Abstract
Steady laminar blood flow protects vessels from atherosclerosis. We showed that flow decreased tumor necrosis factor-α (TNF)-mediated VCAM1 expression in endothelial cells (EC) by inhibiting JNK. Here we determined the relative roles of MEK1, MEK5 and their downstream kinases ERK1/2 and BMK1 (ERK5) in flow-mediated inhibition of JNK activation. Steady laminar flow (shear stress = 12 dynes/cm2) increased BMK1 and ERK1/2 activity in EC. Pre-exposing EC for 10 min to flow inhibited TNF activation of JNK by 58%. A key role for BMK1, but not ERK1/2 was shown. 1) Incubation of EC with PD184352, at concentrations that blocked ERK1/2, but not BMK1, had no effect on flow inhibition of TNF-mediated JNK activation. 2) BIX02188, a MEK5-selective inhibitor, completely reversed the inhibitory effects of flow. These findings indicate that flow inhibits TNF-mediated signaling events in EC by a mechanism dependent on activation of MEK5-BMK1, but not MEK1-ERK1/2. These results support a key role for the MEK5-BMK1 signaling pathway in the atheroprotective effects of blood flow.
Keywords: fluid shear stress, endothelial cells, MAP kinase, TNF, inflammation
Introduction
Substantial evidence exists that physiologic fluid shear stress (flow) exerts atheroprotective effects in vivo, because atherosclerosis preferentially occurs in areas of disturbed flow or low shear stress, whereas regions with steady laminar flow and physiological shear stress are protected [1, 2]. Pathogenic features of atherosclerosis are oxidative stress and inflammation characterized by endothelial expression of VCAM1 [3]. We have proposed that flow inhibits VCAM1 expression by increasing anti-oxidant mechanisms and blocking inflammatory signaling events. Specifically, using in vitro cultured endothelial cells (EC) [4, 5] and ex vivo intact vessels [6, 7], we have demonstrated that inhibiting TNF-mediated activation of the apoptosis signal-regulating kinase (ASK1)-JNK pathway is one mechanism by which steady laminar flow is atheroprotective.
Conceptually there are four ways that flow could inhibit activation of ASK1-JNK. First, flow could inhibit an upstream activator such as the MAP kinase that activates ASK1. To date we have not identified this kinase in EC. Second, flow could inhibit ASK1 directly. We have previously shown that flow inhibits the ASK1-JNK pathway by a redox pathway that involves activation of glutathione reductase[8] and thioredoxin (via increasing reducing equivalents and by downregulating the thioredoxin inhibitor, TXNIP[7]) and inhibition of a phosphatase that dephosphorylates ASK1 (thereby maintaining 14-3-3 binding)[5]. Third, flow could activate phosphatases that dephosphorylate ASK1 and JNK. Likely candidates would be the MAP kinase phosphatases such as MKP-1. Finally, flow could activate kinases that inhibit activation of the ASK1-JNK pathway. Previously we showed that activating MEK5 by transfection with constitutively active MEK5 (CA-MEK5) reproduced the inhibitory effects of flow on TNF-mediated activation of JNK[9]. More recently we showed that flow blocked apoptosis of EC via a pathway that required activation of MEK5-BMK1[10] and inhibition of protein kinase C-ζ activation[11]. Previously we found that the MEK1 and MEK5 inhibitor PD98059 reversed many of the inhibitory effects of flow on TNF signaling[4]. Here we used several specific pharmacologic inhibitors to assay the relative roles of ERK1/2 and BMK1 in flow-mediated inhibition of TNF signaling.
Materials and Methods
Cell Culture and Flow Experiments
Bovine aortic endothelial cells (BAECs) or bovine lung microvascular endothelial cells (BLMECs) were isolated according to previous published protocols[10], and maintained in medium199 (M-199) supplemented with 100 U/mL of penicillin and 100 mg/mL of streptomycin, 1% MEM amino acids, 1% MEM vitamins, 10% fetal clone III (bovine serum incubator at 37°C. Cells at passages 5 to product, HyClone), in a 5% CO2/95% O2 10 were used for experiments. Flow experiments were performed with confluent cells grown in 60-mm dishes after growth-arrest for 6 hr in 0% serum. Cells were exposed to laminar flow (shear stress = 12 dyn/cm2) in a cone and plate viscometer.
Antibodies and Reagents
Antibodies: BMK1, ERK1/2, and JNK (Santa Cruz); phospho (p)-ERK1/2, p-JNK1/2, and p-BMK1 (Cell Signaling). Reagent Sources: TNF-α (Roche); U0126, PD98059 and PD184352 (Calbiochem); BIX02188 Boehringer Ingelheim).
Western Analysis
Cells were rinsed with ice-cold phosphate-buffered saline (PBS; 150 mmol/L NaCl, 20 mmol/L Na2PO4, pH 7.4) on ice and harvested in lysis buffer (50 mmol/L Tirs-HCl pH7.5, 150 mmol/L NaCl, 10 mmol/L Hepes pH7.4, 10 mmol/L EDTA, 1% Triton X-100, 0.5% deoxycholate, 5 mmol/L NaF, 1 mmol/L Na3VO4 plus 1:1000 protein inhibitor cocktail (PIC, Sigma) and clarified by centrifugation. The protein concentration was determined by Bradford assay (Bio-Rad). Total cell lysates were separated by SDS-PAGE and transferred to nitrocellulose membranes, and the membranes were incubated with appropriate primary antibodies. After washing and incubating with secondary antibodies, immunoreactive proteins were visualized by the Odyssey infrared imaging system (LI-COR Biotechnology). Densitometric analyses of immunoblots were performed with ImageJ software.
Statistical analysis
All data are reported as mean±S.E. The IC50 values were calculated by using a computer algorithm that assumes a linear curve between two points around 50% of the maximum response[12]. Comparison for two groups was performed using Student t-test. Differences between 3 or more groups were analyzed by repeated-measures one-way ANOVA and Fisher’s PSLD. Statistical significance was accepted at p<0.05.
Results
Flow activates ERK1/2 and BMK1 with differing time courses
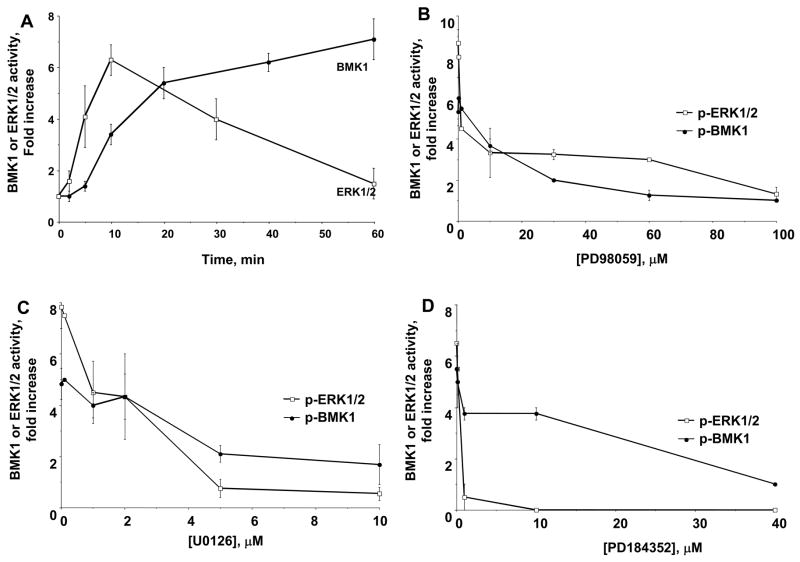
To understand the role of crosstalk between MAP kinase family members in flow-mediated inhibition of TNF signaling we compared the time course for activation. While both ERK1/2 and BMK1 were activated by flow in EC, the time course for ERK1/2 was more rapid with onset at 2 min and peak at 10 min (Fig. 1)[13–15]. Since the ability of flow to inhibit JNK activation by TNF required only 10 min of flow pretreatment[4], the time course data suggested that ERK1/2 was more likely than BMK1 to mediate crosstalk.
Fig. 1. ERK1/2 and BMK1 regulation by flow and inhibitors.
(A) Time course for flow activation of ERK1/2 and BMK1. BAEC and BLMEC were exposed to flow for varying times and cell lysates were prepared for SDS-PAGE. ERK1/2 activation was measured by phospho-specific ERK1/2 antibodies, while BMK1 activity was measured by both band shift and phosphorylation of MEF2C. Results represent mean ± S.E. from three to five independent experiments. Values were normalized by setting the densitometry value of serum-starved static cells to 1.0 in each experiment. (B–D) Effect of U0126 (B), PD98059 (C) and PD184352 (D) on ERK1/2 and BMK1 activity. BAEC and BLMEC were incubated with the indicated concentrations of inhibitors for 30 min, exposed to flow (12 dyn/cm2) for 10 and 20 min to activate ERK1/2 and BMK1 (respectively), and cell lysates were prepared for SDS-PAGE.
Effect of MEK1 inhibitors on flow-mediated activation of ERK1/2 and BMK1
To determine the relative contributions of ERK1/2 and BMK1 to flow-mediated inhibition of TNF, we first characterized the concentration responses for pharmacologic inhibition of ERK1/2 and BMK1 using two well-known MEK1 inhibitors (that also have been reported to inhibit MEK5[16–18]): PD98059 and U0126. Flow alone (10 min, 12 dyn/cm2) activated ERK1/2 by 7.8 ± 0.8-fold. The IC50 for inhibition of ERK1/2 by PD98059 was 2.8 ± 0.4 μM, while for U0126 it was 2.2 ± 0.3 μM (Fig. 1B). Flow alone (20 min, 12 dyn/cm2) activated BMK1 by 4.8 ± 0.6-fold. The IC50 for inhibition of BMK1 by PD98059 was 3.2 ± 0.5 μM, while for U0126 it was 2.2 ± 0.3 μM (Fig. 1C) indicating that these two inhibitors were not specific for MEK1 in EC stimulated by flow.
Therefore, we studied PD184352, a more specific MEK1 inhibitor[19–21]. As shown in Fig. 1D, PD184352 inhibited ERK1/2 activity by 50% at 0.1 μM, with complete inhibition at concentrations ≥10 μM, and an IC50 = 0.7 ± 0.4 μM. In contrast, the IC50 for BMK1 inhibition was 14.4 ± 2.4 μM. This 20-fold difference in IC50 suggested that differential inhibition was possible.
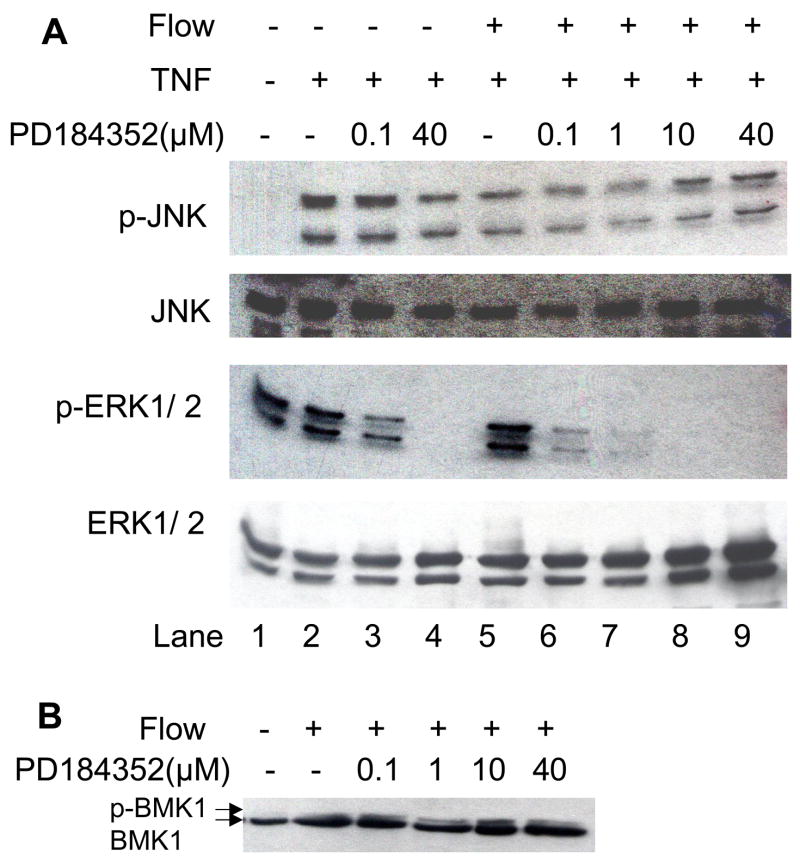
Next, we compared the effects of 0.1–40 μM PD184352 on flow-mediated inhibition of TNF signaling (Fig. 2A). TNF activation of ERK1/2 and JNK served as an internal control. There was no inhibition of JNK activation at 0.1 μM PD184352, but there was a ~50% decrease at 40 μM PD184352 (compare p-JNK in lanes 3&4) indicating likely nonspecific effects on upstream kinases (e.g., MKK4 or MKK7). In contrast there was ~ 90% inhibition of ERK1/2 activation at 0.1 μM (compare p-ERK1/2 in lanes 2&3) suggesting that this concentration of PD184352 was optimal to block ERK1/2 activation specifically without affecting JNK or BMK1. Flow pretreatment blocked TNF activation of JNK by 70% (compare p-JNK in lanes 2&5) without significant effect on ERK1/2 (compare p-ERK1/2 in lanes 2&5). At low concentrations of PD184352 (0.1 μM, lane 6 and 1.0 μM, lane 7), flow still significantly inhibited JNK activation (68± 17% at 0.1 μM, n = 4). At these concentrations there was no significant ERK1/2 activation by flow and TNF. At higher concentrations of PD184352 (10 and 40 μM, lanes 8–9), TNF activated JNK even though there was some inhibition by PD184352 of JNK activation (Fig. 2). The EC lysates exposed to flow and PD184352 were then assayed for activation of BMK1. As shown in Fig. 2B, there was still a significant bandshift representing activated BMK1 (p-BMK1 in Fig. 2B) at 10 μM PD184352 showing that this compound is specific for MEK1-ERK1/2 in EC at the concentrations used. These data indicate that the ability of flow to inhibit JNK activation was not significantly reduced at concentrations of PD184352 that strongly inhibit MEK1 and ERK1/2 (0.1–1 μM). These results suggest that MEK-ERK1/2 do not mediate flow inhibition of TNF signaling.
Fig. 2.
Effect of PD184352 on flow-mediated inhibition of TNF signaling. A. BLMEC were incubated with indicated concentrations of PD184352 for 30 min, exposed to flow (12 dyn/cm2) or left static for 10 min, and then stimulated with10 ng/ml TNF for 15 min. Cell lysates were prepared and SDS-PGE performed followed by western blotting. MAP kinase activation was determined by immunoblotting using phospho-specific antibodies. Equal loading was confirmed with total MAP kinase antibodies. Representative blots from 3 independent experiments are shown. B. Using a similar experimental design as in (A), BMK1 activation was determined after exposure to flow for 15 min and the indicated concentrations of PD184352 for 30 min. Activation was measured by the ratio of the upper band (band shift) to the total BMK1 content by densitometry.
A novel MEK5 inhibitor, BIX02188, selectively inhibits BMK1 phosphorylation
To investigate whether selective inhibition of MEK5-BMK1 would block flow effects on TNF signaling we tested a novel MEK5 inhibitor, BIX02188. It is a potent inhibitor of catalytic function of purified, active MEK5 enzyme. In activated HeLa cells, the inhibitor blocked phosphorylation of ERK5, without affecting phosphorylation of ERK1/2, JNK and p38 MAP kinases (Tetake, manuscript in preparation). To characterize the effects of this inhibitor in cultured EC, we used H2O2 to activate BMK1 as described previously[22]. BLMECs were pretreated with 0.1 – 10 μM BIX02188 for 30 min, and then stimulated with 300 μM H2O2. We previously found that this concentration yielded maximal BMK1 activation in EC[22]. BMK1 was dramatically activated by H2O2, with peak at 20 min (not shown). Phosphorylated BMK1 was inhibited by BIX02188 in a dose-dependent manner, with an IC50 = 0.8 ± 1.0 μM, and maximal inhibition at concentrations > 3 μM (Fig. 3A–B).
Fig. 3.

BIX02188 inhibits MEK5-BMK1 signaling induced by H2O2. A. BLMEC were serum deprived for 6 hr, incubated with the indicated concentrations of BIX02188 for 30 min, and stimulated with 300 μMH2O2 for 20 min. Cell lysates were prepared and SDS-PAGE performed followed by western blotting for phospho-BMK1 or total BMK1, phospho-ERK1/2 or total ERK1/2. B. Results represent mean ± S.E. from three to five independent experiments. Values were normalized by setting the densitometry value of serum-starved cells to 1.0 in each experiment.
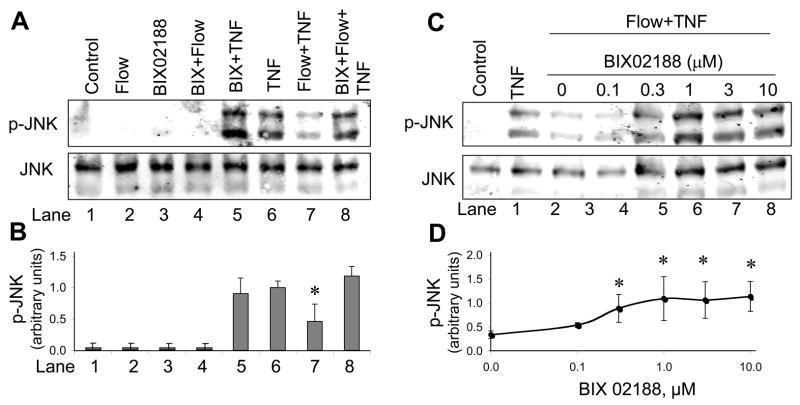
To examine the specificity of BIX02188, we measured the effect of 0.1–10 μM BIX02188 on the activity of ERK1/2 and JNK. There was no significant inhibition of ERK1/2 (Fig. 3A–B) and JNK (Fig. 4A–B, lanes 5&6) at these concentrations. These observations confirmed the selectivity of BIX02188 for MEK5-induced BMK1 phosphorylation.
Fig. 4.
BIX02188 reverses flow inhibition of TNF-induced signaling. A. BLMEC were exposed to 1 μM BIX02188 for 30 min, flow for 10 min, 10 ng/ml TNF for 15 min (or both). Cell lysates were prepared and SDS-PAGE performed followed by western blotting for p-JNK or total JNK. B. Results represent mean ± S.E. from four independent experiments. Values were normalized by setting the densitometry value of serum-starved cells treated with TNF alone to 1.0 in each experiment. C. Dose-dependent restoration of flow-inhibited TNF-induced JNK activation by BIX02188. Using the experimental protocol described in (A), cells were treated with the indicated concentrations of BIX02188 prior to exposure to flow and TNF. D. The results were quantified after setting the densitometry value of serum-starved cells treated with TNF alone to 1.0 in each experiment. *p < 0.01, compared with cells treated with TNF alone, n=4.
Effect of the MEK5 inhibitor BIX02188 on flow-mediated inhibition of TNF signaling
To determine the effect of selectively inhibiting MEK5-BMK1 on TNF-induced JNK signaling, we pretreated BAEC with 1 μM BIX02188 for 30 min, exposed cells to flow for 15 min, and then stimulated with 10 ng/ml TNF for 15 min (Fig. 4A–B). Neither flow nor BIX02188 alone (or together) activated JNK (lanes 2–4). TNF alone activated JNK (1.0-fold, arbitrary units, lane 6), which was not inhibited by BIX02188 (lane 5). In this series of experiments, flow decreased TNF-mediated JNK activation by 58 ± 15% (lane 7, p <0.01). This effect was completely reversed by pre-incubation with BIX02188 (Fig. 4A, lane 8; Fig. 4B, p = NS). To show that the concentration dependence of BIX02188 on TNF-mediated JNK activation paralleled the effect on BMK1 inhibition we performed a dose-response experiment (Fig. 4C–D). At 1 μM BIX02188 the ability of flow to inhibit TNF activation was maximal (Fig. 4C–D), similar to the BIX02188 concentration that maximally inhibited BMK1 (Fig. 3B). These findings demonstrate that flow inhibits TNF signaling through activation of the MEK5-BMK1 signaling pathway.
We attempted to confirm these results by using siRNA to knockdown MEK5 and BMK1. However, the transfection and serum starvation protocols resulted in unacceptable levels of cell death and activation of JNK. This is not surprising given the known role for the MEK5-BMK1 pathway in preventing EC apoptosis induced by serum deprivation[10], and the newly demonstrated role in this paper in limiting JNK activation.
Discussion
The major findings of the present study are that the MEK5-BMK1 pathway mediates flow-dependent inhibition of TNF signaling in EC as shown by a novel MEK5-selective inhibitor BMK1, the MAP kinase downstream of MEK5, was shown previously to play a critical role in EC function since both mek5 and bmk1 knockout mice rapidly die due to disruption of vascular integrity[10, 23, 24]. Our previous studies have demonstrated that BMK1 plays an essential role in the ability of shear stress to prevent EC apoptosis[10]. The present study further supports the key role of BMK1 in flow mediated anti-inflammatory effects. In particular, our data show a significant and specific role of BMK1 in inhibiting TNF-induced JNK activation.
Steady laminar flow inhibits inflammatory responses in EC by both short-term and long-term mechanisms. Flow regulates the activity of several transcription factors including NF-κB, Kruppel-like factor-2 (KLF2) and PPARγ. KLF2 and PPARγ are particularly relevant, since recent reports show these factors are regulated by a MEK5-ERK5 pathway. KLF2 is induced by laminar shear stress in cultured EC[25–27], inhibits induction of VCAM-1, and a MEK5-BMK1-MEF2C pathway is required for up regulation of KLF2 [28]. In EC, laminar flow activates PPARγ activity and activation of PPARγ exerts anti-inflammatory effects since PPARγ ligands reduce TNF induced VCAM-1 expression [29]. We have shown previously that flow-induced PPARγ activation is dependent upon ERK5, and is functionally important because expression of dominant negative PPARγ decreased the ability of ERK5 to decrease VCAM-1 expression [30].
The mechanisms by which MEK5-BMK1 inhibit TNF signaling remain to be elucidated fully. Intriguingly, both MEK5 and PKC-ζ are PB1 domain containing proteins. Thus it is possible that competition between MEK5 and PKC-ζ influences the relative activation of these pathways. Future studies of PB1 domain interacting proteins and MEK5-BMK1 kinase substrates would appear indicated.
The data in this study confirm the selectivity of the novel MEK5 inhibitor, BIX02188 in EC. We also confirmed the selectivity of PD183452 for MEK1 in EC. BIX02188 may be a useful reagent to investigate the role of MEK5-BMK1 signaling in the cardiovascular system, bypassing the mortality associated with MEK5 and BMK1 deficiency. This reagent should enable long term studies of the role of MEK5-BMK1 in cardiovascular diseases such as heart failure, aortic aneurysm and atherosclerosis.
Acknowledgments
This study was supported by the NIH grants (HL-62826 and HL-64839) to Dr. Berk.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gimbrone MA, Jr, Topper JN, Nagel T, Anderson KR, Garcia-Cardena G. Endothelial dysfunction, hemodynamic forces, and atherogenesis. Ann N Y Acad Sci. 2000;902:230–239. doi: 10.1111/j.1749-6632.2000.tb06318.x. discussion 239–240. [DOI] [PubMed] [Google Scholar]
- 2.Traub O, Berk BC. Laminar shear stress: mechanisms by which endothelial cells transduce an atheroprotective force. Arterioscler Thromb Vasc Biol. 1998;18:677–685. doi: 10.1161/01.atv.18.5.677. [DOI] [PubMed] [Google Scholar]
- 3.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 4.Surapisitchat J, Hoefen RJ, Pi X, Yoshizumi M, Yan C, Berk BC. Fluid shear stress inhibits TNF-alpha activation of JNK but not ERK1/2 or p38 in human umbilical vein endothelial cells: Inhibitory crosstalk among MAPK family members. Proc Natl Acad Sci U S A. 2001;98:6476–6481. doi: 10.1073/pnas.101134098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Y, Yin G, Surapisitchat J, Berk BC, Min W. Laminar flow inhibits TNF-induced ASK1 activation by preventing dissociation of ASK1 from its inhibitor 14–33. J Clin Invest. 2001;107:917–923. doi: 10.1172/JCI11947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamawaki H, Lehoux S, Berk BC. Chronic physiological shear stress inhibits tumor necrosis factor-induced proinflammatory responses in rabbit aorta perfused ex vivo. Circulation. 2003;108:1619–1625. doi: 10.1161/01.CIR.0000089373.49941.C4. [DOI] [PubMed] [Google Scholar]
- 7.Yamawaki H, Pan S, Lee RT, Berk BC. Fluid shear stress inhibits vascular inflammation by decreasing thioredoxin-interacting protein in endothelial cells. J Clin Invest. 2005;115:733–738. doi: 10.1172/JCI200523001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hojo Y, Saito Y, Tanimoto T, Hoefen RJ, Baines CP, Yamamoto K, Haendeler J, Asmis R, Berk BC. Fluid shear stress attenuates hydrogen peroxide-induced c-Jun NH2-terminal kinase activation via a glutathione reductase-mediated mechanism. Circ Res. 2002;91:712–718. doi: 10.1161/01.res.0000037981.97541.25. [DOI] [PubMed] [Google Scholar]
- 9.Yoshizumi M, Abe J, Tsuchiya K, Berk BC, Tamaki T. Stress and vascular responses: atheroprotective effect of laminar fluid shear stress in endothelial cells: possible role of mitogen-activated protein kinases. J Pharmacol Sci. 2003;91:172–176. doi: 10.1254/jphs.91.172. [DOI] [PubMed] [Google Scholar]
- 10.Pi X, Yan C, Berk BC. Big Mitogen-Activated Protein Kinase (BMK1)/ERK5 Protects Endothelial Cells From Apoptosis. Circ Res. 2004;94:362–369. doi: 10.1161/01.RES.0000112406.27800.6F. [DOI] [PubMed] [Google Scholar]
- 11.Garin G, Abe JI, Mohan A, Lu W, Yan C, Newby AC, Rhaman A, Berk BC. Flow antagonizes TNF-alpha signaling in endothelial cells by inhibiting caspase-dependent PKC zeta processing. Circ Res. 2007;101:97–105. doi: 10.1161/CIRCRESAHA.107.148270. [DOI] [PubMed] [Google Scholar]
- 12.Chen CY, Korshunov VA, Massett MP, Yan C, Berk BC. Impaired vasorelaxation in inbred mice is associated with alterations in both nitric oxide and superoxide pathways. J Vasc Res. 2007 doi: 10.1159/000106751. in press. [DOI] [PubMed] [Google Scholar]
- 13.Tseng H, Peterson TE, Berk BC. Fluid shear stress stimulates mitogen-activated protein kinase in endothelial cells. Circ Res. 1995;77:869–878. doi: 10.1161/01.res.77.5.869. [DOI] [PubMed] [Google Scholar]
- 14.Yan C, Takahashi M, Okuda M, Lee JD, Berk BC. Fluid shear stress stimulates big mitogen-activated protein kinase 1 (BMK1) activity in endothelial cells. Dependence on tyrosine kinases and intracellular calcium. J Biol Chem. 1999;274:143–150. doi: 10.1074/jbc.274.1.143. [DOI] [PubMed] [Google Scholar]
- 15.Hoefen RJ, Berk BC. The role of MAP kinases in endothelial activation. Vascul Pharmacol. 2002;38:271–273. doi: 10.1016/s1537-1891(02)00251-3. [DOI] [PubMed] [Google Scholar]
- 16.Kamakura S, Moriguchi T, Nishida E. Activation of the protein kinase ERK5/BMK1 by receptor tyrosine kinases. Identification and characterization of a signaling pathway to the nucleus. J Biol Chem. 1999;274:26563–26571. doi: 10.1074/jbc.274.37.26563. [DOI] [PubMed] [Google Scholar]
- 17.Xu B, Stippec LL, Byung-Hoon L, Zhang W, Young-Kyoung L, Cobb MH. WNK1 activates ERK5 by an MEKK2/3-dependent mechanism. Journal of Biological Chemistry. 2003 doi: 10.1074/jbc.M313465200. in press. [DOI] [PubMed] [Google Scholar]
- 18.Cavanaugh JE, Ham J, Hetman M, Poser S, Yan C, Xia Z. Differential regulation of mitogen-activated protein kinases ERK1/2 and ERK5 by neurotrophins, neuronal activity, and cAMP in neurons. J Neurosci. 2001;21:434–443. doi: 10.1523/JNEUROSCI.21-02-00434.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mody N, Leitch J, Armstrong C, Dixon J, Cohen P. Effects of MAP kinase cascade inhibitors on the MKK5/ERK5 pathway. FEBS Lett. 2001;502:21–24. doi: 10.1016/s0014-5793(01)02651-5. [DOI] [PubMed] [Google Scholar]
- 20.Squires MS, Nixon PM, Cook SJ. Cell-cycle arrest by PD184352 requires inhibition of extracellular signal-regulated kinases (ERK) 1/2 but not ERK5/BMK1. Biochem J. 2002;366:673–680. doi: 10.1042/BJ20020372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hii CS, Anson DS, Costabile M, Mukaro V, Dunning K, Ferrante A. Characterization of the MEK5-ERK5 module in human neutrophils and its relationship to ERK1/ERK2 in the chemotactic response. J Biol Chem. 2004;279:49825–49834. doi: 10.1074/jbc.M406892200. [DOI] [PubMed] [Google Scholar]
- 22.Abe J, Kusuhara M, Ulevitch RJ, Berk BC, Lee JD. Big mitogen-activated protein kinase 1 (BMK1) is a redox-sensitive kinase. J Biol Chem. 1996;271:16586–16590. doi: 10.1074/jbc.271.28.16586. [DOI] [PubMed] [Google Scholar]
- 23.Sohn SJ, Sarvis BK, Cado D, Winoto A. ERK5 MAPK Regulates Embryonic Angiogenesis and Acts as a Hypoxia- sensitive Repressor of Vascular Endothelial Growth Factor Expression. J Biol Chem. 2002;277:43344–43351. doi: 10.1074/jbc.M207573200. [DOI] [PubMed] [Google Scholar]
- 24.Hayashi M, Kim SW, Imanaka-Yoshida K, Yoshida T, Abel ED, Eliceiri B, Yang Y, Ulevitch RJ, Lee JD. Targeted deletion of BMK1/ERK5 in adult mice perturbs vascular integrity and leads to endothelial failure. J Clin Invest. 2004;113:1138–1148. doi: 10.1172/JCI19890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dekker RJ, van Soest S, Fontijn RD, Salamanca S, de Groot PG, VanBavel E, Pannekoek H, Horrevoets AJ. Prolonged fluid shear stress induces a distinct set of endothelial cell genes, most specifically lung Kruppel-like factor (KLF2) Blood. 2002;100:1689–1698. doi: 10.1182/blood-2002-01-0046. [DOI] [PubMed] [Google Scholar]
- 26.Dekker RJ, van Thienen JV, Rohlena J, de Jager SC, Elderkamp YW, Seppen J, de Vries CJ, Biessen EA, van Berkel TJ, Pannekoek H, Horrevoets AJ. Endothelial KLF2 links local arterial shear stress levels to the expression of vascular tone-regulating genes. Am J Pathol. 2005;167:609–618. doi: 10.1016/S0002-9440(10)63002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.SenBanerjee S, Lin Z, Atkins GB, Greif DM, Rao RM, Kumar A, Feinberg MW, Chen Z, Simon DI, Luscinskas FW, Michel TM, Gimbrone MA, Jr, Garcia-Cardena G, Jain MK. KLF2 Is a novel transcriptional regulator of endothelial proinflammatory activation. J Exp Med. 2004;199:1305–1315. doi: 10.1084/jem.20031132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parmar KM, Larman HB, Dai G, Zhang Y, Wang ET, Moorthy SN, Kratz JR, Lin Z, Jain MK, Gimbrone MA, Jr, Garcia-Cardena G. Integration of flow-dependent endothelial phenotypes by Kruppel-like factor 2. J Clin Invest. 2006;116:49–58. doi: 10.1172/JCI24787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sasaki M, Jordan P, Welbourne T, Minagar A, Joh T, Itoh M, Elrod JW, Alexander JS. Troglitazone, a PPAR-gamma activator prevents endothelial cell adhesion molecule expression and lymphocyte adhesion mediated by TNF-alpha. BMC Physiol. 2005;5:3. doi: 10.1186/1472-6793-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akaike M, Che W, Marmarosh NL, Ohta S, Osawa M, Ding B, Berk BC, Yan C, Abe JI. The Hinge-Helix 1 Region of Peroxisome Proliferator-Activated Receptor {gamma}1 (PPAR{gamma}1) Mediates Interaction with Extracellular Signal-Regulated Kinase 5 and PPAR{gamma}1 Transcriptional Activation: Involvement in Flow-Induced PPAR{gamma} Activation in Endothelial Cells. Mol Cell Biol. 2004;24:8691–8704. doi: 10.1128/MCB.24.19.8691-8704.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]