Abstract
OBJECTIVES
To find a correlation between the patent foramen ovale (PFO) size measured by the sizing balloon and the appropriate closure device size.
METHODS
The PFO of 57 patients was closed using a sizing balloon. A mathematical model was introduced to relate the PFO balloon waist diameter to the closure device size based on the PFO transformation from a slit-like to a circular form during balloon inflation.
According to this model, PFOs smaller than 8 mm should be closed with a 25 mm device, PFOs 8 mm to 11 mm with a 35 mm device, and PFOs larger than 11 mm with an Amplatzer septal occluder. In the first group, 36 patients (63.2%) received an appropriately sized device and six patients (10.5%) received an oversized device. In the second group, 15 patients (26.3%) received an undersized device.
RESULTS
A comparison of the PFO dimensions in two views showed that the PFO slit was circular when the balloon was inflated. A six-month echocardiography follow-up was obtained in 46 patients (80.7%). Five patients (13.9%) in the group with an appropriately sized device had a discrete residual shunt during Valsalva. In the second group, five patients (33.3%) had a residual shunt (P = 0.06), of which one was considered large.
CONCLUSION
The sizing balloon is helpful in selecting the PFO closure device size. Consequently, the incidence of residual shunt and recurrent events may be reduced.
Keywords: Closure device, Patent foramen ovale, Sizing balloon
Patent foramen ovale (PFO) is likely to be associated with pathological mechanisms leading to paradoxical embolism and unexplained stroke, air embolism in several forms of decompression sickness, and platypnea-orthodeoxia syndrome. Recently, PFO has been implicated in the etiology of migraine (1). The prevalence of PFO is higher in a population with a history of cryptogenic stroke than in a population with apparently healthy subjects (40% to 75% versus 20% to 25%, respectively) (2–4).
The feasibility of procedures for percutaneous closure, with extremely low periprocedural risk, allow the potential avoidance of lifelong anticoagulation, making this treatment an attractive option for patients with PFO-related stroke. Nevertheless, the recurrence rate of cerebrovascular events after PFO closure remains in the range of 0.9% to 4.7% (5,6). Recurrent thromboembolic events may occur because of either thrombus formation on the device surface or persistent residual right-to-left shunt in patients whose devices were too small (6). Thus far, a method for selecting appropriately sized PFO closure devices has not been clearly defined. Currently, PFO device size is determined by the intuition or at the discretion of operators taking into account right atrium size, patient height and weight, or the PFO aspect on echocardiography.
In a recent study by Billinger et al (7) in which a Helex device was used, the PFO was measured using a standard sizing balloon technique, and an arbitrary mean device-defect size ratio of 2.2 was applied. We hypothesized that measuring the PFO diameter using a sizing balloon in two orthogonal projections enables the selection of an appropriately sized PFO closure device, which may result in a lower rate of residual shunt and subsequently in a lower incidence of recurrent events (5,8). Because the PFO anatomically represents a slit-shaped septal defect (9–11), we developed a mathematical model to calculate the dimension of the PFO closure device based on the PFO stretched diameter measured by the sizing balloon technique. By transforming a slit-like PFO defect into a defect of a more circular shape, one can calculate its circumference and subsequently determine the length of the slit. This enables an appropriate choice of a closure device size.
The objective of the present study was to prove that PFO measurement with the sizing balloon enables a more appropriate selection of the size of the closure device, while at the same time providing a preliminary test of whether this could consequently lead to complete PFO closure in a greater number of patients.
METHODS
Patients
A total of 82 patients (mean [± SD] age of 47 ±14 years) with a diagnosed PFO, who were admitted to the University Hospital of Geneva (Geneva, Switzerland) for percutaneous closure in the period from 2002 to 2005, were studied. Patient demographics and PFO characteristics are given in Table 1. All patients had PFO and right-to-left passage of microbubbles diagnosed by contrast transthoracic echocardiography, two-dimensional contrast multiplane transesophageal echocardiography and transcranial Doppler.
TABLE 1.
Patients baseline characteristics
| Characteristic | |
|---|---|
| Age, years, mean ± SD | 47 ± 14 |
| Men, n (%) | 48 (58.5) |
| Hypertension, n (%) | 12 (14.6) |
| Hypercholesterolemia, n (%) | 31 (37.8) |
| Diabetes mellitus, n (%) | 4 (4.9) |
| Smoking, n (%) | 29 (35.4) |
| Family history, n (%) | 5 (6.1) |
| Atrial septal aneurysm plus PFO, n (%) | 25 (30.5) |
| Patent foramen ovale only, n (%) | 57 (69.5) |
| History of stroke, n (%) | 51 (62.2) |
| Transient ischemic attack, n (%) | 12 (14.6) |
| Recurrent stroke, n (%) | 19 (23.2) |
| No anticoagulation or antiaggregation, n (%) | 5 (6.1) |
| Vitamin K antagonists, n (%) | 43 (52.4) |
| Acetylsalicylic acid, n (%) | 17 (20.7) |
| Acetylsalicylic acid plus clopidogrel, n (%) | 14 (17.1) |
| Low molecular weight heparin, n (%) | 3 (3.7) |
In all 82 patients, a transcatheter closure of the PFO was performed. Amplatzer PFO Occluder (AGA Medical Corporation, USA) was used in 63 patients (76.8%), Amplatzer septal occluder in nine patients (11%) and PFO Star device (Cardia, USA) in 10 patients (12.2%).
In 57 patients (69.5%), the size of the PFO was measured using an Amplatzer sizing balloon. Based on the diameter obtained, the size of the device was chosen at the discretion of the operator. However, in January 2005, a mathematical equation, which was intended to directly relate the diameter of the balloon waist to the size of the closure device, was introduced. According to this equation, PFOs with a balloon-stretched diameter smaller than 8 mm should be closed with a 25 mm device, those measuring between 8 mm and 11 mm with a 35 mm device, and any PFO larger than 11 mm should be closed with a correspondingly sized Amplatzer septal occluder.
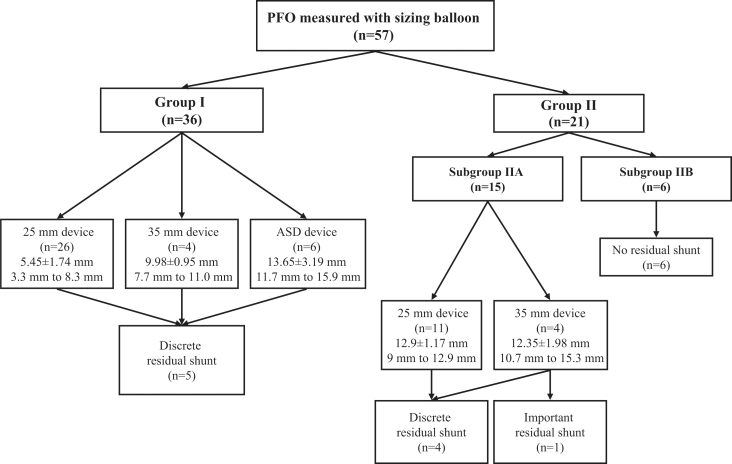
Patients were divided into two groups depending on the closure device used (Figure 1). Group I included 36 patients in whom an appropriately sized closure device was selected by using the mathematical equation. Group II included 21 patients in whom the size of the closure device selected did not correspond with the equation. This group was further divided into subgroup IIA, which included 15 patients in whom the device used was undersized according to the equation, and subgroup IIB, which included six patients in whom the device was oversized.
Figure 1.
Distribution of patients in group I, who were given appropriately sized patent foramen ovale (PFO) closure devices, and group II, who were given devices that did not correspond with those recommended by the equation. Mean (± SD) values of balloon waist diameter and range of device sizes are given for each group and subgroup
Balloon measurement and mathematical equation for selection of the closure device
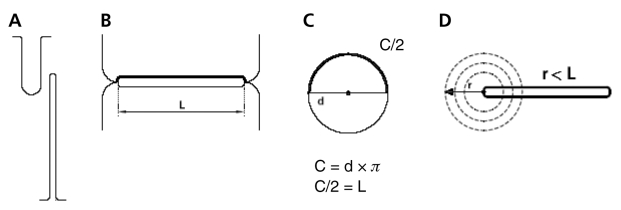
All patients received 50 U/kg of heparin. The right femoral vein was accessed with a 9F sheath. A multipurpose catheter (MPA-1, Super Torque Plus 6F, Cordis, Johnson & Johnson, USA) was used to cross the PFO with exchange guidewire (Standard Wire Guide, William Cook Europe, Denmark). The wire was left in one of the left pulmonary veins to provide better stability for introducing the sizing balloon. A sizing balloon (Amplatzer Sizing Balloon, AGA Medical Corporation, USA), 24 mm or 34 mm in diameter, was advanced across the PFO and inflated with diluted contrast medium. The inflation pressure was operator dependent, and inflation was stopped as soon as the waist appeared on the balloon, while the whole balloon was filled with a dye. In 48 patients, radiography of the inflated balloon was taken in posteroanterior or right anterior oblique projection (20° to 30°), with cranial angulations (20°) to avoid foreshortening of the markers on the balloon shaft, and in left anterior oblique (40° to 60°) or lateral projection (90°). In the remaining nine patients, radiography of the inflated balloon was taken in only one projection. The diameter of the balloon waist created by the PFO was measured by the quantitative coronary analysis technique (Quantcor QCA, Cardiovascular Angiography Analysis System III, Pie Medical Imaging, Netherlands), using the markers of the balloon for calibration. In case of a tubular form of the PFO, the shortest waist diameter measured was taken into account. Then, the size of the device was chosen according to the larger diameters of the balloon waist measured in two projections. Because the diameters in both views were not significantly different, the slit-like form (Figures 2A and 2B) of the PFO was assumed to be approximately circular when the sizing balloon was expanded (Figure 2C). Accordingly, the circumference of the stretched PFO was assumed to be C = d × π, where C stands for the circumference of the balloon waist, and d stands for the measured balloon waist diameter (Figure 2C). Because it was assumed that the balloon-expanded circular PFO goes back to its slit-like form as soon as the balloon is withdrawn (like a button hole), then the length of this slit-like defect could be calculated as C/2 (Figures 2B and 2C). Consequently, to provide complete coverage of the PFO by the device, the radius of the right atrial disc of the device must be at least equal to or greater than the length of the PFO slit, because the PFO closure device may slide from one side of the defect to the other (Figure 2D).
Figure 2.
A,B Two schematic perpendicular cross-sectional views of the patent foramen ovale. A Frontal cross-sectional view. B Transversal cross-sectional view. C Transformation (by sizing balloon) of a slit-shaped patent foramen ovale having a length (L) into a round shape with a diameter (d). One-half of the circumference (C) corresponds to the L. D Undersized device in an extreme position at one of the edges of the patent foramen ovale. Part of the slit remains uncovered
The following equation should therefore be applied:
where r stands for the radius of the right atrial disc of the closure device, and d stands for the measured balloon waist diameter.
The radius of the device must be at least as long as the defect to ensure that at any given moment, should the device slide to one end of the slit, the PFO defect will be covered by the disc fabric.
A simple and practical way of calculating the size of the device can be derived from the aforementioned formula. The diameter of the balloon waist should be multiplied by the coefficient π to obtain the required size of the closure device.
All patients continued platelet inhibition treatment, which consisted of clopidogrel 75 mg per day and acetylsalicylic acid 100 mg per day for six months.
After six months, follow-up of two-dimensional transthoracic contrast echocardiography in 46 patients (80.7%) and transesophageal contrast echocardiography (TEE) in 36 patients (63.1%) were obtained, and the rate of residual shunt was compared in both groups.
Statistical analysis
Continuous variables are presented as mean ± SD and were compared by a two-sided unpaired t test. Categorical data are reported as counts and percentages. Statistical significance was assumed when P<0.05. Correlation between measured PFO balloon waist diameters in both orthogonal projections was calculated by Pearson’s correlation test.
RESULTS
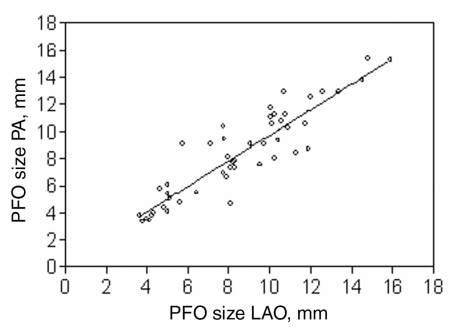
The balloon-stretched diameter of the PFO was measured in two orthogonal projections, and a good correlation was obtained between the two measurements (r = 0.91), with a regression slope of 0.94 (95% CI 0.81 to 1.07; P<0.0001) (Figure 3). This confirmed that the slit of the PFO transforms into a circle with inflation of the sizing balloon.
Figure 3.
Correlation (r = 0.91) with a regression slope of 0.94 (95% CI 0.81 to 1.07; P<0.0001) between two diameters of the inflated sizing balloon, measured in two orthogonal projections, confirming the round shape of the patent foramen ovale (PFO) once the balloon is expanded. LAO Left anterior oblique; PA Posteroanterior
The distribution of the patients into groups, with corresponding PFO dimensions and device sizes, as well as the incidence of residual shunts, is shown in Figure 1.
After six months, compared with the transthoracic contrast echocardiography control obtained in 46 patients (80.7%), a discrete residual shunt during a Valsalva manoeuvre (three to nine microbubbles) (12) was found in five patients (13.9%) in group I. In subgroup IIA, four patients (26.6%) had a discrete residual shunt after six months, while one patient (6.6%) had a discrete residual shunt at rest and a large shunt (more than 30 microbubbles) (12) on Valsalva manoeuvre (P = 0.064). In six patients from subgroup IIB, there was no residual shunt.
DISCUSSION
In our series of patients, we found that the balloon-measured size of the PFO was larger overall (mean stretched diameter 8.6 ±3.5 mm, range 3.8 mm to 14.8 mm) than that of autopsy patients who died of other reasons in the study by Hagen et al (4). This observation is in accordance with other studies (11,13) reporting sizing balloon measurements in patients with a history of cryptogenic stroke. A study by Schuchlenz et al (2) had found that in 100 patients with cryptogenic stroke, the mean balloon size PFO diameter was significantly larger than that in the controls (8.3 ±2.6 mm, range 4 mm to 14 mm versus 5.2 ±1.7 mm, range 2 mm to 10 mm; respectively). In Overell’s meta-analysis, larger PFOs were strongly associated with cryptogenic stroke (RR for stroke patients versus non-stroke controls increased by a factor of 1.83, 95% CI 1.25 to 2.66) and are therefore more likely to be the causative mechanism of recurrences (14). Furthermore, a large PFO was found in 20% of patients with cryptogenic stroke and only in 9.7% of patients with stroke of known cause (15).
It must be emphasized that the values in 965 autopsy patients by Hagen et al (4) may have been underestimated because of the formalin-fixed shrinkage of fibroelastic elements of the interatrial septum. On the other hand, balloon inflation in the slit-like or oval-shaped PFO, as well as introduction of the millimetres probe at autopsy, might have induced stretching of the elastic tissue and therefore overestimated the size of the PFO. In the early 1990s, Mueller et al (16) had measured the PFO in four patients using a guidewire loop technique. This technique used a uniplanar wire loop to measure only the longest axis of the PFO slit, which was crucial information for the selection of a closure device. Nevertheless, the sizing balloon measurement has remained the technique of choice and is superior to the two-dimensional TEE because the latter requires a multiplane probe – which generates cross-sections usually in its shorter axis – making it difficult to obtain a view showing the entire length of the PFO slit or its longitudinal axis (2,11). This limitation will probably be overcome by the introduction of three-dimensinoal TEE.
Consensus on treatment of the PFO has not yet been reached. In the Cryptogenic Stroke Study (PICSS), the presence of a PFO in stroke patients treated with acetylsalicylic acid or warfarin was not associated with an increase in recurrent events (15). No randomized trials have been conducted thus far to prove the superiority of PFO closure versus medical treatment (17). Nevertheless, in a nonrandomized study by Windecker et al (18) comparing percutaneous PFO closure with medical treatment, follow-up after a four-year period had found a nonsignificant trend toward lower risk in combined end point of death, stroke or transient ischemic attack in the invasive group (8.5% versus 24.3% events per year in the invasive and conservative groups, respectively; P = 0.05; RR 0.48, 95% CI 0.23 to 1.01) (18).
The choice of an appropriate closure device size and complete PFO closure may reduce the incidence of recurrence after PFO closure. It is well known that failures are caused either by thrombus formation on the left atrial device disc or by a remaining residual shunt (5,8). In the study by Wahl et al (19), the presence of a residual shunt after percutaneous PFO closure was found to be a predictor of recurrent embolism in patients with PFO and atrial septal aneurysm (RR 5.3, 95% CI 1.3 to 21.0; P = 0.02), which is in accordance with the observation in 80 patients by Windecker at al (5) in which postprocedural shunt tended to be a predictor of recurrent paradoxical embolism (RR 4.2, 95% CI 1.1 to 17.8; P = 0.03). Importantly, in the group of 307 patients reported by Braun et al (20), the risk of stroke recurrence was not associated with small residual shunt, which is in line with the analysis performed by Hung et al (21) (annualized event rate for trivial or no shunt 3.3%, mild or moderate 37.6%; P not significant). However, several studies have reported that recurrent neurological events occurred in the first year after percutaneous closure (5,22,23), which has been ascribed to incomplete closure of the PFO because of a late endothelial-ization process (6,23). This indirectly confirms the role of the residual shunt in the incidence of recurrent neurological events. A sizing balloon was not systematically used in all studies on percutaneous PFO closure, and also the results concerning the incidence of residual shunt vary significantly among the studies (24), which, in part, may be due to the selection of improper device size. In a study of 281 patients reported by Sievert et al (25) the sizing balloon was systematically used, and residual shunt at a mean follow-up of 12 ±16 months was present in 5.5% of the patients, while recurrence of stroke occurred in eight patients (2.8%). Likewise, in a study by Bruch et al (26), there was no residual shunt in 66 patients after six months. On the other hand, Windecker et al (18) reported that 83% of patients had complete occlusion after six months where small, moderate and large shunts persisted in 10%, 4% and 3% of the patients, respectively. In a study by Hung et al (21), in 63 patients who underwent percutaneous PFO closure, a mild shunt persisted in 11% of the cases and a moderate shunt persisted in 3%. In the latter two studies, the sizing balloon was not used during percutaneous PFO closure. The results of our study are in line with those of other studies in which the sizing balloon was used. We had achieved 83.3% complete closure at six months in the group whose device size was selected according to the sizing balloon measurement, while the rest of the patients had a discrete residual shunt, present only on performing a Valsalva manoeuvre. We had developed a mathematical concept for calculating PFO closure device size by measuring the balloon-stretched diameter across the slit-like orifice, which becomes circular once the balloon is expanded. Our assumption that with the inflated balloon, the PFO will adopt a round shape was confirmed by good correlation between two measures of the balloon waist diameter in two orthogonal projections (r = 0.91). This is the basic point of our hypothesis. Once the balloon waist diameter is measured, a precise device size could be selected by simply multiplying the measured waist diameter by the coefficient (p = 3.14). Our results showed a nonsignificant trend in the number of patients with residual shunts after six months in the two groups, favouring the strategy based on balloon measurement (P = 0.064). The only case of a large shunt (more than 30 microbubbles) was found in the group where the large PFO (14.8 mm to 15.3 mm) was closed with a device that was undersized (Amplatzer PFO occluder 35 mm) according to the equation.
Limitations
The present study is too small to show a significant difference in the incidence of residual shunts, although it shows a borderline significant trend in less residual shunts in the group where an appropriately sized closure device was used. Absence of a residual shunt in the group with oversized devices also indirectly emphasizes the importance of device selection. The study is also underpowered to prove the correlation of residual shunt and the incidence of recurrences, and much larger multicentric studies are needed to prove this hypothesis.
CONCLUSIONS
PFO length cannot be easily measured by echocardiography. The present study strongly supports the use of a sizing balloon as a gold standard to measure PFO length because of the capacity of the PFO to transform from a slit-like form to a circular form when the sizing balloon is inflated. Its proper measurement enables an adequate selection of device size based on a simple mathematical calculation. Our study suggests that a reduction in the incidence of residual shunts occurred in the group where the PFO closure device was selected in accordance with the mathematical equation. This may lead to a reduction in the rate of recurrences, although our study was not powered to prove this correlation.
Footnotes
CONFLICT OF INTEREST: In the writing of this article, there was no funding by any financial source and therefore no competing interests or conflicts of interest.
REFERENCES
- 1.Kerut EK, Norfleet WT, Plotnick GD, Giles TD. Patent foramen ovale: A review of associated conditions and the impact of physiological size. J Am Coll Cardiol. 2001;38:613–23. doi: 10.1016/s0735-1097(01)01427-9. [DOI] [PubMed] [Google Scholar]
- 2.Schuchlenz HW, Weihs W, Beitzke A, Stein JI, Gamillscheg A, Rehak P. Transesophageal echocardiography for quantifying size of patent foramen ovale in patients with cryptogenic cerebrovascular events. Stroke. 2002;33:293–6. doi: 10.1161/hs0102.100883. [DOI] [PubMed] [Google Scholar]
- 3.Lechat P, Mass JL, Lascault G, et al. Prevalence of patent foramen ovale in patients with stroke. N Engl J Med. 1988;318:1148–52. doi: 10.1056/NEJM198805053181802. [DOI] [PubMed] [Google Scholar]
- 4.Hagen PT, Scholz DG, Edwards WD. Incidence and size of patent foramen ovale during the first 10 decades of life: An autopsy study of 965 normal hearts. Mayo Clin Proc. 1984;59:17–20. doi: 10.1016/s0025-6196(12)60336-x. [DOI] [PubMed] [Google Scholar]
- 5.Windecker S, Wahl A, Chatterjee T, et al. Percutaneous closure of patent foramen ovale in patients with paradoxical embolism: Long-term risk of recurrent thromboembolic events. Circulation. 2000;101:893–8. doi: 10.1161/01.cir.101.8.893. [DOI] [PubMed] [Google Scholar]
- 6.Varma C, Benson LN, Warr MR, et al. Clinical outcomes of patent foramen ovale closure for paradoxical emboli without echocardiographic guidance. Catheter Cardiovasc Interv. 2004;62:519–25. doi: 10.1002/ccd.20121. [DOI] [PubMed] [Google Scholar]
- 7.Billinger K, Ostermayer SH, Carminati M, et al. HELEX septal occluder for transcatheter closure of patent foramen ovale: Multicentre experience. EuroInterv. 2006;1:465–71. [PubMed] [Google Scholar]
- 8.Schwerzmann M, Windecker S, Wahl A, et al. Percutaneous closure of patent foramen ovale: Impact of device design on safety and efficacy. Heart. 2004;90:186–90. doi: 10.1136/hrt.2002.003111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hara H, Virmani R, Ladich E, et al. Patent foramen ovale: Current pathology, pathophysiology, and clinical status. J Am Coll Cardiol. 2005;46:1768–76. doi: 10.1016/j.jacc.2005.08.038. [DOI] [PubMed] [Google Scholar]
- 10.Ho SY, McCarthy KP, Rigby ML. Morhological features pertinent to interventional closure of patent oval foramen. J Interv Cardiol. 2003;16:33–8. doi: 10.1046/j.1540-8183.2003.08000.x. [DOI] [PubMed] [Google Scholar]
- 11.Schuchlenz HW, Weihs W, Horner S, Quehenberger F. The association between the diameter of a patent foramen ovale and the risk of embolic cerebrovascular events. Am J Med. 2000;109:456–62. doi: 10.1016/s0002-9343(00)00530-1. [DOI] [PubMed] [Google Scholar]
- 12.Mas JL, Arquizan C, Lamy C, et al. Recurrent cerebrovascular events associated with patent foramen ovale, atrial septal aneurysm, or both. N Engl J Med. 2001;345:1740–6. doi: 10.1056/NEJMoa011503. [DOI] [PubMed] [Google Scholar]
- 13.Marshall AC, Lock JE. Structural and compliant anatomy of the patent foramen ovale in patients undergoing transcatheter closure. Am Heart J. 2000;140:303–7. doi: 10.1067/mhj.2000.108236. [DOI] [PubMed] [Google Scholar]
- 14.Overell JR, Bone I, Lees KR. Interatrial septal abnormalities and stroke: A meta-analysis of case-control studies. Neurology. 2000;55:1172–9. doi: 10.1212/wnl.55.8.1172. [DOI] [PubMed] [Google Scholar]
- 15.Homma S, Sacco RL, Di Tullio MR, Sciacca RR, Mohr JP. Effect of medical treatment in stroke patients with patent foramen ovale: Patent foramen ovale in Cryptogenic Stroke Study. Circulation. 2002;105:2625–31. doi: 10.1161/01.cir.0000017498.88393.44. [DOI] [PubMed] [Google Scholar]
- 16.Mueller XM, Sigwart U, Regli F, Kappenberger L. Atrial septal defect measurement with a guidewire loop. J Interv Cardiol. 1990;3:103–8. [Google Scholar]
- 17.Meier B. Closure of patent foramen ovale: Technique, pitfalls, complications, and follow up. Heart. 2005;91:444–8. doi: 10.1136/hrt.2004.052258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Windecker S, Wahl A, Nedeltchev K, et al. Comparison of medical treatment with percutaneous closure of patent foramen ovale in patients with cryptogenic stroke. J Am Coll Cardiol. 2004;44:750–8. doi: 10.1016/j.jacc.2004.05.044. [DOI] [PubMed] [Google Scholar]
- 19.Wahl A, Meier B, Haxel B, et al. Prognosis after percutaneous closure of patent foramen ovale for paradoxical embolism. Neurology. 2001;57:1330–2. doi: 10.1212/wnl.57.7.1330. [DOI] [PubMed] [Google Scholar]
- 20.Braun M, Gliech V, Boscheri A, et al. Transcatheter closure of patent foramen ovale (PFO) in patients with paradoxical embolism. Periprocedural safety and mid-term follow-up results of three different device occluder systems. Eur Heart J. 2004;25:424–30. doi: 10.1016/j.ehj.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 21.Hung J, Landzberg MJ, Jenkins KJ, et al. Closure of patent foramen ovale for paradoxical emboli: Intermediate-term risk of recurrent neurological events following transcatheter device placement. J Am Coll Cardiol. 2000;35:1311–6. doi: 10.1016/s0735-1097(00)00514-3. [DOI] [PubMed] [Google Scholar]
- 22.Martín F, Sánchez PL, Doherty E, et al. Percutaneous transcatheter closure of patent foramen ovale in patients with paradoxical embolism. Circulation. 2002;106:1121–6. doi: 10.1161/01.cir.0000027819.19722.ee. [DOI] [PubMed] [Google Scholar]
- 23.Anzai H, Child J, Natterson B, et al. Incidence of thrombus formation on the CardioSEAL and the Amplatzer interatrial closure devices. Am J Cardiol. 2004;93:426–31. doi: 10.1016/j.amjcard.2003.10.036. [DOI] [PubMed] [Google Scholar]
- 24.Chatterjee T, Petzsch M, Ince H, et al. Interventional closure with Amplatzer PFO occluder of patent foramen ovale in patients with paradoxical cerebral embolism. J Interv Cardiol. 2005;18:173–9. doi: 10.1111/j.1540-8183.2005.04050.x. [DOI] [PubMed] [Google Scholar]
- 25.Sievert H, Horvath K, Zadan E, et al. Patent foramen ovale closure in patients with transient ischemia attack/stroke. J Interv Cardiol. 2001;14:261–6. doi: 10.1111/j.1540-8183.2001.tb00745.x. [DOI] [PubMed] [Google Scholar]
- 26.Bruch L, Parsi A, Grad MO, et al. Transcatheter closure of interatrial communications for secondary prevention of paradoxical embolism: Single-center experience. Circulation. 2002;105:2845–8. doi: 10.1161/01.cir.0000019069.32964.0e. [DOI] [PubMed] [Google Scholar]