Abstract
The directional migration of cells within multicellular organisms is governed by gradients of both chemical attractants and repellents in diverse processes including leukocyte trafficking and neuronal path finding in vivo. These complex extracellular environments direct the orchestrated bidirectional trafficking of leukocytes between the vasculature and tissues. Substantial progress has been made in dissecting the molecular mechanisms involved in orchestrating the directed movement of leukocytes into host tissues, however less is known about the reverse migration of leukocytes from the tissues to the vasculature. In this article, we discuss the functional interplay between chemoattraction and chemorepulsion in the bidirectional movement of cells in complex in vivo environments and the implications of these mechanisms to both normal physiology and human disease.
Understanding reverse leukocyte migration
Immune responses involve the orchestrated trafficking of leukocytes between the vasculature and tissues. Leukocyte migration within lymphoid and non-lymphoid tissues requires both the dynamic interactions between cells and the extracellular matrix and the recognition and directed migration in response to chemoattractants and chemorepellents (see Box 1) [1–4]. The classic steps of leukocyte trafficking within the body involve leukocyte movements through the vasculature, firm adhesion to the endothelium and subsequent transendothelial migration into tissues. This directed movement from the vasculature to the tissues is likely orchestrated by a complex array of soluble factors in combination with the extracellular matrix (ECM) environment. Substantial progress has been made in dissecting the molecular composition of the factors involved in orchestrating the directed movement of leukocytes into host tissues and has been reviewed extensively [4, 5]. However, less is understood about what regulates the reverse movement of leukocytes from tissues to the vasculature or lymphatics, a process known as reverse chemotaxis or intravasation (Figure 1; see Box 1) [6]. This process of reverse migration is a key component of normal physiology including the trafficking of leukocytes from the bone marrow to the vasculature and the process of lymphocyte egress from lymphoid tissue to the vasculature or lymphatics during immune surveillance. The evidence reviewed here supports the existence of both chemoattractants and chemorepellents that guide this process. Studies of leukocyte reverse migration are informed by an increased understanding of how the directional decisions to move either towards or away from a specific agent, guide axonal growth cones during development. Reverse migration is also a likely component of pathologic conditions, including tumor invasion and metastasis and the dissemination of intracellular pathogens from infected tissues into the vasculature.
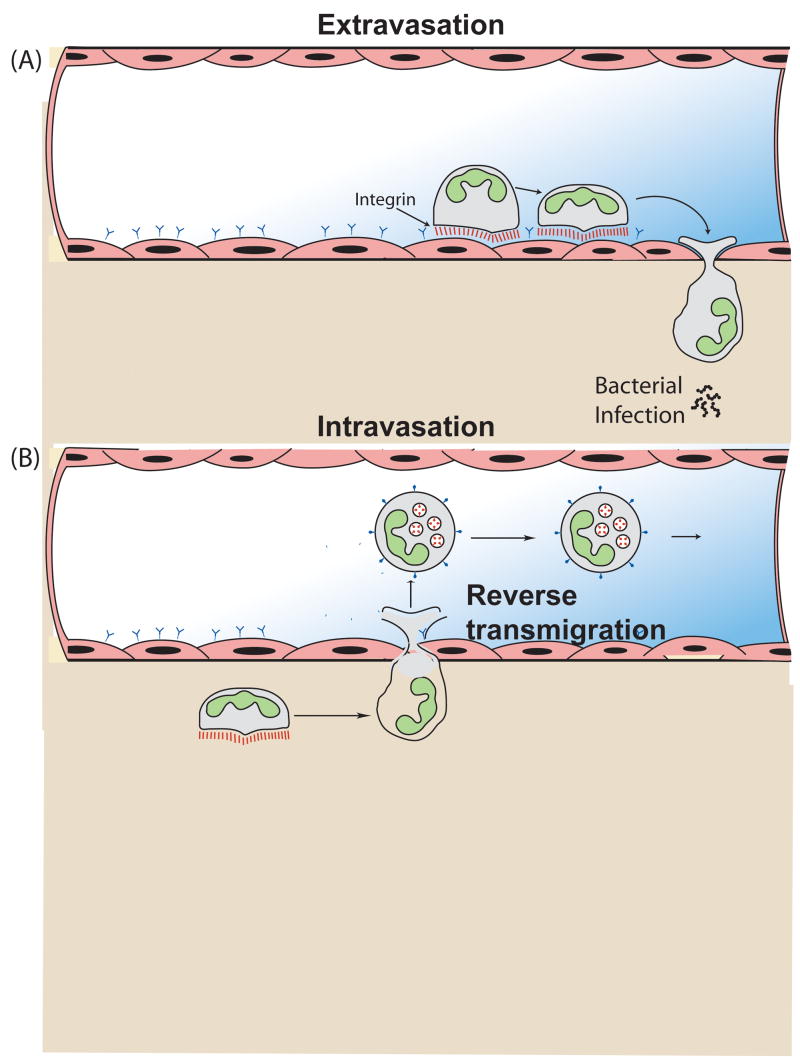
Figure 1.
Schematic of leukocyte bidirectional trafficking in response to inflammatory stimuli. (A) Leukocyte extravasation occurs in response to many inflammatory stimuli including bacterial infection and tissue wounding. The stages of extravasation have been described in detail [4,5], and involve the sequential activation of adhesion receptors on the leukocyte surface including the initial leukocyte rolling mediated by selections. Subsequently firm adhesion is mediated by integrin cell surface receptors that allow for the attachment and transmigration of the leukocyte across the endothelial surface. The final stage of the process involves the directed migration of leukocytes through tissues to the site of inflammation. (B) Leukocyte intravasation or reverse transmigration involves the reverse movement of leukocytes from tissues to the vasculature and their subsequent trafficking through the vasculature. This reverse transmigration is less well characterized and occurs during leukocyte trafficking and lymphocyte egress from the thymus. Recent evidence also indicates that leukocytes in zebrafish models undergo reverse transmigration during the resolution phase of the inflammatory response [9]. Less is understood about the mechanisms that regulate reverse transmigration, but cell surface receptors such as JAM-C are involved in regulating these processes during macrophage reverse transmigration [7]. The mechanisms that contribute to chemorepulsion and reverse transmigration are the focus of this article.
Recent studies implicate reverse chemotaxis and reverse transmigration of leukocytes in the resolution of inflammatory responses involving the innate immune system. For example, blockade of the junctional adhesion protein, JAM-C, at endothelial contact sites, reduced the number of monocytes at extravascular spaces by increasing reverse transmigration of monocytes across the endothelium [7]. Further evidence for reverse migration has been suggested in vivo using mammalian systems and intravital imaging. A recent study demonstrated that rising concentrations of CINC-1 (the rat orthologue of human IL-8) initially serve as a neutrophil chemoattractant in mesentery causing visualized neutrophil extravasation [8]. However, increasing concentrations ultimately result in neutrophil movement in the opposite direction back toward the venule wall. Time-lapse imaging in zebrafish embryos reveals that neutrophil reverse chemotaxis from sites of tissue wounding back to the vasculature is a key mechanism by which inflammation is resolved after acute injury [9], and this process may be impaired under some conditions of chronic inflammation [10]. Thus, reverse migration of leukocytes may be governed by competing gradients of chemical attractants and/or repellents and potentially bidirectional cues including chemokines, in vivo.
Here we review what is known about the molecular mechanisms that mediate the response of cells to chemoattractants, chemorepellents, bidirectional cues that can either attract or repel or the effects of competing gradients, with the aim of providing insight into what may be mediating the reverse movement of leukocytes in complex in vivo environments and the implications of these mechanisms to both normal physiology and human disease.
Signaling in chemoattraction and chemorepulsion
Directional motility in response to chemical gradients, or chemotaxis, is a fundamental and universal mechanism amongst living organisms from prokaryotes to higher vertebrates [11]. Gradient sensing allows a cell to respond to a gradient of a specific chemokinetic agent in its environment [12]. This gradient signal is received through specific receptors on the cell surface and subsequently transduced through intracellular signaling machinery to generate a coherent response causing the cell to move up a gradient of a chemoattractant or down a gradient of a chemorepellent. The sensing and response mechanisms of both chemoattraction and chemorepulsion are found in both prokaryotes and higher eukaryotic cells. From a reductionist standpoint chemoattraction and chemorepulsion are seen as contributing to the ability of a cell to find optimal surroundings for performing specific functions in vivo.
Chemoattraction has been studied extensively in prokaryotes and in simple and complex eukaryotes [11]. These studies have revealed a complex set of chemoattractant-chemoreceptor interactions, intracellular signal transduction pathways and cell migratory processes, which guide this directional choice [13–16]. Furthermore, chemoattraction has been shown in higher vertebrates to play significant roles in embryonic development, development of vascular and neuronal structures and the establishment of an inflammatory or immune response to pathogens or tissue injury. Much of the progress in elucidating the mechanisms by which cells sense and migrate directionally to chemoattractants has benefited from using the genetic model system, Dictyostelium discoideum [17–20].
Chemotaxis involves two separate yet functionally linked events, gradient sensing and cell polarization, that work synergistically to facilitate directional migration [17–19]. Directional sensing is the ability of a cell to detect gradients of chemoattractants and involves the asymmetric recruitment of signaling molecules that mediate chemotaxis, including phosphatidylinositol (3,4,5)-trisphosphate (PtdIns(3,4,5)P3), to the membrane adjacent to the highest concentration of chemoattractant (Figure 2) [21–24]. Gradient sensing does not require cell polarization and is independent of actin polymerization. In contrast, cell polarization is an actin-dependent process that leads to the development of an asymmetric morphology and a leading edge characterized by the accumulation of actin and actin-binding proteins. The generation of a well-defined leading edge is regulated by the asymmetric localization and activation of key signaling molecules including phosphoinostide 3-kinase (PI3K), phospholipase C (PLC) and members of the Rho GTPase family, Cdc42 and Rac, that promote the localized nucleation and polymerization of actin [22,24]. Deficiencies in the activities of these key signaling components at the leading and trailing edge of the cell result in the loss of cell polarity and reduced efficiency of chemotaxis.
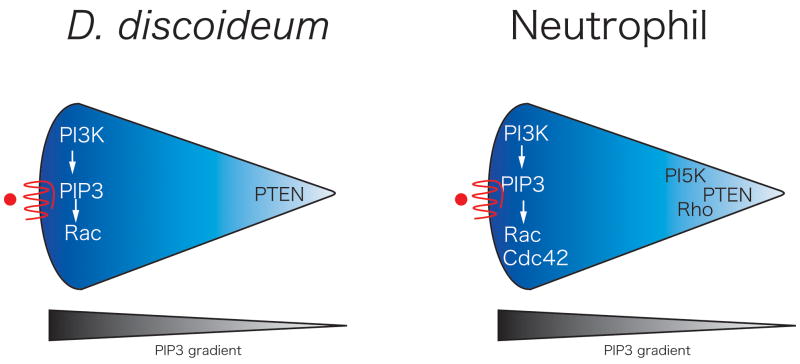
Figure 2.
Schematic of the asymmetric polarization of signaling molecules during chemotaxis. Both Dictyostelium discoideum and neutrophils are common model systems used to study chemotaxis. Substantial evidence supports the asymmetric activation of PI3K at the leading edge of both Dictyostelium discoideum and neutrophils during directed cell migration. Both neutrophils and Dictyostelium also have the localized activation of the small GTPases Rac and/or Cdc42 at the leading edge with the subsequent activation of actin polymerization and pseudopod formation. The uropod contains the phosphatase PTEN (phosphatase and tensin homolog) [12] in both neutrophils and Dictyostelium and recent evidence implicates a role for PI5K [67] and Rho signaling [68] at the uropod of neutrophils.
A recent study suggests that Dictyostelium discoideum may also be instrumental in determining the mechanisms that mediate chemorepulsion. D. discoideum are repelled by substances secreted by starving cells, although the identity of these compounds remains unknown. A recent study suggests that chemorepellents, cAMP analogues, induce chemorepulsion by affecting the polarity of phospholipase C and PI3K signaling [25]. Specifically, the chemorepellents induce localized inhibition of PLC and thereby the accumulation of PtdIns(4,5)P2, while PtdIns(3,4,5)P3 concentrates at the side of the cell farthest from the chemorepellent and mediates pseudopod formation and reverse migration (Figure 3). These findings suggest that chemorepulsion in some cases may represent a reversal of polarity through pathways that are instrumental in normal chemotactic responses, such as PLC and PI3K. This represents a very exciting area for future investigation that will allow the dissection of key pathways that mediate signaling in response to chemorepellents.
Figure 3.
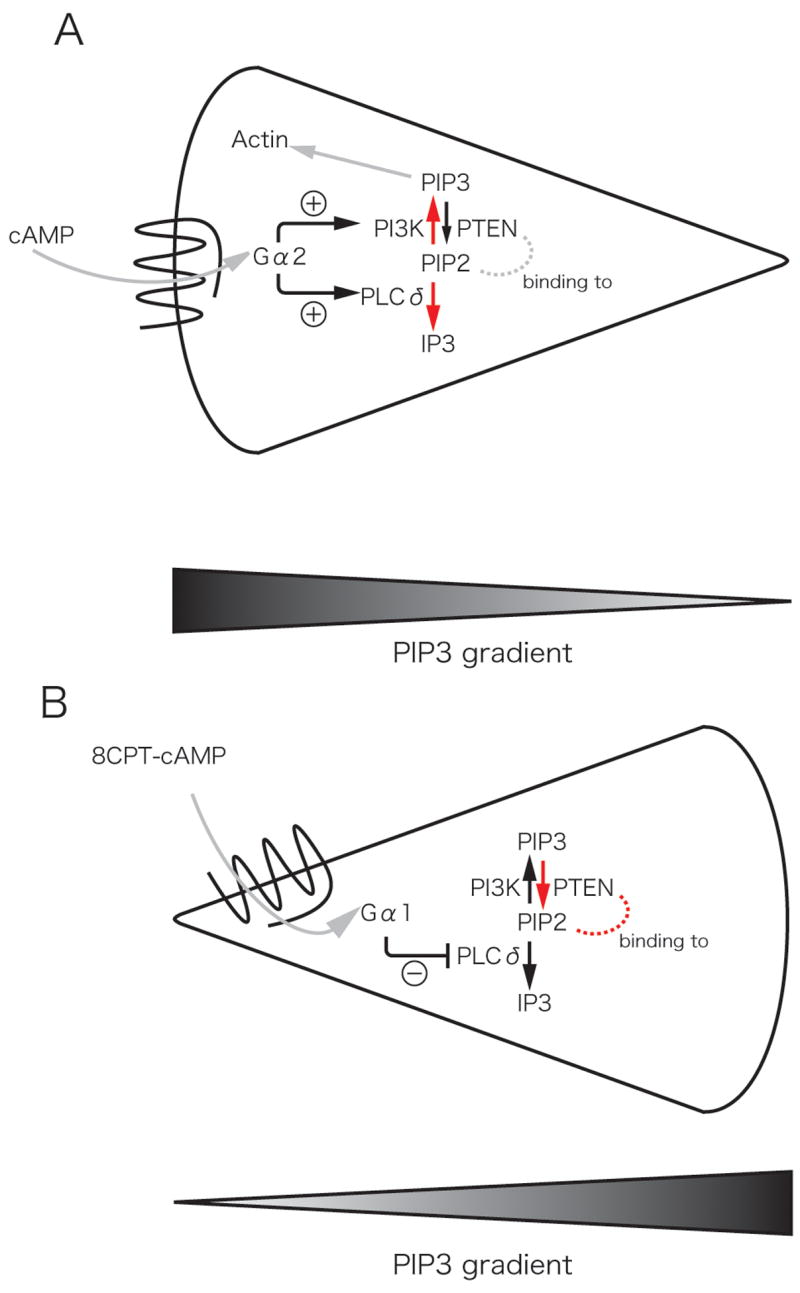
Schematic of reverse polarity induced by cAMP-mediated chemoattraction (A) and 8CPT-cAMP-mediated chemorepulsion (B) in Dictyostelium discoideum [25]. (A) The chemoattractant, cAMP, induces PI3K and PLC activation at the leading edge (red arrows) with localized accumulation of PtdIns(3,4,5)P3 mediated by PI3K and the loss of PtdIns(4,5)P2 at the leading edge through the activation of PLC and the generation of IP3. This generates a PtdIns(3,4,5)P3 gradient with increased levels at the leading edge. (B) The chemorepellant, 8CPT-cAMP, inhibits PLC activity and thereby increases PtdIns(3,4,5)P2 accumulation and activation of PTEN (red arrows). The chemorepellant reverses the polarity of the PtdIns(3,4,5)P3 gradient and induces reverse migration or chemorepulsion.
Leukocytes, including T cells and neutrophils, can exhibit chemorepulsion or fugetaxis in response to chemokines that generally are considered to induce chemoattraction. T cell and neutrophil bidirectional migration in response to chemokines has revealed differential sensitivities of chemoattraction and chemorepulsion to cyclic nucleotide agonists and protein kinase inhibitors [26]. For example, T cell chemoattraction, but not chemorepulsion, to the chemokine, stromal cell derived factor-1 (SDF-1 or CXCL12) is inhibited by the tyrosine kinase inhibitors genestein and herbamycin [26]. Furthermore, the PKC inhibitor GF109203X converted a chemorepellent response of neutrophils to IL-8 into a chemoattractive response [8]. Both T cell and neutrophil chemoattraction to SDF-1 and IL-8, respectively, were shown to be differentially sensitive to cAMP agonists as compared to chemokine induced chemorepulsion [8,26,27]. To date these and other findings, support a model in which intracellular kinase activation, cyclic nucleotide concentrations and possibly intracellular Ca2+ levels determine the directional decision to move towards or away from a chemokine (Figure 4). The precise molecular steps governing the directional decision of T cells and neutrophils to respond to soluble factors by attraction or chemorepulsion remain to be resolved. The further elucidation of mechanistic elements of a chemorepellent signaling pathway that is distinct from that guiding chemoattraction raises the possibility that specific agents could be used therapeutically to augment or antagonize processes by which immune or inflammatory cells traffick to specific anatomic sites in the context of a wide variety of immune and inflammatory diseases.
Figure 4.
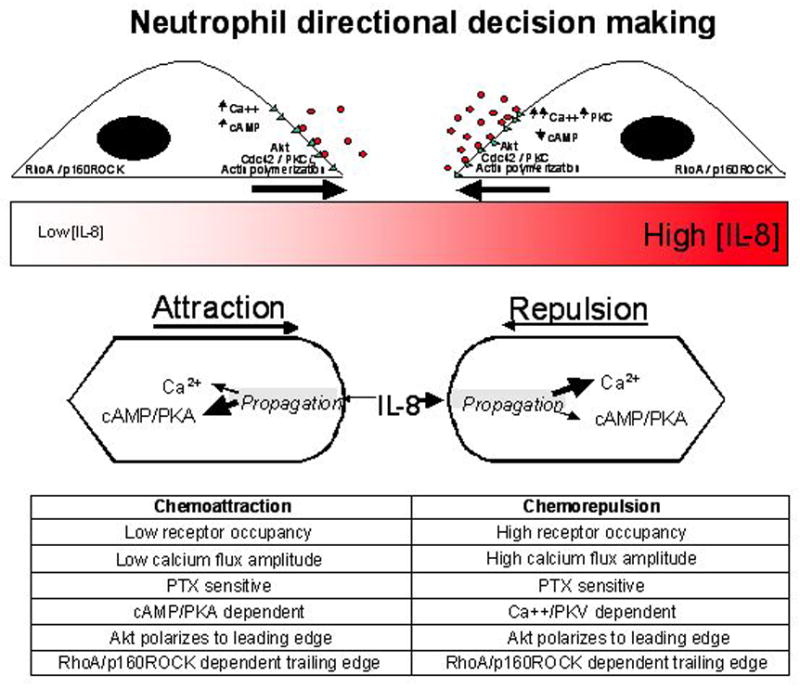
Chemorepulsion and chemoattraction in human neutrophils [8]. Schematic representation of the mechanisms that differ between chemoattraction and chemorepulsion in human neutrophils. Studies of human neutrophils migrating in temporally and spatially continuous gradients of IL-8 within microfluidic devices reveal mechanistic elements involved in signaling that may differentiate a chemoattractive from a chemorepulsive migratory response (indicated by arrows above schematic representation of an IL-8 gradient in red). Receptor occupancy (o = IL-8; Δ = CXCR2). Differential changes in receptor occupancy, Ca2+ flux, cAMP/PKA, Ca2+ /PKC and Akt, Cdc42 and PKCζ localization to the leading edge and RhoA-p160ROCK to the trailing edge associated with chemoattraction and chemorepulsion are shown in the inset table.
Axon growth cone guidance provides mechanistic insights into leukocyte reverse migration
The balance between the action of chemorepellents and chemoattractants has emerged as a critical component that guides the movements of neurons and their growing axons during pathfinding in the developing nervous system. Axon growth cone guidance is the most well studied example of bidirectional cell movement in vertebrate systems. Many of the factors that mediate chemorepulsion can function as bidirectional cues that can either attract or repel including netrins, semaphorins, slit ligands, and ephrins [28–32]. In axon growth cones, the signal transduction mechanism for attraction is molecularly distinct from that guiding repulsion and these agents can serve as a chemoattractant or chemorepellent depending on the developmental stage of the neuron and the specific intracellular signaling cascades that are induced [28,33]. These agents allow a growth cone to migrate towards or away from a bidirectional cue depending on the context. An additional aspect of the diversity of migratory responses in axons is that a repellent response can occur as a result of either diffusible or extracellular matrix bound chemorepellents. For example, both matrix-bound and soluble repellents play a significant role in the development of somites [34].
There is a growing understanding of the signaling pathways that differentiate axon growth cone chemoattraction from chemorepulsion, which may lend clues to similar processes governing leukocyte migration into and out of tissues [35–42]. For example, a number of studies have revealed the mechanistic elements that distinguish between netrin induced chemoattraction and chemorepulsion. Receptors for secreted netrins include DCC (deleted in colorectal cancer) and UNC5 homologues. It has been shown using knock out mouse models that the netrin receptor DCC mediates chemoattraction whereas the DCC-UNC5 complex mediates chemorepulsion. The attractive signaling through DCC is mediated by the GDP-GTP exchange factor DOCK180 and the activation of the small GTPase Rac1 [43]. However, the ratios of cyclic AMP to cyclic GMP probably determines the decision to attract or repel with high ratios associated with attraction and low ratios associated repulsion to netrin-1 [44]. PKCα activation and stimulation of internalization of UNC5 is also important for favoring attraction over repulsion since UNC5A internalization converts netrin-mediated chemorepulsion to attraction [45]. Previous studies have demonstrated that chemorepulsion depends on UNC5A since UNC5A-null axons are not repelled and that the change to chemoattraction requires the adaptor protein PICK1 because PICK1 null axons do not mediate chemoattraction after PKCα activation [45]. Hence the conversion between repellent and attractant responses to netrin-1 is dependent on PKCα and PICK-1. Mechanistic elements involved in second messenger signaling have been shown to influence the netrin-1 induced bidirectional axonal responses. Attraction is converted to repulsion in Xenopus spinal neurons by inhibition of cAMP or protein kinase A or by lowering intracellular Ca2+ [30]. PI3K, mitogen-activated protein kinase (MAPK) and phospholipase C have been shown to influence this process [46]. Further studies of neuronal axon growth cone adaptation have revealed consecutive phases of desensitization and resensitization in the presence of increasing basal concentrations of the guidance factor netrin-1 or brain-derived neurotrophic factor. Furthermore, previous studies have demonstrated that desensitization is accompanied by reduced Ca2+ signaling [46]. In contrast MAPK and local protein synthesis is associated with resensitization. These processes evidently allow the growth cone to re-adjust its sensitivity over a wide range of concentrations of netrin-1 [47]. The role of Ca2+ in signaling the directional decision of growth cones has recently been a focus of attention. The activity of the transient receptor potential (TRP)-like channel in the growth cones of cultured Xenopus neurons is modulated by the chemoattractants, netrin-1 and brain-derived neurotrophic factor [48]. Furthermore independent of changes in intracellular signaling, the concentration or gradient of ligand can also influence the decision to attract or repel [45]. Finally, gradient structure may also influence the directional decision. A recent study demonstrated that steeper gradients of the semaphorin, Sema 2A induce more chemorepulsion than shallow gradients [49].
Axon growth cone chemorepulsion therefore involves specific ligand-receptor interactions and intracellular signaling events that are distinct from those governing chemoattraction. Furthermore, the developmental stage of the cell, gradient structure, Ca2+ flux and the presence of diffusible or matrix-bound gradients of chemokinetic agents may determine the directional response. It is likely that aspects of the signaling cascade that differentiate repulsion from attraction in neuronal growth cones are conserved and may ultimately be relevant to leukocyte migration [35,36,50].
Chemorepulsion in the immune system
There is growing evidence that chemorepulsion is likely a key mechanism involved in regulating leukocyte motility. Some of the cues that regulate neuronal chemorepulsion have also recently been implicated in regulating the responses of leukocytes to chemoattractants. As alluded to above, many of the chemoattractants and chemorepellents that affect neuronal cell migration including netrins, semaphorins, slit ligands, and ephrins, have recently been implicated in the motility of immune cells [35,36,39,40,50]. An example of this conservation is the involvement of Slit, a secreted protein that mediates axonal chemorepulsion, and has also been shown to inhibit the directed migration of leukocytes in response to chemoattractants [51]. These findings indicate that secreted factors may serve as inhibitors of leukocyte chemotaxis and thereby alter the responsiveness of leukocytes in vivo. Furthermore, these findings highlight the cross talk between chemoattractant signaling to seven transmembrane G-protein coupled receptors (GPCRs) and Roundabout (Robo), the single-transmembrane Slit receptor. More recent studies implicate an association between Robo and the CXCR4 receptor on T cells and suggest that Slit/Robo modulates CXCR4-mediated chemotaxis through the regulation of downstream effector pathways such as Src and Lck kinases [38]. In related studies, the guidance molecule netrin has been shown to bind to the netrin receptor UNC5b on leukocytes and inhibit leukocyte chemotaxis both in vitro and in vivo [37]. Recent studies suggest that other neuronal guidance cues, such as semaphorins play important and diverse roles in immune function, including regulating the interactions between T cells and antigen presenting cells [36]. Other factors may also provide chemorepulsive effects on immune cells and this inhibitory effect may be regulated by the tissue microenvironment. An example includes the Ca2+ binding proteins, S100A8 and S100A9, that have recently been reported to repel neutrophil motility, but under conditions of oxidative stress these factors lose their inhibitory functions, suggesting that this inhibitory function may be neutralized [52]. Together, these recent reports suggest that leukocyte migration is regulated, in a manner analogous to the neuronal cells, by the balance of chemorepulsive and chemoattractive signals.
The other aspect of chemorepulsion in the immune system is the active migration of leukocytes away from a factor, such as a chemokine, that is normally considered to stimulate chemoattraction. Previous studies have reported that T cells can migrate away from a high concentration of the chemokine SDF-1 rather than displaying the chemoattraction induced by lower concentrations of the same factor. The SDF-1-induced chemorepulsion is mediated by Gαi-mediated signaling because the process is sensitive to pertussis toxin (PTX), suggesting that the concentration of ligand and receptor occupancy can influence directional decision-making [8,26]. This may be in contrast to chemorepulsion in the nervous system where the decision to attract or repel is generally made by the involvement of different receptors expressed on the cell surface as is the case with the netrin-1/DCC/UNC5A. Similar results have been reported for directional migratory responses of primary human neutrophils to the chemokine, IL-8 [8]. Intriguingly, perturbations of intracellular cyclic nucleotide concentrations in both T cells and neutrophils in the context of chemokine signaling alter directional decision making in these cells in a manner analogous to the effects of netrins on chemoattractant to chemorepellent responses of axon growth cones [8,26].
A final component of the conservation between immune and neuronal cell chemorepulsion likely involves the contribution of Ca2+ signaling. However, the role of Ca2+ signals during the directed migration of leukocytes is controversial, and likely plays a role in both positively and negatively regulating directed cell migration. For example, a recent study suggests that Ca2+ influx is essential for the positive-feedback loop that promotes protrusion of the leading edge of macrophages [53]. Specifically, their findings implicate Ca2+ in the enrichment of PKCα at the leading edge that participates in a positive-feedback mechanism that promotes PI3K activation and F-actin polymerization. However, there is substantial evidence, similar to growth cone guidance, that robust Ca2+ transients are associated with inhibition of cell motility and induction of a stop signal in T cells and other leukocytes [54]. Although Ca2+ signaling has not been directly implicated in mediating chemorepulsion of leukocytes, it is possible that Ca2+-mediated cell stopping and loss of polarity may precede reverse migration in some contexts.
Reverse migration and the role of competing gradients of chemokinetic agents
Leukocytes encounter a combination of chemorepellents and chemoattractants that modify their directional decision making and trafficking in vivo. An additional component that influences the navigation of leukocytes in vivo, is their responses to competing gradients of chemoattractants that can induce distinct signaling pathways. A previous study has in fact demonstrated that there is an intracellular signaling hierarchy that exists in response to different chemoattractants [55]. For example, they showed that end target chemoattractants that are found at sites of infection, such as fMLP, dominate over signals from intermediary chemoattractants such as IL-8 and leukotriene B4 (LTB4). The end target chemoattractants specifically activate signaling through p38 MAPK while the intermediary signals induce PI3K activation, suggesting that these responses are separable. They also show that p38 MAPK inhibitors reverse this hierarchy by promoting preferential migration toward intermediary chemoattractants over end target attractants, providing a mechanism to reverse migration in vivo by altering the activity of different intracellular signaling pathways.
Reverse migration in the context of immune cell development and trafficking: integrating retention, chemoattraction and chemorepulsion
The thymus and other hematopoietic organs have proven to be interesting sites to examine reverse migration since leukocyte populations that develop in these organs must, by the very nature of their developmental pathway, exit the anatomic site in which they develop in order to join the ranks of circulating leukocytes in the vasculature. Furthermore, lymphoid cell trafficking between tissue sites and secondary lymphoid organs also involves reverse migration from tissue into the vasculature or lymphatics. The role of chemoattraction in leukocyte development, maturation and function has been extensively studied [4]. However, the role that chemorepellents play in reverse migration in vivo is less well understood.
The study of thymic emigration has proved to be an interesting model system in which to study the directional cues that contribute to reverse migration. Thymocytes at full maturity migrate from the medulla or other locations in the thymus through the endothelium of post endothelial venules into the vasculature in a process of reverse migration. Chemokinetic agents, including chemokines that signal via Gαi protein-coupled receptors, are thought to be involved in thymic emigration since this process was found to be sensitive to pertussis toxin (PTX) [56]. The chemokine CCL19, which is highly expressed by endothelial venules in the thymic medulla, is thought to act as a chemoattractant for mature T cells generated within the thymus, guiding thymic emigration into venules [57]. In addition, sphingosine 1-phosphate (S1P) and its receptor S1PR have been shown to play a significant role in thymic emigration although the precise mechanism by which this is achieved remains controversial [58–60]. However, there is also evidence that the action of chemoattractants alone does not fully explain the process of thymic emigration. Studies of Cxcr4-knockout mice suggest that both intrathymic chemorepellents and extrathymic chemoattractants govern the egress of mature thymocytes from the thymus, and in particular SP CD4 thymocytes [27, 61]. In these studies, the use of the CXCR4 antagonist AMD3100 suppressed thymic emigration, which was only partially restored by the administration of extrathymic chemoattractant concentrations of S1P. Combinations of cell type specific chemorepellents, chemoattractants and/or chemokinetic agents may therefore exist to guide the reverse migration of cells in the context of thymic emigration.
Studies using intravital microscopy to examine lymphocyte egress from secondary lymph nodes have recently confirmed that reverse migration plays a role in the trafficking of T cells from lymphoid tissue to intranodal vascular and lymphoid spaces [62,63]. Although thymic studies imply a chemoattractant activity for S1P these studies suggest that S1P serves as a “gating” mechanism on endothelial cells that restricts the physical egress of T cells from the lymph node. These findings support a two-step model in which the gate is opened by S1P to permit reverse migration while chemotactic or chemokinetic agents may also actively guide this process. A recent caveat to this hypothesis is raised by the finding that the selective S1PR agonist/antagonist, FTY720, appears to block egress of T cells from the lymph node, which is restored if T cells are PTX treated or are CCR7-deficient [64], suggesting that CCR7 mediates a retention signal in the thymus. These findings support the view that reverse emigration of T cells into medullary venules within the lymph node is the default and can actually occur in the absence of GPCR signaling and thereby raises questions regarding the specific and additional roles that extra lymphoid chemoattractants, intra-lymphoid chemorepellents and/or agents that stimulate chemokinesis might play in the exit of T cells from lymphoid tissue into the vasculature.
Insights from zebrafish models of reverse leukocyte migration
Recent studies suggest that cells of the innate immune system also exhibit reverse chemotaxis to resolve inflammatory responses in zebrafish embryos. Transgenic embryos that express GFP from the leukocyte-specific promoter for the gene encoding myeloperoxidase (MPO) allow the direct visualization of neutrophil responses in the context of acute and chronic injury in vivo [9]. Wounding of the zebrafish fin induces a robust recruitment of neutrophils and macrophages that is resolved at least in part by the reverse chemotaxis of leukocytes to the vasculature [9,65]. Interestingly, the motility to and from the vasculature shows similar speed and directionality, suggesting that the signals that induce forward and reverse chemotaxis are equally robust [9]. A future challenge will be to determine if similar reverse migration occurs in mammalian systems using intravital imaging. Although reverse chemotaxis of neutrophils has not been clearly shown in mammaliam systems to date, this may be because of the limitations associated with the open systems used for intravital imaging that can impact tissue homeostasis or bacterial content, thereby complicating data interpretation. The mechanisms that govern these bidirectional movements in zebrafish systems remain unknown but represent an attractive system to explore the mechanisms that regulate leukocyte chemorepulsion or reverse migration to the vasculature in vivo. It is possible that this reverse migration is induced by competing gradients, or alternatively by the contribution of chemokinetic agents or chemorepellents at the wound site that induce the reverse migration back to the vasculature. In addition, like thymic emigration, retention signals might be shown to play a role in regulating neutrophil reentry into the vasculature. These types of questions will be the focus of future investigations to decipher the complex factors that contribute to reverse chemotaxis in vivo.
Conclusion
In contrast to the mature and detailed study of leukocyte chemoattraction and forward transmigration the study of leukocyte chemorepulsion and reverse migration is in its infancy. The initial provocative studies detailed above argue that exploration of the mechanisms controlling reverse chemotaxis is clearly warranted. What remains unclear is the nature of the precise chemokine/cytokine gradients and intracellular signaling processes that guide these processes in vivo.
What is also potentially exciting is the role that chemorepulsion and reverse migration may play in physiological and pathological processes. The evidence implicates a role for a balance between chemoattraction, chemokinesis and chemorepulsion in the regulation of the emigration of leukocytes from sites in which they develop including the bone marrow, thymus and lymph nodes. Similarly, the possibility that reverse leukocyte migration guided by either chemorepellents and/or chemoattractants might serve as a regulator of an overzealous inflammatory response is highly provocative and potentially paradigm shifting. Finally, we may also speculate about the involvement of reverse migration and chemorepulsion in a diverse range of pathological processes including cancer and infectious diseases. For example, reverse migration and chemorepellents may play a role in the metastatic process in which tumor cells emigrate from the primary tumor into the lymphatics or circulation [66]. Alternatively, one might explore the contribution that reverse chemotaxis and chemorepellents play in the way in which cells, that have been infected with intracellular pathogens such as HIV-1, cytomegalovirus (CMV) and Leishmania subsequently exit tissue sites of primary inoculation and infection with the pathogen and enter and disseminate through the blood stream. The possibility that reverse leukocyte migration plays a role in cancer and infectious diseases raises the possibility of developing new therapeutic approaches that antagonize reverse migration. The corollary to this is that augmentation of reverse migration may also be clinically relevant in immune mediated diseases in which the presence of immune or inflammatory cells at a particular site is detrimental to the individual as in autoimmune disease or in graft versus host disease and the pharmacological manipulation of these cells to undergo reverse migration out of the tissue would be beneficial to the individual.
Box 1 Definition of terms for leukocyte migration
The term chemotaxis is used in this review to describe all directional migration of leukocytes in response to a chemical stimulus. Chemoattraction results from the movement of leukocytes up a gradient and towards a peak concentration of a chemoattractant whereas chemorepulsion results from migration down a gradient away from peak concentrations of the chemorepellent. The term “fugetaxis” has been introduced into the scientific literature and is used interchangeably with the term chemorepulsion but is specifically used in the context of an agent that can either attract or repel cells depending on the concentration of the agent or context [27]. As in neuronal growth cone biology, bidirectional cues exist for leukocyte populations and may serve as both chemoattractants or chemorepellents in a concentration, receptor and/or cell type dependent manner. Whereas transendothelial migration is generally applied to the extravasation of leukocytes from the vascular compartment into the tissues, the term reverse chemotaxis or migration describes the active movement of leukocytes from the tissue space back toward the vasculature [67] and it is this process that is the focus of this review article.
Acknowledgments
This work was supported by NIH Grants R01 GM074827 (A.H.) and by RO1 AI49757, JDRF and Marsha Rivkin Center (MCP). We extend thanks to William G. Tharp, Sa Kan Yoo and Paul Nuzzi for help with the figures and to Jonathan Mathias, John T. Potts and. Michael Santosuosso for their critical reading of the manuscript.
References
- 1.Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392:565–568. doi: 10.1038/33340. [DOI] [PubMed] [Google Scholar]
- 2.Ley K, et al. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 3.Luster AD. Chemokines--chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;338:436–445. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- 4.Luster AD, et al. Immune cell migration in inflammation: present and future therapeutic targets. Nat Immunol. 2005;6:1182–1190. doi: 10.1038/ni1275. [DOI] [PubMed] [Google Scholar]
- 5.Kelly M, et al. Modulating leukocyte recruitment in inflammation. J Allergy Clin Immunol. 2007;120:3–10. doi: 10.1016/j.jaci.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 6.Badolato R. Leukocyte circulation: one-way or round-trip? Lessons from primary immunodeficiency patients. J Leukoc Biol. 2004;76:1–6. doi: 10.1189/jlb.1103529. [DOI] [PubMed] [Google Scholar]
- 7.Bradfield PF, et al. JAM-C regulates unidirectional monocyte transendothelial migration in inflammation. Blood. 2007;110:2545–2555. doi: 10.1182/blood-2007-03-078733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tharp WG, et al. Neutrophil chemorepulsion in defined interleukin-8 gradients in vitro and in vivo. J Leukoc Biol. 2006;79:539–554. doi: 10.1189/jlb.0905516. [DOI] [PubMed] [Google Scholar]
- 9.Mathias JR, et al. Resolution of inflammation by retrograde chemotaxis of neutrophils in transgenic zebrafish. J Leukoc Biol. 2006;80:1281–1288. doi: 10.1189/jlb.0506346. [DOI] [PubMed] [Google Scholar]
- 10.Mathias JR, et al. Live imaging of chronic inflammation caused by mutation of zebrafish Hai1. J Cell Sci. 2007;120:3372–3383. doi: 10.1242/jcs.009159. [DOI] [PubMed] [Google Scholar]
- 11.Eisenbach M. Chemotaxis. Imperial College Press; 2004. [Google Scholar]
- 12.Van Haastert PJ, Devreotes PN. Chemotaxis: signalling the way forward. Nat Rev Mol Cell Biol. 2004;5:626–634. doi: 10.1038/nrm1435. [DOI] [PubMed] [Google Scholar]
- 13.Beckner SK. G-protein activation by chemokines. Methods Enzymol. 1997;288:309–326. doi: 10.1016/s0076-6879(97)88021-4. [DOI] [PubMed] [Google Scholar]
- 14.Wells TN, et al. Definition, function and pathophysiological significance of chemokine receptors. Trends Pharmacol Sci. 1998;19:376–380. doi: 10.1016/s0165-6147(98)01247-4. [DOI] [PubMed] [Google Scholar]
- 15.Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol. 2000;18:217–242. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- 16.Bacon KB. Analysis of signal transduction following lymphocyte activation by chemokines. Methods Enzymol. 1997;288:340–361. doi: 10.1016/s0076-6879(97)88023-8. [DOI] [PubMed] [Google Scholar]
- 17.Bourne HR, Weiner O. A chemical compass. Nature. 2002;419:21. doi: 10.1038/419021a. [DOI] [PubMed] [Google Scholar]
- 18.Devreotes P, Janetopoulos C. Eukaryotic chemotaxis: distinctions between directional sensing and polarization. J Biol Chem. 2003;278:20445–20448. doi: 10.1074/jbc.R300010200. [DOI] [PubMed] [Google Scholar]
- 19.Manahan CL, et al. Chemoattractant signaling in dictyostelium discoideum. Annu Rev Cell Dev Biol. 2004;20:223–253. doi: 10.1146/annurev.cellbio.20.011303.132633. [DOI] [PubMed] [Google Scholar]
- 20.Willard SS, Devreotes PN. Signaling pathways mediating chemotaxis in the social amoeba, Dictyostelium discoideum. Eur J Cell Biol. 2006;85:897–904. doi: 10.1016/j.ejcb.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 21.Comer FI, Parent CA. PI 3-kinases and PTEN: how opposites chemoattract. Cell. 2002;109:541–544. doi: 10.1016/s0092-8674(02)00765-1. [DOI] [PubMed] [Google Scholar]
- 22.Funamoto S, et al. Spatial and temporal regulation of 3-phosphoinositides by PI 3-kinase and PTEN mediates chemotaxis. Cell. 2002;109:611–623. doi: 10.1016/s0092-8674(02)00755-9. [DOI] [PubMed] [Google Scholar]
- 23.Weiner OD. Regulation of cell polarity during eukaryotic chemotaxis: the chemotactic compass. Curr Opin Cell Biol. 2002;14:196–202. doi: 10.1016/s0955-0674(02)00310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weiner OD, et al. A PtdInsP(3)- and Rho GTPase-mediated positive feedback loop regulates neutrophil polarity. Nat Cell Biol. 2002;4:509–513. doi: 10.1038/ncb811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keizer-Gunnink I, et al. Chemoattractants and chemorepellents act by inducing opposite polarity in phospholipase C and PI3-kinase signaling. J Cell Biol. 2007;177:579–585. doi: 10.1083/jcb.200611046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poznansky MC, et al. Active movement of T cells away from a chemokine. Nat Med. 2000;6:543–548. doi: 10.1038/75022. [DOI] [PubMed] [Google Scholar]
- 27.Vianello F, et al. Fugetaxis: active movement of leukocytes away from a chemokinetic agent. J Mol Med. 2005;83:752–763. doi: 10.1007/s00109-005-0675-z. [DOI] [PubMed] [Google Scholar]
- 28.Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- 29.Bashaw GJ, Goodman CS. Chimeric axon guidance receptors: the cytoplasmic domains of slit and netrin receptors specify attraction versus repulsion. Cell. 1999;97:917–926. doi: 10.1016/s0092-8674(00)80803-x. [DOI] [PubMed] [Google Scholar]
- 30.Colamarino SA, Tessier-Lavigne M. The axonal chemoattractant netrin-1 is also a chemorepellent for trochlear motor axons. Cell. 1995;81:621–629. doi: 10.1016/0092-8674(95)90083-7. [DOI] [PubMed] [Google Scholar]
- 31.Goshima Y, et al. Semaphorins as signals for cell repulsion and invasion. J Clin Invest. 2002;109:993–998. doi: 10.1172/JCI15467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goshima Y, et al. Functions of semaphorins in axon guidance and neuronal regeneration. Jpn J Pharmacol. 2000;82:273–279. doi: 10.1254/jjp.82.273. [DOI] [PubMed] [Google Scholar]
- 33.Carmeliet P, Tessier-Lavigne M. Common mechanisms of nerve and blood vessel wiring. Nature. 2005;436:193–200. doi: 10.1038/nature03875. [DOI] [PubMed] [Google Scholar]
- 34.Hernandez-Montiel HL, et al. Diffusible signals and fasciculated growth in reticulospinal axon pathfinding in the hindbrain. Dev Biol. 2003;255:99–112. doi: 10.1016/s0012-1606(02)00033-7. [DOI] [PubMed] [Google Scholar]
- 35.Kikutani H, Kumanogoh A. Semaphorins in interactions between T cells and antigen-presenting cells. Nat Rev Immunol. 2003;3:159–167. doi: 10.1038/nri1003. [DOI] [PubMed] [Google Scholar]
- 36.Kikutani H, et al. Immune semaphorins: increasing members and their diverse roles. Adv Immunol. 2007;93:121–143. doi: 10.1016/S0065-2776(06)93003-X. [DOI] [PubMed] [Google Scholar]
- 37.Ly NP, et al. Netrin-1 inhibits leukocyte migration in vitro and in vivo. Proc Natl Acad Sci U S A. 2005;102:14729–14734. doi: 10.1073/pnas.0506233102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prasad A, et al. Slit-2/Robo-1 modulates the CXCL12/CXCR4-induced chemotaxis of T cells. J Leukoc Biol. 2007;82:465–476. doi: 10.1189/jlb.1106678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suzuki K, et al. Semaphorins and their receptors in immune cell interactions. Nat Immunol. 2008;9:17–23. doi: 10.1038/ni1553. [DOI] [PubMed] [Google Scholar]
- 40.Suzuki K, et al. Semaphorin 7A initiates T-cell-mediated inflammatory responses through alpha1beta1 integrin. Nature. 2007;446:680–684. doi: 10.1038/nature05652. [DOI] [PubMed] [Google Scholar]
- 41.Hong K, et al. A ligand-gated association between cytoplasmic domains of UNC5 and DCC family receptors converts netrin-induced growth cone attraction to repulsion. Cell. 1999;97:927–941. doi: 10.1016/s0092-8674(00)80804-1. [DOI] [PubMed] [Google Scholar]
- 42.Seeger MA, Beattie CE. Attraction versus repulsion: modular receptors make the difference in axon guidance. Cell. 1999;97:821–824. doi: 10.1016/s0092-8674(00)80793-x. [DOI] [PubMed] [Google Scholar]
- 43.Li X, et al. Netrin signal transduction and the guanine nucleotide exchange factor DOCK180 in attractive signaling. Nat Neurosci. 2008;11:28–35. doi: 10.1038/nn2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nishiyama M, et al. Cyclic AMP/GMP-dependent modulation of Ca2+ channels sets the polarity of nerve growth-cone turning. Nature. 2003;423:990–995. doi: 10.1038/nature01751. [DOI] [PubMed] [Google Scholar]
- 45.Bartoe JL, et al. Protein interacting with C-kinase 1/protein kinase Calpha-mediated endocytosis converts netrin-1-mediated repulsion to attraction. J Neurosci. 2006;26:3192–3205. doi: 10.1523/JNEUROSCI.3469-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ming G, et al. Phospholipase C-gamma and phosphoinositide 3-kinase mediate cytoplasmic signaling in nerve growth cone guidance. Neuron. 1999;23:139–148. doi: 10.1016/s0896-6273(00)80760-6. [DOI] [PubMed] [Google Scholar]
- 47.Ming GL, et al. Adaptation in the chemotactic guidance of nerve growth cones. Nature. 2002;417:411–418. doi: 10.1038/nature745. [DOI] [PubMed] [Google Scholar]
- 48.Wang GX, Poo MM. Requirement of TRPC channels in netrin-1-induced chemotropic turning of nerve growth cones. Nature. 2005;434:898–904. doi: 10.1038/nature03478. [DOI] [PubMed] [Google Scholar]
- 49.Isbister CM, et al. Gradient steepness influences the pathfinding decisions of neuronal growth cones in vivo. J Neurosci. 2003;23:193–202. doi: 10.1523/JNEUROSCI.23-01-00193.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Klein RS, Rubin JB. Immune and nervous system CXCL12 and CXCR4: parallel roles in patterning and plasticity. Trends Immunol. 2004;25:306–314. doi: 10.1016/j.it.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 51.Wu JY, et al. The neuronal repellent Slit inhibits leukocyte chemotaxis induced by chemotactic factors. Nature. 2001;410:948–952. doi: 10.1038/35073616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sroussi HY, et al. Oxidation of methionine 63 and 83 regulates the effect of S100A9 on the migration of neutrophils in vitro. J Leukoc Biol. 2007;81:818–824. doi: 10.1189/jlb.0706433. [DOI] [PubMed] [Google Scholar]
- 53.Evans JH, Falke JJ. Ca2+ influx is an essential component of the positive-feedback loop that maintains leading-edge structure and activity in macrophages. Proc Natl Acad Sci U S A. 2007;104:16176–16181. doi: 10.1073/pnas.0707719104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bhakta NR, et al. Calcium oscillations regulate thymocyte motility during positive selection in the three-dimensional thymic environment. Nat Immunol. 2005;6:143–151. doi: 10.1038/ni1161. [DOI] [PubMed] [Google Scholar]
- 55.Heit B, et al. An intracellular signaling hierarchy determines direction of migration in opposing chemotactic gradients. J Cell Biol. 2002;159:91–102. doi: 10.1083/jcb.200202114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chaffin KE, Perlmutter RM. A pertussis toxin-sensitive process controls thymocyte emigration. European Journal of Immunology. 1991;21:2565–2573. doi: 10.1002/eji.1830211038. [DOI] [PubMed] [Google Scholar]
- 57.Ueno T, et al. Role for CCR7 ligands in the emigration of newly generated T lymphocytes from the neonatal thymus. Immunity. 2002;16:205–218. doi: 10.1016/s1074-7613(02)00267-4. [DOI] [PubMed] [Google Scholar]
- 58.Allende ML, et al. Expression of the sphingosine 1-phosphate receptor, S1P1, on T-cells controls thymic emigration. J Biol Chem. 2004;279:15396–15401. doi: 10.1074/jbc.M314291200. [DOI] [PubMed] [Google Scholar]
- 59.Matloubian M, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 60.Vianello F, et al. A CXCR4-dependent chemorepellent signal contributes to the emigration of mature single-positive CD4 cells from the fetal thymus. J Immunol. 2005;175:5115–25. doi: 10.4049/jimmunol.175.8.5115. [DOI] [PubMed] [Google Scholar]
- 61.Rosen H, et al. Rapid induction of medullary thymocyte phenotypic maturation and egress inhibition by nanomolar sphingosine 1-phosphate receptor agonist. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:10907–10912. doi: 10.1073/pnas.1832725100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dustin ML, Chakraborty AK. Tug of war at the exit door. Immunity. 2008;28:15–17. doi: 10.1016/j.immuni.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huang JH, et al. Requirements for T lymphocyte migration in explanted lymph nodes. J Immunol. 2007;178:7747–7755. doi: 10.4049/jimmunol.178.12.7747. [DOI] [PubMed] [Google Scholar]
- 64.Pham TH, et al. S1P1 receptor signaling overrides retention mediated G alpha i-coupled receptors to promote T cell egress. Immunity. 2008;28:122–33. doi: 10.1016/j.immuni.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hall C, et al. The zebrafish lysozyme C promoter drives myeloid-specific expression in transgenic fish. BMC Dev Biol. 2007;7:42. doi: 10.1186/1471-213X-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shields JD, et al. Autologous chemotaxis as a mechanism of tumor cell homing to lymphatics via interstitial flow and autocrine CCR7 signaling. Cancer Cell. 2007;11:526–538. doi: 10.1016/j.ccr.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 67.Lokuta MA, et al. Type Igamma PIP kinase is a novel uropod component that regulates rear retraction during neutrophil chemotaxis. Mol Biol Cell. 2007;18:5069–5080. doi: 10.1091/mbc.E07-05-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alblas J, et al. Activation of RhoA and ROCK are essential for detachment of migrating leukocytes. Mol Biol Cell. 2001;12:2137–2145. doi: 10.1091/mbc.12.7.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]