Abstract
We report what we believe is the 1st case in the medical literature in which an intravenous thrombolytic agent was used successfully—without massive intracranial bleeding—to treat acute stroke induced by atrial myxoma. Our patient, who had biatrial myxomas with a dual blood supply from the right coronary artery, presented with cerebral ischemia. Transesophageal echocardiography was essential in clarifying the diagnosis and in helping to direct surgical treatment.
Key words: Cerebrovascular accident; echocardiography, transesophageal; embolism; heart atria/surgery; heart neoplasms/complications/diagnosis/surgery; intracranial embolism and thrombosis/etiology/drug therapy; myxoma; thrombolytic therapy; tissue plasminogen activator
Cardiac myxomas constitute approximately 50% of all benign cardiac tumors; patients can present with embolic events or sudden death. The challenge for physicians lies in the early recognition, diagnosis, and treatment of these life-threatening neoplasms.
Case Report
In June 2006, a 51-year-old man without known cardiovascular risk factors was hospitalized after developing right-sided weakness and aphasia while jogging in the park. On his arrival at the emergency department, his blood pressure was 148/88 mmHg. An electrocardiogram revealed sinus rhythm, and the chest radiographic findings were normal. His National Institutes of Health Stroke Scale score was 22. In accordance with our institutional protocol, the patient was treated, 84 minutes after the onset of symptoms, with intravenous alteplase (tissue plasminogen activator). Thirteen hours later, computed tomography of the brain revealed no evidence of intracranial hemorrhage or other abnormality.
Two days later, a transesophageal echocardiogram revealed a large (4.7 × 2.7-cm) cerebriform pedunculated homogeneous mass that occupied most of the left atrium and prolapsed through the mitral valve with mild functional mitral stenosis (Fig. 1). A smaller, right atrial mass (2.6 × 1.1 cm) extended into the tricuspid annulus. Each was attached to its respective side of the interatrial septum (Fig. 2).
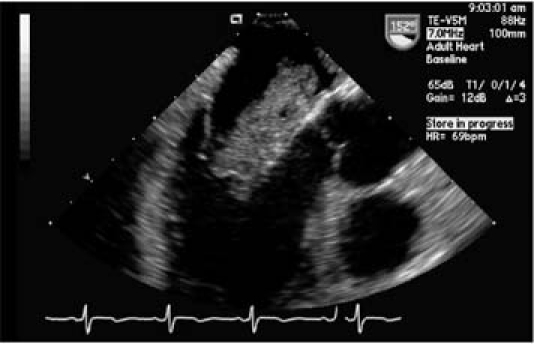
Fig. 1 A transesophageal echocardiogram (mid-esophageal position, long-axis view) shows a left atrial myxoma prolapsing through the mitral valve.
Real-time motion images are available at texasheart.org/journal
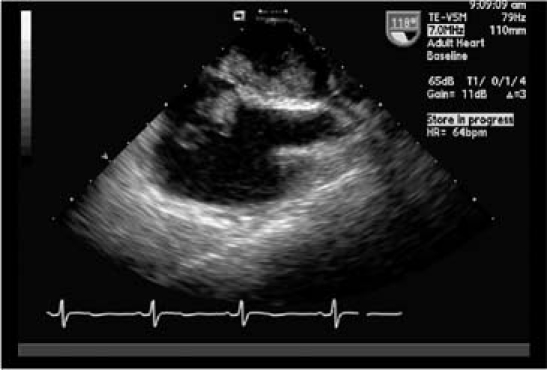
Fig. 2 A transesophageal echocardiogram (mid-esophageal position, bicaval view) shows atrial myxomas on both the left and right aspects of the interatrial septum.
Real-time motion images are available at texasheart.org/journal
Subsequent cardiac catheterization showed tumor vascularity and characteristic “tumor blush,” which originated from the proximal and distal right coronary artery (RCA) for the left- and right-sided tumors, respectively (Fig. 3).
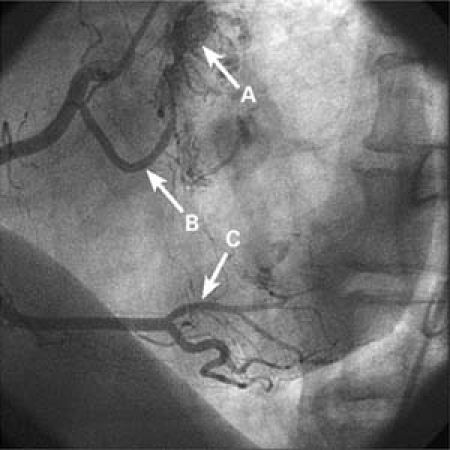
Fig. 3 Right coronary angiogram (left anterior oblique view) shows the independent arterial supply for each tumor (note “tumor blush”): arrow A, the proximal right coronary artery; arrow B, the left atrial myxoma and the distal right coronary artery (posterolateral branch); and arrow C, the right atrial myxoma.
Real-time motion images are available at texasheart.org/journal
At surgery, the biatrial masses were excised, and the interatrial residual defect was covered with a pericardial patch. Postoperatively, the patient had no neurologic symptoms but had a junctional rhythm with a heart rate in the range of 40 to 45 beats/ min, which required permanent pacemaker insertion.
Histologic examination of the biopsy specimen showed cords of small-to-medium tumor cells that contained eosinophilic cytoplasm. The tumor cells were within an abundant myxoid stroma. The findings were diagnostic of atrial myxoma (Fig. 4).
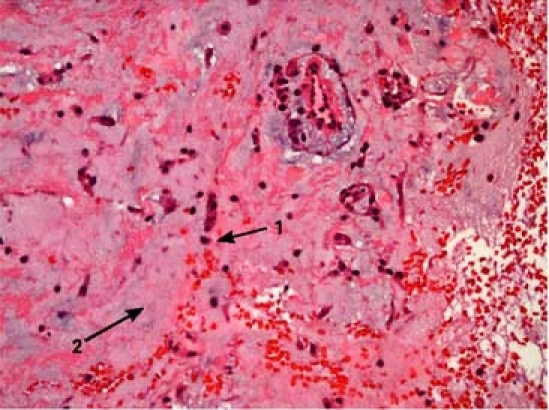
Fig. 4 Photomicrograph of the biopsy specimen shows cords of small-to-medium tumor cells (1) containing eosinophilic cytoplasm within an abundant myxoid stroma (2) (H&E, orig. ×40).
Discussion
More than 75% of primary cardiac tumors are benign, and most of them are myxomas. Other common benign lesions include papillary fibroelastomas and lipomas.1–5 Most myxomas (60%–88%) occur in the left atrium, with smaller percentages in the right atrium (4%–28%), left ventricle (8%), right ventricle (2.5%– 6.1%), 2 or more locations (2.5%), and, in rare cases, in both atria of the same patient (<2.5%).6–7 Most atrial myxomas originate from the limbus fossae ovalis, but approximately 10% arise from other regions, including the posterior and anterior atrial walls or the atrial appendages. When biatrial, the myxomas can arise from mirror-image regions on both sides of the septum.8 A small percentage of primary cardiac tumors have familial penetrance. Recurrence is more frequent in patients with a family history of myxoma, and familial myxomas frequently appear at early ages, with atypical and multicentric locations.8 Our patient had no family history of myxoma and no stigmata of conditions or syndromes that are often associated with familial myxoma. Additionally, all 4 of his sons had normal transthoracic echocardiograms, with no evidence of atrial myxoma.
This patient developed acute cerebral ischemia from his cardiac myxoma. Embolization is one of the major critical complications of myxoma and occurs in about 40% to 50% of patients.6,9,10 Emboli from atrial myxoma may be composed of thrombus, of the tumor itself, or both.11 Tumor emboli from myxomas are unlikely to lyse upon thrombolytic therapy. Our patient's presenting symptoms of loss of consciousness, aphasia, and right-sided weakness made a diagnosis of tumor emboli unlikely. This unlikelihood justified thrombolytic treatment, because our suspicion before the diagnosis of myxoma was that the cause was thrombosis rather than a tumor fragment. Indeed, treatment with a thrombolytic agent was successful. To our knowledge, the use of thrombolytic therapy in atrial myxoma has been reported in only 3 cases, 2 of which were intra-arterial and accompanied by successful recanalization, and the 3rd of which was intravenous and complicated by massive intracranial hemorrhage.12–13 In our patient, there was no evidence of subarachnoid hemorrhage, as shown by the computed tomographic scan of the brain that was performed 13 hours after intravenous administration of tissue plasminogen activator. Our patient still recovered completely, with no residual neurologic deficit. Guidelines for the use of thrombolytic therapy in acute stroke do not deal with myxoma; to the best of our knowledge, ours is the 1st reported case in which an intravenous thrombolytic agent has been used successfully to treat acute stroke induced by atrial myxoma, without massive intracranial bleeding.
Cardiac tumors are most often diagnosed by transthoracic or transesophageal echocardiography. Myxomas are generally homogenous structures with a pedunculated base and a smooth lobulated surface. Some have a villose appearance and are more prone to embolization.
Multifocal atrial myxomas may have blood supply from the circumflex or left anterior descending (LAD) coronary artery, or from both. There is 1 case report of biatrial myxomas with dual coronary arterial blood supply, from the RCA and the LAD.14 Ours, we believe, is the 1st report of a patient whose atrial myxomas had a dual blood supply from the RCA.
The treatment of choice for large and mobile atrial myxomas is prompt resection, to decrease the risk of distal embolization or cardiovascular complications, including sudden death.15–16 Postoperative recovery is generally rapid, but atrial arrhythmias or atrioventricular conduction abnormalities are not uncommon complications; in some series, the reported incidence is up to 26%.17 Follow-up by echocardiography is recommended, because 5% of patients develop recurrent myxomas.10
In conclusion, biatrial myxomas, although rare, may present as an occult neurologic condition. Thrombolytic therapy is the immediate treatment of choice for cerebral ischemia, and it can help to lyse the thrombotic components of myxoma as well. Optimally, rapid imaging with echocardiography should be used to help direct treatment of cerebral ischemia patients, because thrombolytic therapy carries a significant risk. In our patient, transesophageal echocardiography was essential in clarifying the diagnosis and directing surgical treatment.
Acknowledgments
The authors acknowledge Catalin Loghin, MD, for echocardiographic diagnosis; James Grotta, MD, the attending neurologist; Anthony Estrera, MD, of the surgical team; and Ashley Allison, MD, for pathologic diagnosis.
Supplementary Material
Footnotes
Address for reprints: Morhaf Ibrahim, MD, 6431 Fannin St., Suite 1.150, Houston, TX 77030-1501. E-mail: Morhaf.Ibrahim@uth.tmc.edu
References
- 1.Molina JE, Edwards JE, Ward HB. Primary cardiac tumors: experience at the University of Minnesota. Thorac Cardiovasc Surg 1990;38 Suppl 2:183–91. [DOI] [PubMed]
- 2.Tazelaar HD, Locke TJ, McGregor CG. Pathology of surgically excised primary cardiac tumors. Mayo Clin Proc 1992; 67(10):957–65. [DOI] [PubMed]
- 3.Larrieu AJ, Jamieson WR, Tyers GF, Burr LH, Munro AI, Miyagishima RT, et al. Primary cardiac tumors: experience with 25 cases. J Thorac Cardiovasc Surg 1982;83(3):339–48. [PubMed]
- 4.Odim J, Reehal V, Laks H, Mehta U, Fishbein MC. Surgical pathology of cardiac tumors. Two decades at an urban institution. Cardiovasc Pathol 2003;12(5):267–70. [DOI] [PubMed]
- 5.Kamiya H, Yasuda T, Nagamine H, Sakakibara N, Nishida S, Kawasuji M, Watanabe G. Surgical treatment of primary cardiac tumors: 28 years' experience in Kanazawa University Hospital. Jpn Circ J 2001;65(4):315–9. [DOI] [PubMed]
- 6.Reynen K. Cardiac myxomas. N Engl J Med 1995;333(24): 1610–7. [DOI] [PubMed]
- 7.Bjessmo S, Ivert T. Cardiac myxoma: 40 years' experience in 63 patients. Ann Thorac Surg 1997;63(3):697–700. [DOI] [PubMed]
- 8.Tansel T, Harmandar B, Ugurlucan M, Nisli K, Eker R, Sozen AB, et al. Over 14 years of experience with cardiac myxomas. Acta Cardiol 2006;61(3):285–8. [DOI] [PubMed]
- 9.Braun S, Schrotter H, Reynen K, Schwencke C, Strasser RH. Myocardial infarction as complication of left atrial myxoma. Int J Cardiol 2005;101(1):115–21. [DOI] [PubMed]
- 10.Pinede L, Duhaut P, Loire R. Clinical presentation of left atrial cardiac myxoma. A series of 112 consecutive cases. Medicine (Baltimore) 2001;80(3):159–72. [DOI] [PubMed]
- 11.Wold LE, Lie JT. Cardiac myxomas: a clinicopathologic profile. Am J Pathol 1980;101(1):219–40. [PMC free article] [PubMed]
- 12.Bekavac I, Hanna JP, Wallace RC, Powers J, Ratliff NB, Furlan AJ. Intra-arterial thrombolysis of embolic proximal middle cerebral artery occlusion from presumed atrial myxoma. Neurology 1997;49(2):618–20. [DOI] [PubMed]
- 13.Yamanome T, Yoshida K, Miura K, Ogawa A. Superselective fibrinolysis for a middle cerebral artery embolism caused by a left atrial myxoma: case report [in Japanese]. No Shinkei Geka 2000;28(7):653–8. [PubMed]
- 14.Nurkalem Z, Uslu N, Gorgulu S, Yilmaz M, Ozkara S, Eren M. An interatrial mass supplied from dual coronary circulation. Int J Cardiol 2006;113(3):E76–8. [DOI] [PubMed]
- 15.Keeling IM, Oberwalder P, Anelli-Monti M, Schuchlenz H, Demel U, Tilz GP, et al. Cardiac myxomas: 24 years of experience in 49 patients. Eur J Cardiothorac Surg 2002;22(6): 971–7. [DOI] [PubMed]
- 16.Selkane C, Amahzoune B, Chavanis N, Raisky O, Robin J, Ninet J, Obadia JF. Changing management of cardiac myxoma based on a series of 40 cases with long-term follow-up. Ann Thorac Surg 2003;76(6):1935–8. [DOI] [PubMed]
- 17.Cina SJ, Smialek JE, Burke AP, Virmani R, Hutchins GM. Primary cardiac tumors causing sudden death: a review of the literature. Am J Forensic Med Pathol 1996;17(4):271–81. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


