Abstract
Corticosterone hormones mediate the stress response and function in the survival of hippocampal neurons via activation of gluco- (GR) and mineralocorticoid (MR) receptors. Activated GR and MR couple the corticosterone signal through immediate early genes (IEGs) to the late expression of downstream genes, such as neurotrophic factors. The potential importance of IEGs in GR/MR-dependent plasticity in the brain is largely unknown. We examined the region- and time-dependent transcriptional profiles of six IEGs (c-fos, fosB, fra-1, junB, c-jun and egr-1) by in situ hybridization after acute corticosterone challenge in the hippocampus and the primary somatosensory cortex (S1). Adrenalectomized rats and subsequent hormone injections were used as a model system to eliminate interference of endogenous corticosterone on IEG expression.
In the hippocampus, a single corticosterone dose (10 mg/kg, s.c.) caused a widespread and transient reduction of fosB mRNA after 0.8 h, whereas changes in both c-fos and fra-1 mRNA levels were restricted to the dentate gyrus region. Corticosterone treatment gave rise to a delayed and significant reduction of junB mRNA signals after 2 h in all hippocampal regions, which reversed to increase at 4 h. C-jun and egr-1 mRNA levels were unaffected by corticosterone treatment. On the contrary, in the S1, IEG expression seems to be unaffected by corticosterone treatment, with the exception of a transient increase of junB transcripts at 0.8 h.
The early reduction in c-fos family and junB transcripts may contribute to the GR/MR-dependent changes on hippocampal plasticity and may be dependent on rapid corticosteroid signaling.
Keywords: glucocorticoid hormone, transcription factors, fos family proteins, neuroplasticity, in situ hybridization, rat brain
1. Introduction
The adrenocorticosteroid hormone corticosterone (CORT) is a potent modulator of brain function and a critical factor in the mediation of the stress response. The hippocampus is a brain region for ongoing plasticity and functionally important for declarative/spatial learning and memory. Stress, depletion or sustained high levels of CORT affect the morphology and survival of hippocampal neurons (Gould et al., 1990; Sloviter et al., 1989; Watanabe et al., 1992) and have been shown to influence the expression of genes coding for trophic and protective proteins, e.g. basic fibroblast growth factor (bFGF) and brain derived neurotrophic factor (BDNF) (Chao et al., 1998; Chao and McEwen, 1994; Hansson et al., 2000; Hansson et al., 2003; Riva et al., 1995; Schaaf et al., 1998; Smith et al., 1995).
CORT acts via its low affinity receptors (glucocorticoid receptor, GR) and high affinity receptors (mineralocorticoid receptor, MR), which bind as transcription factors to glucocorticoid response elements within promoter regions, thereby modulating the expression of specific genes, e.g. neurotrophic factors (Parrelli et al., 1998). Both receptors are highly prevalent and co-expressed in the majority of the hippocampal neurons (Cintra et al., 1994; Van Eekelen and De Kloet, 1992). Emerging evidence suggests that the inducible transcription factor and immediate early gene (IEG) c-Fos is essential for neuronal cell survival through its regulation of downstream genes such as BDNF (Zhang et al., 2002). The expression of c-fos and the IEG early growth response gene (egr-1, alternatively known as zif268, krox24, NGFI-A or TIS8) have both been used as functional markers of brain activity and neuroplasticity (James et al., 2005; Pfenning et al., 2007; Sheng and Greenberg, 1990). When induced by physiological or pharmacological stimulation, c-Fos proteins dimerize with proteins from the Jun family to form the activator protein-1 (AP-1), which subsequently interacts with AP-1 recognition sequences in the regulatory regions of various genes (Herdegen and Waetzig, 2001). Interactions between GR and the AP-1 complex independent from DNA-binding have been previously described in tissue culture studies (Schule et al., 1990; Yangyen et al., 1990). Using the c-fos antisense oligodeoxynucleotide approach, we recently demonstrated in vivo an antagonistic c-Fos/GR interaction on both bFGF and BDNF gene expression in the CA1 region of the hippocampus (Hansson et al., 2003). Interactions between GR/MR and IEGs other than c-Fos and their potential importance in GR/MR-dependent changes on brain plasticity are largely unknown.
Several laboratories found that stress increases both the protein and mRNA levels of c-fos in the brain (Cullinan et al., 1995; Herrera and Robertson, 1996; Hyder SM, 1994; Kononen et al., 1992; Kovacs and Sawchenko, 1996), resulting in changes in the composition and activity of the transcription factor AP-1 complex (Autelitano, 1998). Notable, however, is that the stress-induced increase in c-fos seems to be independent of circulating levels of CORT (Hansson et al., 2003; Helmreich et al., 1996; Melia et al., 1994; Ryabinin et al., 1999). Depletion of adrenocorticosteroid hormones by adrenalectomy (ADX) increases c-Fos immunoreactivity in the paraventricular nucleus 3 to 6 h after ADX; at 12 h, c-Fos levels have diminished, and are no longer detectable after 24 h (Brown and Sawchenko, 1997).
We previously used the acute 24 h ADX animal model with no detectable levels of endogenous adrenocorticosteroid hormones, under the assumption that GR and MR are largely unoccupied at this time point. Subsequent treatment with low and high doses of CORT hormone allowed us to separately analyze the consequences of GR and MR activation on gene expression profiles without interference from endogenous adrenocorticosteroid hormones potentially pre-occupying GR and MR (Hansson et al., 2000; Hansson et al., 2001; Hansson et al., 2003; Hansson et al., 2006; Hansson and Fuxe, 2002). In a previous paper we established that in the present acutely adrenalectomized rat model there exists an upregulation of GR and MR mRNA levels in the dorsal hippocampus (Hansson et al., 2000). In the study presented here, a high dose of CORT (10 mg/kg, s.c.) was chosen in order to fully activate both GR and MR over several hours (Hansson et al., 2003; Hansson and Fuxe, 2002). This allowed us to analyze the GR/MR-dependent changes on the temporal and regional expression patterns of IEGs (c-fos, fosB, fos related antigen-1 or fra-1, junB, c-jun, egr-1) of ADX rats by in situ hybridization. The aim of the paper was to study the impact of CORT on IEG expression in order to understand how CORT may contribute to stress induced influences on the neuronal networks. The CORT actions on regional hippocampal c-fos mRNA levels have previously been reported in this model (Hansson et al., 2003).
2. Results
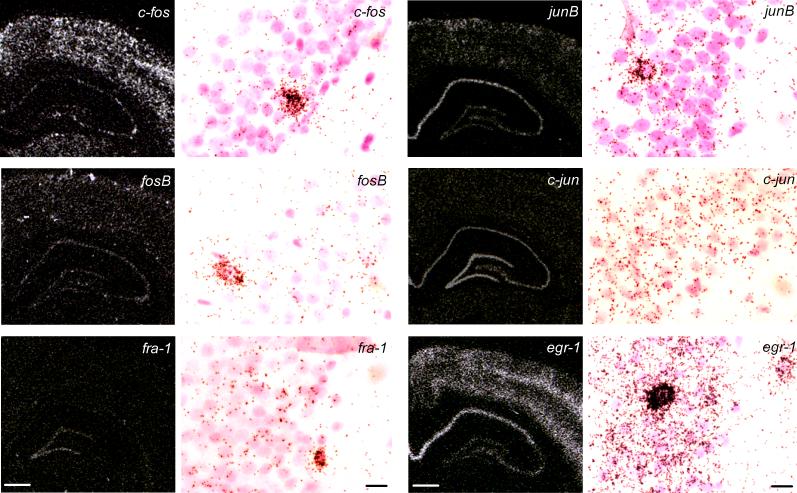
The time course of mRNA levels for six IEGs in the hippocampus and primary somatosensory barrelfield cortex (S1, see Fig. 1) of ADX rats in response to 10 mg/kg CORT (s.c.) as well as plasma CORT levels of the experimental groups are shown in Tables 1 and 2. The regional and cellular expressions for c-fos, fosB, fra-1, junB, cjun and egr-1 mRNA levels are illustrated in Figure 2. IEG expression was mainly linked to nerve cell bodies as revealed in bright-field emulsion autoradiograms counterstained with cresyl violet. In the granular cell layer of the dentate gyrus, we observed a scattered population of nerve cell bodies strongly labelled for all IEG mRNAs with the exception of c-jun (Fig. 2).
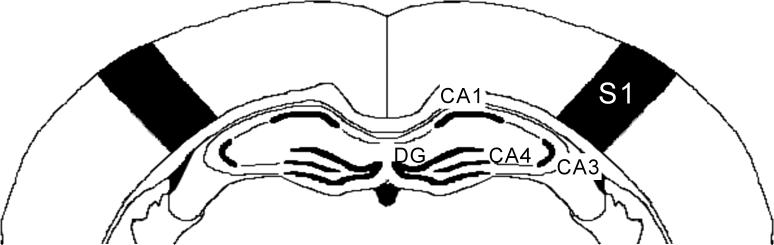
Fig.1.
Schematic representation of the sampled areas for the densitometric evaluation of IEG mRNA in a coronal section through the dorsal hippocampus. (Bregma levels = −2.8 mm). CA1 – CA4: Cornus Ammon areas; DG: granular cell layer of the dentate gyrus; S1: primary somatosensory barrelfield cortex.
Table 1. Radioimmunoassay for plasma CORT.
24 h after ADX rats were injected with either a high dose of CORT (10 mg/kg, s.c.) or saline (s.c., experiment 1) or s.c. injected with saline alone (experiment 2). Data are expressed as CORT (ng/ml, mean ± S.E.M.), n.d. = not detectable.
| time (h) | treatment | (n) | plasma CORT (ng/ml) |
|---|---|---|---|
| Experiment 1 | |||
| 0.8 | ADX + solvent | 4 | n.d. |
| 0.8 | ADX + CORT | 5 | 2638 ± 244 |
| 2 | ADX + solvent | 5 | n.d. |
| 2 | ADX + CORT | 5 | 805 ± 143 |
| 4 | ADX + solvent | 4 | n.d. |
| 4 | ADX + CORT | 5 | 147 ± 27 |
| 8 | ADX + solvent | 5 | n.d. |
| 8 | ADX + CORT | 5 | 29 ±4 |
| 24 | ADX + solvent | 4 | n.d. |
| 24 | ADX + CORT | 5 | n.d. |
| Experiment 2 | |||
| 0.8 | ADX | 4 | n.d. |
| 0.8 | ADX + solvent | 4 | n.d. |
| 4 | ADX | 4 | n.d. |
| 4 | ADX + solvent | 4 | n.d. |
| 24 | ADX | 4 | n.d. |
| 24 | ADX + solvent | 4 | n.d. |
Table 2. Effects of CORT (10 mg/kg, s.c.) on IEG expression in ADX rats.
Data are expressed as optical densities (OD × 10−3, mean ± S.E.M.), statistically analyzed by two-way ANOVA (time and treatment) followed by Fisher's PLSD test and Bonferroni's correction; n = 4−5/group; *p<0.05, **p<0.01, ***p<0.001 CORT vs solvent treated ADX rats per time point; n.d.=not detected, CA1-CA4: Cornus of Ammon areas, DG: Dentate Gyrus, S1: primary somatosensory barrelfield cortex. For details of treatment, see Material and Methods.
| time | S1 | CA1 | CA3 | CA4 | DG | |
|---|---|---|---|---|---|---|
| c-fos mRNA | ||||||
| ADX + solvent | 0.8 | 154 ± 9 | 107 ± 10 | 134 ± 7 | 94 ± 9 | 92 ± 5 |
| ADX + CORT | 0.8 | 173 ± 7 | 85 ± 10 | 120 ± 12 | 74 ± 10 | 57 ± 7 * |
| ADX + solvent | 2 | 80 ± 7 | 41 ± 4 | 102 ± 6 | 67 ± 3 | 56 ± 4 |
| ADX + CORT | 2 | 79 ± 6 | 31 ± 7 | 78 ± 8 | 41 ± 6 * | 30 ± 3 * |
| ADX + solvent | 4 | 53 ± 5 | 39 ± 8 | 62 ± 8 | 34 ± 5 | 40 ± 5 |
| ADX + CORT | 4 | 47 ± 8 | 24 ± 3 | 56 ± 4 | 36 ± 5 | 34 ± 3 |
| ADX + solvent | 8 | 60 ± 9 | 47 ± 7 | 69 ± 14 | 46 ± 5 | 45 ± 7 |
| ADX + CORT | 8 | 76 ± 14 | 50 ± 6 | 69 ± 3 | 61 ± 8 | 42 ± 6 |
| ADX + solvent | 24 | 115 ± 7 | 48 ± 3 | 82 ± 14 | 64 ± 7 | 48 ± 6 |
| ADX + CORT | 24 | 88 ± 3 * | 48 ± 7 | 73 ± 8 | 57 ± 9 | 35 ± 6 |
| fosB mRNA | ||||||
| ADX + solvent | 0.8 | 75 ± 7 | 89 ± 5 | 103 ± 4 | 59 ± 5 | 75 ± 4 |
| ADX + CORT | 0.8 | 59 ± 4 | 54 ± 5 ** | 68 ± 7 * | 45 ± 5 | 37 ± 4 ** |
| ADX + solvent | 2 | 88 ± 7 | 96 ± 3 | 113 ± 8 | 74 ± 9 | 83 ± 7 |
| ADX + CORT | 2 | 96 ± 7 | 118 ± 10 | 125 ± 8 | 98 ± 9 | 102 ± 8 |
| ADX + solvent | 4 | 80 ± 5 | 103 ± 6 | 128 ± 11 | 93 ± 9 | 98 ± 11 |
| ADX + CORT | 4 | 75 ± 9 | 85 ± 13 | 105 ± 24 | 71 ± 15 | 76 ± 12 |
| ADX + solvent | 8 | 57 ± 8 | 76 ± 6 | 86 ± 13 | 59 ± 6 | 68 ± 6 |
| ADX + CORT | 8 | 46 ± 5 | 68 ± 11 | 88 ± 11 | 58 ± 5 | 74 ± 8 |
| ADX + solvent | 24 | 76 ± 12 | 89 ± 14 | 121 ± 20 | 85 ± 18 | 65 ± 11 |
| ADX + CORT | 24 | 33 ± 6 | 38 ± 5 * | 57 ± 10 | 33 ± 6 | 28 ± 5 |
| fra-1 mRNA | ||||||
| ADX + solvent | 0.8 | n.d. | n.d. | n.d. | n.d. | 79 ± 7 |
| ADX + CORT | 0.8 | n.d. | n.d. | n.d. | n.d. | 49 ± 5 * |
| ADX + solvent | 2 | n.d. | n.d. | n.d. | n.d. | 95 ± 8 |
| ADX + CORT | 2 | n.d. | n.d. | n.d. | n.d. | 83 ± 10 |
| ADX + solvent | 4 | n.d. | n.d. | n.d. | n.d. | 64 ± 6 |
| ADX + CORT | 4 | n.d. | n.d. | n.d. | n.d. | 47 ± 10 |
| ADX + solvent | 8 | n.d. | n.d. | n.d. | n.d. | 69 ± 8 |
| ADX + CORT | 8 | n.d. | n.d. | n.d. | n.d. | 68 ± 10 |
| ADX + solvent | 24 | n.d. | n.d. | n.d. | n.d. | 87 ± 11 |
| ADX + CORT | 24 | n.d. | n.d. | n.d. | n.d. | 83 ± 16 |
| junB mRNA | ||||||
| ADX + solvent | 0.8 | 97 ± 6 | 192 ± 9 | 112 ± 9 | 90 ± 6 | 98 ± 2 |
| ADX + CORT | 0.8 | 145 ± 12 * | 194 ± 10 | 122 ± 5 | 110 ± 7 | 98 ± 6 |
| ADX + solvent | 2 | 63 ± 3 | 151 ± 6 | 125 ± 10 | 96 ± 2 | 109 ± 3 |
| ADX + CORT | 2 | 48 ± 4 | 101 ± 10 * | 85 ± 6 * | 72 ± 6 * | 91 ± 5 |
| ADX + solvent | 4 | 62 ± 4 | 122 ± 6 | 94 ± 7 | 72 ± 9 | 104 ± 9 |
| ADX + CORT | 4 | 75 ± 11 | 157 ± 16 | 139 ± 8 * | 100 ± 6 | 141 ± 7 |
| ADX + solvent | 8 | 81 ± 7 | 195 ± 15 | 152 ± 11 | 108 ± 6 | 125 ± 9 |
| ADX + CORT | 8 | 90 ± 8 | 186 ± 10 | 149 ± 15 | 111 ± 9 | 116 ± 9 |
| ADX + solvent | 24 | 74 ± 2 | 175 ± 12 | 143 ± 13 | 124 ± 7 | 121 ± 3 |
| ADX + CORT | 24 | 72 ± 4 | 180 ± 6 | 148 ± 13 | 94 ± 5 * | 107 ± 2 * |
| c-jun mRNA | ||||||
| ADX + solvent | 0.8 | 65 ± 10 | 95 ± 17 | 132 ± 20 | 111 ± 11 | 197 ± 30 |
| ADX + CORT | 0.8 | 58 ± 6 | 88 ± 11 | 108 ± 13 | 95 ± 9 | 135 ± 13 |
| ADX + solvent | 2 | 65 ± 6 | 112 ± 8 | 159 ± 9 | 119 ± 7 | 202 ± 11 |
| ADX + CORT | 2 | 74 ± 6 | 102 ± 12 | 140 ± 23 | 116 ± 6 | 156 ± 15 |
| ADX + solvent | 4 | 58 ± 5 | 87 ± 10 | 125 ± 11 | 98 ± 12 | 165 ± 12 |
| ADX + CORT | 4 | 66 ± 5 | 96 ± 6 | 122 ± 9 | 99 ± 8 | 150 ± 9 |
| ADX + solvent | 8 | 58 ± 9 | 111 ± 15 | 153 ± 12 | 106 ± 7 | 192 ± 15 |
| ADX + CORT | 8 | 75 ± 3 | 101 ± 9 | 128 ± 16 | 99 ± 11 | 155 ± 12 |
| ADX + solvent | 24 | 39 ± 3 | 65 ± 8 | 107 ± 6 | 66 ± 6 | 123 ± 12 |
| ADX + CORT | 24 | 35 ± 3 | 51 ± 14 | 109 ± 17 | 59 ± 11 | 100 ± 20 |
| egr-1 mRNA | ||||||
| ADX + solvent | 0.8 | 176 ± 10 | 234 ± 14 | 125 ± 12 | 127 ± 5 | 78 ± 5 |
| ADX + CORT | 0.8 | 195 ± 14 | 281 ± 7 | 149 ± 7 | 133 ± 13 | 68 ± 6 |
| ADX + solvent | 2 | 180 ± 9 | 268 ± 10 | 155 ± 7 | 137 ± 9 | 97 ± 6 |
| ADX + CORT | 2 | 137 ± 13 | 218 ± 18 | 125 ± 11 | 115 ± 8 | 73 ± 5 |
| ADX + solvent | 4 | 89 ± 6 | 173 ± 8 | 124 ± 10 | 108 ± 10 | 83 ± 4 |
| ADX + CORT | 4 | 78 ± 13 | 195 ± 17 | 118 ± 4 | 92 ± 7 | 89 ± 4 |
| ADX + solvent | 8 | 99 ± 17 | 248 ± 20 | 129 ± 3 | 102 ± 3 | 74 ± 7 |
| ADX + CORT | 8 | 115 ± 15 | 238 ± 14 | 116 ± 7 | 97 ± 8 | 123 ± 29 |
| ADX + solvent | 24 | 191 ± 15 | 307 ± 9 | 146 ± 23 | 121 ± 2 | 59 ± 2 |
| ADX + CORT | 24 | 150 ± 8 | 297 ± 32 | 142 ± 9 | 124 ± 7 | 74 ± 2 ** |
Fig. 2.
Dark-field microphotographs from in situ hybridization of autoradiograms showing the regional distribution of c-fos, fosB, fra-1, junB, c-jun and egr-1 mRNA in the dorsal hippocampus and S1 of saline injected (s.c.) 24 h ADX rats at the 0.8 h time point (left, Bregma = −3 to −4 mm, scale bar = 1 mm), and their respective cellular expression in the dentate gyrus in brightfield emulsion autoradiograms (right, scale bar = 15 μm). CA1 – CA4: Cornus Ammon areas; DG: granular cell layer of the dentate gyrus; S1: primary somatosensory barrelfield cortex. For details on treatment, see Material and Methods.
In a second experiment we addressed the effects of injection stress on the expression of IEG and compared mRNA levels at different time points (0.8 h, 4 h and 24 h) in the above mentioned regions by comparing solvent-injected and non-injected ADX rats. Overall, saline-injection had no effects on fosB, junB, c-jun and egr-1 mRNA levels as compared to non-injected ADX control rats at any time-interval in each analyzed regions (see Table 3). In the same animal experiment injection stress had also no effects on c-fos mRNAs as previous reported in Hansson et al., 2003.
Table 3. Effects of injection stress on IEG expression in the dorsal hippocampus of ADX rats.
Data are expressed as optical densities (OD × 10−3, mean ± S.E.M.), statistically analyzed by two-way ANOVA (time and treatment) followed by Fisher's PLSD test and Bonferroni's correction; n=4/group; There are no statistically significant differences between hormone solvent-injected (ADX + solvent) and non-injected ADX rats at the same time point for each transcript. CA1-CA4: Cornus of Ammon areas, DG: Dentate Gyrus, S1: primary somatosensory barrelfield cortex. For details on treatment, see Material and Methods.
| time | S1 | CA1 | CA3 | CA4 | DG | |
|---|---|---|---|---|---|---|
| fosB mRNA | ||||||
| ADX | 0.8 | 296 ± 16 | 481 ± 30 | 617 ± 23 | 430 ± 14 | 450 ± 31 |
| ADX + solvent | 0.8 | 278 ± 40 | 384 ± 62 | 488 ± 86 | 385 ± 47 | 346 ± 59 |
| ADX | 4 | 258 ± 16 | 373 ± 4 | 501 ± 2 | 389 ± 21 | 366 ± 4 |
| ADX + solvent | 4 | 295 ± 30 | 453 ± 40 | 579 ± 67 | 466 ± 34 | 457 ± 36 |
| ADX | 24 | 311 ± 10 | 430 ± 27 | 578 ± 14 | 455 ± 28 | 416 ± 25 |
| ADX + solvent | 24 | 314 ± 52 | 476 ± 58 | 623 ± 31 | 517 ± 35 | 514 ± 31 |
| junB mRNA | ||||||
| ADX | 0.8 | 38 ± 5 | 107 ± 10 | 78 ± 8 | 60 ± 10 | 63 ± 17 |
| ADX + solvent | 0.8 | 57 ± 9 | 89 ± 11 | 57 ± 12 | 41 ± 3 | 40 ± 7 |
| ADX | 4 | 13 ± 4 | 70 ± 9 | 51 ± 2 | 39 ± 4 | 44 ± 10 |
| ADX + solvent | 4 | 20 ± 2 | 79 ± 6 | 44 ± 13 | 54 ± 8 | 59 ± 8 |
| ADX | 24 | 26 ± 4 | 77 ± 4 | 53 ± 6 | 45 ± 6 | 43 ± 3 |
| ADX + solvent | 24 | 24 ± 8 | 66 ± 3 | 53 ± 7 | 43 ± 5 | 40 ± 5 |
| c-jun mRNA | ||||||
| ADX | 0.8 | 58 ± 12 | 102 ± 15 | 123 ± 13 | 89 ± 11 | 175 ± 23 |
| ADX + solvent | 0.8 | 66 ± 3 | 94 ± 18 | 99 ± 10 | 103 ± 14 | 165 ± 28 |
| ADX | 4 | 75 ± 1 | 136 ± 11 | 110 ± 9 | 111 ± 10 | 201 ± 19 |
| ADX + solvent | 4 | 74 ± 4 | 121 ± 13 | 151 ± 26 | 119 ± 15 | 185 ± 12 |
| ADX | 24 | 74 ± 1 | 108 ± 6 | 114 ± 10 | 106 ± 2 | 158 ± 4 |
| ADX + solvent | 24 | 66 ± 3 | 112 ± 10 | 152 ± 22 | 116 ± 13 | 171 ± 23 |
| egr-1 mRNA | ||||||
| ADX | 0.8 | 105 ± 5 | 176 ± 9 | 101 ± 9 | 95 ± 3 | 61 ± 7 |
| ADX + solvent | 0.8 | 114 ± 10 | 172 ± 6 | 102 ± 6 | 81 ± 7 | 53 ± 5 |
| ADX | 4 | 50 ± 4 | 111 ± 17 | 76 ± 13 | 66 ± 3 | 56 ± 1 |
| ADX + solvent | 4 | 54 ± 4 | 110 ± 18 | 70 ± 10 | 65 ± 5 | 50 ± 10 |
| ADX | 24 | 58 ± 3 | 159 ± 5 | 81 ± 5 | 67 ± 6 | 47 ± 2 |
| ADX + solvent | 24 | 70 ± 9 | 174 ± 14 | 104 ± 17 | 87 ± 8 | 62 ± 8 |
Time-dependent changes of immediate early gene expression after CORT treatment in ADX rats
C-fos mRNA levels
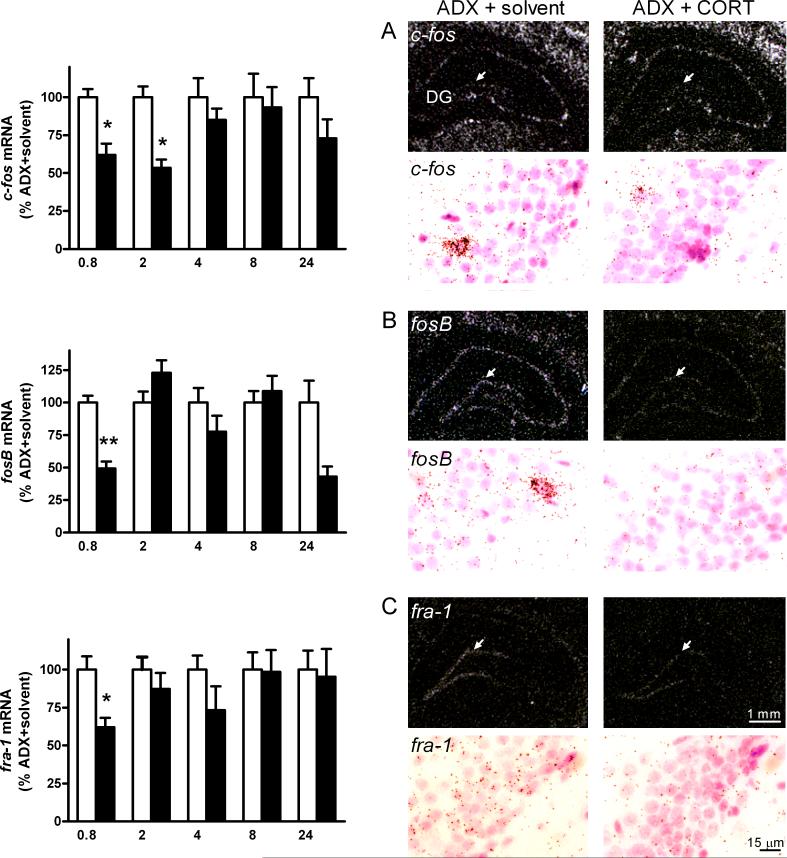
The expression of c-fos in the granule cell layer (DG) and the polymorph layer (CA4) of the dentate gyrus and the S1 demonstrated a time-dependent and significant decrease after CORT treatment as demonstrated by a two-way ANOVA for interaction of time and treatment (DG: F[1, 38] = 3.3, p < 0.05; CA4: F[1, 40] = 2.7, p < 0.05; S1: F[1, 40] = 2.8, p < 0.05). The corrected post-hoc test showed significant decreases of c-fos mRNA levels in the DG between 0.8 h and 2 h (38 % to 46 %, corrected p < 0.05, Table 2, Fig. 3), in the CA4 after 2 h (39 %, corrected p < 0.05, Table 2, Fig. 3) and in the S1 after 24 h (24 %, corrected p < 0.05, Table 2, Fig.3). There was no interaction effect in the CA1 and CA3 hippocampal regions.
Fig. 3.
Left: Densitometric evaluation from autoradiograms of c-fos-, fosB- and fra-1 transcripts in the dentate gyrus of the dorsal hippocampus of CORT (black bars) and solvent (white bars) treated 24 h ADX rats. Values are given in percent of control group (% ADX + solvent, mean ± S.E.M.). Statistical analysis was performed by two-way ANOVA (time x treatment) followed by Fisher's PLSD test and Bonferroni's correction. Corrected values: *p < 0.05, **p < 0.01, n = 4−5/group of CORT vs solvent treated ADX rats. Right: Dark-field microphotographs from in situ hybridization of autoradiograms (upper panel) and brightfield emulsion autoradiograms (lower panel) showing decreased (A) c-fos, (B) fosB, and (C) fra-1 transcripts after acute CORT treatment (10 mg/kg, s.c.) in the granular cell layer of the dentate gyrus at the 0.8 h time interval as compared to solvent treated ADX rats. Bregma = −3 to −4 mm. For abbreviations and details on treatment, see Figure 1.
Furthermore, two-way ANOVA demonstrated a time effect in all subregions in both CORT and solvent treated rats (CA1: F[1, 39] = 33, p < 0.0001; CA3: F[1, 40] = 16.6, p < 0.0001; CA4: F[1, 40] = 12.9, p < 0.0001; DG: F[1, 38] = 16.1, p < 0.0001; S1: F[1, 40] = 62.2, p < 0.0001, Table 2, Fig. 3).
FosB mRNA levels
Two-way ANOVA for interaction of time and treatment demonstrated effects in all regions (CA1: F[1, 40] = 5.2, p < 0.01, CA3: F[1, 40] = 2.8, p < 0.05, CA4: F[1, 40] = 4.2, p < 0.01, DG: (F[1, 40] = 5.0, p < 0.01, S1: F[1, 40] = 3.3, p < 0.05). The corrected post-hoc test showed that CORT (10 mg/kg, s.c.) caused a significant and widespread reduction of fosB mRNA levels in the CA1 (39 %, corrected p < 0.01), CA3 (34 %, corrected p < 0.05), and DG (49 %, corrected p < 0.01) regions after 0.8 h; levels had disappeared as soon as 2 h, as shown in Figure 3 and Table 2. After 24 h, a significant reduction was again observed in the CA1 region (57 %, corrected p < 0.05), and trends towards reduction were also observed at this time point in all other regions, including CA4 and S1 (Table 2).
Fra-1 (fos related antigen-1) mRNA levels
As shown in Table 2 and Fig. 3, CORT produced an early and transient reduction of fra-1 mRNA levels exclusively in the DG (38 %, corrected p < 0.05). The CA1 to CA4 and the S1 did not show any detectable levels of fra-1 mRNA at any time point (see Table 2).
JunB mRNA levels
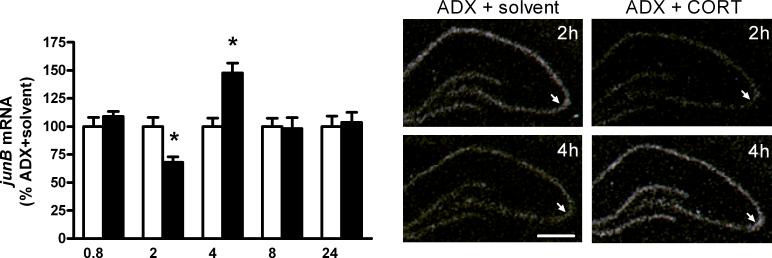
Two-way ANOVA showed an interaction of time and treatment on junB mRNA in the CA1 (F[1,40] = 4.1, p < 0.01), CA3 (F[1,40] = 4.4, p < 0.01), CA4 (F[1,40] = 8.0, p < 0.001), DG (F[1,40] = 6.7, p < 0.001) and S1 (F[1,40] = 6.1, p < 0.001). In the S1, an early significant rise in junB mRNA levels at the 0.8 h time-interval (49 %, corrected p < 0.05) was detected, which disappeared at later time points. Further statistical analysis showed a delayed significant reduction of junB mRNA signals after 2 h in the CA1 (33 %, corrected p < 0.05), CA3 (32 %, corrected p < 0.05) and CA4 (25 %, corrected p < 0.05) regions but not in the DG and the S1 (Table 2 and Fig. 4). This reduction was followed by a rise of transcripts after 4 h in all regions except for the S1; this increase was only statistically significant in the CA3 after correction (48 %, corrected p < 0.05, Table 2 and Fig. 4) and was also transient, having disappeared at the 8 h time interval. In the CA4 and DG regions, a late reduction of junB mRNAs was seen at the 24 h time interval (CA4: 24 %, corrected p < 0.05, DG: 12 %, corrected p < 0.05, Table 2).
Fig. 4.
Left: Densitometric evaluation from autoradiograms of junB mRNAs in the CA3 subregion of the dorsal hippocampus of CORT (black bars) and solvent (white bars) treated 24 h ADX rats. Values are given in percent of control group (% ADX + solvent, mean ± S.E.M.). Statistical analysis was performed by two-way ANOVA (time x treatment) followed by Fisher's PLSD test and Bonferroni's correction. Corrected values: *p < 0.05, n = 4−5/group of CORT vs solvent treated ADX rats. Right: Dark-field microphotographs from in situ hybridization of autoradiograms showing decreased junB mRNA levels after CORT treatment (10 mg/kg, s.c.) at the 2 h time interval followed by increased transcript levels at the 4 h time interval in the dorsal hippocampus as compared to the solvent treated ADX rats. Bregma = −3 to −4 mm, scale bar = 1 mm. Arrows point to the hippocampal CA3 subregion. For abbreviations and details on treatment, see Figure 1.
Overall, there was a strong effect on junB mRNA levels in the CA1 (two-way ANOVA: F[1,40] = 16.1, p < 0.0001), CA3 (two-way ANOVA: F[1,40] = 7.6, p < 0.0001), CA4 (two-way ANOVA: F[1,40] = 7.0, p < 0.001), DG (two-way ANOVA: F[1,40] = 6.7, p < 0.001) and S1 (two-way ANOVA: F[1,40] = 27.4, p < 0.0001, see Table 2) for both CORT and solvent treated ADX rats over time.
C-jun mRNA levels
No changes in c-jun mRNA levels were detected in the hippocampal regions and the S1 at any given time point after CORT treatment as compared to the solvent treated control group (Table 2).
Egr-1 mRNA levels
No changes in egr-1 mRNA levels were observed in the hippocampal regions and the S1 at the different time intervals after CORT treatment, with the exception of the DG region (two-way ANOVA for time and treatment: F[1,39] = 3.2, p < 0.05) at the 24 h interval. In this case, a significant egr-1 mRNA increase was observed (25 %, corrected p < 0.01, Table 2).
The two-way ANOVA showed an overall time effect for both CORT and solvent treated rats in the CA1 (F[1, 39] = 13.4, p < 0.0001) CA4 (F[1, 39] = 6.6, p < 0.001), DG (F[1, 39] = 2.7, p < 0.05) and S1 (F[1, 39] = 25.1, p < 0.0001, Table 2).
3. Discussion
The present paper focused on the differential effects of GR/MR activation on the regional and temporal expression of six IEG genes in the hippocampus and the primary somatosensory cortex of ADX rats to understand the role of corticosterone in mediating the effects of stress on the neuronal networks.
As expected and illustrated in Table 1, 24 hours after ADX, no plasma CORT was found by radioimmunoassay. The plasma levels of CORT were pharmacologically high at 0.8 h after corticosterone injection , while at 2 h after injection they represented levels found at high stress, reaching levels found at the diurnal peak at the 4 h time point (Hansson et al., 2003; Sapolsky et al., 1986). However, we have no evidence for the view that these supramaximal doses produce antagonistic effects in the present model. Thus, in a previous paper (Hansson et al., 2000) it was found that in the present adrenalectomized model a low dose (2 mg/kg, giving similar levels to nadir) and a high dose of corticosterone (10 mg/kg) give a similar rise of bFGF mRNA levels in the dorsal hipopocampus and a similar reduction of BDNF mRNA levels in this region. Thus, a full saturation and activation of intracellular GR and MR (Reul et al., 1987a; Reul et al., 1987b) likely occurs after treatment with this dose of CORT in this time period; by comparison, the plasma levels at the 8 h time point are similar to those as seen at nadir in normal and untreated rats, and may lead most likely to predominant MR activation, but also to some GR activation (Sapolsky et al., 1986; Spencer et al., 1991).
In the hippocampus, a high dose of CORT that activates both GR and MR reduced cfos, fosB and fra-1 transcripts at an early time-point (0.8 h) followed by a decrease and an increase of junB transcripts after 2 h and 4 h, respectively. The effect of CORT on regional hippocampal c-fos expression as well as the lack of influence of the injection stress have previously been reported in this model (Hansson et al., 2003). Hippocampal c-jun and egr-1 transcript levels seemed to be unaffected at an early time point by CORT treatment.
In the S1, IEG expression seemed to be relatively unaffected by CORT treatment. Surprisingly, at 0.8 h CORT stimulated junB transcript levels in the S1. Notably, the S1 expresses predominantly GR and low or no detectable levels of MR (Ahima et al., 1991; Cintra et al., 1994), thus the effects of CORT on IEG expression may be mainly mediated by GR. This suggests that junB may have a special role in mediating GR-induced changes in cortical vs hippocampal plasticity.
Overall, heterogeneity among the hippocampal subregions was observed in the present study in terms of GR/MR-induced changes in IEG expression. The dentate gyrus was the only region in which CORT caused a decrease in the expression level of all analyzed fos family transcripts. These are interesting observations, as they demonstrate for the first time that despite the presence of GR and MR in most of the hippocampal subregions (Cintra et al., 1994; Joels and Dekloet, 1994), CORT can induce a differential early decrease of c-fos, fosB and fra-1 transcripts which may lead to unique plasticity changes in this region compared to the other hippocampal subregions.
Depending on brain region and/or cell population, our data may suggest differential mechanisms underlying the effects of GR and MR on IEG regulation. The mechanisms involved in the GR/MR-dependent dynamics of the fos family and junB expression are unclear. Regulation of IEG transcription occurs very rapidly and is independent of de novo protein synthesis. The ability of GR/MR to decrease IEG transcripts at an early time point may be mediated by post-transcriptional processes (e.g., decreased RNA stability and RNA half-life), post–translational processes (e.g., phosphorylation) and rapid changes in neuronal excitability. Such alterations in neuronal excitability may be brought by rapid CORT signaling via membrane-associated receptors (Tasker et al., 2006). In fact, CORT may act as allosteric modulator of distinct G protein-coupled receptors leading to changes in the intracellular signaling cascades with changes in the expression of the IEG transcripts as observed in the present paper. Alternatively, fos family and junB expression can be reduced by specific self-inhibition processes through the AP-1 complex (Sassone-Corsi et al., 1988).
Under normal or stimulated conditions, Fos family members form heterodimers with Jun family proteins, and the resulting AP-1 complexes bind to AP-1 responsive sequences within promoters to regulate the transcription of a wide panel of genes. A repression of AP-1 by glucocorticoids was previously demonstrated in tissue culture studies (Diamond et al., 1990; Konig et al., 1992; Yangyen et al., 1990) and the interaction of GR with Jun/Fos heterodimers led to both reduced AP-1 activity and cfos expression (Herdegen and Leah, 1998; Reichardt et al., 1998; Teurich and Angel, 1995). The c-fos antisense oligodeoxynucleotide approach also demonstrated the existence of an antagonistic interaction of c-Fos and GR/MR in the hippocampus on the expression of bFGF and BDNF (Hansson et al., 2003). In line with these studies, our results in the hippocampus of ADX rats may suggest that activation of GR and MR reduces the availability of Fos family and JunB proteins, which may affect the composition and activity of AP-1 complexes to further impact changes in neurotrophic factor gene expression and plasticity (Barbany and Persson, 1992; Chao et al., 1998; Hansson et al., 2000; Hansson et al., 2003).
In the ADX model, CORT (at 10 mg/kg) substantially reduces GR and MR mRNA levels in all hippocampal subregions starting at the 2 h time point and persisting up to 24 h (Hansson et al., 2003). It is possible that the proposed changed activity of the AP-1 complexes could also be involved in the CORT autoregulation of GR and MR gene expression. However, using the c-fos antisense oligodeoxynucleotide approach (Hansson et al., 2003), it was not possible to change the strong negative CORT-induced autoregulation of GR and MR gene expression in the hippocampus. Again, this may be explained by the observations presented here indicating that fosB mRNA levels are rapidly and broadly reduced in the hippocampus by this high dose of CORT, thus reducing the possible FosB containing AP-1 complexes. It should also be considered that the possible modulatory influence of such AP-1 complexes on GR/MR autoregulation of their genes may become more pronounced with a lower dose of CORT, leading to less activated GR/MR.
In conclusion, the present study demonstrated that a high dose of CORT that activates both GR and MR produces discrete and specific patterns of IEG expression over time and across multiple brain regions in ADX animals. The major changes after CORT treatment are found in c-fos, fosB, fra-1 and junB mRNA levels within the hippocampus, while c-jun and egr-1 expression is more resistant to the CORT treatment. The CORT-induced early reduction of c-fos, fosB, fra-1 at 0.8 h, which may involve rapid CORT signaling via membrane associated receptors, and delayed reduction of junB transcripts at 2 h may lead to changes in the composition and activity of the AP-1 complexes containing these IEGs, and thus may play a role in the GR/MR mediated changes in regional hippocampal plasticity.
4. Experimental procedures
Animals
Male Sprague-Dawley rats (body weight 200 g), obtained from Alab/Stockholm one week before the start of the experiment, were kept under a standard light/dark cycle (lights on at 6:00 and off at 20:00) and constant room temperature (23 °C) with free access to tap water and food pellets. All animal experiments were approved by the local ethics committee (Stockholm Norra Försöksdjurs Etiska Kommittee). The ADX model used here has been previously described in detail (Hansson et al., 2000; Hansson et al., 2001; Hansson et al., 2003; Hansson et al., 2006; Hansson and Fuxe, 2002). All rats were bilaterally ADX under halothane anesthesia. After surgery, 0.9 % NaCl was added to the drinking water.
24 h after ADX, CORT (10 mg/kg, Sigma, Missouri, USA) or hormone solvent (propylene glycol, Sigma, Missouri, USA) was given subcutaneously (s.c.) and rats were killed 0.8 h, 2 h, 4 h, 8 h and 24 h after injection according to a previous study (Hansson et al., 2003). The experiments with solvent and corticosterone were run absolutely in parallel at the very same time points to avoid an influence of the circadian rhythm. In a second experiment 24 h ADX rats were either injected with hormone solvent alone (s.c., propylene glycol, Sigma, Missouri, USA) or received no injection but handled in the same way as the injected rats. The animal experiment 2 was performed under the same conditions as described above. Rats were killed at 0.8 h, 4 h and 24 h after injection. All surgical procedures and hormone injections (injection volume: 1ml/kg) were performed between 7:00 am and 9:00 am. At various times, trunk blood was collected for plasma CORT measurements and brains were snap-frozen in liquid isopentane (−40 °C) and stored at −70°C.
Radioimmunoassay for CORT
Trunk blood samples were collected in heparin-containing tubes and centrifuged at 4 x g for 20 min at 4°C. Plasma CORT levels were determined by radioimmunoassay (RIA; Coat-a-count, Diagnostic Products Corporation, Los Angeles, CA, USA). The RIA was performed with rat [125I]CORT with a detection limit of ∼ 5.7 ng/ml. Plasma CORT values for all animal groups are summarized in Table 1.
In situ hybridization
The time- and CORT-dependent expression profiles of IEG were studied in the dorsal hippocampus, a classical region for the stress response, and in the primary somatosensory barrelfield cortex (S1), a region which is not primarily involved in the stress response (see Fig. 1). For in situ hybridization, 10 μm coronal brain sections were cryo-sectioned at bregma level −3 mm (Paxinos and Watson, 1986). Rat and mouse specific riboprobes were used as follows: c-fos (position 306 bp to 864 bp on rat cDNA, gene reference sequence: NM_022197.2), egr-1 (position 1384 bp to 1851 bp on rat cDNA, gene reference sequence: NM_012551.1), fosB (positions 1206 bp to 1912 bp and 2051 bp to 2220 bp on mouse cDNA, gene reference sequence: X14897.1, with 96 % sequence homology to rat fosB cDNA), fra-1 (position 1020 bp to 1663 bp on rat cDNA, gene reference sequence: M1965.1), junB (position 457 bp to 931 bp on mouse junB cDNA, gene reference sequence: NM_008416.1, with 97 % sequence homology to rat cDNA) and c-jun (position 1902 bp to 2068 bp on mouse cjun cDNA, gene reference sequence: NM_021835.3, with 91 % sequence homology to rat cDNA). Plasmids for both junB and c-jun riboprobes were kindly provided by Dr. M. Yaniv, Department of Biotechnology, Institute Pasteur, France. Labeling of riboprobes and in situ hybridization have been recently described (Hansson et al., 2003). The hybridized sections were exposed to Kodak BioMax MR film (Eastman Kodak Company, UK). Afterwards, sections were coated with film emulsion (Ilford, Cheshire, UK), stored in desiccated light-tight boxes at 4°C until development, counterstained with cresyl violet and coverslipped with Mountex (Göteborgs Termometerfabrik). Sections were digitized and qualitatively evaluated using a Leica microscope connected with to a high-resolution digital CCD video camera.
Data analysis
Semiquantitative values of mRNA levels were obtained by measuring the gray values of the film autoradiograms according to figure 1 using an SAS Biovision image analyzing system (Avanzati, Milan, Italy). Three measurements were performed for each region: (1) the total value, i.e., measurements of the region in the sections hybridized with [35S]UTP-labeled antisense RNA; (2) the unspecific value, i.e., measurements of the corresponding region in the control sections hybridized with [35S]UTP-labeled sense RNA; and (3) the background value, i.e., measurements of the film background outside the sections. The transmittance percentage values (T%) of specific and unspecific labeling were obtained as described in (Benfenati et al., 1986) and (Zoli et al., 1991). From the T % values the optical density (O.D.) can be obtained (O.D. = −logT %).
All data are expressed as means ± SEM. Data meet assumptions of normality and homogeneity of variances, and were analyzed using standard parametric ANOVA test. Region-wise two-way ANOVA for treatment and time followed by one-way ANOVA (treatment) per time point and Bonferroni's correction (Fisher's p value multiplied by number of brain regions analyzed) was used for the statistical analysis. Significance is indicated at levels for α < 0.05, α < 0.01 or α < 0.001. Bonferroni's correction was used to demonstrate the most robust effects of CORT on expression of IEGs.
Acknowledgements
This work was supported by grants from the Marianne and Marcus Wallenberg Foundation and from the Swedish Medical Research Council (04X-715). We are grateful to Beth Andbjer for technical assistance and Drs. Robert Eskay and Wolfgang Sommer for valuable comments on this manuscript.
Reference List
- 1.Ahima R, Krozowski Z, Harlan R. Type I corticosteroid receptor-like immunoreactivity in the rat CNS: distribution and regulation by corticosteroids. J. Comp Neurol. 1991;313(3):522–538. doi: 10.1002/cne.903130312. [DOI] [PubMed] [Google Scholar]
- 2.Autelitano DJ. Stress-induced stimulation of pituitary POMC gene expression is associated with activation of transcription factor AP-1 in hypothalamus and pituitary. Brain Research Bulletin. 1998;45(1):75–82. doi: 10.1016/s0361-9230(97)00303-1. [DOI] [PubMed] [Google Scholar]
- 3.Barbany G, Persson H. Regulation of Neurotrophin Messenger-Rna Expression in the Rat-Brain by Glucocorticoids. European Journal of Neuroscience. 1992;4(5):396–403. doi: 10.1111/j.1460-9568.1992.tb00888.x. [DOI] [PubMed] [Google Scholar]
- 4.Benfenati F, Cimino M, Agnati LF, Fuxe K. Quantitative autoradiography of central neurotransmitter receptors: methodological and statistical aspects with special reference to computer-assisted image analysis. Acta Physiol. Scand. 1986;128(2):129–146. doi: 10.1111/j.1748-1716.1986.tb07960.x. [DOI] [PubMed] [Google Scholar]
- 5.Brown ER, Sawchenko PE. Hypophysiotropic CRF neurons display a sustained immediate-early gene response to chronic stress but not to adrenalectomy. J. Neuroendocrinol. 1997;9(4):307–316. doi: 10.1046/j.1365-2826.1997.00586.x. [DOI] [PubMed] [Google Scholar]
- 6.Chao HM, McEwen BS. Glucocorticoids and the Expression of Messenger-Rnas for Neurotrophins, Their Receptors and Gap-43 in the Rat Hippocampus. Molecular Brain Research. 1994;26(1−2):271–276. doi: 10.1016/0169-328x(94)90099-x. [DOI] [PubMed] [Google Scholar]
- 7.Chao HM, Sakai RR, Ma LY, McEwen BS. Adrenal steroid regulation of neurotrophic factor expression in the rat hippocampus. Endocrinology. 1998;139(7):3112–3118. doi: 10.1210/endo.139.7.6114. [DOI] [PubMed] [Google Scholar]
- 8.Cintra A, Zoli M, Rosen L, Agnati LF, Okret S, Wikstrom AC, Gustaffsson JA, Fuxe K. Mapping and computer assisted morphometry and microdensitometry of glucocorticoid receptor immunoreactive neurons and glial cells in the rat central nervous system. Neuroscience. 1994;62(3):843–897. doi: 10.1016/0306-4522(94)90481-2. [DOI] [PubMed] [Google Scholar]
- 9.Cullinan WE, Herman JP, Battaglia DF, Akil H, Watson SJ. Pattern and Time-Course of Immediate-Early Gene-Expression in Rat-Brain Following Acute Stress. Neuroscience. 1995;64(2):477–505. doi: 10.1016/0306-4522(94)00355-9. [DOI] [PubMed] [Google Scholar]
- 10.Diamond MI, Miner JN, Yoshinaga SK, Yamamoto KR. Transcription Factor Interactions - Selectors of Positive Or Negative Regulation from A Single Dna Element. Science. 1990;249(4974):1266–1272. doi: 10.1126/science.2119054. [DOI] [PubMed] [Google Scholar]
- 11.Gould E, Woolley CS, McEwen BS. Short-term glucocorticoid manipulations affect neuronal morphology and survival in the adult dentate gyrus. Neuroscience. 1990;37(2):367–375. doi: 10.1016/0306-4522(90)90407-u. [DOI] [PubMed] [Google Scholar]
- 12.Hansson AC, Cintra A, Belluardo N, Sommer W, Bhatnagar M, Bader M, Ganten D, Fuxe K. Gluco- and mineralocorticoid receptor-mediated regulation of neurotrophic factor gene expression in the dorsal hippocampus and the neocortex of the rat. European Journal of Neuroscience. 2000;12(8):2918–2934. doi: 10.1046/j.1460-9568.2000.00185.x. [DOI] [PubMed] [Google Scholar]
- 13.Hansson AC, Fuxe K. Biphasic autoregulation of mineralocorticoid receptor mRNA in the medial septal nucleus by aldosterone. Neuroendocrinology. 2002;75(6):358–366. doi: 10.1159/000059432. [DOI] [PubMed] [Google Scholar]
- 14.Hansson AC, Sommer W, Andbjer B, Bader M, Ganten D, Fuxe K. Induction of hippocampal glial cells expressing basic fibroblast growth factor RNA by corticosterone. Neuroreport. 2001;12(1):141–145. doi: 10.1097/00001756-200101220-00036. [DOI] [PubMed] [Google Scholar]
- 15.Hansson AC, Sommer W, Rimondini R, Andbjer B, Stromberg I, Fuxe K. c-fos reduces corticosterone-mediated effects on neurotrophic factor expression in the rat hippocampal CA1 region. J Neurosci. 2003;23(14):6013–6022. doi: 10.1523/JNEUROSCI.23-14-06013.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hansson AC, Sommer WH, Metsis M, Stromberg I, Agnati LF, Fuxe K. Corticosterone Actions on the Hippocampal Brain-Derived Neurotrophic Factor Expression are Mediated by Exon IV Promoter. Journal of Neuroendocrinol. 2006;18(2):104–114. doi: 10.1111/j.1365-2826.2005.01390.x. [DOI] [PubMed] [Google Scholar]
- 17.Helmreich DL, Cullinan WE, Watson SJ. The effect of adrenalectomy on stress-induced c-fos mRNA expression in the rat brain. Brain Res. 1996;706(1):137–144. doi: 10.1016/0006-8993(95)01215-x. [DOI] [PubMed] [Google Scholar]
- 18.Herdegen T, Leah JD. Inducible and constitutive transcription factors in the mammalian nervous system: control of gene expression by Jun, Fos and Krox, and CREB/ATF proteins. Brain Res Brain Res Rev. 1998;28(3):370–490. doi: 10.1016/s0165-0173(98)00018-6. [DOI] [PubMed] [Google Scholar]
- 19.Herdegen T, Waetzig V. AP-1 proteins in the adult brain: facts and fiction about effectors of neuroprotection and neurodegeneration. Oncogene. 2001;20(19):2424–2437. doi: 10.1038/sj.onc.1204387. [DOI] [PubMed] [Google Scholar]
- 20.Herrera DG, Robertson HA. Activation of c-fos in the brain. Prog. Neurobiol. 1996;50(2−3):83–107. doi: 10.1016/s0301-0082(96)00021-4. [DOI] [PubMed] [Google Scholar]
- 21.Hyder SM, S.G.L.-M.DS. Steroid hormone-induced expression of oncogene encoded nuclear proteins. Crit Rev Eukaryot Gene Expr. 1994;4(1):55–116. doi: 10.1615/critreveukargeneexpr.v4.i1.30. [DOI] [PubMed] [Google Scholar]
- 22.James AB, Conway AM, Morris BJ. Genomic profiling of the neuronal target genes of the plasticity-related transcription factor -- Zif268. J. Neurochem. 2005;95(3):796–810. doi: 10.1111/j.1471-4159.2005.03400.x. [DOI] [PubMed] [Google Scholar]
- 23.Joels M, Dekloet ER. Mineralocorticoid and Glucocorticoid Receptors in the Brain - Implications for Ion Permeability and Transmitter Systems. Prog. Neurobiol. 1994;43(1):1–36. doi: 10.1016/0301-0082(94)90014-0. [DOI] [PubMed] [Google Scholar]
- 24.Konig H, Ponta H, Rahmsdorf HJ, Herrlich P. Interference Between Pathway-Specific Transcription Factors - Glucocorticoids Antagonize Phorbol Ester-Induced Ap-1 Activity Without Altering Ap-1 Site Occupation Invivo. EMBO Journal. 1992;11(6):2241–2246. doi: 10.1002/j.1460-2075.1992.tb05283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kononen J, Honkaniemi J, Alhl H, Koistinaho J, Iadarola M, Pelto-Huikko M. Fos-like immunoreactivity in the rat hypothalamic pituiraty axis after immobilization stress. Endocrinology. 1992;130:3041–3047. doi: 10.1210/endo.130.5.1315265. [DOI] [PubMed] [Google Scholar]
- 26.Kovacs KJ, Sawchenko PE. Regulation of stress-induced transcriptional changes in the hypothalamic neurosecretory neurons. J. Mol. Neurosci. 1996;7(2):125–133. doi: 10.1007/BF02736792. [DOI] [PubMed] [Google Scholar]
- 27.Melia KR, Ryabinin AE, Schroeder R, Bloom FE, Wilson MC. Induction and habituation of immediate early gene expression in rat brain by acute and repeated restraint stress. Journal of Neuroscience. 1994;14(10):5929–5938. doi: 10.1523/JNEUROSCI.14-10-05929.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parrelli JM, Meisler N, Cutroneo KR. Identification of a glucocorticoid response element in the human transforming growth factor beta 1 gene promoter. Int. J. Biochem. Cell Biol. 1998;30(5):623–627. doi: 10.1016/s1357-2725(98)00005-3. [DOI] [PubMed] [Google Scholar]
- 29.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; San Diego: 1986. [DOI] [PubMed] [Google Scholar]
- 30.Pfenning AR, Schwartz R, Barth AL. A comparative genomics approach to identifying the plasticity transcriptome. BMC. Neurosci. 2007;8:20. doi: 10.1186/1471-2202-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reichardt HM, Kaestner KH, Tuckermann J, Kretz O, Wessely O, Bock R, Gass P, Schmid W, Herrlich P, Angel P, Schutz G. DNA binding of the glucocorticoid receptor is not essential for survival. Cell. 1998;93(4):531–541. doi: 10.1016/s0092-8674(00)81183-6. [DOI] [PubMed] [Google Scholar]
- 32.Reul JMHM, Vandenbosch FR, Dekloet ER. Differential Response of Type-I and Type-Ii Corticosteroid Receptors to Changes in Plasma Steroid-Level and Circadian Rhythmicity. Neuroendocrinology. 1987a;45(5):407–412. doi: 10.1159/000124766. [DOI] [PubMed] [Google Scholar]
- 33.Reul JMHM, Vandenbosch FR, Dekloet ER. Relative Occupation of Type-I and Type-Ii Corticosteroid Receptors in Rat-Brain Following Stress and Dexamethasone Treatment - Functional Implications. J. Endocrinol. 1987b;115(3):459–467. doi: 10.1677/joe.0.1150459. [DOI] [PubMed] [Google Scholar]
- 34.Riva MA, Fumagalli F, Racagni G. Opposite Regulation of Basic Fibroblast Growth-Factor and Nerve Growth-Factor Gene-Expression in Rat Cortical Astrocytes Following Dexamethasone Treatment. J. Neurochem. 1995;64(6):2526–2533. doi: 10.1046/j.1471-4159.1995.64062526.x. [DOI] [PubMed] [Google Scholar]
- 35.Ryabinin AE, Wang YM, Finn DA. Different levels of Fos immunoreactivity after repeated handling and injection stress in two inbred strains of mice. Pharmacol. Biochem. Behav. 1999;63(1):143–151. doi: 10.1016/s0091-3057(98)00239-1. [DOI] [PubMed] [Google Scholar]
- 36.Sapolsky RM, Krey LC, McEwen BS. The Neuroendocrinology of Stress and Aging - the Glucocorticoid Cascade Hypothesis. Endocrine Reviews. 1986;7(3):284–301. doi: 10.1210/edrv-7-3-284. [DOI] [PubMed] [Google Scholar]
- 37.Sassone-Corsi P, Sisson JC, Verma IM. Transcriptional autoregulation of the proto-oncogene fos. Nature. 1988;334(6180):314–319. doi: 10.1038/334314a0. [DOI] [PubMed] [Google Scholar]
- 38.Schaaf MJM, de Jong J, De Kloet ER, Vreugdenhil E. Downregulation of BDNF mRNA and protein in the rat hippocampus by corticosterone. Brain Res. 1998;813(1):112–120. doi: 10.1016/s0006-8993(98)01010-5. [DOI] [PubMed] [Google Scholar]
- 39.Schule R, Rangarajan P, Kliewer S, Ransone LJ, Bolado J, Yang N, Verma IM, Evans RM. Functional Antagonism Between Oncoprotein C-Jun and the Glucocorticoid Receptor. Cell. 1990;62(6):1217–1226. doi: 10.1016/0092-8674(90)90397-w. [DOI] [PubMed] [Google Scholar]
- 40.Sheng M, Greenberg ME. The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron. 1990;4(4):477–485. doi: 10.1016/0896-6273(90)90106-p. [DOI] [PubMed] [Google Scholar]
- 41.Sloviter RS, Valiquette G, Abrams GM, Ronk EC, Sollas AL, Paul LA, Neubort S. Selective Loss of Hippocampal Granule Cells in the Mature Rat-Brain After Adrenalectomy. Science. 1989;243(4890):535–538. doi: 10.1126/science.2911756. [DOI] [PubMed] [Google Scholar]
- 42.Smith MA, Makino S, Kvetnansky R, Post RM. Stress and Glucocorticoids Affect the Expression of Brain-Derived Neurotrophic Factor and Neurotrophin-3 Messenger-Rnas in the Hippocampus. J. Neurosci. 1995;15(3):1768–1777. doi: 10.1523/JNEUROSCI.15-03-01768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spencer RL, Miller AH, Stein M, McEwen BS. Corticosterone Regulation of Type-I and Type-Ii Adrenal-Steroid Receptors in Brain, Pituitary, and Immune Tissue. Brain Res. 1991;549(2):236–246. doi: 10.1016/0006-8993(91)90463-6. [DOI] [PubMed] [Google Scholar]
- 44.Tasker JG, Di S, Malcher-Lopes R. Minireview: rapid glucocorticoid signaling via membrane-associated receptors. Endocrinology. 2006;147(12):5549–5556. doi: 10.1210/en.2006-0981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Teurich S, Angel P. The Glucocorticoid Receptor Synergizes with Jun Homodimers to Activate Ap-1-Regulated Promoters Lacking Gr Binding-Sites. Chemical Senses. 1995;20(2):251–255. doi: 10.1093/chemse/20.2.251. [DOI] [PubMed] [Google Scholar]
- 46.Van Eekelen JA, De Kloet ER. Co-localization of brain corticosteroid receptors in the rat hippocampus. Prog. Histochem. Cytochem. 1992;26(1−4):250–258. doi: 10.1016/s0079-6336(11)80102-6. [DOI] [PubMed] [Google Scholar]
- 47.Watanabe Y, Gould E, McEwen BS. Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Res. 1992;588(2):341–345. doi: 10.1016/0006-8993(92)91597-8. [DOI] [PubMed] [Google Scholar]
- 48.Yangyen HF, Chambard JC, Sun YL, Smeal T, Schmidt TJ, Drouin J, Karin M. Transcriptional Interference Between C-Jun and the Glucocorticoid Receptor - Mutual Inhibition of Dna-Binding Due to Direct Protein Protein-Interaction. Cell. 1990;62(6):1205–1215. doi: 10.1016/0092-8674(90)90396-v. [DOI] [PubMed] [Google Scholar]
- 49.Zhang J, Zhang D, McQuade JS, Behbehani M, Tsien JZ, Xu M. c-fos regulates neuronal excitability and survival. Nat. Genet. 2002;30(4):416–420. doi: 10.1038/ng859. [DOI] [PubMed] [Google Scholar]
- 50.Zoli M, Bettuzzi S, Ferraguti F, Ingletti MC, Zini I, Fuxe K, Agnati LF, Corti A. Regional Increases in Ornithine Decarboxylase Messenger-Rna Levels in the Rat-Brain After Partial Mesodiencephalic Hemitransection As Revealed by Insitu Hybridization Histochemistry. Neurochemistry International. 1991;18(3):347–352. doi: 10.1016/0197-0186(91)90165-a. [DOI] [PubMed] [Google Scholar]