Abstract
Pseudoaneurysm formation is a rarely reported phenomenon after percutaneous coronary intervention. The natural course and clinical complications of coronary pseudoaneurysms are not well described, and the possible contribution of drug-eluting stents to the formation of coronary artery pseudoaneurysms is ill defined. Herein, we describe the case of a patient who experienced pseudoaneurysm formation 1 month after deployment of a paclitaxel-eluting stent. Healing was delayed, and there was resolution after 2 years of follow-up.
Key words: Aneurysm, false/complications/diagnosis/etiology/ultrasonography; angioplasty, transluminal, percutaneous coronary; blood vessel prosthesis implantation/adverse effects; coronary aneurysm/diagnosis/etiology/pathology/therapy; coronary vessels/pathology; incidence; paclitaxel/adverse effects; remission, spontaneous; stents/adverse effects; treatment outcome
The development of a coronary pseudoaneurysm after stent deployment is rarely reported, and little is known regarding the prognosis. When a drug-eluting stent (DES) is used, this complication poses a potentially serious problem due to the inhibitory effect of the drug on healing within the surrounding vascular tissue. Some investigators have suggested that a DES can pose an inherent danger in that it delays or prevents vascular healing and might cause a hypersensitivity reaction at the site of contact with the coronary endothelium.1–3 Pseudoaneurysms, of course, can lead to such adverse outcomes as thrombosis with distal embolization, rupture, and cardiac tamponade.4–6 Herein, we report a case of pseudoaneurysm formation 1 month after the deployment of a paclitaxel-eluting stent (TAXUS™, Boston Scientific Corporation Natick, Mass) in a proximal left anterior descending coronary artery (LAD) lesion, with delayed healing over the next 2 years.
Case Report
In June 2005, a 49-year-old man presented with unstable angina after a 6-month history of exertional chest pain. His medical history included obesity, dyslipidemia, hypertension, smoking, and a significant family history of early-onset coronary artery disease.
Transthoracic echocardiography showed normal left ventricular systolic function with no remarkable structural abnormalities. Coronary angiography revealed a focal stenosis in the proximal LAD for which intervention was deemed necessary (Fig. 1). The lesion was predilated with a Maverick® 3.0 × 9-mm balloon (Boston Scientific/Scimed; Maple Grove, Minn) to 6 atm, after which a 3.0 × 16-mm paclitaxel-eluting stent was deployed at a maximal pressure of 18 atm with no residual stenosis.
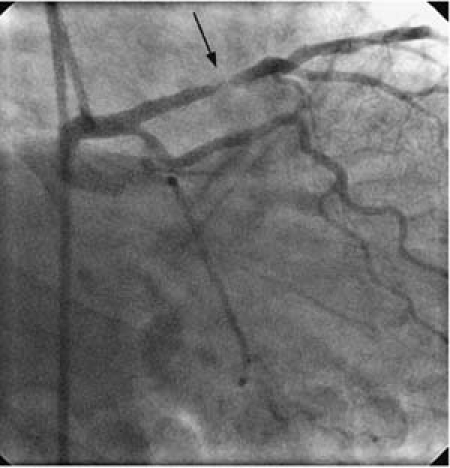
Fig. 1 Coronary angiography (right anterior oblique caudal view) shows a lesion in the proximal left anterior descending coronary artery (arrow).
The patient experienced recurrent chest pain 1 month after the initial percutaneous coronary intervention (PCI), and he was taken for repeat coronary angiography. The LAD stent remained widely patent. However, just outside of the mid-portion of the stent was a small contrast collection, which appeared to reveal a pseudoaneurysm (Fig. 2A). This diagnosis was suspected because of the unusual shape of the contrast collection, which was consistent with the mechanism of focal dissection that typically results from PCI. Subsequent cardiac computed tomography showed a 3 × 2-mm pseudoaneurysm at the mid-portion of the stent (Fig. 3).
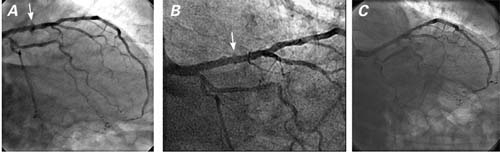
Fig. 2 Coronary angiography (right anterior oblique caudal view) shows the left anterior descending coronary artery A) 1 month after stent placement (arrow denotes pseudoaneurysm in the proximal segment), B) 9 months after percutaneous coronary intervention, and C) at the patient's 2-year follow-up examination.
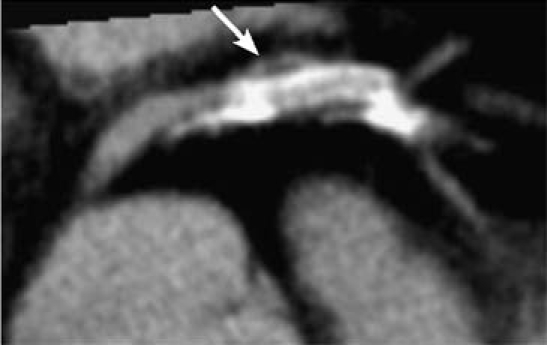
Fig. 3 Axial computed tomographic image of the proximal left anterior descending coronary artery, with pseudoaneurysm (arrow) at the mid-segment of the stented vessel.
During a 2-year period of follow-up, the patient's medical regimen included aspirin, clopidogrel, simvastatin, and metoprolol. Eight months after the initial DES placement, coronary angiography disclosed minimal residual luminal irregularities at the stented segment and near-resolution of the pseudoaneurysm (Fig. 2B). Coronary angiography 2 years after the stent deployment revealed resolution of the pseudoaneurysm (Fig. 2C).
Discussion
Due to the inherent limitations of coronary angiography, pseudoaneurysms that form after PCI are usually overlooked, or they are misdiagnosed as true aneurysms.7 Only a few cases have been reported.8 The incidence of formation of coronary artery aneurysms is 2% to 10% after PCI.9 However, the incidence and natural history of coronary artery pseudoaneurysms after PCI are not well known by coronary angiography alone.10,11 Pseudoaneurysm formation is more commonly a result of traumatic rupture or deep-vessel injury of an artery. The fibrous wall of a pseudoaneurysm forms a continuum with the structure of the adjacent vascular wall but lacks 1 or more of the wall's vascular layers (tunica intima, tunica media, and adventitia). It has been suggested that pseudoaneurysms can progressively enlarge and rupture.12
The role of DES in the formation of coronary aneurysms and pseudoaneurysms remains controversial. Sirolimus has been associated with increased apoptosis in the vascular wall in animal models.1 Higher rates of incomplete stent apposition are associated with sirolimus-eluting stents than with bare-metal stents.2 This suggests that sirolimus-eluting stents inhibit the healing of vessels in cases of large, unsealed arterial dissection. Further, Stabile and associates3 suggest a patient-specific sensitivity to rapamycin (sirolimus) as a cause of aneurysmal dilation and incomplete apposition. In contrast, Wong and colleagues13 reported success in the deployment of sirolimus-eluting stents for complex coronary dissection and appropriate lumen-sealing, both immediately and after 2 months.
The natural history of DES-related pseudoaneurysms has not been clearly defined. Paclitaxel, originally isolated from the Pacific yew tree, Taxus brevifolia, has been used as an antineoplastic agent under the brand name Taxol® (Bristol-Myers Squibb; New York, NY). Its efficacy in reducing neointimal proliferation and restenosis is due to its unique mechanism of action in promoting the assembly of tubulin into extraordinarily stable microtubules, thereby interrupting cellular proliferation, migration, and signal transduction.14 These inhibitory cellular mechanisms have been troubling in the presence of coronary aneurysms. Experiences with sirolimus-eluting stents and the resultant data suggest that paclitaxel-eluting stents could contribute similarly to the late positive remodeling (re-endotheliazation) of aneurysmal or pseudoaneurysmal vascular walls. However, a recent report by Kim and co-authors15 showed spontaneous resolution, at 9-month follow-up, of a neoaneurysm of the coronary artery after paclitaxel-eluting stent implantation. In our patient, the longer delayed healing compared with that previously reported may be attributed to differences in atheroma composition and degree of dissection, in addition to differences in the kinetics of drug elution and inflammation.16 Intravascular ultrasonographic studies have identified factors such as atheroma burden, stent malapposition, and stent dissection that have contributed to the development of pseudoaneurysm. In our patient, we hypothesize that a non-angiographically visible dissection provoked this phenomenon, with resultant thrombosis and negative remodeling over time. To our knowledge, this is the 2nd reported case in the medical literature of pseudoaneurysm formation after TAXUS™ stent implantation, and the 1st report of resolution delayed until the 2-year follow-up.
The case of our patient shows that delayed luminal healing can occur after the formation of a small pseudoaneurysm consequent to the deployment of a paclitaxel-eluting stent, and that the condition can be treated conservatively, with spontaneous resolution expected over time.
Editorial Commentary
The newer drug-eluting coronary stents (DESs) incorporate on their surface an antineoplastic agent within a timed-release polymer. These stents are shown to inhibit or prevent neointimal hyperplasia after placement, thereby reducing restenosis rates to less than 10% across a wide spectrum of clinical and angiographic subsets. Although DESs were initially approved for use in patients in stable condition who had relatively simple coronary lesions, their use has rapidly expanded to more complex coronary lesions and to acute clinical settings.
Although hyperplasia of smooth muscle cells from the arterial medial layers is blocked, recent patho-anatomic studies have raised concerns about incomplete neointimal coverage by endothelial cells and other “healing” cells, with perhaps deleterious consequences in at least some patients. Metal allergy (to nickel or molybdenum) exists during the use of all current stents, but DESs incur the further risk of eosinophilic or heterophilic infiltrates that develop in response to the polymer materials. The combination of vascular injury and dissection, together with delayed healing and hypersensitivity reactions, renders DES more likely than bare-metal stents to promote coronary aneurysm formation. Although this explanation was also advanced to explain the phenomenon of late stent thrombosis, the incidence of late thrombosis now appears to be equivalent for bare-metal stents and DESs; therefore, this view has softened somewhat. Nevertheless, caution is warranted before this explanation is too hastily discarded.
The case report by Chen and colleagues1 points out another important unanswered question—the risk of coronary aneurysm (or pseudoaneurysm) formation after DES implantation.2 Second to stent thrombosis, stent aneurysm is a concern when the long-term safety of DESs is being considered, and one can foresee a parallel trajectory of interest.
According to results from randomized trials,3,4 the incidence of aneurysm formation after DES implantation is low within the first 9 months, with frequencies ranging from 0.2% to 2.3%—a rate similar to that reported after bare-metal stent implantation (0.3%–3.9%). However, beyond those trials, the overall frequency of coronary artery aneurysms after DES implantation is still largely unknown. Only 2 abstracts presented so far have suggested that occurrence rates are low.5,6
When considering this parallel between aneurysm formation and stent thrombosis, one can anticipate some questions. What exactly is a coronary aneurysm? Luminal dilation of more than 20%, or more than 50%, are commonly used cutoffs, but why? The absence of a uniform definition throughout the medical literature and the clinical trials renders any conclusions extremely premature. As for treatment, coronary-stent aneurysms offer more evidence that medicine continues to be both art and science, since most treatment decisions are made on a case-by-case “best clinical judgment” basis. Given the rarity of overall aneurysmal events thus far, and the lack of published data, treatment approaches must be tailored to each patient. Instead of choosing a traditional interventional approach (surgery or coiling), Chen and colleagues1 opted—with success—to observe the condition without applying specific treatment. In their patient, the natural evolution of the aneurysm was toward healing. Whether this is usual is not known.
Because the natural history of coronary-stent aneurysms is almost completely unexplored, more data are needed. Toward this end, clinicians should be encouraged to report aneurysmal occurrences, and keepers of registries and databases encouraged to explore the information that is available to them. While it is to be hoped that unaided healing is the norm, this outcome and its frequency will have to be documented in more complete detail before the caution flag is lifted.
Cezar Iliescu, MD
Cardiology Department, University of Texas M.D. Anderson Cancer Center, Houston
H. Vernon Anderson, MD
Cardiology Division, University of Texas Health Science Center, Houston
References
- 1.Chen D, Chang R, Ho AT, Frivold G, Foster G. Spontaneous resolution of coronary artery pseudoaneurysm after percutaneous intervention with paclitaxel-eluting stent. Tex Heart Inst J 2008;35(2):189–91. [PMC free article] [PubMed]
- 2.Maisel WH. Unanswered questions—drug-eluting stents and the risk of late thrombosis. N Engl J Med 2007;356(10): 981–4. [DOI] [PubMed]
- 3.Aoki J, Kirtane A, Leon MB, Dangas G. Coronary artery aneurysms after drug-eluting stent implantation. J Am Coll Cardiol Intv 2008;1:14–21. [DOI] [PubMed]
- 4.Slota PA, Fischman DL, Savage MP, Rake R, Goldberg S. Frequency and outcome of development of coronary artery aneurysm after intracoronary stent placement and angioplasty. STRESS Trial Investigators. Am J Cardiol 1997;79(8): 1104–6. [DOI] [PubMed]
- 5.Kachru R, Gupta R, Sapra RR, Kumar N, Jetley V, Yadave R, Kaul U. Coronary aneurysms after drug eluting stent implantation—an unusual and potentially life threatening complication [abstract]. Am J Cardiol 2005;96 Suppl 7A:172H.
- 6.Rha SW, Suh SY, Chwe UY, Kim JW, Park CG, Seo HS, Oh DJ. Incidence and characteristics of coronary aneurysm after DES implantation [abstract]. Am J Cardiol 2005;96 Suppl 7A:170H.
Footnotes
Address for reprints: Gary Foster, MD, Division of Cardiology, VA Loma Linda Healthcare System, 11201 Benton St., Loma Linda, CA 92357. E-mail: gary.foster2@va.gov
References
- 1.Roque M, Cordon-Cardo C, Fuster V, Reis ED, Drobnjak M, Badimon JJ. Modulation of apoptosis, proliferation, and p27 expression in a porcine coronary angioplasty model. Atherosclerosis 2000;153(2):315–22. [DOI] [PubMed]
- 2.Serruys PW, Degertekin M, Tanabe K, Abizaid A, Sousa JE, Colombo A, et al. Intravascular ultrasound findings in the multicenter, randomized, double-blind RAVEL (RAndomized study with the sirolimus-eluting VElocity balloon-expandable stent in the treatment of patients with de novo native coronary artery Lesions) trial. Circulation 2002;106(7):798–803. [DOI] [PubMed]
- 3.Stabile E, Escolar E, Weigold G, Weissman NJ, Satler LF, Pichard AD, et al. Marked malapposition and aneurysm formation after sirolimus-eluting coronary stent implantation. Circulation 2004;110(5):e47–8. [DOI] [PubMed]
- 4.Wang KY, Ting CT, St John Sutton M, Chen YT. Coronary artery aneurysms: A 25-patient study. Catheter Cardiovasc Interv 1999;48(1):31–8. [DOI] [PubMed]
- 5.LaMotte LC, Mathur VS. Atherosclerotic coronary artery aneurysms: eight-year angiographic follow-up. Tex Heart Inst J 2000;27(1):72–3. [PMC free article] [PubMed]
- 6.Baman TS, Cole JH, Devireddy CM, Sperling LS. Risk factors and outcomes in patients with coronary artery aneurysms. Am J Cardiol 2004;93(12):1549–51. [DOI] [PubMed]
- 7.Aqel RA, Zoghbi GJ, Iskandrian AE. Spontaneous coronary artery dissection with pseudoaneurysm formation diagnosed by intravascular ultrasound: a case report. Echocardiography 2004;21(2):153–7. [DOI] [PubMed]
- 8.Berkalp B, Kervancioglu C, Oral D. Coronary artery aneurysm formation after balloon angioplasty and stent implantation. Int J Cardiol 1999;69(1):65–70. [DOI] [PubMed]
- 9.Slota PA, Fischman DL, Savage MP, Rake R, Goldberg S. Frequency and outcome of development of coronary artery aneurysm after intracoronary stent placement and angioplasty. STRESS Trial Investigators. Am J Cardiol 1997;79(8): 1104–6. [DOI] [PubMed]
- 10.Garrand TJ, Mintz GS, Popma JJ, Lewis SA, Vaughn NA, Leon MB. Intravascular ultrasound diagnosis of a coronary artery pseudoaneurysm following percutaneous transluminal coronary angioplasty. Am Heart J 1993;125(3):880–2. [PubMed]
- 11.Rodriguez O, Baim DS. Coronary aneurysms after catheter interventions: an exception to “bigger is better”. Cathet Cardiovasc Diagn 1997;41(4):411–2. [DOI] [PubMed]
- 12.Regar E, Klauss V, Henneke KH, Werner F, Theisen K, Mudra H. Coronary aneurysm after bailout stent implantation: diagnosis of a false lumen with intravascular ultrasound. Cathet Cardiovasc Diagn 1997;41(4):407–10. [DOI] [PubMed]
- 13.Wong EM, Pawsey C, Lowe HC. Evidence for “lumen sealing” with sirolimus eluting stents in the treatment of complex coronary artery dissection. Heart 2004;90(3):e13. [DOI] [PMC free article] [PubMed]
- 14.Axel DI, Kunert W, Goggelmann C, Oberhoff M, Herdeg C, Kuttner A, et al. Paclitaxel inhibits arterial smooth muscle cell proliferation and migration in vitro and in vivo using local drug delivery. Circulation 1997;96(2):636–45. [DOI] [PubMed]
- 15.Kim JW, Seo HS, Suh SY, Rha SW, Park CG, Oh DJ. Spontaneous resolution of neoaneurysm following implantation of a paclitaxel-eluting coronary stent. Int J Cardiol 2006;112 (2):e12–3. [DOI] [PubMed]
- 16.Finn AV, Kolodgie FD, Harnek J, Guerrero LJ, Acampado E, Tefera K, et al. Differential response of delayed healing and persistent inflammation at sites of overlapping sirolimus- or paclitaxel-eluting stents. Circulation 2005;112(2):270–8. [DOI] [PubMed]


