Abstract
We analyzed the postoperative short- and mid-term outcomes of a series of patients with annuloaortic ectasia who underwent a modified Bentall operation in our clinic from September 2000 through March 2006.
The study included 44 patients. Their average age was 53.4 ± 14.1 years. The underlying disease was degenerative aortic aneurysm in 42 patients (95.5%) and acute aortic dissection in 2 patients (4.5%). Six patients (13.6%) had Marfan phenotype. Aortic insufficiency was moderate in 30 patients (68.2%) and severe in 14 patients (31.8%).
In our modification of the Bentall technique, we completed the resection of the aortic root while leaving 5 to 10 mm of native aortic wall tissue to support the anastomosis. A long piece of Teflon felt (width, 0.5–1 cm) was laid on the annulus, and nonpledgeted 2–0 polyester sutures were passed in turn through the Teflon felt, the preserved aortic tissue, and the aortic annulus. A thin piece of Teflon felt was also used in the coronary artery reimplantation sites. Fibrin glue was routinely applied to all anastomoses.
There were no intraoperative deaths. One patient died in the hospital after surgery for acute type I aortic dissection. Another patient died 1 year after the operation from prosthetic-valve endocarditis. No patient required surgical correction of excessive postoperative bleeding. Kaplan-Meier curves showed overall survival of 0.94 (95% confidence intervals, 0.9–0.99).
We consider our approach an easy, effective way to minimize bleeding from the anastomoses and at the aortic root—a common challenge in aortic surgery.
Key words: Anastomosis, surgical; aneurysm, dissecting/complications/surgery; aorta, thoracic/surgery; aortic valve insufficiency/surgery; cardiac surgical procedures/methods; coronary vessels/surgery; heart valve prosthesis implantation/methods; retrospective studies; survival analysis; suture techniques; treatment outcome
The surgical correction of aortic root aneurysms was first described by Bentall and De Bono1 in 1968; later, modifications were reported by Cabrol2 and Kouchoukos3 and their associates. Aortic root surgery is the procedure most frequently performed to correct thoracic aortic disorders.4 In early series, the hospital mortality rate for the procedure was relatively high.5 The incidence of serious postoperative hemorrhage has been reduced by increased operative experience, preclotting of grafts with albumin, improvements in pump oxygenator systems, and accurate heparin/protamin titration3; excellent results have been reported.3,6,7
Positive outcomes of modified Bentall procedures include the prevention of excessive bleeding, less frequent development of pseudoaneurysms, reduction of tension on coronary button anastomoses, and complete restoration of the aortic root, with low morbidity and mortality rates.8–10
Herein, we review the outcomes of 44 patients who underwent modified Bentall operations in our clinic, and we discuss our surgical technique. A chief element of our technique involves a single Teflon modification to reduce bleeding at the aortic root.
Patients and Methods
From September 2000 through March 2006, 44 consecutive patients underwent modified Bentall operations for aortic aneurysm or dissection. Using a standard protocol (with some evolution in the approach to arterial cannulation), the same surgical team operated on all 44 patients. The study data were collected retrospectively from operative records and from early and mid-term postoperative documentation.
The average age of the patients was 53.4 ± 14.1 years (range, 21–71 yr). The underlying disease was degenerative aortic aneurysm in 42 patients (95.4%) and acute aortic dissection in 2 patients (4.5%). Six patients (13.6%) had the Marfan phenotype. Aortic insufficiency was moderate in 30 patients (68.2%) and severe in 14 patients (31.8%). Preoperative risk factors are shown in Table I; Table II shows the aortic and cardiac diseases of the patients.
TABLE I. Preoperative Risk Factors of the 44 Patients
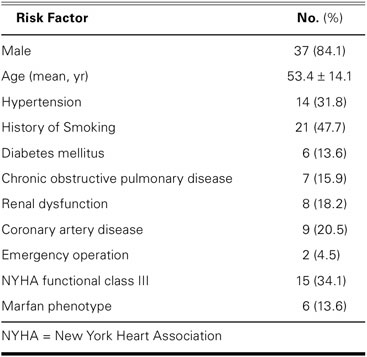
TABLE II. Preoperative Aortic and Cardiac Disease of the Patients

Surgical Technique
All patients underwent median sternotomy. At the outset of our series, systemic heparinization was followed by cannulation of either the ascending aorta or the femoral artery; toward the end, our standard approach for arterial cannulation was to use the axillary artery instead of the femoral artery. The right atrium was routinely used for venous cannulation. Except in the patients who were placed under hypothermic circulatory arrest, the ascending aorta was clamped just below the brachiocephalic artery, and the operations were performed with the patients under hypothermia at 32 °C rectal temperature. During the cooling period, cold-blood cardioplegic solution was administered antegrade directly through the coronary ostia. Myocardial protection was maintained by intermittent administration of cold cardioplegic solution retrograde through the coronary sinus. The right superior pulmonary vein was used to vent and decompress the left heart. A Dacron composite graft with a mechanical heart valve (St. Jude Medical, Inc.; St. Paul, Minn) was used in all patients. Valsalva modification of the graft was not performed.
The ascending aorta and the aortic valve leaflets were resected in a fashion that left approximately 5 to 10 mm of native aortic tissue, and the coronary buttons were prepared. A long piece of polytetrafluoroethylene felt (IMPRA, Inc., a subsidiary of C.R. Bard, Inc.; Tempe, Ariz), about 1 cm wide, was placed all around the aortic annulus (Fig. 1). The composite graft was sutured to the annulus with nonpledgeted Atramat® premium tapering 2–0 polyester sutures (Internacional Farmacéutica S.A. de C.V. [IFSA]; Coyoacan, Mexico), which were passed in turn through the Teflon, the native aortic tissue, and the prosthetic aortic annulus. The left and then the right coronary button was anastomosed—in an end-to-side fashion with running 5–0 polypropylene suture (IFSA)—to openings that had been prepared on the composite graft. For the additional control of bleeding, a long piece of Teflon, about 2 mm thick, was used circumferentially on each coronary button anastomosis. In addition, fibrin glue was routinely applied to all anastomoses. The distal end of the tubular graft was closed manually, and a cardioplegia cannula was inserted through a small opening. The aortic root and the coronary button anastomoses were checked for possible bleeding, under high-pressure administration of cardioplegic solution. The composite graft was then measured and cut to the appropriate length; under aortic cross-clamping, the distal anastomosis was performed with the use of a running 3–0 Premilene® polypropylene suture (Aesculap AG & Co. KG, a division of B. Braun Melsungen AG; Tuttlingen, Germany) in an end-to-end fashion, with the support of Teflon felt. Each patient was rewarmed, air was evacuated through the aortic graft, and cardiopulmonary bypass (CPB) was terminated.
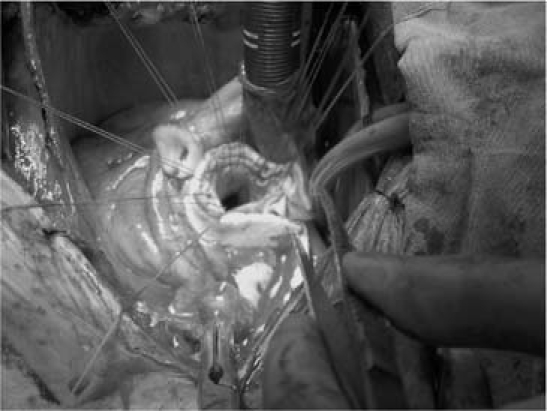
Fig. 1 Intraoperative photograph shows the use of a single piece of Teflon felt in the aortic root to reduce bleeding.
Intraoperative Characteristics
Every patient received a Dacron composite graft with a mechanical valve. The most commonly used graft size was 25 mm (20 patients, 45.4%). Thirteen patients (29.5%) were cannulated via the femoral artery; in 26 patients (59.1%), the ascending aorta was used for arterial cannulation. Late in our series, antegrade perfusion by axillary artery cannulation was used in 5 patients (11.4%). Hypothermic circulatory arrest was performed in 8 patients (18.2%); all other operations were performed with use of CPB (36 patients, 81.8%). The average aortic cross-clamp time was 108.1 ± 24.3 min; the average time on CPB, 132.3 ± 28.3 min. All of the patients received oral warfarin for anticoagulation (target international normalized ratio, 2.0–2.5).
Statistical Analysis
Statistical analysis was performed by use of the Statistical Package for the Social Sciences, version 10.0 (SPSS Inc.; Chicago, Ill). All results were expressed as mean ± SD. Values of P <0.05 were considered statistically significant. Analysis of the actuarial survival curve was performed by use of the Kaplan-Meier estimation for freedom from death. The Kaplan-Meier curves showed overall survival of 0.94 (95% confidence intervals, 0.9–0.99).
Results
Short-Term Postoperative Outcome
One patient, who had acute aortic dissection and presented with rupture, died in the hospital of multi-organ failure on the 4th postoperative day (mortality rate, 2.3%).
No patient required additional surgery to correct excessive bleeding. The average volume of postoperative drainage was 790.4 ± 277.6 cc. The average intubation time was 10.2 ± 3 hr. Twenty-five patients (56.8%) were discharged from the surgical intensive care unit on the 1st postoperative day, and 16 (36.4%) were discharged on the 2nd day. Only 3 patients (6.8%) needed more time in the intensive care unit because of hemodynamic or respiratory problems. The average hospital stay was 10 ± 5.3 days.
Follow-Up of the Surviving Patients
The mean estimated follow-up time was 57.7 months. Survival status was determined by telephone contact or upon a patient's visit to our surgical department. Periodic follow-up was conducted in all of the patients who survived hospitalization. Our follow-up schedule consisted of routine laboratory tests, chest radiography 1 week and 1 month after surgery, and annually performed echocardiography and computed tomography. Of the 43, only 1 patient (2.3%) died—1 year after the operation, from infective endocarditis. The overall survival rate was 94%. The actuarial survival of the hospital survivors is shown in Figure 2.

Fig. 2 The actuarial survival rate of hospital survivors (Kaplan-Meier curve).
Except for the death at 1 year from infective endocarditis, no late complications related to the operation occurred or were detected during follow-up. Routine computed tomographic and echocardiographic examinations of the patients showed no abnormalities, and no patient needed additional surgical intervention.
Discussion
Composite valve graft replacement is highly recommended as the preferred treatment for diseases that involve the aortic root, especially for annuloaortic ectasia or dissection. This operation is performed widely, with acceptable morbidity and mortality rates; however, associated bleeding, perioperative myocardial infarction, and right ventricular dysfunction still cause death.11
Emergency operation for acute aortic dissection was cited by Kouchoukos and colleagues12 as the only independent predictor of hospital death in aortic root surgery. Our patient who underwent surgery for acute type I aortic dissection died during the early postoperative period.
In 2 different series,3,7 prosthetic-valve endocarditis was the most common late sequela of composite-graft aortic root replacements; that condition usually occurs in 4% to 5% of patients within 14 to 17 years. Prosthetic-valve endocarditis occurred in 1 of our patients (2.3%) 1 year after the operation, which led to the only late death in our series.
The advantages of aortic root replacement with a composite graft are the elimination of all diseased aortic tissue and a well-defined operative technique with good results. The most frequent sequela of the Bentall operation is probably the development of a pseudoaneurysm at the coronary artery reimplantation site, due to coronary detachment from the composite graft.13 In our series, no patient developed a pseudoaneurysm. We attribute this to well-performed surgery on the aortic root and to the routine application of tissue adhesives.
Teflon reinforcement in the aortic root has been used by others in operations for acute type A aortic dissection. However, the Teflon felt was not used as an outer supportive layer; instead, the felt was placed between the dissected layers of the aorta to form a neo-intima.14 As we describe in our operative technique, we used Teflon strips and tissue adhesives in all 44 operations, and no patient required correction for excessive bleeding from the anastomoses or elsewhere.
To improve hemostasis, pericardial strip reinforcement has been used to buttress the inflow (annular) and outflow (ascending aortic) suture lines during free-standing root replacement (Ross operation). Luciani and coworkers15 considered the absence of pericardial strip buttressing to be an important predictor of autograft dilation. This reinforcement technique is also used during repair of ascending aortic aneurysms with aortic valve regurgitation when the stentless Freestyle® aortic bioprosthesis (Medtronic Inc.; Minneapolis, Minn) is used for aortic root replacement.16
Our surgical team's preferences regarding aortic cannulation have evolved. Our current approach is via the ascending aorta, if possible, or the axillary artery, because use of the femoral artery increases the risk of retrograde cerebral embolism from aortic debris. We do not interpose a graft; we directly cannulate the axillary artery and have not yet experienced any complications.
The suitability of patients with connective-tissue disease for valve-sparing operations remains controversial.17 The high risk of aneurysm formation or dissection obliges surgeons to perform an early operation and replace the entire aortic root. No randomized clinical trial has been conducted to compare the outcomes of valve-sparing operations with the outcomes of the Bentall procedure; however, retrospective studies of patients with Marfan syndrome suggest that the outcomes are similar.18 David and co-authors19 reported better long-term survival and freedom from morbid events with the reimplantation technique than with aortic root replacement that involves mechanical and tissue valves, and Birks and colleagues20 reported an 83% freedom from additional procedures over 10 years. Nevertheless, composite replacement of the aortic root with a biologic or mechanical valve may be a better choice, especially in Marfan patients who have stress fenestration or severe prolapse of cusps, which may negatively influence outcome after reconstruction.21
The recent interest in valve-sparing techniques notwithstanding, the Bentall operation is a well-defined, safe, and easy procedure for aortic root replacement. The use of Teflon will strengthen the aortic root and anastomotic sites. In particular, we recommend our single Teflon modification to further buttress the aortic root in order to avoid excessive bleeding. We consider our approach an easy, effective way to prevent bleeding from the anastomoses, which is a chief problem in aortic surgery.
Footnotes
Address for reprints: Onur Sokullu, MD, 66 Ada, Kardelen 3/3 No: 42, Atasehir, 34758 Kadikoy, Istanbul, Turkey. E-mail: onursokullu@gmail.com
References
- 1.Bentall H, De Bono A. A technique for complete replacement of the ascending aorta. Thorax 1968;23(4):338–9. [DOI] [PMC free article] [PubMed]
- 2.Cabrol C, Pavie A, Gandjbakhch I, Villemot JP, Guiraudon G, Laughlin L, et al. Complete replacement of the ascending aorta with reimplantation of the coronary arteries: new surgical approach. J Thorac Cardiovasc Surg 1981;81(2):309–15. [PubMed]
- 3.Kouchoukos NT, Wareing TH, Murphy SF, Perrillo JB. Sixteen-year experience with aortic root replacement. Results of 172 operations. Ann Surg 1991;214(3):308–20. [DOI] [PMC free article] [PubMed]
- 4.Gillum RF. Epidemiology of aortic aneurysm in the United States. J Clin Epidemiol 1995;48(11):1289–98. [DOI] [PubMed]
- 5.Aoyagi S, Kosuga K, Akashi H, Oryoji A, Oishi K. Aortic root replacement with a composite graft: results of 69 operations in 66 patients. Ann Thorac Surg 1994;58(5):1469–75. [DOI] [PubMed]
- 6.Baumgartner WA, Cameron DE, Redmond JM, Greene PS, Gott VL. Operative management of Marfan syndrome: the Johns Hopkins experience. Ann Thorac Surg 1999;67(6): 1859–60; discussion 1868–70. [DOI] [PubMed]
- 7.Gott VL, Gillinov AM, Pyeritz RE, Cameron DE, Reitz BA, Greene PS, et al. Aortic root replacement. Risk factor analysis of a seventeen-year experience with 270 patients. J Thorac Cardiovasc Surg 1995;109(3):536–45. [DOI] [PubMed]
- 8.Ehrlich MP, Ergin MA, McCullough JN, Lansman SL, Galla JD, Bodian CA, Griepp RB. Favorable outcome after composite valve-graft replacement in patients older than 65 years. Ann Thorac Surg 2001;71(5):1454–9. [DOI] [PubMed]
- 9.Westaby S, Katsumata T, Vaccari G. Aortic root replacement with coronary button re-implantation: low risk and predictable outcome. Eur J Cardiothorac Surg 2000;17(3):259–65. [DOI] [PubMed]
- 10.Dossche KM, Schepens MA, Morshuis WJ, de la Riviere AB, Knaepen PJ, Vermeulen FE. A 23-year experience with composite valve graft replacement of the aortic root. Ann Thorac Surg 1999;67(4):1070–7. [DOI] [PubMed]
- 11.Kirali K, Mansuroglu D, Omeroglu SN, Erentug V, Mataraci I, Ipek G, et al. Five-year experience in aortic root replacement with the flanged composite graft. Ann Thorac Surg 2002;73 (4):1130–7. [DOI] [PubMed]
- 12.Kouchoukos NT, Marshall WG Jr, Wedige-Stecher TA. Eleven-year experience with composite graft replacement of the ascending aorta and aortic valve. J Thorac Cardiovasc Surg 1986;92(4):691–705. [PubMed]
- 13.Svensson LG, Crawford ES, Hess KR, Coselli JS, Safi HJ. Composite valve graft replacement of the proximal aorta: comparison of techniques in 348 patients. Ann Thorac Surg 1992;54(3):427–37; discussion 438–9. [DOI] [PubMed]
- 14.Bavaria JE, Pochettino A, Brinster DR, Gorman RC, McGarvey ML, Gorman JH, et al. New paradigms and improved results for the surgical treatment of acute type A dissection. Ann Surg 2001;234(3):336–43. [DOI] [PMC free article] [PubMed]
- 15.Luciani GB, Favaro A, Casali G, Santini F, Mazzucco A. Ross operation in the young: a ten-year experience. Ann Thorac Surg 2005;80(6):2271–7. [DOI] [PubMed]
- 16.Akpinar B, Guden M, Aytekin S, Sanisoglu I, Sagbas E, Ozbek U, et al. The use of stentless valves for root replacement during repair of ascending aortic aneurysms with aortic valve regurgitation. Heart Surg Forum 2002;5(1):52–6. [PubMed]
- 17.Hagl C, Strauch JT, Spielvogel D, Galla JD, Lansman SL, Squitieri R, et al. Is the Bentall procedure for ascending aorta or aortic valve replacement the best approach for long-term event-free survival? Ann Thorac Surg 2003;76(3):698–703. [DOI] [PubMed]
- 18.Karck M, Kallenbach K, Hagl C, Rhein C, Leyh R, Haverich A. Aortic root surgery in Marfan syndrome: comparison of aortic valve-sparing reimplantation versus composite grafting. J Thorac Cardiovasc Surg 2004;127(2):391–8. [DOI] [PubMed]
- 19.David TE, Feindel CM, Webb GD, Colman JM, Armstrong S, Maganti M. Aortic valve preservation in patients with aortic root aneurysm: results of the reimplantation technique. Ann Thorac Surg 2007;83(2):S732–5; discussion S785–90. [DOI] [PubMed]
- 20.Birks EJ, Webb C, Child A, Radley-Smith R, Yacoub MH. Early and long-term results of a valve-sparing operation for Marfan syndrome. Circulation 1999;100(19 Suppl):II29–35. [DOI] [PubMed]
- 21.Kallenbach K, Baraki H, Khaladj N, Kamiya H, Hagl C, Haverich A, Karck M. Aortic valve-sparing operation in Marfan syndrome: what do we know after a decade? Ann Thorac Surg 2007;83(2):S764–8; discussion S785–90. [DOI] [PubMed]


