ABSTRACT
Objective: The aim of this study was to determine the use and safety of the endoscope as an adjunct during trigeminal and facial nerve decompression procedures performed under keyhole conditions in the posterior fossa. Method: We performed 67 surgeries in 65 patients with symptomatic trigeminal and facial nerve compression syndromes. The diagnosis was made mainly on the basis of clinical history, examination, and magnetic resonance imaging scans. Surgery was performed in all cases under endoscope-assisted keyhole conditions. The follow-up was 1 week postoperatively, 6 months, and then yearly up to 7 years. All 34 patients with trigeminal neuralgia received preoperative medication treatment and experienced failure with it. Eighteen patients out of 30 with hemifacial spasm had been previously treated with botulinum toxin injections. One patient suffered from both trigeminal neuralgia and facial spasm, because of a megadolichobasilar and vertebral artery with compression of both cranial nerves. Results: Sixty-four of the 65 patients became symptom free after surgical treatment; one revision surgery was necessary because of disappearance of the decompression muscle piece. No mortalities or minor morbidities were observed in this series. Conclusion: A precise planned keyhole craniotomy and the simultaneous use of the microscope and the endoscope render the procedure of the decompression less traumatic.
Keywords: Trigeminal neuralgia, hemifacial spasm, endoscope-assisted neurosurgery, neurovascular compression syndromes
Trigeminal neuralgia, hemifacial spasm, glossopharyngeal neuralgia, tinnitus, and disabling positional vertigo have all been associated with vascular compression.1,2,3 In 1934, Dandy hypothesized that vascular compression of the trigeminal nerve by aberrant vessels of the posterior circulation may be responsible for this disorder, and ~30 years later Jannetta stated that compression and distortion of the root entry zone of the trigeminal nerve by aberrant venous and arterial structures were a causative factor in the etiology of trigeminal neuralgia.1,4 Microvascular decompression surgery involves separation of the compressive vessel from its contact point with the cranial nerve and interposition of a prosthesis to avoid further compression of the involved cranial nerve. Over the past 30 years, several large series of such surgeries have documented very good results with the use of the microscope as a single visualization tool with minimal morbidity.5,6,7,8,9 For this reason, microscopic vascular decompression became the gold standard for surgical treatment of neurovascular compression syndromes in the posterior fossa. The results in large series demonstrated a 70 to 95% success rate with minimal morbidity and almost no mortality.5,6,7,8,10,11,12 Furthermore, documented failures, recurrences, and complications inherent to this surgery and a drive toward less-invasive operations have led to a search for ways to improve and refine surgical outcomes by further reducing the morbidity of this procedure.13,14,15 Still, some failure after microscopic decompression might occur because of the tubular view of the operating microscope and the limitation of vision of the pathoanatomic situation in hidden areas.13,15
The introduction of the endoscope in neurosurgical procedures has brought a further new dimension into the field of intraoperative visualization.16,17 It provides, in contrast to the microscope, a panoramic view of the cerebellopontine angle (CPA) anatomy (especially with angled endoscopes) and shows exactly the differences between the pathological and the normal anatomy.18,19,20,21
In the present study we retrospectively analyzed the indication, the surgical technique, the outcome, and the role of the endoscope in 65 patients with neurovascular compression diseases of the trigeminal and facial nerve in the posterior fossa treated under endoscope-assisted keyhole microsurgical conditions in our department.
MATERIAL AND METHOD
Patient Population
At the Department of Neurosurgery, University of Mainz, Mainz, Germany, 67 surgeries were performed in 65 patients (33 female and 32 male patients) with symptomatic trigeminal and facial nerve compression syndromes, between January 2000 and December 2006. The most common symptoms were, respectively, trigeminal neuralgia and hemifacial spasm. The diagnosis was made mainly on the basis of clinical history, examination, and magnetic resonance imaging (MRI). The mean patient age at the date of surgery was 58 years (range, 38 to 77 years). In all patients, the target of the surgical therapy was to identify the neurovascular conflict and decompress the cranial nerve involved in this conflict. The follow-up was 1 week postoperatively, 6 months, and then yearly up to 7 years. The findings from the follow-up examinations were used to evaluate the results of the vascular decompression. Clinical outcome was recorded as “excellent” if complete absence of symptoms had occurred, “good” if an improvement of the symptoms was observed, and as “failure” if the preoperative clinical status has been unchanged or became worse.
All 34 patients with trigeminal neuralgia received preoperative medication treatment and experienced failure with it. Two patients underwent preoperative percutaneous radiofrequency lesioning. Eighteen patients out of 30 with hemifacial spasm had been previously treated with botulinum toxin injections. One patient suffered from both trigeminal neuralgia and facial spasm, because of a megadolichobasilar and vertebral artery that extended along the brainstem with compression of different cranial nerves.
The average hospital stay postoperatively for those patients was ~5 to 7 days.
Surgical Procedure
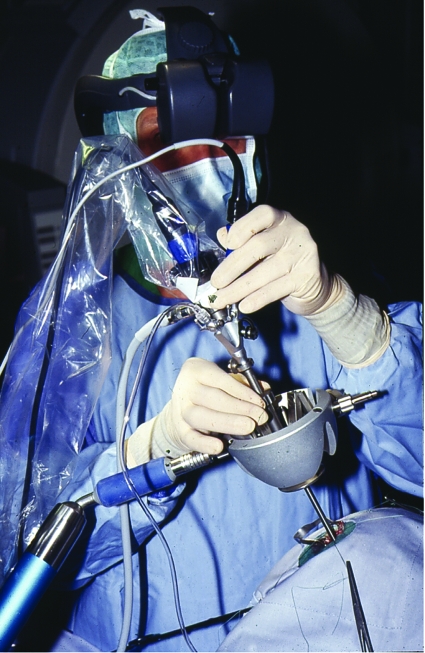
The surgical approaches were chosen individually for each patient according to the anatomic and pathological relationships that were based on the preoperative imaging. Precise planning of the surgical corridor, including skin incision, trephination, and exact surgical pathway toward the center of the neurovascular conflict, was performed according to the preoperative image data. In all patients, the surgery was performed under general anesthesia. The patient was positioned supine. The head was rotated to the contralateral site of the surgical field, and a fixation in the Mayfield clamp was almost never used. Depending on the location of the vascular compression and the craniotomy planning, the skin incision was retroauricular 3 to 4 cm long. Subsequently, a craniotomy was performed of ~1 to 1.5 cm in diameter (Fig. 1). The special approaches were selected individually for each patient according to the target point of interest. To reach the trigeminal nerve, a craniotomy close to the transverse and sigmoid sinus angle was performed with exposure of the caudal site of the sinuses. If the target point was vascular conflicts of the facial nerve, a more caudal approach was performed. After opening the dura, the CPA was explored by a 0-degree endoscope that gives a panoramic view of the pathoanatomic situation in the region of interest (Fig. 2A). The nerve root entry zone was then systematically inspected for nerve compression, using the microscope (Fig. 2B) as well as different angled endoscopes (30 or 45 degrees), allowing the identification of the precise location of the site of conflict—Meckel's cave (MC) or the internal auditory canal (IAC). Mobilization of the offending vessel and placement of a muscle piece between vessel and nerve were performed under the microscope or the endoscope (only by using Olympus Endo Arm [Olympus Co., Tokyo, Japan]) (Fig. 2C) to keep them separated. Before closure, a search with an angled (30 or 45 degrees) endoscope was performed to check the stability of the prosthesis and ensure complete decompression (Fig. 2D).
Figure 1.
Keyhole craniotomies ~1 to 1.5 cm in diameter were individually planned and performed.
Figure 2.
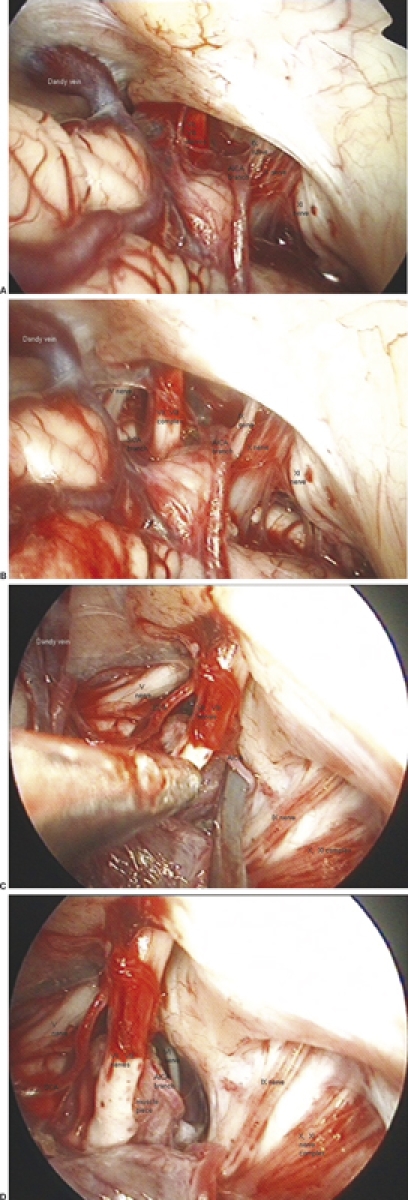
(A) After opening the dura, the cerebellopontine angle (CPA) was explored by a 0-degree endoscope that gave a panoramic view of the pathoanatomic situation in the region of interest. (B) Exploration of the pathology showing the facial nerve from its origin in the brainstem to the entry point in the internal auditory canal and the conflicting vessels, a superior cerebellar artery (SCA) branch compressing the nerve cranially and an anterior inferior cerebellar artery (AICA) branch compressing the nerve caudally. (C) Mobilization of the offending vessel and placement of a muscle piece between vessel and nerve. (D) Before dura closure, a search with an angled endoscope was performed to check the stability of the prosthesis and ensure complete decompression.
Surgical Equipment
ENDOSCOPES
Two types of endoscopes were used to perform the neurovascular decompression.
MINOP (Minimal Invasive Neurosurgical Operation technique) Endoscopes
The rigid endoscopes (Aesculap, Tuttlingen, Germany) used by the decompression surgery have a 0-degree, 30-degree, or 45-degree direction of view, 2.7-mm shaft diameter, lateral camera connection, and light source. An unobstructed view of the working area parallel to the shaft allows simultaneous use of the microscope and microinstruments. The endoscope either was held by the operating surgeon alone or by the assistant or was rigidly fixed to the operating table. As a holding device, we used the Unitrac (Aesculap, Tuttlingen, Germany); the optics used in this system are 0 degrees, 30 degrees, and 45 degrees. The Unitrac is a pneumatically operated universal holding device (Fig. 3).
Figure 3.
The Unitrac (Aesculap, Tuttlingen, Germany) is a pneumatically operated universal holding device. We used it to stabilize the endoscopes; the optics used in those systems were 0, 30, and 45 degrees.
Olympus Endo Arm
The Olympus Endo Arm (Olympus Co., Tokyo, Japan) counterbalance arm is designed to deliver smooth endoscope movement and steady fixation (Fig. 4A) by use of compressed nitrogen gas to control the arm movement. The shape of the stand differs from the other endoscopic devices in that the device is a bedside fixed design and subject device is a floor-stand type. This allows the surgeon to move the endoscope near the target site. The illumination is supplied by the neuroendoscope through the light guide cable connected to the separate high-intensity light source. Another significant difference from other endoscopes is the ability of the Endo Arm to manage image data from the neuroendoscope (like a microscope device) and allow the user to adjust zoom, focus, and rotate images (Fig. 4B). The image from the distal end of the neuroendoscope is transmitted to the monitor via a charge coupled device camera connected with a head-mounted liquid crystal display (LCD) screen before the surgeon'eyes (Fig. 4C).
Figure 4.
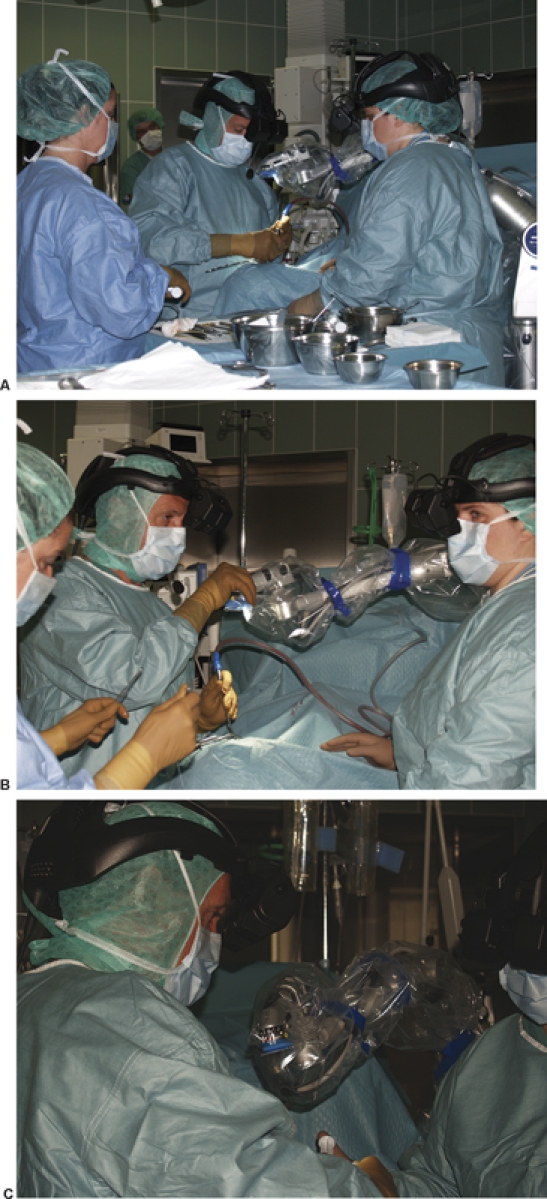
(A) The Olympus Endo Arm (Olympus Co., Tokyo, Japan) can be used as a microscopic device to illuminate the operating field (with focus, zoom, and image rotation) and as an endoscope placed inside on the surgical corridor. (B) The Endo Arm has the ability to manage image data from the neuroendoscope, like a microscopic device, and allow the user to adjust zoom, focus, and rotate the image. (C) The image from the distal end of the neuroendoscope is transmitted to the monitor via a charge coupled device camera connected with a head-mounted liquid crystal display (LCD) screen within the surgeon's view.
HEAD-MOUNTED LCD SCREEN
Using the endoscope as an additional viewing instrument during microscope-based microsurgery allows the use of two sets of visual information, microscopic and endoscopic, that must be integrated simultaneously by the surgeon to gain complete information about the operating field. The alternation of microscopic and endoscopic viewing and also the fusion of the microscopic and endoscopic pictures by special technical devices are the two possibilities to coordinate visual information.16 Therefore, to provide a simultaneous observation of both endoscopic and microscopic images, we displayed the images in a picture-in-picture mode on a screen placed in front of the surgeon.16,22 The best and most comfortable method to achieve this was to display both images (or only the endoscopic image without using video screen—Olympus Endo Arm) on a head-mounted LCD screen for simultaneous viewing (Figs. 3, 4).
RESULTS
We studied sixty-five patients with compression of cranial nerves in the posterior fossa who were decompressed by endoscope-assisted microsurgical keyhole conditions. Of these, 34 patients suffered from trigeminal neuralgia, 30 suffered from hemifacial spasm, and 1 suffered from both. We analyzed the results of the decompression surgery for each disease separately according to the compressive vessels, the behavior of symptoms after surgery, the complications during and after surgery, and the role of the endoscopy.
Trigeminal Neuralgia
Of 35 patients, 1 of them associated with hemifacial spasm, 3 patients felt pain in all three areas corresponding to the three divisions of the trigeminal nerve. Eleven patients felt pain in the V2 (maxillary) area alone, and 10 patients felt it in the V3 (mandibular) area. Several combinations between several areas were observed. Two patients felt pain in the areas of V1and V2, and 8 patients felt it in the V2 and V3 areas (Table 1). One patient suffered from pain in the areas of V2 and V3 associated with facial spasm (Table 1).
Table 1.
Distribution of Pain by Trigeminal Neuralgia
| Nerve Affected | Number of Cases |
|---|---|
| V-2 | 11 |
| V-3 | 10 |
| V1–3 | 3 |
| V1–2 | 2 |
| V2–3 | 8 |
| V2–3 and VII | 1 |
The superior cerebellar artery (SCA) alone as a compressive vessel was involved in 17 patients, and a vein alone was involved in 5 patients (Table 2). The SCA and vein were involved in 4 patients. The vertebral artery alone was involved in 1 case, the anterior inferior cerebellar artery (AICA) alone was involved in 1 case; a double existing SCA was the compression vessel in another 1 case. The AICA and SCA were involved in 3 cases. In 1 patient the AICA, vertebral artery, and posterior inferior cerebellar artery (PICA) were the conflict vessels. One patient suffered from a petrosal sinus fistula with a big superior petrosal vein compressed from above the trigeminal nerve. One patient showed clinically typical trigeminal neuralgia and on an MRI scan an elongated vertebral artery, but in the following surgery the conflict vessel was an elongated fusiform AICA. The site of conflict was at the root entry zone of the nerve in 28 patients and at Meckel's cave in 3 patients. In 2 patients both sides, the root entry zone and Meckel's cave, were affected by vessels. The patient who suffered from trigeminal neuralgia and hemifacial spasm has the site of conflict with the vessels on the root entry zone of both nerves V and VII. No cases showed intraoperative complications.
Table 2.
Vessels Involved in the Compression of Nerves V and VII
| Vessels | Vth Nerve | VIIth Nerve |
|---|---|---|
| SCA, superior cerebellar artery; AICA, anterior inferior cerebellar artery; PICA, posterior inferior cerebellar artery. | ||
| SCA | 17 | |
| Vein | 5 | 1 |
| SCA and vein | 4 | |
| SCA and AICA | 3 | |
| Double SCA | 1 | |
| Vertebral artery | 1 | 4 |
| AICA | 2 | 11 |
| PICA | 5 | |
| Vertebrobasilar complex | 1 | |
| AICA, PICA, vertebral artery | 1 | |
| Petrosal sinus fistula | 1 | |
| AICA and vein | 1 | |
| AICA and vertebral artery | 3 | |
| PICA and vertebral artery | 2 | |
| AICA and labyrinth artery | 2 | |
In 26 cases, the type of contact between the nerve and the vessel was simple because only one vessel compressed the nerve in one site. In 9 cases, it was a multiple type of contact because two or more vessels touched the nerve at different points.
At the first week after surgery, 32 patients showed complete absence of pain, 2 showed improvement of their conditions (the pain was reduced in intensity and in the daily number of attacks), and 1 showed unchanged pain relief.
After 6 months' follow-up, 34 of 35 patients (98%) showed complete absence of pain, and 1 (2%) unchanged pain relief (Table 3). In this patient the MRI scan showed disappearance of the decompression muscle piece. We performed a second surgery and the pain disappeared (Table 3).
Table 3.
Results of Endoscope-Assisted Microvascular Decompression According to Clinical Symptoms after 6 Months' Follow-Up
| Symptom | Excellent | Unchanged |
|---|---|---|
| Trigeminal neuralgia | 34 (98%) | 1 (2%) |
| Hemifacial spasm | 30 (100%) |
Postoperative complications were rare. One patient developed a cerebrospinal fluid (CSF) leak and was treated first conservatively with lumbar drainage. One week later, after failure of the drainage, a revision surgery was performed to close the fistula in the petrous bone (Table 4).
Table 4.
Postoperative Complications and Recurrence after Neurovascular Decompression
| Complication | Number of Cases |
|
|---|---|---|
| First Week after Surgery | 6 Months after Surgery | |
| Trigeminal neuralgia | ||
| CSF leak | 1 | |
| Recurrence (first treated in our department) | 1 | |
| Hemifacial spasm | ||
| Hypoacusis | 4 | 0 |
| Facial paralysis | 2 | 0 |
Preoperative MRI scans were performed and correlated with the intraoperative endoscopic findings in all patients. In 5 of 34 patients (15%) with trigeminal neuralgia where the MRI showed no evidence of vascular compression, the conflict was clearly identified by endoscopy. Therefore, endoscopy was successful in identifying a neurovascular conflict in all cases (100%).
Hemifacial Spasm
Out of 30 patients, clinically the origin of the hemifacial spasm was in the palpebral region in 20 patients, in the labial region in 6 patients, and in both regions in 4 patients. The spasm was associated with facial paralysis due to botulinum toxin injections in 7 cases. The mean duration of symptoms was 7 years. Eighteen patients received botulinum toxin injections before surgery, 9 received no treatment (neither conservative nor surgical), and 4 received decompression surgery (2 of those patients received two surgeries) in other hospitals.
The AICA alone as a compressive vessel was involved in 11 patients, and a vein alone was involved in 1 patient (Table 2). The AICA and vein were involved in 1 patient. The vertebral artery alone was involved in 4 cases, and in combination with the AICA in 3 cases.
The PICA alone was in five cases the conflict vessel and the vertebral artery and PICA in combination appeared in two cases. The AICA together with the labyrinth artery, as conflict vessels, were involved in two cases. The vertebrobasilar complex alone was responsible for facial nerve compression in one case.
The site of conflict was at the root entry zone of the nerve in 29 patients and at the IAC in 1 patient. In 14 cases, the type of contact between nerve and vessel was a simple type. In 16 cases, it was a multiple type. Intraoperative complications did not occur.
At the first week postoperatively, 29 patients showed complete absence of spasm, and 1 patient showed improvement of the conditions (the spasm was reduced in the daily number of attacks). After 6 months of follow-up, all 30 (100%) patients showed complete absence of spasm (Table 3).
Preoperative MRI scans were performed to correlate with the intraoperative endoscopic findings in all patients. In 6 of 30 patients (20%), where the MRI showed no evidence of vascular compression, the conflict was clearly identified by endoscopy. Therefore, endoscopy was successful in identifying a neurovascular conflict in all cases (100%).
Two patients, treated microscopically in other departments, underwent a second surgery and two others underwent a third surgery, in our department, after failure of the microscopic procedures. All patients showed complete cure of the spasms after the revision surgery performed in our department. Intraoperatively, we found total absence of the prosthesis in one case, and conflict vessels left unrecognized during the initial surgery in three cases.
In two cases, facial paralysis in the labial region occurred immediately postoperatively. In the 6-month follow-up, the paralysis was no longer observed. Five of seven patients with preoperative facial paralysis due to botulinum toxin injections recovered totally after the decompression surgery. Postoperative hearing loss was observed in four patients, caused by the passage of CSF in the mastoid cells during the surgery; the CSF absorbed rapidly and hearing returned to normal (Table 4).
DISCUSSION
Microscopic vascular decompression for various compression syndromes in the posterior fossa are today a routine procedure performed with very high safety and a low morbidity rate.6,9,15,23 Therefore, the question may arise whether there is an indication at all for the intraoperative use of endoscopes. Indeed, most of the microvascular decompressions may be performed without the endoscope-assisted technique. However, the complex syndromes of more than one vessel or cranial nerve involved in the conflict in the same patient due to a megadolichobasilar or megadolichovertebral artery may be treated in a safer fashion with the application of intraoperative endoscopy. Studies performed for various compression syndromes in the posterior fossa showed the importance and the high identification rate (almost 100%) of the conflicts with the use of different angled endoscopes for inspecting the pathoanatomic situation in the CPA.18,19,20,21 We showed in our study that the involvement of hidden parts of small arteries and especially veins in the compression conflict can be better visualized with an angled endoscope placed in front of the pathology. Angled endoscopes have the ability to look around the corner and inside of cavities like the Meckel's cave or the auditory canal to identify compression vessels, which cannot be seen with the tubular view of the operating microscope.
In our study, endoscopy of the CPA was able to identify in all cases a neurovascular conflict. In the literature, the percentage of microscopic conflict identification varied between 70% and 95%.6,23,24 Our results show that the capacity of endoscopy to detect a neurovascular conflict is higher than the best results obtained with the operating microscope.
Furthermore, we presented several cases with recurrent symptoms requiring reoperations. We again performed a microvascular decompression under endoscope-assisted conditions with the endoscope placed in front of the nerve entry zone and discovered a vessel loop that was not seen during the first surgery performed with the microscope. Furthermore, visualization behind big vessels, like the basilar or vertebral arteries, without retraction of the vessels, is with the endoscope a very safe procedure, which is not possible with the operating microscope. Therefore, in our department it is our policy to plan all microvascular decompressions as endoscope-assisted microsurgeries to avoid second-look surgeries due to hidden arterial loops. The use of the endoscope as a visualization tool in addition to the microscope in the daily routine in the past decades in our department led us to the development of the keyhole concept for a less traumatic approach to the CPA and the important neurovascular structures in the area. Keyhole surgery does not imply that the size of the craniotomy is a keyhole, but that the choice of the correct individual craniotomy has a key function to enter a particular intracranial room and to work there with minimum trauma and maximum effectiveness.16 The keyhole concept is defined by two principles: (1) the intracranial optical field widens with increasing distance from the keyhole, and (2) contralateral structures are well visualized.16 Therefore, lesions (compressed cranial nerves and aberrant vessels of the posterior fossa) located far away from the entry point (retrosigmoidal craniotomy) can be visualized through a small keyhole. At this point enters the big advantage of the endoscope: to bring light inside the CPA cavity to help the surgeon to see hidden areas not visible with the microscope. The illumination of the narrow surgical field may be poor due to a smaller craniotomy, but it can be increased by continuous adjustment of the microscope and the simultaneous use of the endoscope within the surgical field. In recent years, vascular decompression of cranial nerves in the posterior fossa has been more and more frequently performed under endoscope-assisted keyhole conditions. The additional visualization of the endoscope and the resulting panoramic view in the region of interest reveal difficult corners which cannot be seen with the operating microscope.18,19,20,21
The endoscope-assisted keyhole techniques enable a wide surgical corridor, according to the fisheye like widening of the visual field, as needed to enhance the neurosurgeon's ability to maneuver and simultaneously reduce the injury of normal brain tissue in the surrounding region. In this study, no intraoperative complications occurred, and a very low rate of postoperative complications was registered. Only one case of CSF leak was reported. In comparison with the complications reported in the literature, like deaths, CPA hematomas, brain infarction, acute hydrocephalus, meningitis, multiple cranial nerve paresis, and so on,5,6,7,9,10,11,13,14,15,23 this study demonstrates that in experienced hands the performance of minimal invasive surgery, like a precisely planned keyhole craniotomy and simultaneous use of the microscope with the endoscope, renders the decompression procedure less traumatic. Although we cannot suggest that the outcomes of our technique are much superior to the microsurgical procedure, we think that there are benefits of reduced morbidity and enhanced efficiency from using endoscopy in cases of vascular decompression. Furthermore, the reduction of the craniotomy size, the minimal exploration of the healthy brain without retraction of the cerebellum or manipulation on the neural structures due to a spatula, the circumferential examination of the nerve of interest without manipulation on it, all reduce the complication rates enormously.
Because of the disadvantages of the endoscopes, like the lack of three-dimensionality, lenses obscured by excessive blood, thermal injuries to the nerves, and so on, further technical developments will be necessary to make complex surgeries in the CPA region a success. Developments like the Olympus Endo Arm, with the ability to focus, zoom, rotate images, function like both endoscope and microscope simultaneously giving image information on an LCD screen placed directly in front of the surgeon's eyes, demonstrate future possibilities for helping to further improve minimally invasive operative techniques. Small high-resolution visualization tools, which given the optical information on a three-dimensional way on a virtual reality corridor, could be placed in front of the surgeon's eyes. By this information, microscopic and endoscopic views can be matched in virtual pathoanatomic space, respecting the individual anatomy of the patient. The surgeon could perform the procedure inside this virtual anatomic corridor, getting the real-time information of the progress of the surgery through the LCD screen on the head-mount display. This could be a further step in the progress of minimally invasive keyhole neurosurgery during the 21st century.
CONCLUSION
The application of the endoscope in CPA surgical procedures is an important tool to help recognize hidden and deep-lying structures like small arteries and veins responsible for vascular compression on the surface of cranial nerves. Simultaneous display of both microscopic and endoscopic information on one screen is an innovation that has helped to improve surgical outcomes in difficult regions like the CPA, and has made decompression surgeries less traumatic.
REFERENCES
- Jannetta P J. Gross (mesoscopic) description of the human trigeminal nerve and ganglion. J Neurosurg. 1967;26(suppl):109–111. doi: 10.3171/jns.1967.26.1part2.0109. [DOI] [PubMed] [Google Scholar]
- Payner T D, Tew J M., Jr Recurrence of hemifacial spasm after microvascular decompression. Neurosurgery. 1996;38:686–690. discussion 690–691. [PubMed] [Google Scholar]
- Resnick D K, Jannetta P J, Bissonnette D, Jho H D, Lanzino G. Microvascular decompression for glossopharyngeal neuralgia. Neurosurgery. 1995;36:64–68. discussion 68–69. doi: 10.1227/00006123-199501000-00008. [DOI] [PubMed] [Google Scholar]
- Jannetta P J. Arterial compression of the trigeminal nerve at the pons in patients with trigeminal neuralgia. J Neurosurg. 1967;26(suppl):159–162. doi: 10.3171/jns.1967.26.1part2.0159. [DOI] [PubMed] [Google Scholar]
- Barker F G, II, Jannetta P J, Bissonette D J, Larkins M V, Jho H D. The long-term outcome of microvascular decompression for trigeminal neuralgia. N Engl J Med. 1996;334:1077–1083. doi: 10.1056/NEJM199604253341701. [DOI] [PubMed] [Google Scholar]
- McLaughlin M R, Jannetta P J, Clyde B L, Subach B R, Comey C H, Resnick D K. Microvascular decompression of cranial nerves: lessons learned after 4400 operations. J Neurosurg. 1999;90:1–8. doi: 10.3171/jns.1999.90.1.0001. [DOI] [PubMed] [Google Scholar]
- Bederson J B, Wilson C B. Evaluation of microvascular decompression and partial sensory rhizotomy in 252 cases of trigeminal neuralgia. J Neurosurg. 1989;71:359–367. doi: 10.3171/jns.1989.71.3.0359. [DOI] [PubMed] [Google Scholar]
- Cutbush K, Atkinson R L. Treatment of trigeminal neuralgia by posterior fossa microvascular decompression. Aust N Z J Surg. 1994;64:173–176. doi: 10.1111/j.1445-2197.1994.tb02171.x. [DOI] [PubMed] [Google Scholar]
- Lovely T J, Jannetta P J. Microvascular decompression for trigeminal neuralgia: surgical technique and long-term results. Neurosurg Clin N Am. 1997;8:11–29. [PubMed] [Google Scholar]
- Aksik I. Microneural decompression operations in the treatment of some forms of cranial rhizopathy. Acta Neurochir (Wien) 1993;125:64–74. doi: 10.1007/BF01401830. [DOI] [PubMed] [Google Scholar]
- Hanakita J, Kondo A. Serious complications of microvascular decompression operations for trigeminal neuralgia and hemifacial spasm. Neurosurgery. 1988;22:348–352. doi: 10.1227/00006123-198802000-00012. [DOI] [PubMed] [Google Scholar]
- Kureshi S A, Wilkins R H. Posterior fossa reexploration for persistent or recurrent trigeminal neuralgia or hemifacial spasm: surgical findings and therapeutic implications. Neurosurgery. 1998;43:1111–1117. doi: 10.1097/00006123-199811000-00061. [DOI] [PubMed] [Google Scholar]
- Lee S H, Levy E I, Scarrow A M, Kassam A, Jannetta P J. Recurrent trigeminal neuralgia attributable to veins after microvascular decompression. Neurosurgery. 2000;46:356–361. discussion 361–362. doi: 10.1097/00006123-200002000-00019. [DOI] [PubMed] [Google Scholar]
- Liao J J, Cheng W C, Chang C N, et al. Reoperation for recurrent trigeminal neuralgia after microvascular decompression. Surg Neurol. 1997;47:562–568. discussion 568–570. doi: 10.1016/s0090-3019(96)00250-9. [DOI] [PubMed] [Google Scholar]
- Rath S A, Klein H J, Richter H P. Findings and long-term results of subsequent operations after failed microvascular decompression for trigeminal neuralgia. Neurosurgery. 1996;39:933–938. discussion 938–940. doi: 10.1097/00006123-199611000-00010. [DOI] [PubMed] [Google Scholar]
- Perneczky A, Fries G. Endoscope-assisted brain surgery: part 1—evolution, basic concept, and current technique. Neurosurgery. 1998;42:219–224. discussion 224–225. doi: 10.1097/00006123-199802000-00001. [DOI] [PubMed] [Google Scholar]
- Charalampaki P, Filippi R, Welschehold S, Conrad J, Perneczky A. Tumors of the lateral and third ventricle: removal under endoscope-assisted keyhole conditions. Neurosurgery. 2005;57:302–311. discussion 302–311. doi: 10.1227/01.neu.0000176638.86772.2d. [DOI] [PubMed] [Google Scholar]
- Badr-El-Dine M, El-Garem H F, Talaat A M, Magnan J. Endoscopically assisted minimally invasive microvascular decompression of hemifacial spasm. Otol Neurotol. 2002;23:122–128. doi: 10.1097/00129492-200203000-00002. [DOI] [PubMed] [Google Scholar]
- El-Garem H F, Badr-El-Dine M, Talaat A M, Magnan J. Endoscopy as a tool in minimally invasive trigeminal neuralgia surgery. Otol Neurotol. 2002;23:132–135. doi: 10.1097/00129492-200203000-00004. [DOI] [PubMed] [Google Scholar]
- Kabil M S, Eby J B, Shahinian H K. Endoscopic vascular decompression versus microvascular decompression of the trigeminal nerve. Minim Invasive Neurosurg. 2005;48:207–212. doi: 10.1055/s-2005-870928. [DOI] [PubMed] [Google Scholar]
- King W A, Wackym P A, Sen C, Meyer G A, Shiau J, Deutsch H. Adjunctive use of endoscopy during posterior fossa surgery to treat cranial neuropathies. Neurosurgery. 2001;49:108–115. discussion 115–116. doi: 10.1097/00006123-200107000-00017. [DOI] [PubMed] [Google Scholar]
- Fries G, Perneczky A. Endoscope-assisted brain surgery: part 2—analysis of 380 procedures. Neurosurgery. 1998;42:226–231. discussion 231–232. doi: 10.1097/00006123-199802000-00008. [DOI] [PubMed] [Google Scholar]
- Kalkanis S N, Eskandar E N, Carter B S, Barker F G., II Microvascular decompression surgery in the United States, 1996 to 2000: mortality rates, morbidity rates, and the effects of hospital and surgeon volumes. Neurosurgery. 2003;52:1251–1261. discussion 1261–1262. doi: 10.1227/01.neu.0000065129.25359.ee. [DOI] [PubMed] [Google Scholar]
- Kaye A H, Adams C B. Hemifacial spasm: a long-term follow-up of patients treated by posterior fossa surgery and facial nerve wrapping. J Neurol Neurosurg Psychiatry. 1981;44:1100–1103. doi: 10.1136/jnnp.44.12.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]