Abstract
Our aim was to investigate, in patients with heart failure, the relationship between left atrial size and exercise capacity and cardiovascular events.
Seventy-five patients (67 men and 8 women; mean age, 53.4 ± 8.8 yr) with left ventricular ejection fractions of ≤0.45 (New York Heart Association functional classes I–III) were matched by age and sex with 20 healthy control subjects. Echocardiographic examinations were performed, as was exercise testing by the modified Bruce protocol. Patients were monitored for a period of 330 to 480 days for cardiac death or for heart failure that required hospitalization.
The indexed left atrial diastolic size (β level = −0.534, P <0.001) and left ventricular late diastolic filling velocity (β level = 0.247, P <0.017) were the most important values in predicting low exercise capacity. The only independent predictor of low exercise capacity (<5 METS) was the indexed left atrial diastolic size (odds ratio, 1.428; 95% confidence interval, 1.09–1.702; P <0.001). Every 1 mm/m2 increase in indexed left atrial diastolic dimension caused a 42.8% increase in the risk of severe heart failure (exercise capacity, <5 METS). Independent predictors for cardiovascular events were indexed as left atrial systolic size (odds ratio, 1.383; 95% confidence interval, 1.145–1.671; P <0.001) and left ventricular early diastolic/late diastolic filling velocity (odds ratio, 1.096; 95% confidence interval, 1.010–1.189; P <0.027). Indexed left atrial diastolic and left atrial systolic size predict exercise capacity and cardiovascular events, respectively, in New York Heart Association functional class I through III heart failure patients.
Key words: Atrial function, left/physiology; cardiac output; cardiomegaly, dilated/ultrasonography; diastole; exertion; exercise test; exercise tolerance/physiology; heart atria; heart failure; myocardial contraction; predictive value of tests; prognosis; ROC curve; stroke volume; systole
New York Heart Association (NYHA) functional class, brain natriuretic peptide (BNP) levels, and echocardiographic examination are used to evaluate exercise capacity and predict cardiovascular (CV) events in patients with heart failure. However, NYHA functional classification can be subjective and misleading; and exercise testing and the measurement of BNP levels are not easily repeated. Echocardiography contributes substantially to the diagnostic, hemodynamic, and prognostic evaluation of heart failure patients. A major focus of echocardiography in such patients is left ventricular (LV) function as evaluated by ejection fraction (LVEF). Although the degree of systolic dysfunction is an important measure of heart failure, the correlation between degree of systolic dysfunction and exercise capacity is weak.1–3
Recently, there has been renewed interest in the relationship between the left atrium (LA) and several CV diseases and conditions. It has been shown, for example, that LA dilation negatively affects clinical outcomes in patients who have systolic and diastolic heart failure, mitral regurgitation, stroke, atrial fibrillation, and myocardial infarction.4–6 This present study investigates the relationship between exercise capacity and echocardiographic values in patients with heart failure and also reveals the role of LA size as a predictor of exercise capacity and CV events.
Patients and Methods
From March through August 2004, we enrolled 75 patients (67 men and 8 women; mean age, 53.4 ± 8.8 yr) who presented with left ventricular ejection fractions ≤0.45 and whose echocardiographic images were of adequate quality. Twenty sex- and age-matched healthy subjects were included as members of a control group. We excluded patients with mitral and aortic valve disease, recent myocardial infarction (<6 mo), congenital heart disease, pacemakers, bundle branch block, or atrial fibrillation. Patients were divided into groups I, II, and III, according to their NYHA functional class. All subjects provided written, informed consent in order to participate in the study. The study was approved by our local ethics committee. All patients were monitored for a period of 330 to 480 days (420 ± 45 days), for cardiac death or hospitalization for worsening heart failure.
Echocardiographic Examination
All echocardiographic measurements were obtained at rest. Standard echocardiographic examination and pulsed-wave Doppler and tissue-Doppler imaging were performed by use of an ACUSON Sequoia™ (Siemens Medical Solutions USA, Inc.; Malvern, Pa) with a 2.5- or 3.5-MHz phased-array transducer. We used the mean of all recordings from 3 consecutive cycles.
M-Mode measurements of end-diastolic and end-systolic dimensions and of interventricular septum and posterior wall thicknesses were obtained in accordance with the recommendations of the American Society of Echocardiography.7 Left ventricular mass was calculated by use of the Devereux formula8 and was indexed to body surface area. Simpson's method was used to calculate LVEF. Left atrial size was measured at ventricular end-systole (when the LA chamber was at diastole = LAd) and at ventricular end-diastole (when the LA chamber was at systole = LAs) and also was indexed to body surface area, according to current American Society of Echocardiography guidelines.9 In this manner, the timings of diastole and systole for the LA in this study were related to the ventricular cardiac cycle. Left atrial fractional shortening was calculated by using the same formula that is used for calculating LV fractional shortening.
Left ventricular diastolic function was evaluated by means of pulsed-wave Doppler and tissue-Doppler imaging. Pulsed-wave Doppler was performed by measurement of transmitral flow values, including early (E) and late (A) diastolic filling velocities, the E/A ratio, E deceleration time, and isovolumetric relaxation time in the apical 4-chamber view. Sample volume was ascertained at the tips of the mitral and tricuspid valves. Tissue-Doppler imaging was also performed in the apical 4-chamber view, and sample volume was ascertained at the lateral walls of both ventricles. Velocities of a systolic wave (S) and of early (Em) and late (Am) diastolic waves, together with their ratios (Em/Am), were obtained at the end of expiration.
Exercise Test
Exercise testing was conducted on a Marquette T-2000 treadmill using the modified Bruce protocol. A preliminary familiarization procedure identified patients who were not able to exercise for reasons other than cardiac limitation, and these patients were excluded. The same supervisor conducted the exercise tests throughout the study. All patients performed symptom-limited exercise tests unless termination was indicated for reasons such as fatigue or dizziness. Patients did not terminate exercise testing as a consequence of myocardial ischemia. Patients were exercised after a 4-hour postprandial interlude, were asked not to consume alcohol or caffeine in the 12 hours preceding exercise, and were advised to take all prescribed medications. Electrocardiograms and blood pressure recordings were monitored throughout. Exercise time, exercise stage, maximum workload (metabolic equivalents, or METS), peak exercise, and resting heart rate were recorded.
Statistical Analysis
All analyses were performed by the SPSS 14.0 statistical software package (SPSS Inc.; Chicago, Ill). Continuous variables were defined as mean ± SD. In the analysis of these variables in the control groups and in patients grouped in accordance with their NYHA classifications, the one-way analysis of variance (ANOVA) or the Kruskal-Wallis test was used, and for multiple comparisons in ANOVA, post hoc tests (Scheffé or Tamhane) were applied. When the dependent variable was binary, the nonparametric Mann-Whitney test or independent samples of the t test were applied for comparison. Discrete variables were compared using χ2 analysis. Correlations between continuous variables were analyzed by means of the Pearson product moment or Spearman rank correlation. Two multivariate logistic regression analyses were performed to determine significant predictors of CV events and of exercise capacity <5 METS. In univariate analysis, variables significant at the P <0.1 level were entered in our logistic regression analysis. Moreover, a linear regression analysis was applied for exercise capacity. A receiver operator characteristic (ROC) curve analysis was performed in order to identify the optimal cutoff point of LA size (at which sensitivity and specificity are maximal) for the prediction of exercise capacity and CV events. The area under the ROC (AUROC) value was calculated as a measure of the accuracy of the test. A value of P <0.05 was considered statistically significant.
Results
Patients' Characteristics
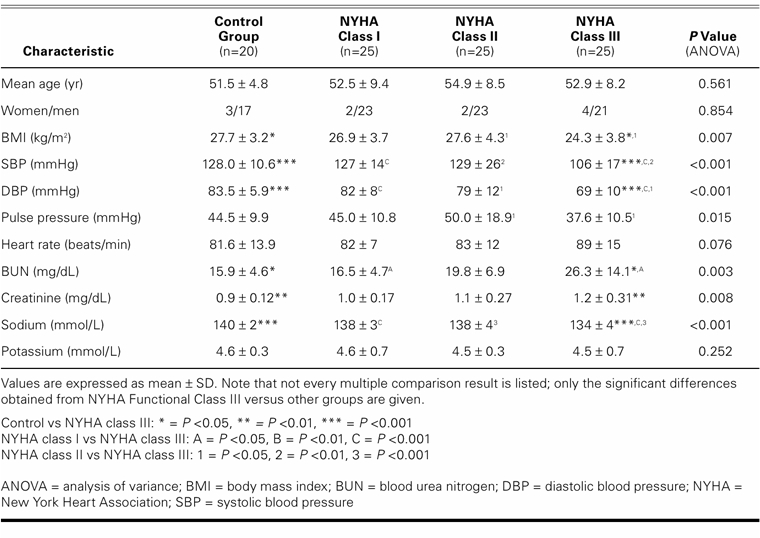
Systolic and diastolic blood pressures, body mass index, and sodium levels were lower, and pulse pressure, blood urea nitrogen, and creatinine levels were higher in the NYHA class III patients than in the control subjects and in NYHA class I and II patients. Clinical and laboratory features of the control group and of the patient groups are shown in Table I. All 75 patients were taking either angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, 41 (54.7%) of the patients were taking diuretics, 59 (78.7%) were on β-blockers, 22 (29.3%) were on digitalis, and 32 (42.7%) were on spironolactone. Almost every patient was on 2 or more antihypertensive agents.
TABLE I. Clinical and Laboratory Characteristics of Control and Study Participants
Echocardiographic and Exercise Test Findings
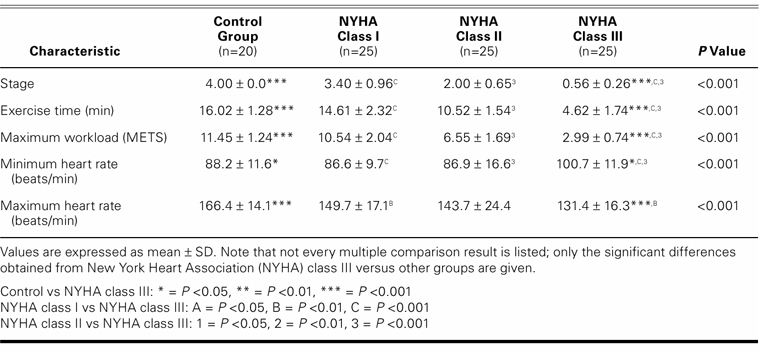
Except for LV-isovolumetric relaxation times, there was a significant difference between the control group and the patients regarding echocardiographic findings. The higher the NYHA class, the worse the echocardiographic measurements (Table II). Total exercise time, METS, and maximum heart rate all decreased in accordance with worsening in NYHA numerical classification (Table III).
TABLE II. Echocardiographic Findings in the Control and Study Groups
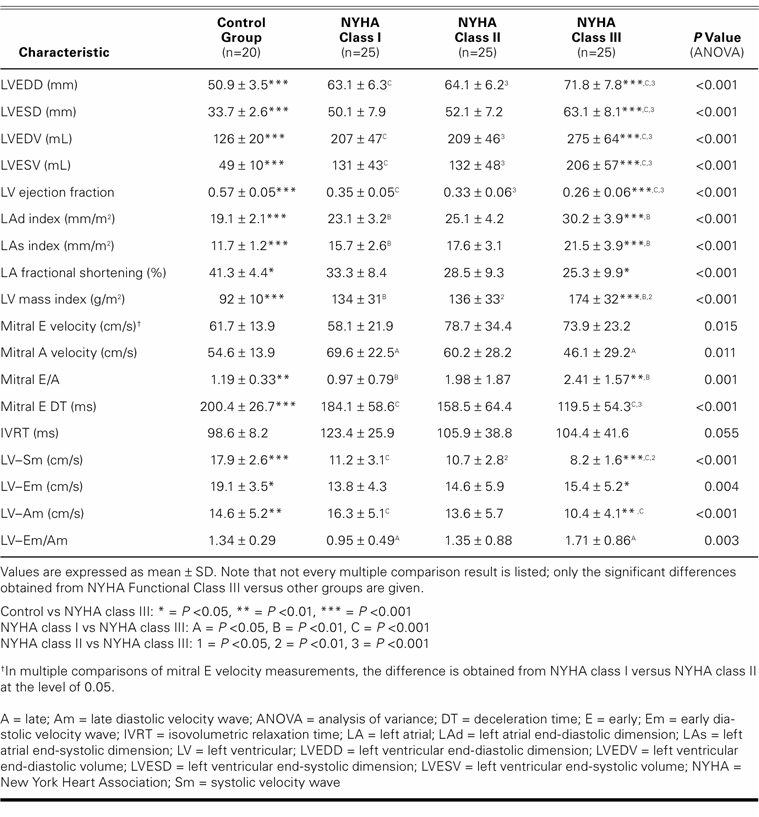
TABLE III. Exercise Test Findings in the Control and Study Groups
Correlates of Echocardiographic Variables and Exercise Capacity
Except for LVEF, A wave, E wave, DT, Sm, and Am waves, all echocardiographic variables showed a negative correlation with exercise capacity as determined by METS (Table IV). Multivariate linear regression analysis showed that indexed LAd size and LV-Am were the most important variables in predicting exercise capacity (β level = −0.534, P <0.001 and β level = 0.247, P=0.017, respectively; the explained variance of exercise capacity [R2] was 0.447). The only independent predictor of exercise capacity <5 METS was the indexed LAd size (P <0.0001). Logistic regression analysis showed that every 1 mm/m2 increase in indexed LAd size caused a 42.8% increase in the risk of the presence of exercise capacity <5 METS (odds ratio, 1.428; 95% confidence interval, 1.09–1.702). Receiver operating characteristic curve analysis also showed that when 25 mm/m2 was accepted as a cutoff value for the prediction of exercise capacity <5 METS, sensitivity and specificity were 86.2% and 67%, respectively. However, sensitivity decreased and specificity increased when 26 mm/m2 was used as a cutoff value (75.9% and 75%, respectively). The AUROC was calculated as 0.853 (0.767–0.940), which indicates good discriminatory power (Fig. 1A).
TABLE IV. Correlation Analysis of Echocardiographic Variables in Relation to Variables in Exercise Capacity
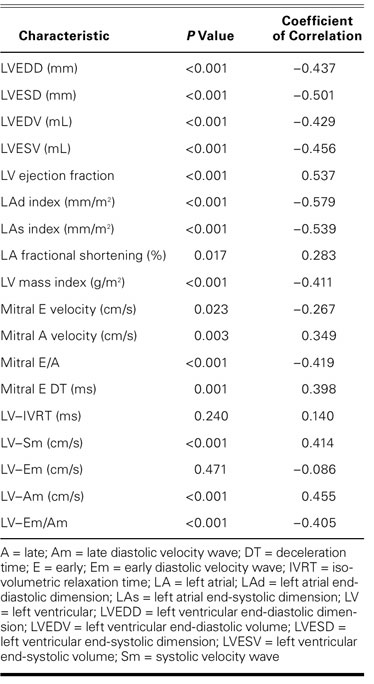
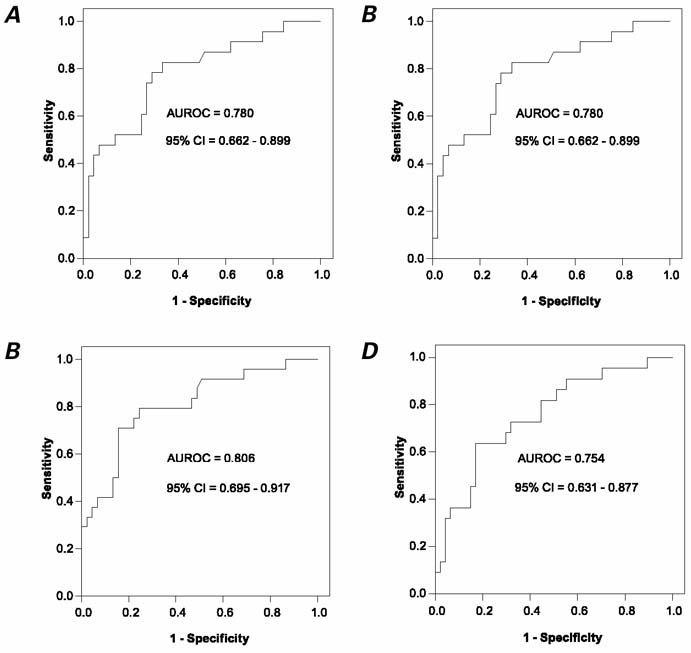
Fig. 1 A) Receiver operating characteristic analysis for indexed LAd dimension, as used in predicting exercise capacity, B) indexed LAd dimension, in predicting cardiovascular events, C) indexed LAs dimension, in predicting cardiovascular events, and D) left ventricular Em/Am, in predicting cardiovascular events.
AUROC = area under receiver operating characteristic curve; CI = confidence interval; Em/Am = ratio of velocities of early (Em) and late (Am) diastolic waves; LA = left atrium; LAd = LA chamber at its greatest dimension (i.e., LA is at diastole and LV is at end-systole); LAs = LA chamber at its smallest dimension (i.e., LA is at systole and LV is at end-diastole); LV = left ventricle
Correlates of Clinical and Echocardiographic Variables and Cardiovascular Events
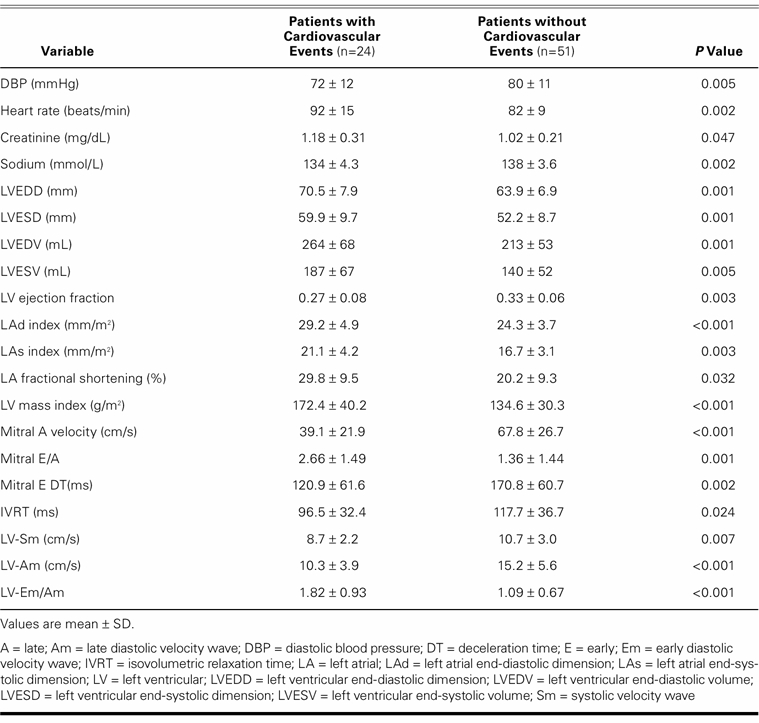
During the 330 to 480 days of follow-up, 6 cardiac-related deaths and 18 hospitalizations for worsening of heart failure occurred. Patients who developed CV events had a larger LA and LV size and a lower LVEF and LA fractional shortening. They also had diastolic function values indicative of greater impairment (Table V). Independent predictors of CV events were indexed LAs size (P=0.001) and LV–Em/Am (P=0.027). Logistic regression analysis showed that every 1 mm/m2 increase in indexed LAs size was accompanied by a 38% increase in the occurrence of CV events (odds ratio, 1.383; 95% confidence interval, 1.145–1.671). When the LV–Em/Am ratio increased 0.1, the risk of CV events increased 9.6% (odds ratio, 1.096; 95% confidence interval, 1.010–1.189).
TABLE V. Significant Variables of Patients, With and Without Cardiovascular Events
Receiver operating characteristic curve analysis showed that when the indexed LAs size of 18 mm/m2 was accepted as a cutoff value (for the prediction of CV events) sensitivity and specificity were 79.2% and 73.3%, respectively. However, when 19 mm/m2 was accepted as a cutoff value, sensitivity decreased and specificity increased, to 70.2% and 82.2%, respectively. For indexed LAd size (Fig. 1B), the AUROC was 0.780 (95% confidence interval, 0.66–0.90). For discriminatory power (Fig. 1C), the AUROC was calculated as 0.806 (95% confidence interval, 0.70–0.92). For LV–Em/Am ratio, the AUROC was 0.756 (95% confidence interval, 0.63–0.88) (Fig. 1D). Although these analyses showed that indexed LAs size determined CV events with a higher predictive value than did indexed LAd size and LV–Em/Am ratio, this finding was not significant.
Discussion
Among the several measurable variables that can be used to predict exercise capacity and CV events in patients with heart failure, clinical, echocardiographic, and biochemical variables have been proposed as most useful. Some of them have good sensitivity and specificity, and some do not. Recently, the relationship between CV disease and LA dimension and function has gained greater recognition.4–6
Jikuhara and colleagues10 showed that there was a strong correlation, in recent myocardial infarction patients, between LA fractional shortening at rest and maximum exercise capacity. In addition, they found strong relationships between preserved LA function and LV filling, exercise capacity, and increased cardiac output. The same findings have been reported in patients with idiopathic dilated cardiomyopathy, hypertrophic cardiomyopathy, chronic congestive heart failure, and hypertension.11–13 Terzi and associates14 found that decrease in left atrial ejection fraction and increase in LA size were related to the decrease in peak oxygen consumption. In our study, all echocardiographic variables except for LVEF and mitral A wave were related to exercise capacity. However, regression analysis showed that indexed LAd size and Em/Am ratio were the most important variables. In contrast with investigators who considered left atrial fractional shortening and LA volume, we emphasized indexed LA dimensions. Most prior studies point out that diastolic function is more strongly related to exercise capacity than to systolic function in heart failure.10–14 Our findings are in accord with that conclusion. During isotonic exercise in normal subjects, the Frank-Starling curve contributes to increases in contractility, EF, stroke volume, and LV end-diastolic volume. However, the increase in stroke volume depends mainly on the Frank-Starling mechanism, and this dependence has implications for diastolic filling properties in heart failure patients with systolic dysfunction.15,16 Left atrial function contributes to LV diastolic filling, and this contribution is more prominent in patients with LV dysfunction.17 In advanced heart failure, structural changes develop in the LA wall due to the increased LV end-diastolic pressure, and diminished LA function ensues.18,19 The decrease in LA function impairs late LV diastolic filling, which means that cardiac output cannot increase during exercise.10 A recent study showed that the most important predictor of LA volume is the degree of LV diastolic dysfunction.20 However, the mitral filling pattern can be an early indicator of LV diastolic dysfunction. We found that the Em/Am ratio, as an important mitral filling pattern, correlated significantly with exercise capacity. This finding supports the observation that exercise capacity is affected before LA dysfunction becomes manifest.
Left atrial dimension was previously shown as a prognostic indicator in patients with aortic stenosis and restrictive cardiomyopathy.21,22 On the other hand, the LA volume index has been shown to be the most important echocardiographic variable for survival in idiopathic dilated cardiomyopathy patients.23 Dini and associates5 reported that LA diastolic size index was the only independent predictor of cardiac death or of worsening heart failure in patients with dilated cardiomyopathy who were over 70 years of age. Indexed LA diastolic size may help to predict functional capacity and CV events in patients with heart failure. In subjects with sinus rhythm, de Groote and colleagues6 showed that LA volume is a more robust marker of CV events than is area or diameter; they also showed that the patients who developed CV events had a larger LA size. Moreover, LA enlargement has been shown to be an independent prognostic value in elderly patients who already have LV dysfunction.7 We found that indexed LA systolic size is an independent parameter for predicting CV events. In addition, our study showed that LV filling as revealed by the tissue Doppler Em/Am ratio was also an independent prognostic marker in heart failure patients.
Limitations of the Study
The most important limitation of our study was the lack of maximum oxygen consumption measurement. The sample size of the study was small. Patients were taking different drugs at different dosages. We did not consider the medications that might have some influence on LA size. Another important limitation was our lack of LA volume measurements. The determination of LA volume and also tissue-Doppler variables of the LA wall might be more reliable in the evaluation of prognosis.
Conclusion
Measurement of LA size is neither sufficiently sensitive nor sufficiently specific to alter treatment decisions. However, indexed LAd and LAs size predict exercise capacity and CV events, respectively, in patients with heart failure (NYHA functional class I–III). Measurement of LA size is an easy, simple, and reliable method of predicting exercise capacity and CV events in daily clinical practice.
Footnotes
Address for reprints: Esmeray Acarturk, MD, FESC, Cardiology Department, School of Medicine, Cukurova University, 01330 Adana, Turkey E-mail: kanarya@cu.edu.tr
References
- 1.Franciosa JA, Park M, Levine TB. Lack of correlation between exercise capacity and indexes of resting left ventricular performance in heart failure. Am J Cardiol 1981;47(1):33–9. [DOI] [PubMed]
- 2.Benge W, Litchfield RL, Marcus ML. Exercise capacity in patients with severe left ventricular dysfunction. Circulation 1980;61(5):955–9. [DOI] [PubMed]
- 3.Port S, McEwan P, Cobb FR, Jones RH. Influence of resting left ventricular function on the left ventricular response to exercise in patients with coronary artery disease. Circulation 1981;63(4):856–63. [DOI] [PubMed]
- 4.Tsang TS, Abhayaratna WP, Barnes ME, Miyasaka Y, Gersh BJ, Bailey KR, et al. Prediction of cardiovascular outcomes with left atrial size: is volume superior to area or diameter? J Am Coll Cardiol 2006;47(5):1018–23. [DOI] [PubMed]
- 5.Dini FL, Cortigiani L, Baldini U, Boni A, Nuti R, Barsotti L, Micheli G. Prognostic value of left atrial enlargement in patients with idiopathic dilated cardiomyopathy and ischemic cardiomyopathy. Am J Cardiol 2002;89(5):518–23. [DOI] [PubMed]
- 6.de Groote P, Soudan B, Lamblin N, Rouaix-Emery N, Mc-Fadden E, Meurice T, et al. Is hormonal activation during exercise useful for risk stratification in patients with moderate congestive heart failure? Am Heart J 2004;148(2):349–55. [DOI] [PubMed]
- 7.Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation 1978;58(6):1072–83. [DOI] [PubMed]
- 8.Devereux RB, Reichek N. Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation 1977;55(4):613–8. [DOI] [PubMed]
- 9.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 2005;18(12):1440–63. [DOI] [PubMed]
- 10.Jikuhara T, Sumimoto T, Tarumi N, Yuasa F, Hattori T, Sugiura T, Iwasaka T. Left atrial function as a reliable predictor of exercise capacity in patients with recent myocardial infarction. Chest 1997;111(4):922–8. [DOI] [PubMed]
- 11.Iriarte M, Murga N, Sagastagoitia D, Molinero E, Morillas M, Salcedo A, et al. Congestive heart failure from left ventricular diastolic dysfunction in systemic hypertension. Am J Cardiol 1993;71(4):308–12. [DOI] [PubMed]
- 12.Chikamori T, Counihan PJ, Doi YL, Takata J, Stewart JT, Frenneaux MP, McKenna WJ. Mechanisms of exercise limitation in hypertrophic cardiomyopathy. J Am Coll Cardiol 1992;19(3):507–12. [DOI] [PubMed]
- 13.Kitzman DW, Higginbotham MB, Cobb FR, Sheikh KH, Sullivan MJ. Exercise intolerance in patients with heart failure and preserved left ventricular systolic function: failure of the Frank-Starling mechanism. J Am Coll Cardiol 1991;17(5): 1065–72. [DOI] [PubMed]
- 14.Terzi S, Dayi SU, Akbulut T, Sayar N, Bilsel T, Tangurek B, et al. Value of left atrial function in predicting exercise capacity in heart failure with moderate to severe left ventricular systolic dysfunction. Int Heart J 2005;46(1):123–31. [DOI] [PubMed]
- 15.Dahan M, Aubry N, Baleynaud S, Ferreira B, Yu J, Gourgon R. Influence of preload reserve on stroke volume response to exercise in patients with left ventricular systolic dysfunction: a Doppler echocardiographic study. J Am Coll Cardiol 1995;25(3):680–6. [DOI] [PubMed]
- 16.Tomai F, Ciavolella M, Crea F, Gaspardone A, Versaci F, Giannitti C, et al. Left ventricular volumes during exercise in normal subjects and patients with dilated cardiomyopathy assessed by first-pass radionuclide angiography. Am J Cardiol 1993;72(15):1167–71. [DOI] [PubMed]
- 17.Matsuda M, Matsuda Y. Mechanism of left atrial enlargement related to ventricular diastolic impairment in hypertension. Clin Cardiol 1996;19(12):954–9. [DOI] [PubMed]
- 18.Dernellis JM, Stefanadis CI, Zacharoulis AA, Toutouzas PK. Left atrial mechanical adaptation to long-standing hemodynamic loads based on pressure-volume relations. Am J Cardiol 1998;81(9):1138–43. [DOI] [PubMed]
- 19.Hoit BD, Shao Y, Tsai LM, Patel R, Gabel M, Walsh RA. Altered left atrial compliance after atrial appendectomy. Influence on left atrial and ventricular filling. Circ Res 1993;72(1): 167–75. [DOI] [PubMed]
- 20.Tsang TS, Barnes ME, Gersh BJ, Bailey KR, Seward JB. Left atrial volume as a morphophysiologic expression of left ventricular diastolic dysfunction and relation to cardiovascular risk burden. Am J Cardiol 2002;90(12):1284–9. [DOI] [PubMed]
- 21.Rossi A, Tomaino M, Golia G, Anselmi M, Fuca G, Zardini P. Echocardiographic prediction of clinical outcome in medically treated patients with aortic stenosis. Am Heart J 2000; 140(5):766–71. [DOI] [PubMed]
- 22.Ammash NM, Seward JB, Bailey KR, Edwards WD, Tajik AJ. Clinical profile and outcome of idiopathic restrictive cardiomyopathy. Circulation 2000;101(21):2490–6. [DOI] [PubMed]
- 23.Rossi A, Cicoira M, Zanolla L, Sandrini R, Golia G, Zardini P, Enriquez-Sarano M. Determinants and prognostic value of left atrial volume in patients with dilated cardiomyopathy. J Am Coll Cardiol 2002;40(8):1425. [DOI] [PubMed]


