Abstract
OBJECTIVE
To elucidate the influence of drug-eluting stents (DESs) on interventional therapy of de novo unprotected left main stem (LMS) lesions in a hospital with on-site cardiac surgery.
METHODS AND RESULTS
A retrospective study of all patients with unprotected LMS angioplasty from 1999 to 2005 was conducted with regard to clinical and procedural data, and follow-up data.
Fifty-four patients with unprotected LMS stenosis were treated inter-ventionally. Of these patients, 16 were treated with DESs. Seven patients presented with cardiogenic shock. During their hospital stay, four patients died (all treated with bare metal stents [BMSs], three initially presenting with cardiogenic shock).
Follow-up data for 53 patients (98%) were obtained. Median follow-up time was 24 months (25th percentile, 12 months; 75th percentile, 35 months). Survival after nine months was 87% (81% from the BMS-treated group, and 100% from the DES-treated group). Control angiography had been performed in 36 patients (67%). Patients with unprotected LMS with an angiographic follow-up had a higher nine-month survival rate than patients without (36 of 36 patients [100%] versus 10 of 17 patients [59%], respectively; P<0.0001). Target lesion revascularization rate was 19% in both the BMS and the DES groups. Methods of revascularization did not vary significantly between the groups.
CONCLUSIONS
In the present study of selected patients with LMS stenosis, the use of DESs showed a low mortality rate but did not have a clear effect on target lesion revascularization rate compared with BMSs. A close follow-up appears to be mandatory to achieve acceptable results.
Keywords: Angioplasty, Drug-eluting stents, Left main stem
Treatment of left main stem (LMS) stenosis used to be dominated by cardiac surgery (1). Few clinical studies comparing interventional treatment of LMS stenosis with bypass surgery are currently available (2–4).
We attempted to elucidate the results of catheter-based interventional treatment of de novo unprotected LMS stenosis in daily practice in a community hospital in Germany from 1999 to 2005, and how these results were influenced by the introduction of drug-eluting stents (DESs) into the German stent market in early 2002.
METHODS
A retrospective analysis of patients who underwent angioplasty of de novo unprotected LMS lesions in a community hospital with on-site cardiac surgery was conducted. Data from 1999 to 2005 (n=54) were used.
Baseline data included patient medical history, clinical presentation, interventional procedure data and clinical course during hospital stay. Clinical follow-up was conducted during hospital stay or by telephone.
Patients were advised to have angiographic follow-up at three months and at six to eight months after the index procedure. Follow-up data included major adverse cardiovascular or cerebrovascular events (MACCEs). Patients were advised to take lifelong monotherapy with 100 mg acetylsalicylic acid in combination with 75 mg clopidogrel for one month in case of receiving a bare metal stent (BMS) or for six months in case of receiving a DES.
Definitions
Patients were classified as ‘suboptimal candidates for cardiac surgery’ in cases with unsuitable distal targets for bypass surgery, jointly assessed by both a cardiologist and a cardiac surgeon.
Cardiogenic shock was defined as having systolic blood pressure below 90 mmHg and a heart rate of more than 100 beats/min for longer than 30 min, requiring hemodynamic support by catecholamines and/or intra-aortic counterpulsation (5).
Renal function was calculated by the abbreviated Modification of Diet in Renal Disease formula. An estimated glomerular filtration rate of 60 mL/min/1.73 m2 or lower was regarded as a relevant impairment (6).
MACCEs comprise death from both cardiac and noncardiac causes, myocardial infarction (MI), target lesion revascularization (TLR), stroke, and transient and prolonged ischemic attacks. MI was defined in accordance with the guidelines of the European Society of Cardiology, including both ST elevation and non-ST elevation MI (7).
TLR comprises any revascularization performed on the treated segment, ie, ±5 mm to the stented area. It includes both catheter-based interventional therapy and (repeated) coronary artery bypass grafting.
Angiographic analysis
Quantitative analysis of angiographies was performed retrospectively using an automated biplane quantitative coronary angiography analysis (AXIOM Artis, Siemens, Germany). Reference vessel diameter was calculated by averaging the proximal and distal vessel diameters.
Left ventricular ejection fraction (LVEF) was calculated using left ventricular angiography. Biplane two-dimensional transthoracic echocardiography was used when left ventricular angiography was not available.
Statistical analysis
Continuous variables are presented as mean ± SD, or quartiles (25th and 75th percentiles) when appropriate. For comparison of continuous values between two groups, the Mann-Whitney U test was used. Bivariate cross-tabulations were compared using Fisher’s exact test; otherwise, for multiple categories, Pearson’s χ2 test was calculated. Time until death is presented as Kaplan-Meier curves and compared using the log-rank test.
All tests were performed two-tailed and with exact method. P values were nominally interpreted, and differences were regarded as statistically significant when P<0.05.
Statistics were calculated using SPSS for Windows 11.5.2.1 (2003, SPSS Inc, USA).
RESULTS
Baseline
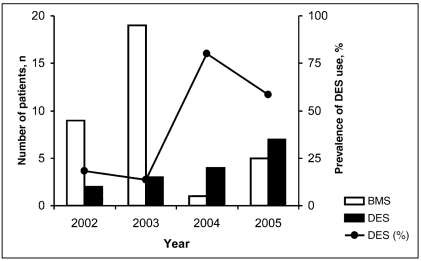
The distribution of the different brands of DESs is as follows: 15 Cypher (Cordis Corporation, USA) (94% ), and one Taxus Express (Boston Scientific Corporation, USA) (6%). The relative frequency of the implantation of DES from 2002 to 2005 predominately rose: 18% (two of 11 patients) in 2002 and 58% (seven of 12 patients) in 2005 (Figure 1).
Figure 1.
Use of a bare metal stent (BMS) or drug-eluting stent (DES) from 2002 to 2005
Most decisions in favour of percutaneous coronary intervention instead of bypass surgery in unprotected LMS were taken because of patients’ refusal of cardiac surgery or emergency situations (Table 1).
TABLE 1.
Reasons for percutaneous coronary intervention in patients (n=54) with unprotected left main disease
| Bare metal stent (n=38) n (%) | Drug-eluting stent (n=16) n (%) | |
|---|---|---|
| Emergency | 15 (39) | 4 (25) |
| Suboptimal candidates for cardiac surgery | 6 (16) | 1 (6) |
| Age or comorbidity | 4 (11) | 2 (13) |
| Patients’ refusal of surgery | 13 (34) | 9 (56) |
Patient characteristics and interventional data are presented in Table 2.
TABLE 2.
Patient and procedure baseline data
| Unprotected left main stem (n=54)
|
|||
|---|---|---|---|
| Criteria | BMS, n=38 | DES, n=16 | P |
| Age, years, mean ± SD | 66.8±11.9 | 67.0±10.5 | 0.786 |
| Women/men, n (%) | 12 (32)/26 (68) | 7 (44)/9 (56) | 0.534 |
| Diabetes, n (%) | 10 (26) | 4 (25) | 1.000 |
| Renal insufficiency, n (%) | 14 (37) | 6 (38) | 1.000 |
| Previous MI, n (%) | 19 (50) | 7 (44) | 0.770 |
| LVEF, %, mean ± SD | 49±16 | 54±14 | 0.295 |
| Previous cardiac surgery, n (%) | 3 (8) | 1 (6) | 1.000 |
| Cardiogenic shock, n (%) | 7 (18) | 0 (0) | 0.012* |
| Reference vessel diameter, mm, mean ± SD | 3.7±0.4 | 3.5±0.3 | 0.008* |
| Location of stent placement, n (%) | |||
| Ostial | 6 (16) | 4 (25) | 0.459 |
| Bifurcation | 18 (47) | 6 (38) | 0.561 |
| Predilation, n (%) | 31 (82) | 12 (75) | 0.714 |
| Stent diameter, mm, mean ± SD | 3.6±0.4 | 3.3±0.3 | 0.003* |
| Stent length, mm, mean ± SD | 13.0±3.7 | 13.9±9.9 | 0.038* |
| Use of glycoprotein IIb/IIIa antagonist, n (%) | 29 (76) | 13 (81) | 1.000 |
| Postprocedure stay, days, (Q1, Q3) | 5.5 (3.8, 13.0) | 4.0 (2.3, 5.0) | 0.017* |
| In-hospital events, n (%) | |||
| New MI | 0 (0) | 0 (0) | – |
| Stroke | 0 (0) | 0 (0) | – |
| Death | 4 (10.5) | 0 (0) | 0.306 |
P<0.05. BMS Bare metal stent; DES Drug-eluting stent; LVEF Left ventricular ejection fraction; MI Myocardial infarction; Q1 Twenty-fifth percentile; Q3 Seventy-fifth percentile
Patients in both groups did not differ significantly in age, sex distribution and comorbidities, and had similar left ventricular function. All patients in the DES group were hemodynamically stable, whereas in the BMS group, seven of the 38 patients (18%) admitted had cardiogenic shock (P=0.012).
Reference vessel and stent diameters of the BMS group were significantly larger than those in the DES group; however, the stents tended to be longer in the DES group. The rate of in-hospital events and the duration of postinterventional in-hospital stay were smaller in DES-treated patients (in-hospital MACCE rate of 0% for DES-treated patients, and 10.5% for BMS-treated patients; P=0.306). Four patients died; all BMS-treated. Three of the patients were admitted in cardiogenic shock, and one died of a sudden cardiac death. Localization of stent placement, stenting technique and application of glycoprotein IIb/IIIa antagonists were not significantly different between the two groups. In only one case, two DESs were implanted using the ‘crushing technique’. All other patients with unprotected LMS received one stent, followed by postdilation of the distal bifurcation using the ‘kissing balloon technique’ if necessary (8,9).
Follow-up
Clinical follow-up for 53 patients (98%) was obtained (Table 3). The overall median time interval between primary procedure and clinical follow-up was 24 months (25th percentile, 12 months; 75th percentile, 35 months). No relevant bleeding complications or cerebrovascular events were reported in both groups of patients.
TABLE 3.
Follow-up data
| Unprotected left main stem (n=54)
|
|||
|---|---|---|---|
| Criteria | BMS | DES | P |
| Clinical follow-up | |||
| Clinical follow-up available, n (%) | 37 (97) | 16 (100) | 1.000 |
| Median follow-up time, months (Q1, Q3) | 24 (12, 35) | 22 (15, 38) | 0.457 |
| Overall death rate, n (%) | 10 (26) | 1 (6) | 0.262 |
| Cardiac death within nine months | 6 (16) | 0 (0) | 0.176 |
| Noncardiac death within nine months | 1 (3) | 0 (0) | 1.000 |
| Angiographic follow-up | |||
| Angiographic follow-up available, n (%) | 23 (61) | 13 (81) | 0.209 |
| Angiographic follow-up, months (Q1; Q3) | 7 (4, 22) | 7 (5, 13) | 0.691 |
| Target lesion revascularization rate, n (%) | 7 (18) | 3 (19) | 1.000 |
| Balloon angioplasty | 0 (0) | 0 (0) | – |
| PCI plus DES | 4 (11) | 1 (6) | – |
| PCI plus PTFE-covered stent | 1 (3) | 0 (0) | – |
| CABG operation | 2 (5) | 2 (13) | – |
BMS Bare metal stent; CABG Coronary artery bypass graft; DES Drug-eluting stent; PCI Percutaneous coronary intervention; PTFE Polytetrafluoroethylene; Q1 Twenty-fifth percentile; Q3 Seventy-fifth percentile;
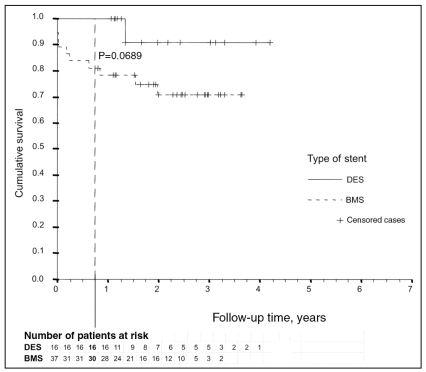
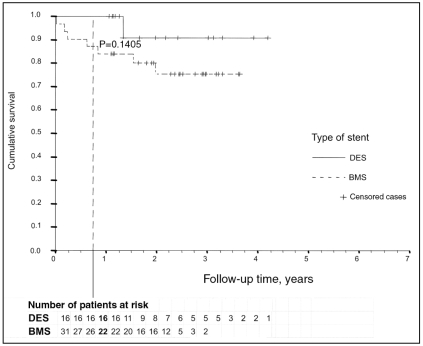
Mortality within a period of nine months was solely experienced by patients with BMSs; however, this was not statistically significant compared with DES-treated patients (0% versus 19%; P=0.0689) (Figure 2). Survival analysis with exclusion of the seven patients with initial cardiogenic shock (which affected only the BMS-treated group) revealed a lower difference in nine-month mortality between the two groups (0% versus 16%; P=0.1405). One DES-treated patient died after 16 months – a sudden cardiac death was suspected (Figure 3). Six of the BMS-treated patients died after hospital discharge (two of malignancy, four of cardiac or unknown cause).
Figure 2.
Kaplan-Meier curve comparing bare metal stent (BMS)- or drug-eluting stent (DES)-treated patients
Figure 3.
Kaplan-Meier curve comparing bare metal stent (BMS)- or drug-eluting stent (DES)-treated patients, excluding those patients with cardiogenic shock
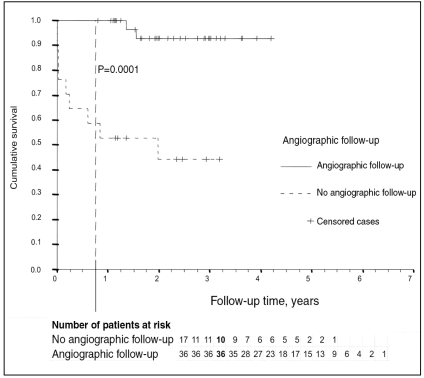
Survival analysis comparing control patients who had undergone angiography with patients without angiography revealed a significantly higher nine-month mortality (0% versus 41%; P=0.0001) in patients without angiographic follow-up (Figure 4). Of the five patients (four with BMS, one with DES) who died after hospital discharge because of cardiac or unknown cause, only the DES-treated patient had angiographic follow-up.
Figure 4.
Kaplan-Meier curve comparing patients with angiographic follow-up to patients without angiographic follow-up
During follow-up, TLR was performed in 10 of 53 patients (19%). There was no difference (P=1.000) between DES- and BMS-treated patients (three of 16 patients [19%] versus seven of 37 patients [19%], respectively).
DISCUSSION
The present investigation was aimed at elucidating the influence of DESs on daily practice and results of angioplasty of LMS stenosis in a community hospital in Germany, representing a ‘real world scenario’ (10).
Because of higher costs, the use of DESs had been limited to willing participants. Patients with a high likelihood of in-hospital death (ie, patients in cardiogenic shock) were regarded as poor candidates for DES implantation. Also, in patients with reference vessel diameters of 4.0 mm or larger (ie, a suspected low restenosis rate), BMSs were preferred over DESs. Initially, DESs were available only up to a diameter of 3.0 mm to 3.5 mm, thus also leading to a smaller average diameter of implanted stents, a selection of appropriate candidates and higher inflation pressures (11).
In daily practice, interventional therapy was limited to a certain selection of patients because of patients’ refusal of cardiac surgery, age, comorbidity, poor candidates for cardiac surgery, cardiogenic shock, etc. These limitations partially explain the high nine-month mortality rate (13%) of patients with unprotected LMS in the present study. Patients presenting with cardiogenic shock had a reduced prognosis irrespective of the method of revascularization (5,12,13).
These results are comparable with other trials (11,14–16). In recent publications, the one-year mortality rate of patients with unprotected LMS treated with bypass surgery was approximately 5% to 10% (4,17,18). In the present population, DESs offered a comparable or even lower rate (12-month mortality rate of 0%). Compared with other trials of unprotected LMS percutaneous coronary intervention, the use of side-branch stenting in a case of bifurcational stent placement was lower (one of 16 DES-treated patients) (15,16,19–21). This could be another explanation for the low death rate observed in the DES group in comparison with other studies (16,22).
The fact that LVEF between BMS- and DES-treated patients did not differ significantly, despite the different hemodynamic situations, may be due to the limited number of patients and the use of different techniques (ie, echocardiography or left ventricular angiography) to calculate LVEF, at different time points (23).
After the introduction of DESs, patients tended to demand interventional instead of surgical coronary revascularization more frequently (24). However, in our data, TLR rates of both BMSs and DESs came up to approximately 20%. While the revascularization rate in BMS-treated patients is comparable with other trials, results for the DES-treated patients are rather unsatisfactory. The smaller stent diameter in our DES-treated patients (3.3 mm versus 3.6 mm in BMS-treated patients) may offer one explanation for the results (25). Whether a closer angiographic follow-up would have resulted in a higher TLR rate could not be answered (19,22).
Although control angiography had been recommended in all patients, it was performed in only 67%. This is assumed to be the result of multiple reasons, such as age, comorbidity, unwillingness because of lack of symptoms, recommendations by family doctors, etc. It appears that patients’ adherence to a close angiographic follow-up is important for survival after unprotected LMS angioplasty.
Limitations
Our data are based on a single-centre clinical experience and represent retrospective results, not a randomized, controlled study. Because the number of patients is very limited, statistical differences between the different groups of patients may have been missed. Control angiography had been performed in only 36 patients (67%); therefore, a precise analysis of restenosis rate cannot be made.
Different brands of stents (two different DESs, seven different BMSs) were used, which may have had an influence on restenosis rate (25). Furthermore, the use of DESs has not only been influenced by medical reasons, but also economic and technical constraints (eg, availability of distinct stent sizes), leading to an imbalance in the different groups of patients.
CONCLUSIONS
With the introduction of DESs, the interventional treatment of left main stenosis outside of clinical trials shows promising results in selected cases, even with significant comorbidity. In unprotected LMS treatment, the need for repeated revascularization is still considerable, even with DESs. However, in-hospital event rates and one-year mortality rates in hemodynamically stable DES-treated patients are low and comparable with bypass surgery.
A close clinical and angiographic follow-up is important to achieve acceptable long-term results.
ACKNOWLEDGEMENTS
The authors thank Aoife Bhreatnach, Peter Hertting and Liana Patzelt for their indefatigable support. Also, we highly appreciate the great help of John Lowell (Stereotaxis Inc, USA)
Footnotes
CONFLICT OF INTERESTS: The authors have no conflicts of interest to declare.
REFERENCES
- 1.Kappetein AP, Dawkins KD, Mohr FW, et al. Current percutaneous coronary intervention and coronary artery bypass grafting practices for three-vessel and left main coronary artery disease. Insights from the SYNTAX run-in phase. Eur J Cardiothorac Surg. 2006;29:486–91. doi: 10.1016/j.ejcts.2006.01.047. [DOI] [PubMed] [Google Scholar]
- 2.Chieffo A, Morici N, Maisano F, et al. Percutaneous treatment with drug-eluting stent implantation versus bypass surgery for unprotected left main stenosis: A single-center experience. Circulation. 2006;113:2542–7. doi: 10.1161/CIRCULATIONAHA.105.595694. [DOI] [PubMed] [Google Scholar]
- 3.Lee MS, Kapoor N, Jamal F, et al. Comparison of coronary artery bypass surgery with percutaneous coronary intervention with drug-eluting stents for unprotected left main coronary artery disease. J Am Coll Cardiol. 2006;47:864–70. doi: 10.1016/j.jacc.2005.09.072. [DOI] [PubMed] [Google Scholar]
- 4.Palmerini T, Marzocchi A, Marrozzini C, et al. Comparison between coronary angioplasty and coronary artery bypass surgery for the treatment of unprotected left main coronary artery stenosis (the Bologna Registry) Am J Cardiol. 2006;98:54–9. doi: 10.1016/j.amjcard.2006.01.070. [DOI] [PubMed] [Google Scholar]
- 5.Hochman JS, Sleeper LA, White HD, et al. One-year survival following early revascularization for cardiogenic shock. JAMA. 2001;285:190–2. doi: 10.1001/jama.285.2.190. [DOI] [PubMed] [Google Scholar]
- 6.Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–47. doi: 10.7326/0003-4819-139-2-200307150-00013. Erratum in 2003;139:605. [DOI] [PubMed] [Google Scholar]
- 7.Silber S, Albertsson P, Avilés FF, et al. Guidelines for percutaneous coronary interventions. The Task Force for Percutaneous Coronary Interventions of the European Society of Cardiology. Eur Heart J. 2005;26:804–47. doi: 10.1093/eurheartj/ehi138. [DOI] [PubMed] [Google Scholar]
- 8.Melikian N, Di Mario C. Treatment of bifurcation coronary lesions: A review of current techniques and outcome. J Interv Cardiol. 2003;16:507–13. doi: 10.1046/j.1540-8183.2003.01056.x. [DOI] [PubMed] [Google Scholar]
- 9.Wood F, Bazemore E, Schneider JE, Jobe RL, Mann T. Technique of left main stenting is dependent on lesion location and distal branch protection. Catheter Cardiovasc Interv. 2005;65:499–503. doi: 10.1002/ccd.20426. [DOI] [PubMed] [Google Scholar]
- 10.Hordijk-Trion M, Lenzen M, Wijns W, et al. Patients enrolled in coronary intervention trials are not representative of patients in clinical practice: Results from the Euro Heart Survey on Coronary Revascularization. Eur Heart J. 2006;27:671–8. doi: 10.1093/eurheartj/ehi731. [DOI] [PubMed] [Google Scholar]
- 11.de Lezo JS, Medina A, Pan M, et al. Rapamycin-eluting stents for the treatment of unprotected left main coronary disease. Am Heart J. 2004;148:481–5. doi: 10.1016/j.ahj.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 12.Hata M, Shiono M, Sezai A, et al. Outcome of emergency conventional coronary surgery for acute coronary syndrome due to left main coronary disease. Ann Thorac Cardiovasc Surg. 2006;12:28–31. [PubMed] [Google Scholar]
- 13.De Luca G, Suryapranata H, Thomas K, et al. Outcome in patients treated with primary angioplasty for acute myocardial infarction due to left main coronary artery occlusion. Am J Cardiol. 2003;91:235–8. doi: 10.1016/s0002-9149(02)03115-6. [DOI] [PubMed] [Google Scholar]
- 14.Lee RJ, Lee SH, Shyu KG, et al. Immediate and long-term outcomes of stent implantation for unprotected left main coronary artery disease. Int J Cardiol. 2001;80:173–7. doi: 10.1016/s0167-5273(01)00478-8. [DOI] [PubMed] [Google Scholar]
- 15.Park SJ, Lee CW, Kim YH, et al. Technical feasibility, safety, and clinical outcome of stenting of unprotected left main coronary artery bifurcation narrowing. Am J Cardiol. 2002;90:374–8. doi: 10.1016/s0002-9149(02)02492-x. [DOI] [PubMed] [Google Scholar]
- 16.Chieffo A, Stankovic G, Bonizzoni E, et al. Early and mid-term results of drug-eluting stent implantation in unprotected left main. Circulation. 2005;111:791–5. doi: 10.1161/01.CIR.0000155256.88940.F8. [DOI] [PubMed] [Google Scholar]
- 17.Lu JC, Grayson AD, Pullan DM. On-pump versus off-pump surgical revascularization for left main stem stenosis: Risk adjusted outcomes. Ann Thorac Surg. 2005;80:136–42. doi: 10.1016/j.athoracsur.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 18.Yeatman M, Caputo M, Ascione R, Ciulli F, Angelini GD. Off-pump coronary artery bypass surgery for critical left main stem disease: Safety, efficacy and outcome. Eur J Cardiothorac Surg. 2001;19:239–44. doi: 10.1016/s1010-7940(01)00572-3. [DOI] [PubMed] [Google Scholar]
- 19.Price MJ, Cristea E, Sawhney N, et al. Serial angiographic follow-up of sirolimus-eluting stents for unprotected left main coronary artery revascularization. J Am Coll Cardiol. 2006;47:871–7. doi: 10.1016/j.jacc.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 20.Lee SH, Ko YG, Jang Y, et al. Sirolimus- versus paclitaxel-eluting stent implantation for unprotected left main coronary artery stenosis. Cardiology. 2005;104:181–5. doi: 10.1159/000088106. [DOI] [PubMed] [Google Scholar]
- 21.Valgimigli M, Malagutti P, Aoki J, et al. Sirolimus-eluting versus paclitaxel-eluting stent implantation for the percutaneous treatment of left main coronary artery disease: A combined RESEARCH and T-SEARCH long-term analysis. J Am Coll Cardiol. 2006;47:507–14. doi: 10.1016/j.jacc.2005.09.040. [DOI] [PubMed] [Google Scholar]
- 22.Schühlen H, Kastrati A, Mehilli J, et al. Restenosis detected by routine angiographic follow-up and late mortality after coronary stent placement. Am Heart J. 2004;147:317–22. doi: 10.1016/j.ahj.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 23.Lopez-Jimenez F, Goraya TY, Hellermann JP, et al. Measurement of ejection fraction after myocardial infarction in the population. Chest. 2004;125:397–403. doi: 10.1378/chest.125.2.397. [DOI] [PubMed] [Google Scholar]
- 24.van Domburg RT, Lemos PA, Takkenberg JJ, et al. The impact of the introduction of drug-eluting stents on the clinical practice of surgical and percutaneous treatment of coronary artery disease. Eur Heart J. 2005;26:675–81. doi: 10.1093/eurheartj/ehi088. [DOI] [PubMed] [Google Scholar]
- 25.Serruys PW, Kutryk MJ, Ong AT. Coronary-artery stents. N Engl J Med. 2006;354:483–95. doi: 10.1056/NEJMra051091. [DOI] [PubMed] [Google Scholar]