Abstract
BACKGROUND
Increased proinflammatory cytokines have mainly been studied in younger patients with heart failure and are regarded as prognostic markers. However, whether this holds true in elderly patients with heart failure remains uncertain.
OBJECTIVES
To determine whether inflammation is equally important in the progression of heart failure in the elderly as has been previously reported in younger patients, and whether cytokine level can predict mortality in this population of elderly heart failure patients.
METHODS
The cytokine profile in an elderly patient group with severe heart failure (n=54, mean [± SD] age of 80.1±5.0 years, New York Heart Association class III or IV) was compared with that of age-matched healthy individuals (n=70). Of the 54 study patients, 46% were hypertensive, 54% had coronary artery disease, 43% had atrial fibrillation and 24% had a previous stroke. One-year mortality was 24%.
RESULTS
The results showed increased levels of interleukin-6 (IL-6), tumour necrosis factor-alpha and epidermal growth factor in the heart failure patients compared with those in the control group. Moreover, IL-6, tumour necrosis factor-alpha and vascular endothelial growth factor were significantly increased in patients who died within one year. Further logistic regression analyses showed that IL-6 was the only significant predictor of one-year mortality. In a subgroup of heart failure patients with atrial fibrillation, there were significant cytokine activations, whereas in a subgroup with ischemia or diabetes, cytokines were less activated.
CONCLUSIONS
In the present octogenarian group with heart failure, there were significant increases of inflammatory cytokines that were associated with mortality, and IL-6 was the only cytokine to predict one-year mortality. Cytokine activation was more pronounced in the subgroup of patients with heart failure and concomitant atrial fibrillation.
Keywords: Cytokine, Elderly, Heart failure
There is ample evidence that shows inflammation playing an important role in the progression of heart failure, particularly in younger patients (1–11). Circulating markers of inflammation, such as tumour necrosis factor-alpha (TNF-α), interleukin-6 (IL-6) and C-reactive protein, may be useful in establishing a diagnosis and in gauging a prognosis in patients with heart failure. In addition, inflammatory cytokines have been investigated as targets of heart failure therapy. Although results from clinical trials directed against specific cytokines (such as TNF-α) have thus far been disappointing (12–14), studies continue to address the importance and therapeutic potential of modulating the immune response in heart failure.
The normal myocardium is composed of cardiomyocytes and noncardiomyocytes, which includes endothelial smooth muscle cells, vascular smooth muscle cells and fibroblasts. Cytokines are believed to be involved in structural remodelling of muscular and nonmuscular compartments. For example, epidermal growth factor (EGF) has previously been implicated in cardiac hypertrophy and heart failure (15).
Chronic heart failure (CHF) is partly characterized by tissue ischemia as well as endothelial dysfunction (16). Conditions that induce tissue ischemia or endothelial dysfunction, eg, myocardial infarction, are associated with a release of angiogenic factors, including vascular endothelial growth factor (VEGF), which promotes mobilization of endothelial progenitor cells from the bone marrow to the peripheral circulation (17).
The present study aimed to determine whether inflammation is equally important in the progression of heart failure in the elderly as has been previously reported in younger patients, and whether cytokine level can predict mortality in this population of elderly heart failure patients.
METHODS
Subjects
Patients (n=54) with CHF, New York Heart Association (NYHA) class III or IV, who were admitted to the Heart Failure Unit at Sahlgrenska University Hospital (Gothenburg, Sweden), were recruited to participate in the present study during 2005. A diagnosis of CHF was based on the European Society of Cardiology (18) definition. For a diagnosis of systolic heart failure, left ventricular ejection fraction (LVEF) needed to be less than 40%, as assessed by conventional echocardiography. For a diagnosis of heart failure with preserved systolic function (PSF), two criteria were required: LVEF greater than 50% by echocardiography, and B-type natri-uretic peptide (BNP) (Biosite, USA) greater than 400 pg/L. To avoid bias because of possible overdiagnosis of heart failure PSF (ie, including those with CHF symptoms but without diastolic dysfunction), patients only required a reduced LVEF. This was because many cases of diastolic dysfunction was unable to be reliably determined, such as in the presence of atrial fibrillation. Patients were followed as frequently as dictated by the patients’ clinical status. Healthy age-matched control subjects (n=70) were included. All healthy subjects had a physical examination, blood analysis and echocardiography. The present study complied with the Declaration of Helsinki. The study protocol was approved by a Human Ethical Committee at Gothenburg University (Gothenburg, Sweden), and all patients gave written informed consent to participate in the present study.
Clinical variables
Age, sex, NYHA functional class and LVEF data, as well as one-year mortality data, were collected. Echocardiography was performed using a commercially available probe and system to assess cardiac function. Blood samples were collected from a peripheral vein after the patient had rested in the supine position for at least 10 min. Blood samples were placed on ice and spun in a refrigerated centrifuge to separate the plasma. Samples were stored at −80° C until further analysis by appropriate techniques.
Analyses of cytokines
Cytokines were measured by protein array biochip technology using the Evidence analyzer, which is a fully automated system from Randox Laboratories Ltd (catalogue number EV 3508, Randox Laboratories Ltd, United Kingdom).
Statistical analyses
Data are expressed as mean ± SD. Cytokine levels were evaluated by Student’s t test. Logistic regression analysis was used to assess predictors of one-year mortality. Variables with P=0.05 by univariable analysis were further assessed by multivariable logistic regression analysis. Statistical significance was set at P<0.05. StatView 5.0 software (SAS Institute Inc, USA) was used for statistical analyses.
RESULTS
Clinical profiles
Baseline clinical characteristics are shown in Table 1. The CHF patients had a mean (± SD) age of 80.1±5.0 years. These patients were representative of a typical elderly population with severe heart failure. The most common comorbidities were hypertension (46%), coronary artery disease (54%), atrial fibrillation (43%) and stroke (24%). As stated previously, most of the CHF patients had systolic heart failure. Only a very limited number of heart failure patients with PSF was enrolled. One-year mortality was 24%.
TABLE 1.
Clinical characteristics of chronic heart failure (CHF) patients and healthy volunteers
| Characteristic | CHF (n=54) | Control (n=70) |
|---|---|---|
| Age, years, mean ± SD | 80.1±5.0 | 75.2±4.1 |
| Men, % | 74 | 63 |
| New York Heart Association class | III or IV | Not relevant |
| Hypertension, % | 46 | 0 |
| Diabetes, % | 25 | 0 |
| Coronary artery diseases, % | 54 | 0 |
| Atrial fibrillation, % | 43 | 0 |
| Chronic obstructive pulmonary disease, % | 17 | 0 |
| Stroke, % | 24 | 0 |
| ACE inhibitors or ARB, % | 74 | 0 |
| Beta-blockers, % | 78 | 0 |
| Spironolactone, % | 30 | 0 |
| Statin, % | 24 | 0 |
ACE Angiotensin-converting enzyme; ARB Angiotensin II receptor subtype 1 blocker
Cytokine profile and correlation with clinical variables
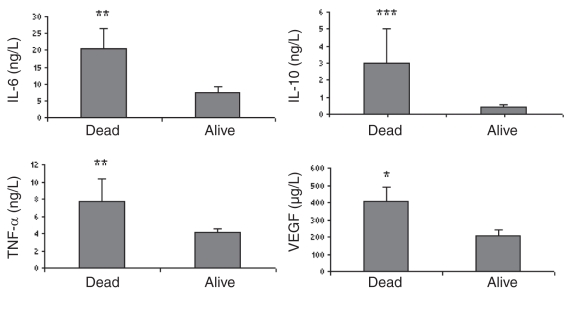
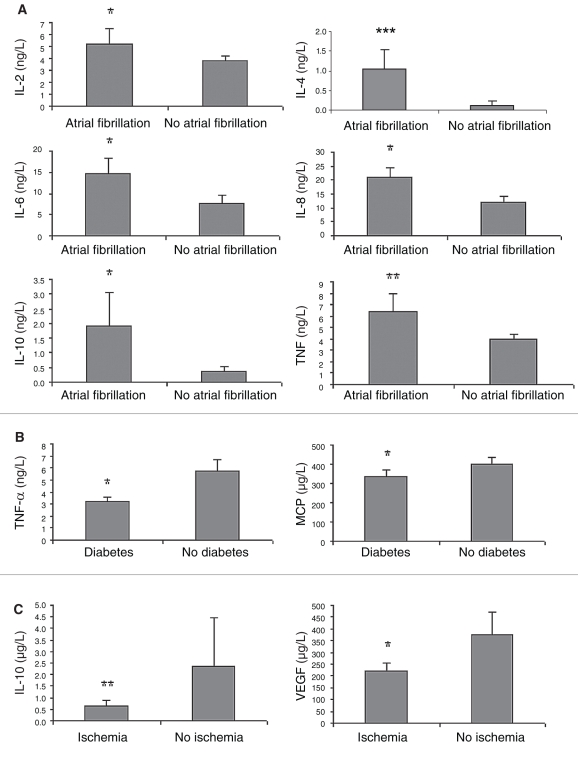
The results showed increased levels of IL-6, IL-8, IL-10, IL-1b, TNF-α and EGF in heart failure patients compared with those in the control group (Table 2). Moreover, IL-6, IL-10, TNF-α and VEGF were significantly increased in those patients who did not survive within one year (Figure 1). In a subgroup of heart failure patients with atrial fibrillation (paroxysmal, persistent or permanent), there were significant increases in IL-2, IL-4, IL-6, IL-8, IL-10 and TNF-α (Figure 2A). However, in subgroups with coronary artery diseases and diabetes, cytokines were less activated (Figures 2B and 2C). No significant difference was found between sexes or among different medications.
TABLE 2.
Cytokine profile in chronic heart failure (CHF) patients and controls
| Cytokine profile | CHF (n=54), mean ± SD | Control (n=70), mean ± SD |
|---|---|---|
| Interleukin-2, ng/L | 4.39±4.49 | 5.78±9.00 |
| Interleukin-4, ng/L | 0.50±1.69 | 0.40±1.25 |
| Interleukin-6, ng/L | 10.59±15.01*** | 1.52±0.94 |
| Interleukin-8, ng/L | 15.92±14.21*** | 8.21±5.48 |
| Interleukin-10, ng/L | 1.02±3.65* | 0.24±1.05 |
| Vascular endothelial growth factor, μg/L | 256.90±237.66 | 281.17±230.37 |
| Interferon-gamma, ng/L | 3.53±4.45 | 4.45±4.07 |
| Tumour necrosis factor-alpha, ng/L | 4.96±5.43** | 1.30±1.57 |
| Interleukin-1a, ng/L | 0.61±1.85 | 0.42±1.15 |
| Interleukin-1b, ng/L | 1.40±6.42* | 0.47±1.27 |
| Monocyte chemoattractant protein, μg/L | 380.47±196.59 | 413.66± 156.96 |
| Epidermal growth factor, ng/L | 23.10±33.01*** | 14.68±14.43 |
P<0.05;
P<0.01;
P<0.001 compared with controls
Figure 1.
Cytokine profile in chronic heart failure patients between those who survived and those who died within one year. *P<0.05; **P<0.01; ***P<0.001. IL Interleukin; TNF-α Tumour necrosis factor-alpha; VEGF Vascular endothelial growth factor
Figure 2.
Cytokine profile in chronic heart failure pathients between those with or without atrial fibrillation (A), diabetes (B) and ischemia (C). *P<0.05; **P<0.01; ***P<0.001. IL Interleukin; MCP Monocyte chemoattractant protein; TNF-α Tumour necrosis factor-alpha; VEGF Vascular endothelial growth factor
Logistic regression analyses
Among the 54 patients with CHF, 13 died within one year. Univariate analyses of available risk factors (37 in total) demonstrated that IL-6 and IL-8 were significant risk factors. Other factors such as VEGF and monocyte chemoattractant protein had a tendency toward significance. Further multivariate analyses, after adjustment for imbalance in other risk factors (so-called independent risk factors), showed that only IL-6 was significant (Table 3). However, the relationship between IL-6 level and one-year mortality was not linear. By dividing the 54 patients into quartiles, there was significantly increased mortality in IL-6 levels greater than 10 ng/L compared with those less than 10 ng/L (Table 4).
TABLE 3.
Logistic regression analyses of cytokines in patients with heart failure who died within one year
| Univariate, P | Multivariate, P | |
|---|---|---|
| Interleukin-6 | 0.010 | 0.002 |
| Interleukin-8 | 0.021 | |
| Tumour necrosis factor-alpha | 0.121 | |
| Interferon-gamma | 0.029 | |
| Vascular endothelial growth factor | 0.054 | |
| Monocyte chemoattractant protein | 0.068 |
TABLE 4.
Quartile distribution of patients with chronic heart failure (CHF) based on interleukin-6 (IL-6) level
| CHF, n | IL-6, ng/L | Died within one year, n | Mortality, % | Fisher’s exact test |
|---|---|---|---|---|
| 14 | 0.5–2 | 2 | 14 | |
| 13 | 2–4 | 2 | 15 | |
| 13 | 4–10 | 2 | 15 | |
| 4 | >10 | 8 | 57 | 0.002 |
DISCUSSION
Our study has demonstrated that in elderly patients with heart failure, a significant increase of inflammatory cytokines is associated with one-year mortality.
Heart failure in octogenarians
The majority of the population with heart failure are elderly, and they often have multiple diseases. Despite the vast number of elderly heart failure patients, this group has not been well studied (particularly octogenarians) compared with younger heart failure patients. For instance, there is increasing evidence that inflammation is involved in the progression of heart failure, mostly in younger patients (1–11). This raises the question of whether inflammation is equally important in heart failure in the elderly, and whether cytokines can predict mortality in this population of elderly heart failure patients. All CHF patients included in the present study had severe heart failure with NYHA class III or IV. Diastolic function was difficult to determine in approximately 40% to 50% of patients because of the presence of either atrial fibrillation or other technical limitations. To avoid bias because of possible over diagnosis of heart failure with PSF – when diastolic function measurement was impossible – we chose mostly CHF patients with reduced LVEF. In fact, in the present elderly group with severe heart failure, diastolic dysfunction was believed to be often present more or less concomitantly with decreased systolic function. Our heart failure registry study (unpublished data) showed that approximately 40% of this senior patient group with CHF had PSF.
Cytokines in heart failure
Our results have shown increased IL-6, TNF-α and EGF in elderly heart failure patients. Moreover, IL-6, TNF-α and VEGF were significantly increased in those patients who died within one year. These results are in line with previous findings in younger heart failure patients. Interestingly, in the present study, we demonstrated increased VEGF in CHF in the elderly, implying the occurrence of endothelial dysfunction. Logistic analyses of available risk factors (37 in total), after adjustment for imbalance, showed that only IL-6 is a significant predictor of one-year mortality. To our knowledge, this is the first study to show that octogenarian heart failure patients displayed increased proinflammatory cytokines, but in a different pattern from those reported in the younger heart failure patients. By dividing patients into quartiles, there was an approximately fourfold increase in one-year mortality in patients with IL-6 levels greater than 10 ng/L, compared with those who had less than 10 ng/L.
It is well known that hypertension is one of the main causes of heart failure, particularly in the elderly. Approximately 91% of heart failure patients have current or previous hypertension (19). In the present study, 46% of patients had hypertension. However, this does not exclude the possibility that these elderly heart failure patients may have had hypertension previously. There is evidence showing that plasma IL-6 level is strongly associated with hypertension (20). Recently, Coles et al (21) reported that IL-6 knockout was able to prevent angiotensin II-induced hypertension in mice. Our current results, which showed increased inflammatory cytokines in CHF patients with hypertension, were also in accordance with our previous publication on increased inflammation in the early stages of heart failure in spontaneously hypertensive rats (22).
Further analyses of subgroups with different comorbidities in CHF in the elderly showed heterogeneous patterns. For instance, in a subgroup of heart failure patients with atrial fibrillation there were significant increases in IL-2, IL-4, IL-6, IL-8, IL-10 and TNF-α. However, in subgroups with coronary artery diseases and diabetes, cytokines were less activated. This is particularly interesting and clinically relevant because heart failure in the elderly is a heterogeneous group complicated by diversified etiologies, clinical symptoms, cardiac dysfunctions, coexistence of multiple diseases, and higher incidences of both morbidity and mortality. Accordingly, in a subgroup with CHF as described above, it does not necessarily mean that the CHF subgroup only has one underlying cause. In most cases, the etiology of CHF in the elderly is multifactorial.
Studies have indicated that there is an association between systemic inflammation and atrial fibrillation. High-sensitivity C-reactive protein level determined before cardioversion represents an independent predictor of both successful cardioversion for atrial fibrillation and maintenance of sinus rhythm after conversion (23). Recently, a meta-analysis (24) suggested that increased C-reactive protein levels were associated with greater risk of recurrence of atrial fibrillation, although there was significant heterogeneity across the studies. An experimental study (25) using whole-cell patch clamp and indo-1 fluorometric ratio techniques, in single cardiomyocytes isolated from rabbit pulmonary veins, showed that TNF-α increased the pulmonary vein arrhythmogenicity and induced an abnormal calcium homeostasis, thereby causing inflammation-related atrial fibrillation. Our current study in CHF patients with atrial fibrillation is in line with the above observations.
We have also observed in our study that IL-10, which is often regarded as an anti-inflammatory cytokine, was also increased. This was probably due to a compensatory mechanism secondary to increased proinflammatory cytokines, such as IL-6 and TNF.
The heterogeneous phenotype of CHF, particularly in the very elderly, makes clinical judgement difficult and unreliable. To find a suitable marker for this group will no doubt facilitate clinical practice. An increase in proinflammatory cytokines is one useful marker. In some cases, it is complementary to BNP, eg, in patients who are overweight. It is believed that a combination of BNP and increases in inflammatory cytokines may have additional prognostic values.
Limitations
In the present study, we included only a very limited number of CHF patients with PSF, because measurement of diastolic dysfunction was difficult, thus avoiding bias. Therefore, the CHF group mostly consisted of patients with systolic heart failure. Moreover, because of the relatively limited sample size, the interpretation of results should be cautious. For example, the present study did not have statistical power to rule out other prognostic indicators of CHF in the elderly, except IL-6. BNP level is a well-recognized prognostic marker of CHF in younger patients. In the present study, BNP was not routinely measured when the study was initiated. Therefore, we did not have BNP data for all the patients, except for those with PSF.
CONCLUSION
In elderly patients with heart failure, there was a significant increase in inflammatory cytokines associated with mortality. Therefore, cytokine activation may be regarded as a prognostic marker for heart failure in the elderly in the same way as is considered in younger patients.
ACKNOWLEDGEMENTS
This project is supported by the Swedish Heart-Lung Foundation and the Swedish Medical Research Council. Dr Michael Fu is the holder of The Lars Werkö Distinguished Research Fellowship from the Swedish Heart-Lung Foundation
REFERENCES
- 1.Mann DL. Inflammatory mediators and the failing heart: Past, present, and the foreseeable future. Circ Res. 2002;91:988–98. doi: 10.1161/01.res.0000043825.01705.1b. [DOI] [PubMed] [Google Scholar]
- 2.Tracey KJ, Beutler B, Lowry SF, et al. Shock and tissue injury induced by recombinant human cachectin. Science. 1986;234:470–4. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]
- 3.Natanson C, Eichenholz PW, Danner RL, et al. Endotoxin and tumor necrosis factor challenges in dogs simulate the cardiovascular profile of human septic shock. J Exp Med. 1989;169:823–32. doi: 10.1084/jem.169.3.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pagani FD, Baker LS, Hsi C, Knox M, Fink MP, Visner MS. Left ventricular systolic and diastolic dysfunction after infusion of tumor necrosis factor-alpha in conscious dogs. J Clin Invest. 1992;90:389–98. doi: 10.1172/JCI115873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franco F, Thomas GD, Giroir BP, et al. Magnetic resonance imaging and invasive evaluation of development of heart failure in transgenic mice with myocardial expression of tumor necrosis factor-alpha. Circulation. 1999;99:448–54. doi: 10.1161/01.cir.99.3.448. [DOI] [PubMed] [Google Scholar]
- 6.Rauchhaus M, Doehner W, Francis DP, et al. Plasma cytokine parameters and mortality in patients with chronic heart failure. Circulation. 2000;102:3060–7. doi: 10.1161/01.cir.102.25.3060. [DOI] [PubMed] [Google Scholar]
- 7.Deswal A, Petersen NJ, Feldman AM, Young JB, White BG, Mann DL. Cytokines and cytokine receptors in advanced heart failure: An analysis of the cytokine database from the Vesnarinone trial (VEST) Circulation. 2001;103:2055–9. doi: 10.1161/01.cir.103.16.2055. [DOI] [PubMed] [Google Scholar]
- 8.Torre-Amione G. Immune activation in chronic heart failure. Am J Cardiol. 2005;95:3C–8C. doi: 10.1016/j.amjcard.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 9.Torre-Amione G, Kapadia S, Benedict C, Oral H, Young JB, Mann DL. Proinflammatory cytokine levels in patients with depressed left ventricular ejection fraction: A report from the Studies of Left Ventricular Dysfunction (SOLVD) J Am Coll Cardiol. 1996;27:1201–6. doi: 10.1016/0735-1097(95)00589-7. [DOI] [PubMed] [Google Scholar]
- 10.Bozkurt B, Torre-Amione G, Warren MS, et al. Results of targeted anti-tumor necrosis factor therapy with etanercept (ENBREL) in patients with advanced heart failure. Circulation. 2001;103:1044–7. doi: 10.1161/01.cir.103.8.1044. [DOI] [PubMed] [Google Scholar]
- 11.Deswal A, Bozkurt B, Seta Y, et al. Safety and efficacy of a soluble P75 tumor necrosis factor receptor (Enbrel, etanercept) in patients with advanced heart failure. Circulation. 1999;99:3224–6. doi: 10.1161/01.cir.99.25.3224. [DOI] [PubMed] [Google Scholar]
- 12.Anker SD, Coats AJ. How to RECOVER from RENAISSANCE? The significance of the results of RECOVER, RENAISSANCE, RENEWAL and ATTACH. Int J Cardiol. 2002;86:123–30. doi: 10.1016/s0167-5273(02)00470-9. [DOI] [PubMed] [Google Scholar]
- 13.Chung ES, Packer M, Lo KH, Fasanmade AA, Willerson JT. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: Results of the anti-TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation. 2003;107:3133–40. doi: 10.1161/01.CIR.0000077913.60364.D2. [DOI] [PubMed] [Google Scholar]
- 14.Mann DL, McMurray JJ, Packer M, et al. Targeted anticytokine therapy in patients with chronic heart failure: Results of the Randomized Etanercept Worldwide Evaluation (RENEWAL) Circulation. 2004;109:1594–602. doi: 10.1161/01.CIR.0000124490.27666.B2. [DOI] [PubMed] [Google Scholar]
- 15.Weber KT. Fibrosis and hypertensive heart disease. Curr Opin Cardiol. 2000;15:264–72. doi: 10.1097/00001573-200007000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Fischer D, Rossa S, Landmesser U, et al. Endothelial dysfunction in patients with chronic heart failure is independently associated with increased incidence of hospitalization, cardiac transplantation, or death. Eur Heart J. 2005;26:65–9. doi: 10.1093/eurheartj/ehi001. [DOI] [PubMed] [Google Scholar]
- 17.Shintani S, Murohara T, Ikeda H, et al. Mobilization of endothelial progenitor cells in patients with acute myocardial infarction. Circulation. 2001;103:2776–9. doi: 10.1161/hc2301.092122. [DOI] [PubMed] [Google Scholar]
- 18.Swedberg K, Cleland J, Dargie H, et al. Guidelines for the diagnosis and treatment of chronic heart failure: Executive summary (update 2005): The Task Force for the Diagnosis and Treatment of Chronic Heart Failure of the European Society of Cardiology. Eur Heart J. 2005;26:1115–40. doi: 10.1093/eurheartj/ehi204. [DOI] [PubMed] [Google Scholar]
- 19.Levy D, Larson MG, Vasan RS, Kannel WB, Ho KK. The progression from hypertension to congestive heart failure. JAMA. 1996;275:1557–62. [PubMed] [Google Scholar]
- 20.Vázuqez-Oliva G, Fernández-Real JM, Zamora A, Vilaseca M, Badimón L. Lowering of blood pressure leads to decreased circulating interleukin-6 in hypertensive subjects. J Hum Hypertens. 2005;19:457–62. doi: 10.1038/sj.jhh.1001845. [DOI] [PubMed] [Google Scholar]
- 21.Coles B, Fielding CA, Rose-John S, Scheller J, Jones SA, O’Donnell VB. Classic interleukin-6 receptor signaling and interleukin-6 trans-signaling differentially control angiotensin II-dependent hypertension, cardiac signal transducer and activator of transcription-3 activation, and vascular hypertrophy in vivo. Am J Pathol. 2007;171:315–25. doi: 10.2353/ajpath.2007.061078. Erratum in 2007;171:1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haugen E, Chen J, Wikström J, Grönros J, Gan LM, Fu LX. Parallel gene expressions of IL-6 and BNP during cardiac hypertrophy complicated with diastolic dysfunction in spontaneously hypertensive rats. Int J Cardiol. 2007;115:24–8. doi: 10.1016/j.ijcard.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 23.Watanabe E, Arakawa T, Uchiyama T, Kodama I, Hishida H. High-sensitivity C-reactive protein is predictive of successful cardioversion for atrial fibrillation and maintenance of sinus rhythm after conversion. Int J Cardiol. 2006;108:346–53. doi: 10.1016/j.ijcard.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 24.Liu T, Li G, Li L, Korantzopoulos P. Association between C-reactive protein and recurrence of atrial fibrillation after successful electrical cardioversion: A meta-analysis. J Am Coll Cardiol. 2007;49:1642–8. doi: 10.1016/j.jacc.2006.12.042. [DOI] [PubMed] [Google Scholar]
- 25.Lee SH, Chen YC, Chen YJ, et al. Tumor necrosis factor-alpha alters calcium handling and increases arrhythmogenesis of pulmonary vein cardiomyocytes. Life Sci. 2007;80:1806–15. doi: 10.1016/j.lfs.2007.02.029. [DOI] [PubMed] [Google Scholar]