HSV-1. Mice were weighed immediately prior to the start of the experiment and then administered vehicle or 6OHDA (50 mg/kg, i.p.) daily for three days. The mice were then infected with HSV-1 (1,000 pfu/eye) and placed into metabolic cages for daily monitoring of urine and fecal output. At the end of the experiment, the mice were removed from the cages, anesthetized, weighed, and subsequently euthanized for analysis of cytokine/chemokine detection in infected tissue.
2.11 Statistics
A Bonferoni corrected T-test was used to determine significance (p<.05) comparing the vehicle- (WT) to 6OHDA-treated mice using the GBSTAT program (Dynamic Microsystems, Silver Spring, MD). The Mann-Whitney rank order test was performed to determine significance (p<.05) in survival comparing vehicle- to 6OHDA-treated, HSV-1-infected mice over a 9 day survey period.
3. Results
3.1. 6OHDA treatment reduces tyrosine hydroxylase expressing sympathetic nerve fibers in the cornea
The daily treatment of mice with 6OHDA (80 mg/kg, i.p.) for three consecutive days has previously been found to sympathectomize mice as measured by norepinephrine content in the spleen (Härle et al., 2005). However, whether systemic treatment with 6OHDA resulted in a loss of sympathetic innervation in the cornea was unknown. Consequently, the corneas of vehicle- and 6OHDA-treated mice were evaluated for existence of sympathetic nerve fibers. Whereas there was an impressive array of tyrosine hydroxylase-expressing fibers in the basal aspects of the cornea of vehicle-treated mice, 6OHDA-treated animals displayed a significant reduction (Fig. 1). To the best of our knowledge, this finding is the first report by which systemic 6OHDA treatment can dramatically reduce sympathetic innervation of the cornea.
Figure 1.
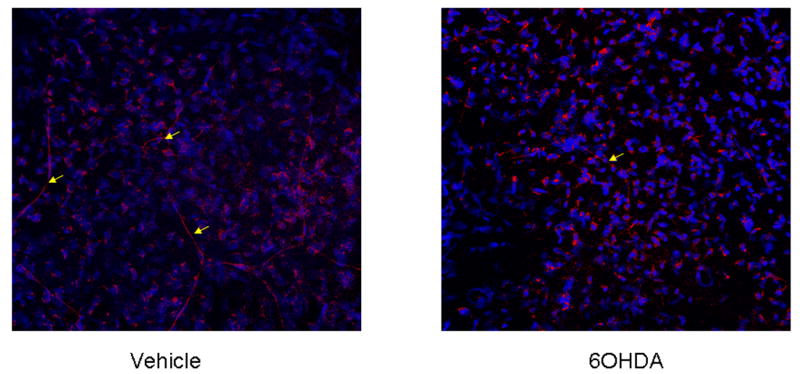
6OHDA treatment reduces tyrosine hydroxylase-expressing sympathetic fibers in the cornea of mice. Mice (n=2/group) were administered vehicle (0.1% ascorbic acid in PBS) or 6OHDA (80 mg/kg) intraperitoneally daily for 3 days. Mice were exsanguinated, and the corneas were removed and stained for tyrosine hydroxylase expression by whole mount staining. The corneas were evaluated by laser scanning confocal microscopy. A representative image of a cornea flat mount at 400x is shown for a vehicle- and 6OHDA-treated mouse. Yellow arrows denote tyrosine hydroxylase-expressing sympathetic nerve fibers.
3.2. Chemical sympathectomy results in an increase in susceptibility to HSV-1 infection
Since we found 6OHDA could reduce corneal sympathetic innervation, we anticipated sympathectomized mice would show changes in susceptibility to ocular HSV-1 infection. To test this hypothesis, vehicle- and 6OHDA-treated mice were evaluated for HSV-1 in infected tissue over the course of acute infection. Assessment of virus titer in the cornea of mice found a modest but insignificant increase in HSV-1 in the cornea of 6OHDA-treated mice over the 9 day period following infection (Fig. 2A). In contrast, there was a transient but significant rise in HSV-1 recovered in the iris, TG, and BS of 6OHDA-treated animals by day 7 pi (Fig. 2A). The increase in virus titer in the nervous system correlated with cumulative survival measured over 9 days pi in which 11/21 6OHDA-treated mice succumbed to infection compared to 2/21 of the vehicle-treated group (Fig. 2B).
Figure 2.
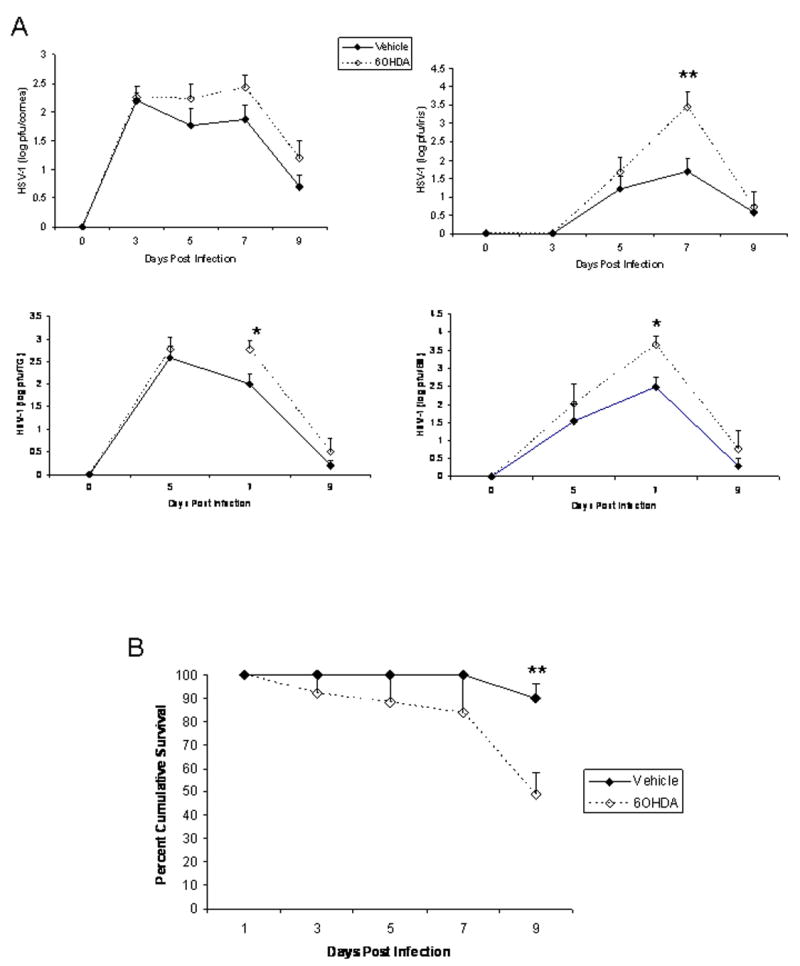
6OHDA increases susceptibility to HSV-1 infection. Vehicle- or 6OHDA-treated mice were infected with HSV-1 (1,000 pfu/eye). (A) At the indicated time post infection, the mice were euthanized, and the corneas, irises, trigeminal ganglia (TG), and brain stems were removed and homogenized. Clarified supernatants from the homogenate were assayed for virus content by plaque assay. The results are a summary of 2-4 experiments for each time point (n=3 mice/group/experiment). The results are expressed as mean log pfu ± SEM. **p<.01, *p<.05 comparing the vehicle- to 6OHDA-treated groups at the 7 day post infection time point. (B) Mice were surveyed for survival over a 9 day period post infection. The results are a summary of 5 experiments (n=4-5 mice/experiment) expressed as mean percent survival ± SEM. **p<.01 comparing the vehicle- to 6OHDA-treated mice.
3.3. Chemical sympathectomy does not alter recruitment of leukocyte populations to infected tissue following HSV-1 infection
We have previously found a change in leukocyte migration is associated with increases in virus titer in the nervous system (i.e., TG and BS) during acute HSV-1 infection (Carr et al., 2006). Since we found a significant increase in virus residing in the TG and BS by day 7 pi as well as an increase in mortality in 6OHDA-treated, HSV-1-infected mice, we investigated changes in leukocyte migration recovered from the vehicle- and 6OHDA-treated mice at day 7 pi. In contrast to what was anticipated, there was no difference in the absolute number of NK cell, NKT cell, CD4+ T cell, CD8+ T cell, HSV-specific CD8+ T cell, neutrophil (F4/80-Gr1+), macrophage (CD45hi, F4/80+Gr1-), or activated macrophage (CD45hiF4/80+Gr1+) populations residing in the TG or BS comparing vehicle- to 6OHDA-treated animals following HSV-1 infection (Fig. 3). Similar numbers of these cell populations were also found in the draining lymph nodes as well as the corneas comparing the two treated groups of mice (data not shown).
Figure 3.
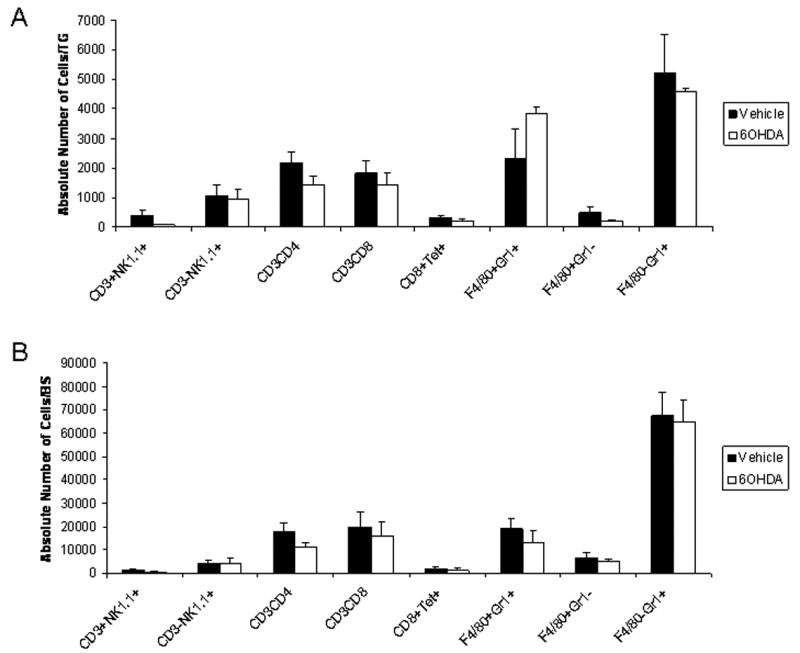
Leukocyte trafficking to the nervous system following ocular HSV-1 infection is not modified in sympathectomized mice. Vehicle- and 6OHDA-treated, HSV-1-infected mice were exsanguinated seven days pi. The trigeminal ganglia (TG) and brain stems (BS) were removed, processed, and analyzed for the indicated leukocyte subpopulations. Bars represent the mean absolute number of cells/TG (A) or cells/BS (B) ± SEM, n=10/group for each phenotype.
3.4 Sympathectomized mice show no change in draining lymph node CTL activity
6OHDA-treatment has been reported to significantly reduce the generation of CTLs following intraperitoneal infection with HSV-1 (Leo and Bonneau, 2000). As a means to explain our findings, we next sought to measure CTL function. Due to technical limitations, we were unable to recover sufficient cells from the TG and BS to measure CTL activity. However, effector cells obtained from the draining lymph nodes of vehicle- and 6OHDA-treated, HSV-1-infected mice showed similar levels of cytolytic activity (Fig. 4A) and expressed similar levels of granzyme B (Fig. 4B) and perforin (data not shown). Likewise, CD8+ T cells from the TG and BS also expressed similar levels of granzyme B (Fig. 4B) and perforin (data not shown).
Figure 4.
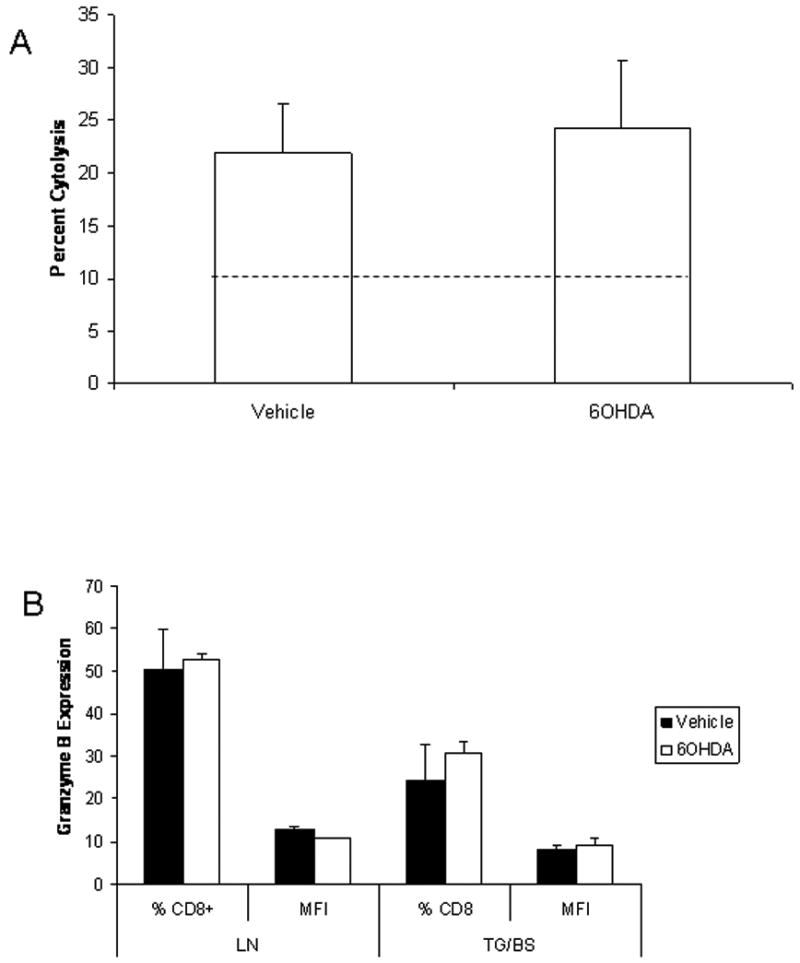
Cytolytic activity is similar comparing vehicle- and 6OHDA-treated, HSV-1 infected mice. Chemically-sympathectomized or control mice infected with HSV-1 were exsanguinated seven days pi. The cervical/mandibular lymph nodes were removed and single cell suspensions of lymph node samples were prepared and counted. (A) HSV-2 infected target cells (MC57G) were labeled with CSFE dye and incubated with lymph nodes cells at a E:T ratio of 10:1 for 4 h at 37° C. Propidium iodide (PI) was added after the 4 h incubation and the percentage of lysis was determined using flow cytometry. Percent cytolysis from labeled target cells incubated with spleen cells from uninfected mice or incubated alone ranged from 4.2 - 12.0. (B) Percoll-enriched CD8+ leukocytes from the trigeminal ganglia (TG) and brain stem (BS) or lymph node (LN) cells were analyzed for granzyme B expression by flow cytometry. The results show the mean percentage of CD8+ T cells (% CD8+) expressing granzyme B as well as the amount expressed per cell (mean fluorescence intensity, MFI) ± SEM, n=5/group.
3.5 IFN-γ levels are reduced in the TG of sympathectomized mice following HSV-1 infection
An elevation in cytokine and chemokine levels is reportedly correlative with virus titers in the nervous system but not cornea following ocular HSV-1 infection (Carr and Campbell, 2006). Therefore, we investigated select cytokine (TNF-α and IFN-γ) and chemokine (CXCL1 and CCL2) levels in the TG and BS of vehicle- and 6OHDA-treated mice at day 7 pi. Consistent with a lack of change in leukocyte trafficking to infected tissues, there were no significant differences in the level of CXCL1 or CCL2 found in the TG or BS of HSV-1-infected mice comparing vehicle- to 6OHDA-treated animals (data not shown). Likewise, there was no difference in the level of TNF-α in the TG or BS or IFN-γ in the BS comparing the treated groups of HSV-1-infected animals (data not shown). However, there was a 2-fold decrease in the amount of IFN-γ expressed in the TG of 6OHDA-treated, HSV-1-infected mice compared to the vehicle-treated group (Fig. 5A).
Figure 5.
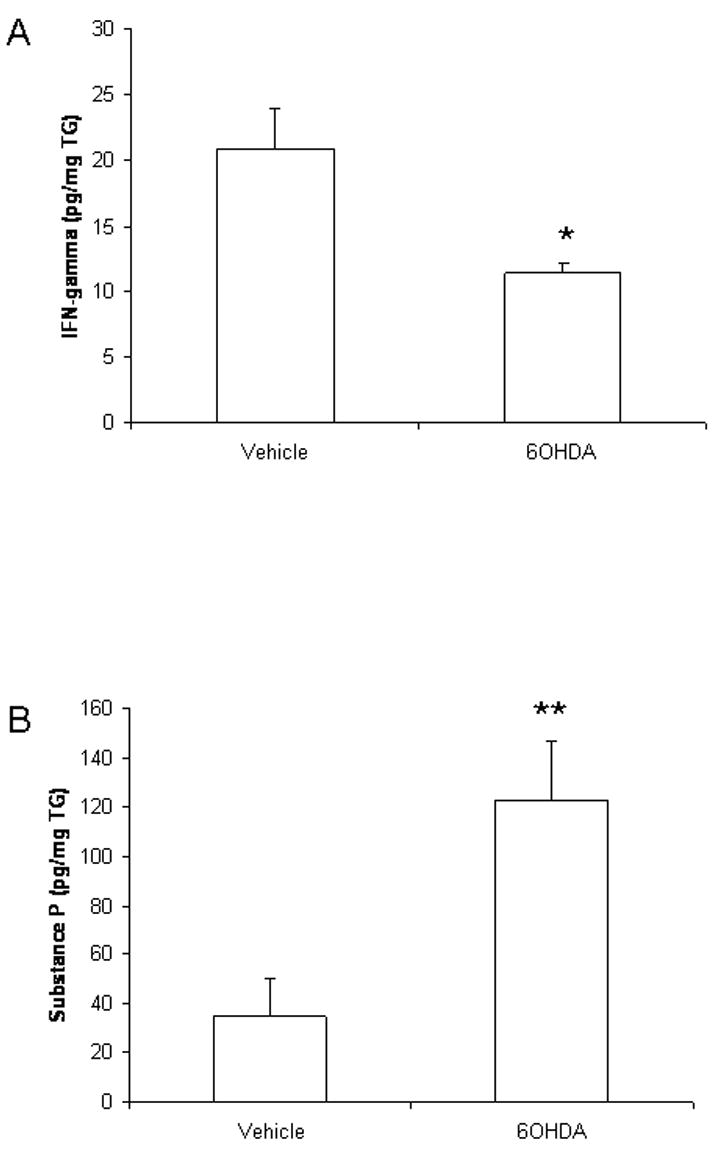
IFN-γ is suppressed and substance P elevated in the TG of sympathectomized, HSV-1-infected mice. Control (vehicle-treated) and sympathectomized, HSV-1-infected mice were exsanguinated 7 days pi. The trigeminal ganglia (TG) were removed, weighed, and homogenized in PBS containing a cocktail of protease inhibitors. Following removal of cell debri (10,000 × g, 1 min), the supernatant was assayed for IFN-γ or substance P content by ELISA. The results are displayed as mean pg/mg TG ± SEM, n=6-8/group. **p<.01, *p<.05 comparing vehicle- to 6OHDA-treated mice.
3.6 Substance P is elevated in the TG but not BS of 6OHDA-treated mice
The absence of sympathetic fibers has been found to result in a transient increase in sensory fiber innervation including substance P-expressing fibers (Ruocco et al., 2001). Moreover, substance P contributes to the inflammatory process in the nervous system (Marriott, 2004). Therefore, we investigated the presence of substance P in the TG and BS in mice infected with HSV-1. Whereas there was no difference in substance P levels found in the BS comparing vehicle- to 6OHDA-treated mice, there was a significant rise in substance P recovered in the TG of 6OHDA-treated animals following HSV-1 infection (Fig. 5B).
3.7 Sympathectomy modification of metabolism
Since substance P was found to be elevated in the TG of 6OHDA-treated, HSV-1-infected mice, we reasoned these animals would be suffering from heightened pain perception (Greco et al., 2008). Relative to the current model, we have found the chronic treatment of mice morphine resulted in greater survival compared to saline-treated animals following ocular HSV-1 infection (Alonzo and Carr, 1999). Consequently, we compared HSV-1-infected mice treated with or without 6OHDA for changes in excretion (urine and fecal mass) as a means to measure pain reasoning that elevated levels of substance P would result in an increase in pain leading to a decrease in food/water intake, and subsequent death. The results show a noticeable decrease in urinary output in HSV-1-infected mice that tends to be exaggerated in the sympathectomized mice starting at day 5 pi (6A). However, there was no change in fecal output in either group showing similar levels from day 1 thru day 7 pi (Fig. 6B). Vehicle-treated mice lost an average weight of 3.96 ± 0.64 gms compared to 4.2 ± 0.8 gms for the 6OHDA-treated mice over the course of the infection.
Figure 6.
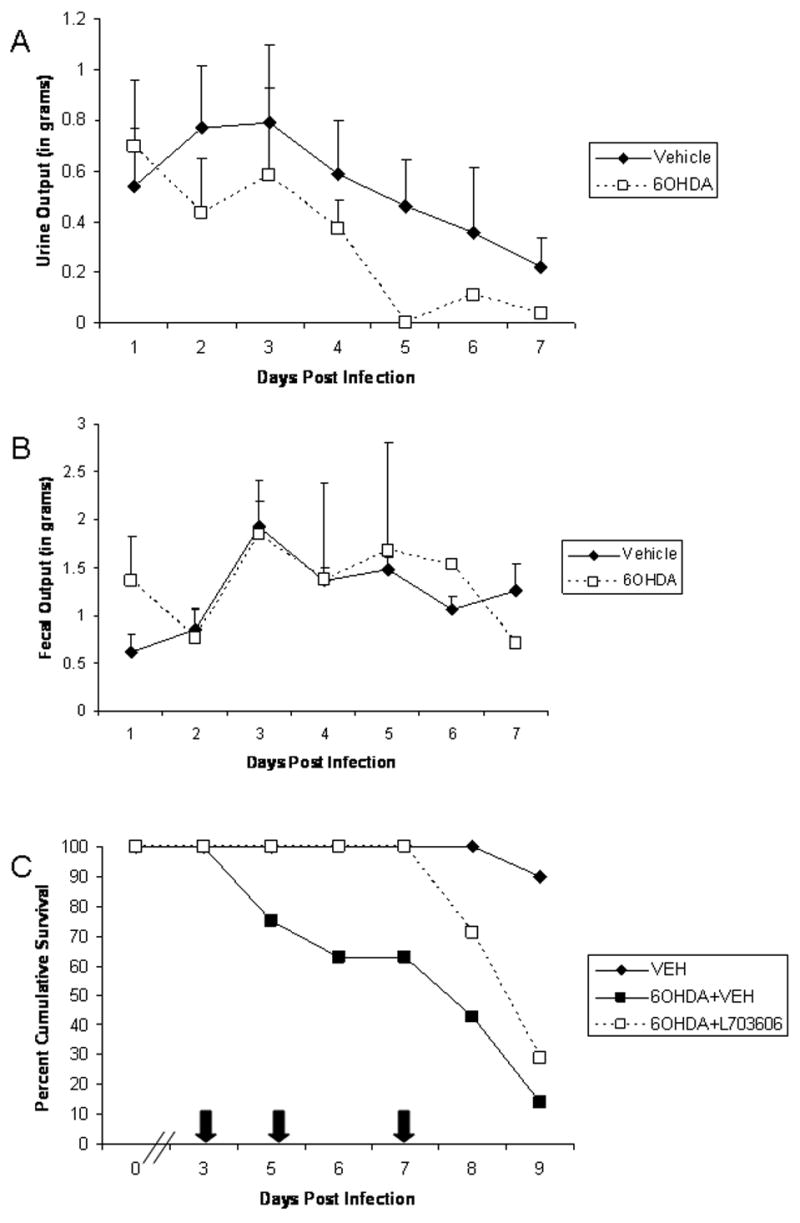
Substance P affects urinary output and mortality in 6OHDA-treated mice. Vehicle- or 6OHDA-treated, HSV-1-infected mice were housed singly in metabolic cages. Daily urine and fecal output was collected, measured, and recorded. A summary of the urinary (A) and fecal (B) output is displayed as mean ± SEM, n = 5/group. (C) Vehicle- and 6OHDA-treated, HSV-1-infected mice were treated with vehicle (PBS) or the neurokinin-1 receptor, L703,606 (5.0 mg/kg) at the indicated times (arrows) pi. The mice were monitored for survival and time of death recorded. The data is displayed as mean survival with 7 mice/group.
Since the data up to now did not directly indicate immune compromise but some association with a rise in substance P in the infected sensory ganglia, we further pursued involvement of substance P thru the use of the neurokinin-1 receptor (NK-1R) antagonist, L703,606. Sympathectomized, HSV-1-infected mice were treated at times pi with L703,606 or vehicle and monitored for survival over 9. The results show the application of the substance P receptor antagonist delayed mortality by 3 days over the course of the evaluation (Fig. 6C). However, sympathectomized mice treated with L703,606 inevitably succumbed to the infection at levels similar to vehicle-treated, sympathectomized animals.
4. Discussion
Chemical sympathectomy induced through the neurotoxin 6OHDA has been used to study the impact of innervation on the immune system including antibody production (Cross et al., 1986), NK cell activity (Cross and Roszman, 1988), and TH1 cytokine production (Madden et al., 2000). Relative to HSV-1 infection, a previous study found 6OHDA-mediated sympathectomy reduced the generation of CTLs specific for HSV-1 through the activation of the hypothalamic pituitary adrenal axis and end product corticosterone as well as the release of catecholamines (Leo et al., 1998; Leo and Bonneau, 2000). While the results were convincing, the study infected mice with a high inoculum (107 plaque forming units) of HSV-1 intraperitoneally and employed a single dose of 6OHDA (200 mg/kg) that we have found results in 40-100% mortality of our C57BL/6 mice (data not shown).
We chose to use an ocular HSV-1 model for our study as an established model that more closely mimics the human condition and in which we could explore the ramifications of sympathectomy using a lower dose (80 mg/kg daily over 3 days) on a highly innervated tissue (Whitear, 1960; Luhtala and Uusitalo, 1991; Ohara et al., 2000). Chemical sympathectomy of the cornea led to a transient rise in HSV-1 titer recovered in the iris, TG, and BS of mice with no significant difference found in the cornea by day 7 pi. The loss of 50% of 6OHDA-treated mice as a result of virus infection by day 9 pi may have skewed the titer recovered in the tissue masking any significant difference comparing the sympathectomized to vehicle-treated animals at that time point. Based on previously cited studies and the mortality rate suffered by the 6OHDA-treated, HSV-1-infected mice in the current study, we predicted the increase in sensitivity of sympathectomized mice to ocular HSV-1 infection would correlate with changes in the host immune response to the pathogen. However, with the exception of a decrease in IFN-γ levels in the TG of 6OHDA-treated mice, there were no significant changes in CTL effector number or function, the infiltration of PMNs, NK cells, T cells, or macrophages into infected tissue, or expression of selective cytokines (TNF-α and IFN-γ) or chemokines (CXCL1 and CCL2) found in the cornea, TG, or BS comparing sympathectomized versus non-sympathectomized mice. However, there was a noticeable increase in the level of substance P found in the TG of 6OHDA-treated, HSV-1-infected mice which might explain the moribund behavior of the sympathectomized animals. In fact, urine output dramatically dropped in the 6OHDA-treated mice by day 5 pi. This drop correlated with the death rate displayed by the sympathectomized mice as well.
As a means to understand the contribution of substance P in the sensitivity of 6OHDA-treated mice to ocular HSV-1 infection, the neurokinin 1 receptor (NK1R) antagonist, L703,606 (Shepherd et al., 2005) was administered to 6OHDA-treated mice at times after HSV-1 infection. Treatment of sympathectomized mice with the NK1R antagonist was found to delay mortality by 3 days although eventually the survival rate was similar to vehicle-treated, sympathectomized mice. The incomplete protection based on survival in the NK1R antagonist-treated animals may be due to the incomplete action of the NK1R antagonist as the levels of substance P rise in the infected, sympathectomized mice outcompetes for NK1R activation or the dual role of substance P associated with pain perception (Lundy and Linden, 2004) as well as an immunomodulator. For example, substance P elicits the production of cytokines including TNF-α (Lotz et al., 1988), and IFN-γ (Lighvani et al., 2005) which are linked to the control of HSV-1 infection. As T and B lymphocytes and monocyte/macrophages express the NK1R (Stanisz et al., 1987; Pascual et al., 1992; Marriott and Bost, 2000), it has been suggested substance P may serve to optimize the local inflammatory response (Marriott and Bost, 2000) which may facilitate the clearance of pathogens (Brogden et al., 2005; Svensson et al., 2005) but have detrimental consequences relative to sites sensitive to inflammation as is the case of rheumatoid arthritis (Miller et al., 2000) and most likely, the CNS. In contrast to what has been reported, we found an elevation in substance P was associated with a reduction of IFN-γ in the TG but not BS of HSV-1-infected, sympathectomized mice. Consequently, the change in IFN-γ within the TG but not CNS of 6OHDA-treated, HSV-1-infected mice would not explain the increased sensitivity to the virus infection. Although there is currently no explanation for the reduction in IFN-γ found in the TG of 6OHDA-treated mice infected with HSV-1, it is tempting to speculate changes in migration of effector T or NK cells into the TG of sympathectomized mice may not allow for direct apposition to HSV-1-infected cells resulting in less than optimal conditions to trigger T cell receptor activation (in the case of T cells) and IFN-γ release.
The relationship between substance P, other neuropeptides, and HSV-1 is not fully understood. Previous work reported CGRP-expressing neurons are infected with HSV-1 following corneal inoculation (LaVail et al., 1991) with expression of latency associated transcripts inducing substance P expression (Hamza et al., 2006). Eliminating CGRP- and substance P sensory fibers in the cornea prevents or greatly reduces the establishment of latency and efficient reactivation (Herbort et al., 1989). Therefore, further exploration in characterizing the relationship between neuropeptides including substance P and the consequences of its expression on the host response to HSV-1 as well as the capacity of the virus to establish latency and reactivate is appropriate with the anticipated outcome including the development of novel therapeutic applications.
Acknowledgments
The authors would like to thank Manoj Thapa and Todd Wuest for their technical help with flow cytometry. This work was supported by USPHS NIH grants AI053108 (to D.J.J.C.), NEI core grant EY12190, DFG Research Unit FOR696 (to R.H.S.) as well as a Jules and Doris Stein Research to Prevent Blindness research professorship award (to DJJC).
References
- Alonzo NC, Carr DJJ. Morphine reduces mortality in mice following ocular infection with HSV-1. Immunopharmacol. 1999;41:187–197. doi: 10.1016/s0162-3109(99)00003-x. [DOI] [PubMed] [Google Scholar]
- Ackerman KD, Bellinger DL, Felten SY, Felten DL. Ontogeny and senescence of noradrenergic innervation of the rodent thymus and spleen. In: Ader R, Felten DL, Cohen N, editors. Psychoneuroimmunology. 2nd. Academic Press; San Diego: 1991. pp. 71–126. [Google Scholar]
- Brogden KA, Guthmiller JM, Salzet M, Zasloff M. The nervous system and innate immunity: the neuropeptide connection. Nat Immunol. 2005;6:558–564. doi: 10.1038/ni1209. [DOI] [PubMed] [Google Scholar]
- Carr DJJ, Campbell IL. Herpes simplex virus type 1 induction of chemokine production is unrelated to viral load in the cornea but not in the nervous system. Viral Immunol. 2006;19:741–746. doi: 10.1089/vim.2006.19.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DJJ, Ash J, Lane TE, Kuziel WA. Abnormal immune response of CCR5-deficient mice to ocular infection with herpes simplex virus type 1. J Gen Virol. 2006;87:489–499. doi: 10.1099/vir.0.81339-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DJ, Tomanek L. Herpes simplex virus and the chemokines that mediate the inflammation. Current Topics in Microbiology & Immunology. 2006;303:47–65. doi: 10.1007/978-3-540-33397-5_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelmicka-Schorr E, Checinski M, Arnason BGW. Chemical sympathectomy augments the severity of experimental allergic encephalomyelitis. J Neuroimmunol. 1988;17:347–350. doi: 10.1016/0165-5728(88)90125-7. [DOI] [PubMed] [Google Scholar]
- Cross RJ, Roszman TL. Central catecholamine depletion impairs in vivo immunity but not in vitro lymphocyte activation. J Neuroimmunol. 1988;19:33–45. doi: 10.1016/0165-5728(88)90033-1. [DOI] [PubMed] [Google Scholar]
- Cross RJ, Jackson JC, Brooks WH, Sparks DL, Markesbery WR, Roszman TL. Neuroimmunomodulation: impairment of humoral immune responsiveness by 6-hydroxydopamine treatment. Immunol. 1986;57:145–152. [PMC free article] [PubMed] [Google Scholar]
- Felten DL, Felten SY, Belinger DL, Lorton D. Noradrenergic and peptidergic innervation of secondary lymphoid organs: role in experimental rheumatoid arthritis. Eur J Clin Invest. 1992;22:37–41. [PubMed] [Google Scholar]
- Greco R, Tassorelli C, Sandrini G, Di Bella P, Buscone S, Nappi G. Role of calcitonin gene-related peptide and substance P in different models of pain. Cephalalgia. 2008;28:114–126. doi: 10.1111/j.1468-2982.2007.01468.x. [DOI] [PubMed] [Google Scholar]
- Hamza MA, Higgins DM, Ruyechan WT. Herpes simplex virus type-1 latency-associated transcript-induced immunoreactivity of substance P in trigeminal neurons is reversed by bone morphogenetic protein-7. Neurosci Lett. 2006;413:31–35. doi: 10.1016/j.neulet.2006.11.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Härle P, Möbius D, Carr DJJ, Schölmerich J, Straub RH. An opposing time-dependent immune-modulating effect of the sympathetic nervous system conferred by altering the cytokine profile in the local lymph nodes and spleen of mice with type II collagen-induced arthritis. Arthritis Rheum. 2005;52:1305–1313. doi: 10.1002/art.20987. [DOI] [PubMed] [Google Scholar]
- Herbort CP, Weissman SS, Payan DG. Role of peptidergic neurons in ocular herpes simplex infection in mice. FASEB J. 1989;3:2537–2541. doi: 10.1096/fasebj.3.13.2553522. [DOI] [PubMed] [Google Scholar]
- Kohm AP, Sanders VM. Suppression of antigen-specific Th2 cell-dependent IgM and IgG1 production following norepinephrine depletion in vivo. J Immunol. 1999;162:5299–5308. [PubMed] [Google Scholar]
- LaVail JH, Meade LB, Dawson CR. Ultrastructural immunocytochemical localization of herpes simplex virus (type 1) in trigeminal ganglion neurons. Curr Eye Res. 1991;10S:23–29. doi: 10.3109/02713689109020354. [DOI] [PubMed] [Google Scholar]
- Leo NA, Callahan TA, Bonneau RH. Peripheral sympathetic denervation alters both the primary and memory cellular immune responses to herpes simplex virus infection. Neuroimmunomodulation. 1998;5:22–35. doi: 10.1159/000026323. [DOI] [PubMed] [Google Scholar]
- Leo NA, Bonneau RH. Mechanisms underlying chemical sympathectomy-induced suppression of herpes simplex virus-specific cytotoxic T lymphocyte activation and function. J Neuroimmunol. 2000;110:45–56. doi: 10.1016/s0165-5728(00)00336-2. [DOI] [PubMed] [Google Scholar]
- Lepisto AJ, Frank GM, Hendricks RL. How herpes simplex virus type 1 rescinds corneal privilege. In: Niederkorn JY, Kaplan HJ, editors. Immune response and the eye, Chem Immunol Allergy. Vol. 92. Karger; Basel: 2007. pp. 203–212. [DOI] [PubMed] [Google Scholar]
- Lighvani S, Huang X, Trivedi PP, Swanborg RH, Hazlett LD. Substance P regulates natural killer cell interferon-gamma production and resistance to Psuedomonas aeruginosa infection. Eur J Immunol. 2005;35:1567–1575. doi: 10.1002/eji.200425902. [DOI] [PubMed] [Google Scholar]
- Livnat S, Felten SY, Carlson SL, Bellinger DL, Felten DL. Involvement of peripheral and central catecholamine systems in neural-immune interactions. J Neuroimmunol. 1985;10:5–30. doi: 10.1016/0165-5728(85)90031-1. [DOI] [PubMed] [Google Scholar]
- Lotz M, Vaughan JH, Carson DA. Effect of neuropeptides on production of inflammatory cytokines by human monocytes. Science. 1988;241:1218–1221. doi: 10.1126/science.2457950. [DOI] [PubMed] [Google Scholar]
- Luhtala J, Uusitalo H. The distribution and origin of substance P immunoreactive nerve fibres in the rat conjunctiva. Exp Eye Res. 1991;53:641–646. doi: 10.1016/0014-4835(91)90224-3. [DOI] [PubMed] [Google Scholar]
- Lundy FT, Linden GJ. Neuropeptides and neurogenic mechanisms in oral and periodontal inflammation. Crit Rev Oral Biol Med. 2004;15:82–98. doi: 10.1177/154411130401500203. [DOI] [PubMed] [Google Scholar]
- Madden KS, Felten SY, Felten DL, Sundaresan PR, Livnat S. Sympathetic neural modulation of the immune system. I. Depression of T cell immunity in vivo and in vitro following chemical sympathectomy. Brain Behavior Immun. 1989;3:72–89. doi: 10.1016/0889-1591(89)90007-x. [DOI] [PubMed] [Google Scholar]
- Madden KS, Stevens SY, Felten DL, Bellinger DL. Alterations in T lymphocyte activity following chemical sympathectomy in young and old Fischer 344 rats. J Neuroimmunol. 2000;103:131–145. doi: 10.1016/s0165-5728(99)00243-x. [DOI] [PubMed] [Google Scholar]
- Marriott I, Bost KL. IL-4 and IFN-gamma up-regulate substance P receptor expression in murine peritoneal macrophages. J Immunol. 2000;165:182–191. doi: 10.4049/jimmunol.165.1.182. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Biron CA, Brunson KW, Bulloch K, Chambers WH, Dhabhar FS, Goldfarb RH, Kitson RP, Miller AH, Spencer RL, Weiss JM. The role of adrenocorticoids as modulators of immune function in health and disease: neural, endocrine, and immune interactions. Brain Res Brain Res Rev. 1997;23:79–133. doi: 10.1016/s0165-0173(96)00012-4. [DOI] [PubMed] [Google Scholar]
- Miller LE, Justen HP, Schölmerich J, Straub RH. The loss of sympathetic nerve fibers in the synovial tissue of patients with rheumatoid arthritis is accompanied by increased norepinephrine release from synovial macrophages. FASEB J. 2000;14:2097–2107. doi: 10.1096/fj.99-1082com. [DOI] [PubMed] [Google Scholar]
- Miura T, Kudo T, Matsuki A, Sekikawa K, Tagawa YI, Iwakura Y, Nakane A. Effecto fo 6-hydroxydopamine on host resistance against Listeria monocytogenes infection. Infect Immun. 2001;69:7234–7241. doi: 10.1128/IAI.69.12.7234-7241.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moynihan J, Kruszewska B, Madden K, Callahan T. Sympathetic nervous system regulation of immunity. J Neuroimmunol. 2004;147:87–90. doi: 10.1016/j.jneuroim.2003.10.018. [DOI] [PubMed] [Google Scholar]
- Ohara PT, Chin MS, LaVail JH. The spread of herpes simplex virus type 1 from trigeminal neurons to the murine cornea: an immunoelectron microscopy study. J Virol. 2000;74:4776–4786. doi: 10.1128/jvi.74.10.4776-4786.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual DW, Bost KL, Xu-Amano J, Kiyono H, McGhee JR. The cytokine-like action of substance P upon B cell differentiation. Reg Immunol. 1992;4:100–104. [PubMed] [Google Scholar]
- Picklo MJ. Methods of sympathetic degeneration and alteration. J Auton Nerv Syst. 1997;62:111–125. doi: 10.1016/s0165-1838(96)00121-x. [DOI] [PubMed] [Google Scholar]
- Reder A, Checinski M, Chelmicka-Schorr E. The effect of chemical sympathectomy on natural killer cells in mice. 1989 doi: 10.1016/0889-1591(89)90011-1. [DOI] [PubMed] [Google Scholar]
- Ruocco I, Cuello AC, Shigemoto R, Riveiro-da-Silva A. Sympathectomies lead to transient substance P-immunoreactive sensory fibre plasticity in the rat skin. Neurosci. 2001;108:157–166. doi: 10.1016/s0306-4522(01)00158-0. [DOI] [PubMed] [Google Scholar]
- Shepherd AJ, Beresford LJ, Bell EB, Miyan JA. Mobilization of specific T cells from lymph nodes in contact sensitivity requires substance. P J Neuroimmunol. 2005;164:115–123. doi: 10.1016/j.jneuroim.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Shimeld C, Whiteland JL, Nicholls SM, Grinfeld E, Easty DL, Gao H, Hills TJ. Immune cell infiltration and persistence in the mouse trigeminal ganglion after infection of the cornea with herpes simplex virus type 1. J Neuroimmunol. 1995;61:7–16. doi: 10.1016/0165-5728(95)00068-d. [DOI] [PubMed] [Google Scholar]
- Stanisz AM, Scicchitano R, Dazin P, Bienenstock J, Payan DG. Distribution of substance P receptors on murine spleen and Peyer's patch T and B cells. J Immunol. 1987;139:749–754. [PubMed] [Google Scholar]
- Svensson A, Kaim J, Mallard C, Olsson A, Brodin E, Hökfelt T, Eriksson K. Neurokinin 1 receptor signaling affects the local innate immune defense against genital herpes virus infection. J Immunol. 2005;175:6802–6811. doi: 10.4049/jimmunol.175.10.6802. [DOI] [PubMed] [Google Scholar]
- Turner SL, Jenkins FJ. The roles of herpes simplex virus in neuroscience. J Neurovirol. 1997;3:110–125. doi: 10.3109/13550289709015801. [DOI] [PubMed] [Google Scholar]
- Whitear M. An electron microscope study of the cornea in mice, with special reference to the innervation. J Anat. 1960;94:387–409. [PMC free article] [PubMed] [Google Scholar]
- Wuest T, Austin BA, Uematsu S, Thapa M, Akira S, Carr DJJ. Intact TLR9 and type I interferon signaling pathways are required to augment HSV-1 induced corneal CXCL9 and CXCL10. J Neuroimmunol. 2006;179:46–52. doi: 10.1016/j.jneuroim.2006.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]


