ABSTRACT
We compared the surgical outcomes of recent patients with cerebellopontine angle (CPA) epidermoids treated with advanced surgical tools with those of patients treated in earlier series. From November 2000 to June 2004, we treated 12 patients with epidermoid tumors. One patient had a strict CPA lesion. Tumors extended into the prepontine region in seven cases and supratentorially in two. In two cases the CPA was involved bilaterally. All patients but one underwent a lateral suboccipital approach in a semi-sitting position with microsurgical technique. Endoscopic assistance was used in cases with extensions beyond the CPA. In one case, a subtemporal route was used. The mean follow-up was 27 months (range, 8 to 50 months). There were no deaths. Total removal was achieved in 7 of the 10 patients with unilateral CPA epidermoids. Preoperative status improved in eight (80%) patients, particularly the function of cranial nerves (CNs) V and VII. Only two patients had permanent CN deficits. Complete excision with preservation of CN function should be the goals of management of epidermoids of the CPA. In some cases, these goals can be difficult to achieve, even with contemporary surgical equipment. Bilateral and extensive tumors should be removed in staged procedures. The function of CN V and CN VII may recover after decompression, but the outcome of symptoms related to CN VIII is less certain. The endoscope is a reliable tool for assessing the extension of epidermoids, but it cannot be used for tumor removal.
Keywords: Epidermoid, cerebellopontine angle, cranial nerve deficits, suboccipital, subtemporal
Epidermoid tumors are dysontogenic lesions that represent 0.2 to 1.8% of intracranial tumors.1,2 They typically occur in the cerebellopontine angle (CPA), which is involved in 40 to 60% of the cases.3 Epidermoids represent 4.6 to 6.3% of all CPA lesions.4,5
The clinical presentation and symptoms of CPA epidermoids depend on the presence of adhesions and compression of adjacent nerves, brainstem, and vascular structures. They tend to spread along the subarachnoid space and envelop adjacent neurovascular structures. Their growth along the cisterns of the CPA can produce a chronic inflammatory reaction that increases their adherence to vascular and neural structures.6
In the premicrosurgical era, the operative mortality associated with epidermoids ranged from 20 to 57%.1,7,8,9 This rate has been greatly reduced. However, the rate of permanent deficits, which depends on the degree that the tumor adheres to the neurovascular structures, is still relatively high.2,10,11 Despite the need for complete excision of the tumor, including the capsule, to avoid a recurrence,2,4,12,13,14,15,16 total removal may not be possible without inducing severe deficits. We therefore compared the senior author's (M.S.) recent experience with epidermoids to assess whether contemporary surgical techniques and instrumentation have improved the outcomes of patients harboring these challenging lesions.
MATERIALS AND METHODS
From November 2000 to June 2004, 12 patients (8 women and 4 men; mean age, 47 years; range, 24 to 71 years (Table 1)) with CPA epidermoids were operated on by the senior author at the International Neuroscience Institute of Hannover, Germany. One patient, who had been operated on by the senior author 20 years earlier, presented with a late recurrence. Two other patients had undergone surgery at other institutions 1 year and 12 years before admission to the facility in Hannover. All patients underwent computed tomography (CT) and magnetic resonance imaging (MRI) of the head and brain. Eleven patients underwent a lateral suboccipital approach. A subtemporal approach was used in one patient whose tumor primarily extended into the middle cranial fossa. The procedures were performed with patients in a semi-sitting position. In patients with extensive tumors, an endoscope was used to assist the microsurgical removal of the lesion. Intraoperatively, acoustic evoked potentials (AEPs), somatosensory evoked potentials (SEPs), and facial nerve function were monitored continuously by electromyography (EMG). Patient follow-up was based on outpatient and phone interviews and repeated brain MRI studies.
Table 1.
Recent Publications on Epidermoids of the Cerebellopontine Angle
| Reference | No. of Patients | M/F | Mean Age (y) | Duration of Symptoms (mo) |
|---|---|---|---|---|
| NA, not available. | ||||
| Gagliardi et al,4 1980 | 8 | 4/4 | 41.7 | 69 |
| Fisher et al,23 1984 | 6 | 3/3 | 38.6 | 112.6 |
| Berger et al,2 1985 | 13 | 7/6 | 47 | 4.3 |
| Sabin et al,25 1987 | 20 | 12/8 | 42 | 54 |
| Salazar et al,15 1987 | 17 | 10/7 | 15–60 | 45.6 |
| Morard et al,24 1988 | 6 | 4/2 | 42 | 66.8 |
| Rubin et al,31 1989 | 7 | 4/3 | 40.7 | 202.2 |
| de Souza et al,22 1989 | 30 | 19/11 | 27 | NA |
| Yamakawa et al,5 1989 | 15 | 9/6 | 33 | 94 |
| Altschuler et al,14 1990 | 11 | NA/NA | NA | 44.4 |
| Lunardi et al,13 1990 | 17 | 8/9 | 40 | 84 |
| Vinchon et al,11 1995 | 9 | 6/3 | 40.7 | 47 |
| Samii et al,10 1996 | 40 | NA/NA | 42 | 36 |
| Mohanty et al,29 1997 | 25 | 15/10 | NA | 35 |
| Mallucci et al,19 1999 | 12 | 2/10 | 42 | 24 |
| Kobata et al,18 2002 | 30 | 7/23 | 37.8 | 137 |
| Lakhdar et al,20 2002 | 10 | 6/4 | 31.7 | 31.2 |
| Schroeder et al,26 2004 | 8 | 5/3 | 36 | NA |
| Safavi-Abbasi et al, 2005 (current study) | 12 | 4/8 | 47.3 | 42 |
RESULTS
Ten patients presented with signs and symptoms related to mass compression and presented with headache (3 cases), pyramidal or cerebellar signs (6 cases), or hydrocephalus (1 case) (Tables 2 and 3). One patient's primary complaint was headache. Ten patients had some degree of cranial nerve (CN) involvement: CN V in six patients, CN VIII in seven, CN VII in four, CN VI in four, CN XII in three, and one patient each for CN III and CN IX. In these 11 patients at least two CNs were involved (Table 2). In the remaining patient, the epidermoid tumor was incidentally detected after he underwent CT for minor head trauma. The mean duration of symptoms before surgery in the 11 patients was 42 months (range, 2 months to 10 years).
Table 2.
Cranial Nerve Deficits at Presentation
| Reference | VIII |
VII |
V |
IX, X |
III, IV, VI |
II |
XII |
||
|---|---|---|---|---|---|---|---|---|---|
| Hearing Impairment | Dizziness | Tinnitus | Diplopia | ||||||
| NA, not available. | |||||||||
| Gagliardi et al,4 1980 | 4 | NA | NA | 5 | 4 | 1 | 2 | 2 | 0 |
| Fisher et al,23 1984 | 3 | 3 | NA | 3 | 6 | 1 | 2 | 0 | 0 |
| Sabin et al,25 1987 | 8 | 9 | 5 | 12 | 13 | 10 | 6 | 0 | NA |
| Salazar et al,15 1987 | 8 | 6 | 2 | 0 | 8 | 7 | 6 | 0 | NA |
| Morard et al,24 1988 | 3 | 0 | 1 | 3 | 4 | 0 | 0 | 0 | 0 |
| Rubin et al,31 1989 | 5 | NA | 1 | 4 | 6 | 1 | 2 | 1 | 1 |
| de Souza et al,22 1989 | 15 | NA | 7 | 16 | 11 | 5 | 6 | 8 | 0 |
| Yasargil et al,30 1989 | 12 | 6 | 0 | 9 | 13 | 5 | 7 | 1 | 1 |
| Yamakawa et al,5 1989 | 10 | 2 | 9 | 12 | 11 | 7 | 4 | 0 | 4 |
| Vinchon et al,11 1995 | 5 | 2 | 3 | 1 | 1 | 0 | 1 | 0 | 0 |
| Samii et al,10 1996 | 22 | 16 | 5 | 7 | 17 | 4 | 4 | 2 | 1 |
| Mohanty et al,29 1997 | 12 | 0 | 0 | 13 | 17 | 4 | 0 | 6 | 0 |
| Talacchi et al,12 1998 | 7 | 3 | 2 | 9 | 5 | 4 | 7 | 1 | 1 |
| Mallucci et al,19 1999 | 6 | NA | 0 | 4 | 5 | 1 | 1 | 0 | 0 |
| Kobata et al,18 2002 | 2 | 0 | 2 | 2 | 29 | 1 | 1 | 0 | 0 |
| Lakhdar et al,20 2002 | 8 | 6 | 1 | 4 | 2 | 2 | 0 | 5 | 0 |
| Schroeder et al,26 2004 | 0 | 0 | 1 | 1 | 3 | 0 | 1 | 0 | 0 |
| Our series, 2005 | 4 | 1 | 2 | 4 | 6 | 1 | 4 | 0 | 3 |
Table 3.
Preoperative Signs and Symptoms of Patients with CPA Epidermoids
| Reference | Headache | Cerebellar Signs | Pyramidal Signs | Seizures | Hydrocephalus |
|---|---|---|---|---|---|
| NA, not available. | |||||
| Gagliardi et al,4 1980 | 2 | 4 | 2 | 1 | NA |
| Sabin et al,25 1987 | 6 | 17 | 10 | 3 | NA |
| Salazar et al,15 1987 | 9 | 10 | 8 | 2 | 5 |
| Morard et al,24 1988 | 1 | 2 | 0 | 0 | 0 |
| Rubin et al,31 1989 | 3 | 4 | 0 | 0 | 0 |
| de Souza et al,22 1989 | 16 | 12 | 4 | 3 | NA |
| Yamakawa et al,5 1989 | 9 | 10 | 4 | 0 | NA |
| Vinchon et al,11 1995 | 2 | 1 | 0 | 0 | 1 |
| Samii et al,10 1996 | NA | 7 | 0 | 1 | NA |
| Mohanty et al,29 1997 | NA | 16 | 5 | 2 | 13 |
| Talacchi et al,12 1998 | 7 | 10 | 4 | 2 | 4 |
| Mallucci et al,19 1999 | 0 | 4 | 0 | 0 | 1 |
| Kobata et al,18 2002 | 1 | 1 | 0 | 0 | NA |
| Lakhdar et al,20 2002 | 8 | 7 | 1 | 0 | 4 |
| Schroeder et al,26 2004 | 3 | 2 | 1 | 2 | NA |
| Our series, 2005 | 3 | 3 | 3 | 0 | 1 |
In two patients, the tumor was limited only to the CPA. In the remaining 10 patients, the tumor extended to the surrounding cisterns or compressed the neural structures. In seven cases, the mass extended into the prepontine region. The tumor was associated with radiological compression of the brainstem in five cases and of the cerebellum in two cases. One patient had obstructive hydrocephalus. In two patients, supratentorial extension was associated with temporal lobe compression. The CPA was involved bilaterally in two patients.
The mean follow-up period was 27 months (range, 8 to 50 months). No patients died (Table 4). The tumors were removed completely (Fig. 1) in 9 of the 12 patients (Table 5). In two cases, small capsular remnants could not be removed completely because of their tight adherence to vessels and nerves. The remnants were coagulated with the utmost care to avoid damage to the underlying structures. The tumor was removed completely in the patient who underwent the subtemporal approach.
Table 4.
Complications of Cerebellopontine Angle Epidermoid Surgery
| Reference | CSF Leak | Aseptic Meningitis | Septic Meningitis Abscess | Hematoma | Venous Thrombosis | Long Tract and Cerebellar Deficit | Epilepsy | Hydrocephalus | CN Worsening* | No. of Deaths |
|---|---|---|---|---|---|---|---|---|---|---|
| NA, not available; EVD, external ventricular drainage; VP, ventriculoperitoneal, CN, cranial nerve. | ||||||||||
| Fisher et al,23 1984 | – | – | – | – | 1 | – | – | – | NA | – |
| Berger et al,2 1985 | – | 2 | 2 | – | – | NA | – | 2 | NA | – |
| Sabin et al,25 1987 | 1 | 3 | 1 | – | 2 | 2 | 1 | 3 (2 shunts and 1 EVD) | 3 | 1 |
| Salazar et al,15 1987 | NA | NA | NA | NA | NA | 2 | 1 | 5 (VP shunt) | NA | 1 |
| Morard et al,24 1988 | – | 1 | – | – | – | – | – | – | NA | 1 |
| Rubin et al,31 1989 | – | 1 | – | – | – | – | – | – | 5 | 5 |
| de Souza et al,22 1989 | 3 | – | 3 | – | – | – | – | 12 (shunted prior to surgery) | 5 | 2 |
| Lunardi et al,13 1990 | – | 3 | – | – | – | – | – | 3 | NA | 2 |
| Vinchon et al,11 1995 | – | – | 2 | 1 | 1 | – | 1 | – | 6 | 2 |
| Samii et al,10 1996 | 2 | 1 | – | – | – | – | – | 1 | 19 | 1 |
| Mohanty et al,29 1997 | – | 2 | – | – | – | 2 | – | 1 | 11 | 2 |
| Talacchi et al,12 1998 | – | – | – | – | – | 1 | – | – | 11 | – |
| Mallucci et al,19 1999 | 1 | – | – | – | – | – | – | – | 2 | – |
| Kobata et al,18 2002 | 1 | 2 | 1 | – | – | 1 | – | – | NA | – |
| Lakhdar et al,20 2002 | 3 | 1 | 1 | – | – | – | – | 2 (VP shunt) | NA | – |
| Schroeder et al,26 2004 | – | – | – | – | – | – | – | – | 5 | – |
| Our series 2005 | 1 | – | – | – | – | – | – | 1 (EVD) | 6 | – |
Includes new onset of cranial nerve deficit, remaining or worsening of an existing deficit, and unchanged deficits.
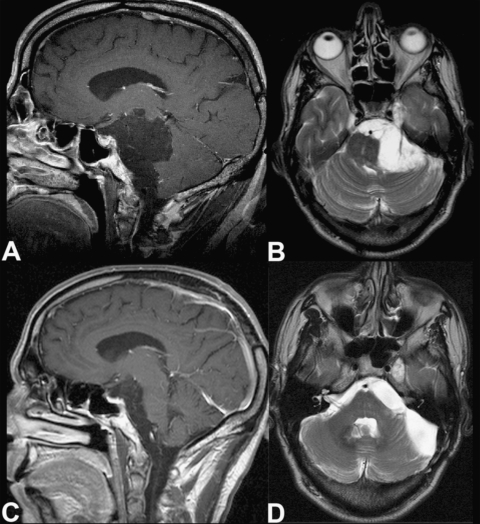
Figure 1.
Preoperative (A) sagittal T1-weighted and (B) axial T2-weighted magnetic resonance (MR) images show a CPA epidermoid mass dislocating the brainstem and extending to the prepontine region and temporal fossa. Postoperative (C) sagittal T1-weighted and (D) axial T2-weighted MRIs show the decompression of neurovascular structures and confirm removal of the infratentorial component. The lesion site has filled with cerebrospinal fluid. The right temporal remnant of the tumor was stable at the patient's 24-month follow-up examination.
Table 5.
Radical Removal of CPA Epidermoids
| Reference | Radical Removal of CPA Epidermoids (%) |
|---|---|
| Berger et al,2 1985 | 0 |
| Sabin et al,25 1987 | 5 |
| Salazar et al,15 1987 | 0 |
| Rubin et al,31 1989 | 57 |
| de Souza et al,22 1989 | 18 |
| Yasargil et al,30 1989 | 97 |
| Yamakawa et al,5 1989 | 47 |
| Lunardi et al,13 1990 | 35 |
| Vinchon et al,11 1995 | 55.50 |
| Samii et al,10 1996 | 75 |
| Mohanty et al,29 1997 | 48 |
| Talacchi et al,12 1998 | 55 |
| Mallucci et al,19 1999 | 62.50 |
| Kobata et al,18 2002 | 56.70 |
| Lakhdar et al,20 2002 | 40 |
| Schroeder et al,26 2004 | 37.50 |
| Our series, 2005 | 75 |
A two-step surgery was planned for the patient with the large CPA tumor that extended supratentorially. The CPA component was totally removed through the suboccipital route. The tentorium was incised to extend the removal supratentorially. However, this component was only partially removed. The patient recovered, his symptoms improved, and the temporal residual has been stable on serial MRI examinations (24 months of follow-up) (Fig. 2).
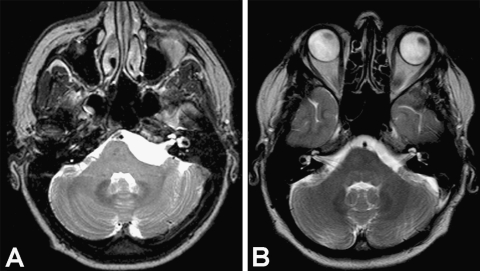
Figure 2.
(A) Preoperative axial T2-weighted image shows a CPA epidermoid mass compressing the brainstem and extending to the basilar artery. (B) Postoperative axial T2-weighted MRI shows the decompression of neurovascular structures and confirms the complete removal of the lesion 24 months after surgery.
In the two patients with bilateral epidermoids, the side of the operation was selected based on the patients' clinical complaints (in particular, trigeminal neuralgia or hemifacial spasm). Each patient underwent a unilateral approach on the symptomatic side. No attempt was made to remove the contralateral component of the tumor completely to avoid damage to neurovascular structures and to prevent acute bilateral deficits of the lower CNs.
Compared with their preoperative clinical status, eight patients (67%) improved. In two patients, CN function was unchanged after surgery, and two patients had CN deficits (17%). Postoperatively in one of the patients that experienced deficits, a preexisting facial palsy worsened to grade IV on the House-Brackmann (H-B) scale (from grade II).1 Six months after surgery, her nerve function improved moderately (grade III on the H-B scale). Postoperatively, the other patient developed a new partial deficit affecting CN IX and CN X; the deficit had recovered completely at the second clinical follow-up 12 months after surgery. Postoperatively, one patient had a cerebrospinal fluid (CSF) fistula, which was treated by wound revision and external drainage. In another case, transient hydrocephalus was treated by external ventricular drainage.
DISCUSSION
First reported by Cruveilhier in 1829,8,17 epidermoid cysts originate from inclusion of ectodermal elements within the neural groove. Classically, these anomalies were thought to occur during closure of the neural tube at the third or fifth week of gestation. Recently, some authors have suggested that an earlier error during gastrulation would explain the different types of dysontogenic lesions.9 The lateral location of these lesions is thought to reflect the lateral displacement of the included ectodermal cells by the otic and optic vesicles,7 which develop later.
Epidermoid tumors grow slowly from the desquamation of cells and from the breakdown of keratin.7 After acoustic neurinomas and meningiomas, these lesions are the third most common lesions in the CPA.12,18,19
Clinical Presentation
The peak age of occurrence of epidermoids is between the third and fifth decades of life (Table 1).2,4,5,7,10,11,14,18,19,20,21,22,23,24,25,26 The most common symptoms are hearing impairment and trigeminal neuralgia. In fact, as indicated by Guidetti et al, Dandy and Olivecrona considered the latter to be the typical manifestation of epidermoids in this location.27
The course of illness is often prolonged, and patients may present at a late stage. When trigeminal neuralgia is the predominant complaint, the mean duration of symptoms is usually shorter.2 Trigeminal neuralgia is more often associated with epidermoid cysts than with acoustic neurinomas or meningiomas of the CPA.18 In some series, the incidence of trigeminal neuralgia in patients with CPA epidermoids has been as high as 90.6%.18 However, in a recent meta-analysis of the literature, the incidence of hearing impairment was 37.6% and that of trigeminal neuralgia was 29.7%.19 The incidence of diminished hearing increases when formal testings are performed.18 In our series, the trigeminal and acoustic nerves were both clinically impaired in 50% of the patients.
CPA epidermoids cause trigeminal neuralgia more often than hypesthesia.19 Nonetheless, in our series, those symptoms were evenly distributed, with each occurring in three cases. Conversely, CPA epidermoids involve the facial nerve less often than the trigeminal nerve (facial paresis, 19.4%, and hemifacial spasm, 4.9%).18 In our series, however, 4 patients had CN VII deficits (Table 2).
Depending on the size of the tumor at diagnosis, diplopia, involvement of the lower CNs by cranial or caudal extension of the mass, or both are often found. Of our 12 patients, 5 had diplopia and 4 had lower CN deficits at admission. Epidermoids may also be associated with symptoms and signs related to compression of the brainstem (pyramidal signs, seen in 3 for our patients) or cerebellum (cerebellar signs, also seen in 3 patients) (Table 3).
Surgical Considerations
As described elsewhere,10,15,28 the retrosigmoid approach allows good visualization and resection of lesions involving the CPA without requiring excessive cerebellar retraction (Figs. 3–5). It has most often been used to resect epidermoids in the posterior fossa.10,12,18,22,29,30 This approach facilitates identification of the structures of the CPA (Fig. 4). It is often possible to resect the tumor from neurovascular structures while preserving them (Figs. 3–5). Even large tumors extending beyond the CPA can be managed with this approach.10,29
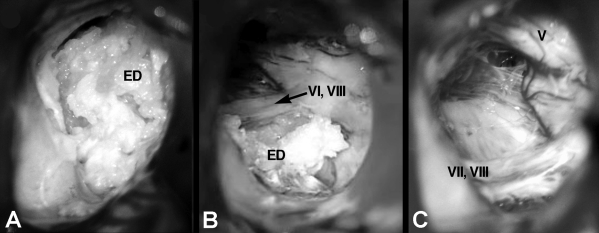
Figure 3.
(A) Microscopic intraoperative photograph shows the pearly appearance of a CPA epidermoid. (B) Partial resection of the tumor reveals the CN VII and CN VIII cranial nerve complex. (C) The surgical field after its removal. CN V, CN VII, and CN VIII were preserved.
Figure 4.
Microscopic intraoperative photographs show (A) CPA epidermoid with a thick bright capsule, (B) the surgical field during its removal, and (C) the involvement of CN VI. (D) A view “around the corner” was obtained by using an angulated mirror. (E) The lesion was removed, and CN VII, CN VIII, and the lower CNs were preserved.
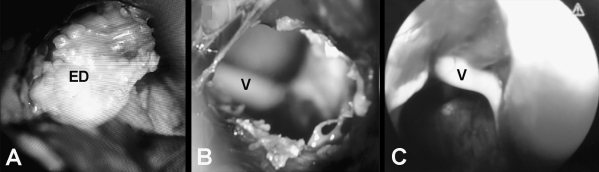
Figure 5.
(A) Microscopic intraoperative photograph shows a CPA epidermoid and (B) the surgical field during its removal. CN V is visible in the background. (C) An endoscope provides a different angle of view.
Usually, the selection of a surgical approach depends on the surgeon's experience and preference. However, performing the approach in the semisitting position depends on the surgical team's level of expertise and experience with this approach. We prefer the semisitting position and have used it in almost all cases of our previous and recent resections of epidermoid tumors. Our neurosurgery and neuroanesthesiology team has many years of experience with this approach and is comfortable with it. Nonetheless, the park-bench or prone position can also be used depending on the surgeon's experience and preference. We do not believe that this position per se affects outcome in the management of epidermoid tumors.
Acute venous air embolism is a well-known intraoperative complication of the sitting position. Therefore, in all of our cases precordial Doppler intrasonograhy is used to monitor signs of air embolism. Furthermore, this risk is decreased by elevating the legs to the level of the right atrium of the heart. Adding preoperative thin-cut bone window CT and intraoperative volumetric 3D neuronavigation to magnetic resonance tomography can help locate the emissary veins and increase awareness of air embolism. These novel tools provide information on the exact location of the sigmoid and transverse sinuses and emissary and bridging veins. In the current series, we avoided air embolism completely.
Hearing may improve after resection of epidermoid tumors.19 Consequently, the translabyrinthine approach should usually be preserved for patients with an irreversible hearing loss. Of course, the approach must be tailored to the location and extent of each patient's lesions to ensure optimal exposure. As advised by Berger and Wilson,2 a subtemporal approach can be used if a lesion extends supratentorially into the middle cranial fossa. Other authors have recommended a two-stage approach, using the suboccipital route first followed by the subtemporal approach.11,15,29 However, seizure disorders are a postoperative risk of the subtemporal approach.11,15 Even after a combined approach, only partial removal may be feasible if the capsule densely adheres to the adjacent structures.11,29 When only partial resection is possible, careful severance and coagulation of the tumor capsule may reduce the risk of regrowth and symptom recurrence.22,29
Epidermoid Extension and Radical Removal
The rates of radical removal have ranged from 0 to 97% (Table 5).2,5,10,15,18,19,25,30,31 Our resection rate is comparable and the same as in our previous series (75% in both). The extent of resection is not only influenced by the tumor's adhesion to surrounding structures but also by its pattern of extension. In our patient with extensive supratentorial invasion who underwent a suboccipital approach, a radical excision could not be obtained even though the tentorium was opened. A small tumor remnant in the middle fossa has remained stable (last follow-up examination, 24 months; Fig. 2).
In both cases with bilateral tumors, the ipsilateral mass was removed completely. However, the contralateral component of the tumor was left in place to avoid damaging neurovascular structures. We recommend staged resection of bilateral masses with considerable time between the two procedures to allow possible deficits to recover. This strategy is particularly advisable to avoid complications from injuries to the lower CNs.18,19,22,25
Management of Recurrence
Most series report good control of tumor growth after resection of CPA epidermoid cysts.19,27 Nonetheless, these lesions are prone to regrowth.19,21 A second operation may be planned when the tumor remnant becomes symptomatic or extends beyond its original site.12,25
In our series, three patients had already been operated on (1, 12, and 20 years earlier, respectively) and had experienced a recurrence. Scars and adherences related to a previous surgery can constitute a further obstacle to achieving complete removal. However, radical excision was possible in our two patients with a late recurrence.
Radiological confirmation of a recurrence can be challenging. After a tumor is excised, the remaining defect can fill with CFS. Thus the signal can resemble that of a recurrent lesion.25 These MR imaging characteristics make it difficult to differentiate epidermoid tumors from surrounding CSF and also to detect small, residual, or recurrent epidermoid tumors. However, fast fluid-attenuated inversion recovery (fast-FLAIR) MR imaging and echo-planar diffusion-weighted imaging may be superior to conventional MR imaging in detecting such lesions.3 To avoid recurrences and to evaluate the extent of resection all corners of the CPA should be inspected as a final operative step. The surgical field should then be irrigated to ensure removal of any remnants. We evaluated the extent of resection on immediate and follow-up postoperative MR images.
Surgical Outcome of CNs
Epidermoids of the CPA are surgically challenging.2,17 In fact, their benign nature and slow growth rate contrast with their capacity to adhere to neurovascular structures.2,17 Although the surgeon's goal should be radical removal, in some cases it may be achieved only with significant morbidity.
Worsening of a preexisting CN deficit is common.2,11,17,19,22,25,26,29 Vinchon and coworkers found postoperative CN deterioration, such as diplopia, facial palsy or swallowing difficulties, in 55% of patients in their series.11 especially, involvement of the lower CNs may constitute a significant surgical difficulty and postoperative management problem. Of the 10 patients reported by Sabin et al., 2 patients required temporary nasogastric feeding and 1 patient needed a tracheostomy to protect the airway.25
Furthermore, new postoperative deficits from previously functioning CN also may develop.2,19,26,29 Mallucci et al.19 reported the postoperative appearance of gag-reflex anomalies in two patients after surgery. De Souza and colleagues22 reported the development of a CN IX or CN X palsy in 5 patients. Mohanty et al. described two new cases of CN VII and VIII dysfunction.29 Postoperatively, 11 patients (44%) of the 25 reported cases experienced a worsening of CN function during the postoperative period.29 In their series, Kobata and coworkers found that facial hypesthesia was the most common postoperative complication.18
After surgery the CN status (16%) of two of our patients, a preoperative CN VII deficit in one and a new CN IX deficit in the other, worsened. Postoperatively, the first patient's preexisting facial palsy worsened (grade IV H-B), but it recovered partially during the follow-up period (grade III H-B). The second patient developed postoperative vocal cord weakness that recovered completely. Postoperatively, in all other cases, CN function was unchanged or improved. Compared with our previous series, our rates of postoperative CN dysfunction, surgical morbidity, and mortality improved significantly (Table 6). At discharge only one (8%) patient with CN dysfunction could not return to his/her (which?) level of premorbid activity compared with 20% in our previous series. Nevertheless, on long-term follow-up, more than 90% of the patients in both series had excellent outcomes. Overall, our data show that immediate postoperative outcomes improved with improvements in microsurgical technique although the long-term outcomes remained the same. Notwithstanding advances in neuroradiological and diagnostic techniques, the reported duration of symptoms before presentation has not decreased in the current literature (Table 1). In fact, the duration of symptoms in our current series was longer than in our previous series. Thus, it seems that the improvements in postoperative outcomes may likely reflect refinements in microsurgical technique and use of technical tools for pre- and intraoperative planning, such as localization of venous structures and other variable anatomic structures.
Table 6.
CN Outcome After Surgery in our Series
| Series | CN VIII* | CN VII | CN V | Other CN |
|---|---|---|---|---|
| Current | ||||
| 0 new | 0 new | 0 new | 1 new† | |
| 1 improved | 2 improved | 5 improved | 7 improved | |
| 3 unchanged | 1 unchanged | 1 unchanged | 0 unchanged | |
| 0 worsened | 1 worsened | 0 worsened | 0 worsened | |
| Samii et al10 | ||||
| 0 new | 0 new | 0 new | 3 new | |
| 11 improved | 4 improved | 16 improved | 1 improved | |
| 11 unchanged | 1 unchanged | 1 unchanged | 3 unchanged | |
| 0 worsened | 2 worsened | 0 worsened | 1 worsened |
Includes only patients with hearing deficiencies and not other CN VIII symptoms.
After surgery, one patient complained of transient swallowing disturbances that completely regressed during the follow-up period.
Intraoperative AEP and facial EMG, which was used during all procedures, is important for monitoring and preserving CN VII and CN VIII functions. The response of CNs to tumor compression and surgical decompression is variable. The function of CN V improved in five of our patients. This finding is consistent with previous reports in which epidermoid-associated trigeminal neuralgia was associated with a good prognosis.16 After surgery facial palsy improved in two patients. Only one patient with CN VIII disturbances (i.e., tinnitus) improved after surgery. Patients with a preoperative hearing loss had no change after surgery. In earlier reports, however, hearing has improved after resection of CPA epidermoids.19
Other Surgical Complications
A typical complication of cystic tumors with fat content, such as epidermoid cysts, is aseptic meningitis (Table 4). This problem is caused by the spillage of cholesterol breakdown products into the CSF.17,29,32 Hydrocephalus and cerebral vasospasm can also be present.7,17,30,32,33
The subarachnoid dissemination of fat may occur spontaneously or after surgical removal of epidermoid tumors.15,32 This complication seems most common after incomplete resection, especially when the capsule is not removed.27 Continuous irrigation of the operative field and steroid therapy may diminish the risk of postoperative aseptic meningitis.2,29,30,32
The incidence of aseptic meningitis has ranged from 10 to 40%; in recent series this rate has decreased.11,18,20,33 None of our patients had this complication, most likely because all patients were administered corticosteroids (dexamethasone, 16 to 24 mg/day) for 7 days after surgery.
Obstructive hydrocephalus can occur as a result of the tumor itself.25,29 De Souza et al. reported 30 patients with CPA epidermoids, 12 of whom underwent shunt placement to treat the hydrocephalus before surgery.22 In their 25 patients, Mohanty et al. reported a 50% occurrence of hydrocephalus associated with 4 cases of preoperative CSF diversion.29 Although one of our patients had transient postoperative obstruction of CSF flow, it resolved after 10 days of external ventricular drainage. No shunt was required.
Subarachnoid hemorrhage and brainstem ischemia are uncommon but serious complications of epidermoid tumors.6,33 Malignant transformations of epidermoid tumors have also been reported.15,24 More commonly, CSF fistulas, infections, seizures, or postoperative hematomas can follow epidermoid surgery (Table 4).11,19 One of our patients presented with a CSF fistula that was treated by wound revision and placement of transitory lumbar drainage.
Endoscopic Assistance
Videoendoscope-assisted microsurgery is a new microsurgical technique that uses endoscopes as surgical instruments under microscopic control.34 In contrast to our previous series, we used endoscopic assistance in all current cases. In all patients, the endoscope was used free-hand. Use of the endoscope allows inspection of obscured but important corners of the operating field without the need to retract neurovascular structures or to resect dura and bone edges. Compared with a microscopic surgical field alone, the endoscope provides a view of the operative field from a different angle (Fig. 5). This feature improves assessment of the extent of the surgical excision. In 1998 Fries and Perneczky evaluated their experience with endoscope-assisted microsurgery in the treatment of 205 brain tumors.34 The authors did not report whether their patient population included CPA epidermoids or other CPA tumors. However, they concluded that endoscope-assistance may reduce the trauma related to resection and improve operative outcomes. Comparing our current data with our previous data, we found that immediate postoperative outcomes significantly improved in concert with a less traumatic and more sophisticated resection.
In particular, the endoscope could provide views of infra- and supratentorial extensions via the suboccipital approach and improved control of the extent of the surgical removal. In the patient operated on by a subtemporal route, the endoscope was also used at the end of the procedure to confirm excision of the lesion. Thus, a more complete resection may be assured and the possibility of recurrence reduced. Schroeder and colleagues reported endoscopic removal of CPA epidermoids without the use of the microscope in 8 patients.26 No complications were attributed to the direct application of the endoscope. In three of their patients, the capsule was removed completely, resulting in permanent CN deficits in two. Two other patients developed transient CN palsies. These authors concluded that enlargement of the craniotomy is unnecessary.
We agree that a routine craniotomy need not be enlarged to use endoscope. We would add that reducing the size of the craniotomy is also infeasible. Endoscopic removal can become difficult and hazardous when epidermoid tumors tend adhere to adjacent neurovascular tissue. The high fat content of the tumor can disturb clear endoscopic visualization. Based on our current level of experience, we do not believe that purely endoscopic removal is superior to microsurgical technique and we do not advocate endoscopic removal of CPA epidermoids. In our series all tumors were resected only under the view of the operating microscope. Overall, the endoscope is a reliable tool for assessing the extent of a tumor. However, epidermoids and tumor remnants should be removed via microsurgical technique. No attempt should be made to remove either the lesion or its capsule with the endoscope alone.
That the rate of resection between our two series did not change shows that endoscope-assisted removal of CPA epidermoids in recent series did not improve the rate of complete resection. Nonetheless, we believe that the combined application of all advanced tools is responsible for improving postoperative outcomes and decreasing morbidity rates.
CONCLUSION
Despite recent improvements in postoperative outcomes, CPA epidermoids remain challenging entities. The aim of the treatment should be complete removal and preservation of the CNs. Such outcomes can be obtained in a significant proportion of the cases. The CN V and CN VII may recover after decompression. Whether symptoms related to CN VIII dysfunction will improve remains uncertain. Meticulous surgical technique with the aid of neurophysiological monitoring is crucial to achieve safe and effective total or subtotal removal of these lesions. When complete excision of the cyst capsule cannot be obtained, its remnants should be severed cautiously and coagulated thoroughly to reduce the risk of recurrence as much as possible. Endoscopy may be a useful tool for tumor visualization, particularly for supratentorial or bilateral extension, but it should not be used for tumor removal. Bilateral and extensive tumors should be removed in staged procedures with enough time between the two procedures to allow recovery.
ACKNOWLEDGMENTS
We thank Mrs. Frommhold for her help in the neurophysiological monitoring of the patients and the Neuroscience Publications Office of Barrow Neurological Institute for assistance in preparing this manuscript.
REFERENCES
- Caldarelli M, Massimi L, Kondageski C, Di Rocco C. Intracranial midline dermoid and epidermoid cysts in children. J Neurosurg. 2004;100:473–480. doi: 10.3171/ped.2004.100.5.0473. [DOI] [PubMed] [Google Scholar]
- Berger M S, Wilson C B. Epidermoid cysts of the posterior fossa. J Neurosurg. 1985;62:214–219. doi: 10.3171/jns.1985.62.2.0214. [DOI] [PubMed] [Google Scholar]
- Chen S, Ikawa F, Kurisu K, Arita K, Takaba J, Kanou Y. Quantitative MR evaluation of intracranial epidermoid tumors by fast fluid-attenuated inversion recovery imaging and echo-planar diffusion-weighted imaging. AJNR Am J Neuroradiol. 2001;22:1089–1096. [PMC free article] [PubMed] [Google Scholar]
- Gagliardi F M, Vagnozzi R, Caruso R, Delfini R. Epidermoids of the cerebellopontine angle (cpa): usefulness of CT scan. Acta Neurochir (Wien) 1980;54:271–281. doi: 10.1007/BF01407095. [DOI] [PubMed] [Google Scholar]
- Yamakawa K, Shitara N, Genka S, Manaka S, Takakura K. Clinical course and surgical prognosis of 33 cases of intracranial epidermoid tumors. Neurosurgery. 1989;24:568–573. doi: 10.1227/00006123-198904000-00013. [DOI] [PubMed] [Google Scholar]
- Yilmazlar S, Kocaeli H, Cordan T. Brainstem stroke associated with epidermoid tumours: report of two cases. J Neurol Neurosurg Psychiatry. 2004;75:1340–1342. doi: 10.1136/jnnp.2003.029520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldarelli M, Colosimo C, Di Rocco C. Intra-axial dermoid/epidermoid tumors of the brainstem in children. Surg Neurol. 2001;56:97–105. doi: 10.1016/s0090-3019(01)00542-0. [DOI] [PubMed] [Google Scholar]
- Kachhara R, Bhattacharya R N, Radhakrishnan V V. Epidermoid cyst involving the brainstem. Acta Neurochir (Wien) 2000;142:97–100. doi: 10.1007/s007010050013. [DOI] [PubMed] [Google Scholar]
- Dias M S, Walker M L. The embryogenesis of complex dysraphic malformations: a disorder of gastrulation? Pediatr Neurosurg. 1992;18:229–253. doi: 10.1159/000120670. [DOI] [PubMed] [Google Scholar]
- Samii M, Tatagiba M, Piquer J, Carvalho G A. Surgical treatment of epidermoid cysts of the cerebellopontine angle. J Neurosurg. 1996;84:14–19. doi: 10.3171/jns.1996.84.1.0014. [DOI] [PubMed] [Google Scholar]
- Vinchon M, Pertuzon B, Lejeune J P, Assaker R, Pruvo J P, Christiaens J L. Intradural epidermoid cysts of the cerebellopontine angle: diagnosis and surgery. Neurosurgery. 1995;36:52–56. doi: 10.1227/00006123-199501000-00006. [DOI] [PubMed] [Google Scholar]
- Talacchi A, Sala F, Alessandrini F, Turazzi S, Bricolo A. Assessment and surgical management of posterior fossa epidermoid tumors: report of 28 cases. Neurosurgery. 1998;42:242–251. doi: 10.1097/00006123-199802000-00020. [DOI] [PubMed] [Google Scholar]
- Lunardi P, Missori P, Innocenzi G, Gagliardi F M, Fortuna A. Long-term results of surgical treatment of cerebello-pontine angle epidermoids. Acta Neurochir (Wien) 1990;103:105–108. doi: 10.1007/BF01407514. [DOI] [PubMed] [Google Scholar]
- Altschuler E M, Jungreis C A, Sekhar L N, Jannetta P J, Sheptak P E. Operative treatment of intracranial epidermoid cysts and cholesterol granulomas: report of 21 cases. Neurosurgery. 1990;26:606–613. doi: 10.1097/00006123-199004000-00008. [DOI] [PubMed] [Google Scholar]
- Salazar J, Vaquero J, Saucedo G, Bravo G. Posterior fossa epidermoid cysts. Acta Neurochir (Wien) 1987;85:34–39. doi: 10.1007/BF01402367. [DOI] [PubMed] [Google Scholar]
- Samii M, Tatagiba M. Comments on: Kobata H, Kondo A, Iwasaki K. Cerebellopontine angle epidermoids presenting with cranial nerve hyperactive dysfunction: pathogenesis and long-term surgical results in 30 patients. Neurosurgery 2002;50:276–286. Neurosurgery. 2002;50:286. doi: 10.1097/00006123-200202000-00008. [DOI] [PubMed] [Google Scholar]
- Love J G, Kernohan J W. Dermoid and epidermod tumors (cholesteatomas) of the central nervous system. JAMA. 1936;107:1876–1883. [Google Scholar]
- Kobata H, Kondo A, Iwasaki K. Cerebellopontine angle epidermoids presenting with cranial nerve hyperactive dysfunction: pathogenesis and long-term surgical results in 30 patients. Neurosurgery. 2002;50:276–285. doi: 10.1097/00006123-200202000-00008. [DOI] [PubMed] [Google Scholar]
- Mallucci C L, Ward V, Carney A S, O'Donoghue G M, Robertson I. Clinical features and outcomes in patients with non-acoustic cerebellopontine angle tumours. J Neurol Neurosurg Psychiatry. 1999;66:768–771. doi: 10.1136/jnnp.66.6.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakhdar A, Sami A, Naja A, et al. (Epidermoid cyst of the cerebellopontine angle: a surgical series of 10 cases and review of the literature) Neurochirurgie. 2003;49:13–24. [PubMed] [Google Scholar]
- De Micheli E, Bricolo A. The long history of a cerebello-pontine angle epidermoid tumour: a case report and lessons learned. Acta Neurochir (Wien) 1996;138:350–354. doi: 10.1007/BF01411748. [DOI] [PubMed] [Google Scholar]
- deSouza C E, deSouza R, da Costa S, et al. Cerebellopontine angle epidermoid cysts: a report on 30 cases. J Neurol Neurosurg Psychiatry. 1989;52:986–990. doi: 10.1136/jnnp.52.8.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer G, Bret P, Hor F, Pialat J, Massini B. (Epidermoid cysts of the cerebellopontile angle: 6 cases) Neurochirurgie. 1984;30:365–372. [PubMed] [Google Scholar]
- Morard M, De Tribolet N. (Epidermal cysts of the cerebellopontile angle) Neurochirurgie. 1988;34:253–257. [PubMed] [Google Scholar]
- Sabin H I, Bordi L T, Symon L. Epidermoid cysts and cholesterol granulomas centered on the posterior fossa: twenty years of diagnosis and management. Neurosurgery. 1987;21:798–805. doi: 10.1227/00006123-198712000-00004. [DOI] [PubMed] [Google Scholar]
- Schroeder H W, Oertel J, Gaab M R. Endoscope-assisted microsurgical resection of epidermoid tumors of the cerebellopontine angle. J Neurosurg. 2004;101:227–232. doi: 10.3171/jns.2004.101.2.0227. [DOI] [PubMed] [Google Scholar]
- Guidetti B, Gagliardi F M. Epidermoid and dermoid cysts: clinical evaluation and late surgical results. J Neurosurg. 1977;47:12–18. doi: 10.3171/jns.1977.47.1.0012. [DOI] [PubMed] [Google Scholar]
- Samii M, Carvalho G A, Schuhmann M U, Matthies C. Arachnoid cysts of the posterior fossa. Surg Neurol. 1999;51:376–382. doi: 10.1016/s0090-3019(98)00095-0. [DOI] [PubMed] [Google Scholar]
- Mohanty A, Venkatrama S K, Rao B R, Chandramouli B A, Jayakumar P N, Das B S. Experience with cerebellopontine angle epidermoids. Neurosurgery. 1997;40:24–29. doi: 10.1097/00006123-199701000-00004. [DOI] [PubMed] [Google Scholar]
- Yasargil M G, Abernathey C D, Sarioglu A C. Microneurosurgical treatment of intracranial dermoid and epidermoid tumors. Neurosurgery. 1989;24:561–567. doi: 10.1227/00006123-198904000-00012. [DOI] [PubMed] [Google Scholar]
- Rubin G, Scienza R, Pasqualin A, Rosta L, Da Pian R. Craniocerebral epidermoids and dermoids: a review of 44 cases. Acta Neurochir (Wien) 1989;97:1–16. doi: 10.1007/BF01577734. [DOI] [PubMed] [Google Scholar]
- Carvalho G A, Cervio A, Matthies C, Samii M. Subarachnoid fat dissemination after resection of a cerebellopontine angle dysontogenic cyst: case report and review of the literature. Neurosurgery. 2000;47:760–763. doi: 10.1097/00006123-200009000-00047. [DOI] [PubMed] [Google Scholar]
- Yan P X, Yu C J. Minicraniotomy treatment of an intracerebral epidermoid cyst. Minim Invasive Neurosurg. 2004;47:245–248. doi: 10.1055/s-2004-818518. [DOI] [PubMed] [Google Scholar]
- Fries G, Perneczky A. Endoscope-assisted brain surgery: part 2—analysis of 380 procedures. Neurosurgery. 1998;42:226–231. doi: 10.1097/00006123-199802000-00008. [DOI] [PubMed] [Google Scholar]