Abstract
Though generalization of conditioned fear has been implicated as a central feature of pathological anxiety, surprisingly little is known about the psychobiology of this learning phenomenon in humans. Whereas animal work has frequently applied methods to examine generalization gradients to study the gradual weakening of the conditioned-fear response as the test stimulus increasingly differs from the conditioned stimulus (CS), to our knowledge no psychobiological studies of such gradients have been conducted in humans over the last 40 years. The current effort validates an updated generalization paradigm incorporating more recent methods for the objective measurement of anxiety (fear-potentiated startle). The paradigm employs 10, quasi-randomly presented, rings of gradually-increasing size with extremes serving as CS+ and CS-. The eight rings of intermediary size serve as generalization stimuli (GS’s) and create a continuum-of-similarity from CS+ to CS-. Both startle data and online self-report ratings demonstrate continuous decreases in generalization as the presented stimulus becomes less similar to the CS+. The current paradigm represents an updated and efficacious tool with which to study fear generalization—a central, yet understudied conditioning-correlate of pathologic anxiety.
Keywords: fear-potentiated startle, stimulus generalization, fear-conditioning, anxiety disorders
1. Introduction
Classical fear-conditioning, the cross-species learning process by which a neutral conditioned stimulus (CS) comes to evoke fear following its repeated pairing with an aversive unconditioned stimulus (US), has long been implicated in the development of pathologic anxiety (for a review, see Mineka & Zinbarg, 2006). A recent meta-analysis of lab-based, fear-conditioning studies in the anxiety disorders implicates heightened anxious reactivity to conditioned stimuli (CS’s) signaling safety as an important conditioning correlate of clinical anxiety (Lissek et al., 2005). A closer look at this literature reveals that studies of posttraumatic stress disorder (PTSD) contribute prominently to this pattern. Whereas healthy individuals display anxious reactivity to CS’s paired (CS+: danger cue) but not unpaired (CS-: safety cue) with the aversive US, PTSD patients tend to display fear responses to both CS+ and CS-. Given that CS+ and CS- employed by this literature share many stimulus properties (e.g., size, shape, duration), such findings implicate an enhanced tendency among PTSD patients to generalize conditioned fear from danger cues to safety cues with overlapping features. In the context of PTSD, such generalization of fear represents an experimental analogue of the pathologic generalization process by which fear during a traumatic event transfers to safe conditions that ‘resemble’ the distressing event (American Psychiatric Association, 2000).
Though such generalization of conditioned fear is a promising marker of pathological anxiety responses, very little is known about the psychophysiology, pharmacology, neurobiology, and genetics of this learning phenomenon in humans. Indeed, systematic tests of generalization of conditioned responses developed and tested in animals (for reviews, see Honig & Urcuioli, 1981; Kalish, 1969; Mackintosh, 1974) have sparsely been applied to study generalization of conditioned fear in humans over the years (Bass & Hull, 1934; Grant & Schiller, 1953; Hovland, 1937; Kopp, Schlimm, & Hermann, 2005; Mednick, 1957; Mednick & Wild, 1962; Vervliet, Vansteenwegen, Baeyens, Hermans, & Eelen, 2005; Bram Vervliet, Vansteenwegen, & Eelen, 2004)1. These systematic tests of generalization in animals assess conditioned fear responses to both CS+ and generalization-stimuli (GS) parametrically varying in similarity to the CS+, and document generalization gradients, or slopes, with the highest level of fear responding to the CS+ and gradually decreasing levels of fear generalization to GS’s of decreasing similarity to the CS+ (e.g., Armony, Servan-Schreiber, Romanski, Cohen, & LeDoux, 1997). The steepness of this gradient indexes generalization, with steeper downward gradients indicating less generalization.
Unfortunately, the few human studies applying this method are not adequate for present purposes. Specifically, the early work from Bass and Hull (1934) and Hovland (1937) employs methods that diverge considerably from current experimental practice. Additionally, studies by Martha and Sarnoff Mednick (1957, 1962) employ words associatively linked to the CS+ as generalization stimuli and thus do not form an interval scale of generalization. Furthermore, the paradigm by Kopp et al. (2005) has not been psychophysiologically validated and used animal-fear relevant stimuli that are largely unrelated to anxiety disorders other than specific phobia. Finally, whereas conditioned fear-generalization studies by Vervliet and colleagues (2004, 2005) elegantly demonstrate the persistence of conditioned responding following extinction to generalization stimuli, such studies do not take as their focus the generalization of fear-acquisition to stimuli approximating the CS+. The difference between studies by the Vervliet group and the current effort can be viewed in terms of a fear inhibition versus excitation focus, with the former studying inhibition of fear to a CS+ following extinction to a CS+ approximate, and the current effort assessing the transfer of fear excitation to CS+ approximates.
In addition to the above, existing human paradigms applying psychophysiological techniques measure generalization with skin conductance responses (SCR), and it is yet to be demonstrated whether such generalization can be captured by fear-potentiated startle (FPS: the reliable enhancement of the startle reflex when an organism is in a state of fear [Davis & Astrachan, 1978]). Measuring conditioned fear and its generalization with FPS may be advantageous over SCR for a few important reasons. Whereas SCR is highly sensitive to attentional processes (e.g., Filion, Dawson, Schell, & Hazlett, 1991) and reflects increases in general sympathetic arousal, attentional effects on FPS—though present— may well be smaller than emotional effects (e.g., Bocker, Baas, Kenemans, & Verbaten, 2004) and FPS is more valence specific (for a review, see Lang, Bradley, & Cuthbert, 1998). Additionally, the construct validity of FPS as a measure of fear is supported by the central role played by amygdala-based “fear circuits” in the potentiation of startle in both rodents (e.g., Hitchcock & Davis, 1986) and humans (e.g., Pissiota et al., 2003). Because of these advantages, FPS is increasingly used to measure psychophysiological correlates of pathologic anxiety and to test the anxiolytic properties of pharmaceutical compounds (for a review, see Grillon, in press). Given the strong relevance of conditioned fear generalization to anxiety disorders (e.g., Foa, Steketee, & Rothbaum, 1989; Grillon & Morgan, 1999; Keane, Zimmering, & Caddell, 1985; Lissek et al., 2005; Mineka, 1992), conditioned startle-potentiated paradigms capable of eliciting continuous gradients of fear generalization may be a particularly powerful translational tool. The current study tests a novel conditioned startle-potentiation paradigm designed for this purpose in 20 healthy participants.
2. Methods
2.1. Participants
Twenty healthy participants (50% female) with a mean age of 26.10 (SD=8.16), and average State and Trait Anxiety Inventory scores (Spielberger, Gorsuch, Lushene, Vagg, & Jacobs, 1983) of 26.30 (SD=7.02) and 29.41 (SD=6.38), were recruited from the community and reimbursed for their time. Prior to testing, participants gave written informed consent that had been approved by the NIMH-IRB. Inclusion criteria included: (1) no past or current Axis-I psychiatric disorder as per Structured Clinical Interview for DSM-IV, (SCID-I/NP: First, Gibbon, Spitzer, & Williams, 2001), (2) no major medical condition that interfered with the objectives of the study, (3) no current use of medications altering central nervous system function, and (4) no current use of illicit drugs as per urine test.
2.2. Physiological apparatus
Stimulation and recording were controlled by a commercial system (Contact Precision Instruments). Startle-blink EMG was recorded with two 6-mm tin cup electrodes filled with a standard electrolyte (SignaGel, bio-medical.com[CG04]) placed under the left eye. The EMG signal was sampled at 1000 Hz and amplifier band width was set to 30-500 Hz. Startle was elicited by a 40-ms duration, 102 dB(A) burst of white-noise with a near instantaneous rise-time presented binaurally through headphones.
2.3. Conditioned, Unconditioned, and Generalization Stimuli
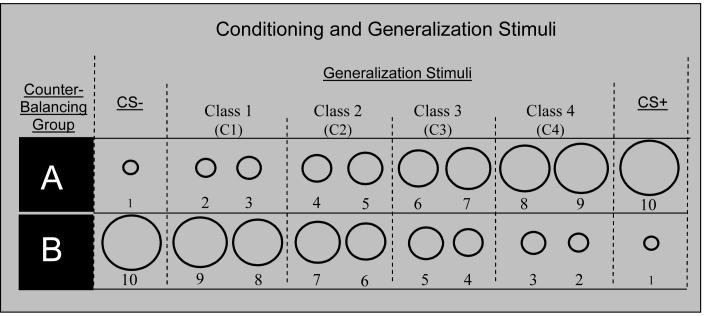
Ten rings of continuously increasing size (see Figure 1) served as conditioned stimuli (CS+, CS-) and generalization stimuli (GS’s). The dimensions and size increments for employed rings are described in Figure 1. For half of participants the smallest ring was the CS+ and the largest was the CS-; for the other half this was reversed. The eight intermediately-sized rings served as GS’s and formed a continuum-of-size between the CS+ and CS-. All CS’s and GS’s were presented for 8 s on a computer monitor at a viewing distance of approximately 46 cm. The unconditioned stimulus (US) was a 100-ms electric shock delivered to the left wrist (3-5 mA) that was rated by participants as being ‘highly uncomfortable but not painful’.
Figure 1.
Conditioning and generalization stimuli for counterbalancing groups A and B. Half of participants were assigned to counterbalancing group A and half to B. The numbers 1-10 at the bottom of the rings label the stimuli from smallest (1) to largest (10) but did not appear when the rings were presented to participants. For both counterbalancing groups A and B, Class 4 consisted of the two rings closest in size to the CS+ and Classes 3-1 gradually decreased in similarity to the CS+. Prior to data analysis, startle and behavioral responses to every two neighboring intermediaries were averaged to form the mean level of responding for that class of ring (e.g., [Ring 2 + Ring 3] / 2 = Class 1). This allowed for the capture of a more gradual slope of generalization—produced by eight rather than four gradations— while requiring half the number of trials per intermediary. This methodological decision was made to avoid an unrealistically long experiment (leading to undue habituation of the startle reflex and subject fatigue) while achieving a gradual continuum of similarity. The diameter for the smallest ring (Ring #1) was 2.00 inches and subsequent rings increased by 20% with Ring #2 increasing 20% from Ring#1 (2.40 inches), Ring #3 increasing 40% from Ring #1 (2.80 inches), Ring #4 increasing 60% from #1 (3.20 inches), and so on. Such size increments resulted in ring diameters, from smallest to largest, of 2.00, 2.40, 2.80, 3.20, 3.60, 4.00, 4.40, 4.80, 5.20, and 5.60. CS+ = conditioned stimulus paired with shock, CS- = conditioned stimulus unpaired with shock.
2.4. Behavioral Ratings
During half of the of CS+ and CS- trials at acquisition, and during half of CS+, CS-, and GS trials during generalization, the question “Level of risk?” appeared on the computer monitor above the presented stimulus at 1- or 2-s post trial-onset and cued participants to rate their perceived level of risk for shock. Similar to other studies assessing subjective risk (e.g., Foster et al., 2002; Jackson, Hobfoll, Jackson, & Lavin, 2001), participants completed risk ratings using a 3-point Likert scale, where 1=“no risk”, 2=“moderate risk”, and 3=“high risk”. Participants were instructed to answer based on their ‘gut feeling’ of risk and to respond as quickly as possible using their index finger to depress buttons 1, 2 or 3 on a computer keyboard. Risk ratings and corresponding response latencies were recorded with Presentation software (Neurobehavioral Systems) and reaction times exceeding 2.5 standard deviations above the average were considered outliers and discarded (Ratcliff, 1993). These behavioral ratings and response times were elicited on odd trials and startle responses were probed on even trials. This separation of behavioral and startle responses was applied due to concerns that behavioral ratings would alter startle magnitudes via attentional demands (Lipp, Siddle, & Dall, 2000) or preparation of motor movements (Valls-Sole, Valldeoriola, Tolosa, & Nobbe, 1997).
2.5. Design
The generalization paradigm included three phases: 1) pre-acquisition— consisted of 6 CS+ (3 startle, 3 behavioral), 6 CS- (3 startle, 3 behavioral), and 6 inter-trial-interval (ITI) measures (3 startle, 3 behavioral) all presented in the absence of any shock US’s; 2) acquisition— included 12 CS+ (6 startle, 6 behavioral), 12 CS- (6 startle, 6 behavioral), and 12 ITI (6 startle, 6 behavioral), with 9 of 12 CS+’s coterminating with shock delivery (75% reinforcement schedule); and 3) generalization test— included 12 CS+ (6 startle, 6 behavioral), 12 CS- (6 startle, 6 behavioral), and 12 ITI (6 startle, 6 behavioral), as well as 6 trials from each of the eight GS sizes (3 startle and 3 behavioral). Because every two sizes of GS’s formed a single class of GS (see Figure 1), there were 12 GS’s from each class (6 startle and 6 behavioral) providing balanced numbers of trials across GS classes, CS+, CS-, and ITI. Though all eight GS’s were presented to participants, prior to analyses, responses to every two GS’s were averaged resulting in four classes of responses to GS’s. This decision to collapse every two intermediaries was due to concerns that treating each of eight GS’s as a separate class would require an unrealistically long experiment (leading to excessive startle habituation and subject fatigue), but having only four gradients-of-size would not allow a gradual enough continuum between CS+ and CS-. Having four classes of intermediaries with two ring sizes in each class seemed a good compromise as the generalization slope was a derivative of eight gradations of CS+ similarity while each intermediary required half as many trials. As displayed in Figure 1, classes of GS’s were numbered such that Class 4 consisted of the two rings closest in size to the CS+ (rings 8 and 9 for counterbalancing group A, rings 3 and 2 for group B), and Classes 3 through 1 consisted of rings progressively increasing in similarity to the CS-. Finally, during the generalization test, 6 of 12 CS+’s coterminated with shock delivery (50% reinforcement schedule) to prevent extinction of the conditioned response during the generalization sequence.
Trials for all 3 phases of the study were arranged in quasi-random order such that no more than two stimuli of the same class occurred consecutively. An additional constraint placed on the ordering of the generalization sequence was the arrangement of trials into 6 blocks of 14 trials (2 CS+, 2 CS-, 2 ITI, and 1 each of rings 2-9) to ensure an even distribution of trial types throughout the generalization run. Finally on odd CS+, CS-, GS, and ITI trials across phases, startle probes were delivered at either 4- or 5-s post-trial onset, and the inter-probe interval was maintained between 18-25 s throughout.
2.6. Procedure
Participants were not instructed of the CS/US contingency but were told that they might learn to predict the shock if they attend to the presented stimuli. Shock electrodes were then attached and a shock workup procedure was completed. Next, EMG electrodes and headphones were placed and a habituation sequence consisting of nine startle probes (ITI = 18-25 s) was run. The three phases of the experiment were then completed with pre-acquisition followed by acquisition and generalization test, with a ten-minute break separating acquisition and generalization test. After acquisition and generalization phases, participants rated levels of anxiety and arousal evoked by CS+ and CS- using 10-point Likert scales (1=none, 5=some, 10=a lot).
2.7. Data Analysis
Startle EMG was rectified and then smoothed (20-ms moving window average). The onset latency window for the blink reflex was 20-100-ms and the peak magnitude was determined within a window of time extending from the time of response onset to 120 ms. Additionally, the average baseline EMG level for the 50 ms immediately preceding delivery of the startle stimulus was subtracted from the peak magnitude. EMG magnitudes across all phases of the study were standardized together using within subject T-score conversions to normalize data and to reduce the influence of between subjects variability unrelated to psychological processes. Because similar results were obtained with the raw and T-scored data, only the results of the T-scored data are presented though descriptive statistics for raw startle magnitudes are available in Table 1. Acquisition of conditioning was analyzed with a 2 (CS-type: CS+, CS-) × 2 (Size: CS+=largest ring vs. CS+=smallest ring) multivariate analysis of variance (MANOVA) with repeated measures. Additionally, generalization effects were analyzed using a 6 (Trial-type: CS+, CS-, Class1, Class 2, Class 3, Class 4) × 2 (Size: CS+=largest ring vs. CS+=smallest ring) MANOVA with repeated measures. MANOVAs were computed using Wilk’s Lambda and were followed, when necessary, by paired samples t-tests. Alpha was set at .05 for all statistical tests and effect sizes were estimated using the unbiased estimator d (Hedges & Olkin, 1985).
Table 1. Means (and standard errors of the mean) for raw startle EMG values in microvolts.
| Testing Phase | ITI | CS- | C1 | C2 | C3 | C4 | CS+ |
|---|---|---|---|---|---|---|---|
| Pre-Acquisition | 34.43 (8.51) |
29.67 (8.28) |
-- | -- | -- | -- | 32.92 (8.23) |
| Acquisition | 26.68 (6.74) |
27.36 (7.51) |
-- | -- | -- | -- | 36.09 (7.37) |
| Generalization | 16.64 (4.45) |
16.14 (3.84) |
19.80 (4.75) |
18.63 (4.63) |
21.94 (5.29) |
26.66 (5.99) |
32.80 (7.64) |
Note: CS+ = conditioned stimulus paired with shock; CS- = conditioned stimulus unpaired with shock; C1, C2, C3, and C4 = generalization stimulus Classes 1, 2, 3, and 4; ITI = inter-trial-interval.
Because CS+ size was not found to interact with dependent measures (startle EMG, reported risk, reaction times) at either pre-acquisition, acquisition, or generalization (all p’s>.20, all d’s<.33), such effects are not reported below. Nevertheless these null effects of CS+ size should be interpreted with caution as the small number of participants receiving the smallest (n=10) or largest ring (n=10) conferred poor statistical power (ß) to such analyses with ß ranging from .10 to .48, indicating false negative rates (i.e., 1 - ß) ranging from .52 to .90. Thus, larger sample sizes will be needed before effects of circle size on conditioning and generalization can be ruled out.
3. Results
3.1. Pre-Acquisition
Prior to conditioning, there was no main effect of CS-type for startle magnitudes (p=.82, d=.07), online risk ratings (p=.24, d=.36), and reaction times (p=.20, d=.40).
3.2. Acquisition
During acquisition, startle was potentiated by the CS+ (M=52.66, SD=4.18) relative to CS- (M=47.35, SD=3.15), F(1,18)=22.69, p=.0002, d=1.02. Consistent with startle data, online ratings of shock risk where higher for CS+ (M=2.75, SD=.24) compared to CS- (M=1.17, SD=.22), F(1,18)=343.68, p<.0001, d=3.98, and CS+ relative to CS- was retrospectively rated as more anxiety provoking (M=8.15, SD=1.69 vs. M=1.85, SD=1.31; t(19)=14.26, p < .0001, d=3.06) and arousing (M=6.35, SD=2.85 vs. M=2.20, SD=1.32; t(19)=7.03, p<.0001, d=1.51). Finally, a trend for shorter reaction times to report risk to the CS+ relative to CS- was found (p=.08, d=.55).
3.3. Generalization Test
3.3.1. Startle EMG
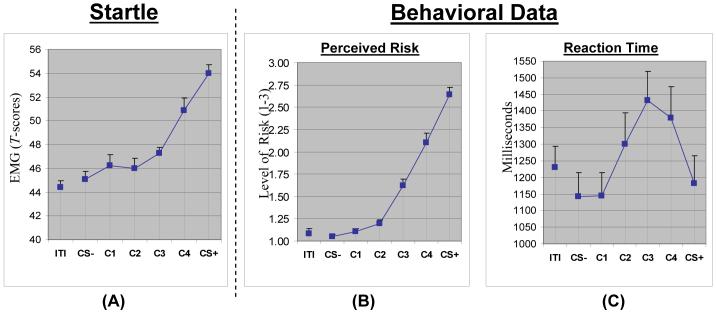
Robust enhancement of startle during CS+ relative to CS- persisted during the generalization sequence, t(90)=7.13, p<.0001, d=1.53 (see Figure 2A). Additionally, a main effect of trial-type was found, F(5, 14)=13.18, p<.0001, d=.78, and trend analyses of this effect revealed both linear, F(1,18)=36.18, p<.0001, d=1.29, and quadratic decreases in startle, F(1,18)=19.45, p<.001, d=.95, with decreasing GS similarity to the CS+ (see Figure 2A). Such results demonstrate the efficacy of the current paradigm for eliciting continuous generalization gradients of conditioned fear-potentiated startle.
Figure 2.
Results for both psychophysiological (standardized startle EMG) and behavioral indices (risk ratings [1= no risk, 2 = some risk, 3 = high risk], reaction times [ms]) of conditioned fear generalization. Error bars reflect the standard error of the mean. CS+ = conditioned stimulus paired with shock; CS- = conditioned stimulus unpaired with shock; C1, C2, C3, and C4 = generalization stimulus Classes 1, 2, 3, and 4; ITI = inter-trial-interval.
In order to identify the point on the continuum-of-similarity at which startle potentiation ceased to generalize, paired samples t-tests were computed with CS- as the reference condition compared against CS+, C4, C3, C2, and C1. The Bonferroni-corrected criterion of significance for these tests was set at p< .05/5 = .01. Results indicate startle potentiation to the CS+ (p<.0001, d=1.49), generalization of this potentiation to C4 (p=.0004, d=.91), a trend for generalization to C3 (p=.015, d=.57), and no generalization to C2 (p=.36, d=.19) or C1 (p=.32, d=.22). Thus generalization of startle potentiation for healthy controls in the current paradigm can be described as extending to C4 but not beyond C3.
3.3.2. Online risk ratings
Consistent with startle data, a main effect of trial-type, F(5,14)=52.44, p<.0001, d=1.55, was found). Additionally, the main effect of trial-type consisted of both linear, F(1,18)=211.04, p<.0001, d=3.12, and quadratic gradients, F(1,18)=42.00, p<.0001, d=1.39, with increasing levels of risk from CS- to C1 to C2 to C3 to C4 to CS+ (see Figure 2B). As was done with startle data, paired samples t-tests were computed with CS- as the reference condition compared against CS+, C4, C3, C2, and C1. Results indicate elevated risk perception to the CS+ (p<.00001, d=3.80) and generalization of this elevation to C4 (p<.00001, d=2.09), C3 (p<.00001, d=1.65), and C2 (p=.001, d=.79), but not C1 (p=.20, d=.29). Thus generalization of risk perception in the current paradigm can be described as extending to C4, C3, and C2 but not C1.
3.3.3. Reaction times
A main effect of trial-type, F(5,14)=10.38, p<.0001, d=.69, was found and consisted of linear, F(1,18)=10.96, p=.003, d=.71, quadratic, F(1,18)=18.72, p<.0004, d=.93, and cubic components, F(1,18)=15.75, p=.001, d=.85 (see Figure 2C). Additionally, CS- was associated with the quickest reaction times, with responses to C1 being equally fast to CS- (p=.96, d=.02); responses to C2, C3, and C4 each significantly slower than CS- (all p’s<.005, d’s>.97); equally fast reaction times for CS+ relative to CS- (p=.36, d=.28); and significantly slower reaction times to C4 and C3 versus CS+ (both p’s<.008, d’s>.90). This pattern of results suggests that participants were more certain of their level of risk (or lack thereof) during CS+ and CS- presentations and less certain during presentations of rings in Classes 4, 3, and 2.
3.3.4. Retrospective ratings
CS+ relative to CS- was rated as more anxiety provoking (M=7.45, SD=1.85 vs. M=1.25, SD=.44; t(19)=13.58, p<.0001, d=2.92) and arousing (M=6.20, SD=3.05 vs. M=2.00, SD=1.12; t(19)=6.70, p<.0001, d=1.44), suggesting that robust aversive conditioning persisted during the generalization sequence.
4. Discussion
Current findings demonstrate the sensitivity of fear-potentiated startle for measuring continuous gradients of conditioned fear-generalization. In particular, startle magnitudes were potentiated by CS+ relative to CS- and gradually decreased when elicited during stimuli falling on a continuum-of-similarity from CS+ to the intermediary classes of generalization stimuli (Class 4, 3 to 2 to 1) to CS-. Similarly, online ratings of perceived risk for shock fell along a downward gradient, with highest ratings to the CS+ and continuous decreases in such ratings corresponding with a gradual decrease in similarity to the CS+. Finally, reaction times for risk ratings indicated quickest response latencies for CS+ and CS-, and significantly longer latencies for stimuli from Classes 4, 3, and 2. This final result suggests that reaction times capture the ‘threat ambiguity’ of the presented stimulus, with faster risk ratings for stimuli with unambiguous safe/threat information (CS+, CS-) and slower response times for stimuli with more uncertain signal value (Classes 2, 3, and 4).
The generalization gradient of conditioned startle potentiation in the current study is consistent with the curvelinear shape of such gradients found in animals: fear responding is highest to the CS+, curves downward for the first and second closest approximations of the CS+, hits floor levels of responding at the third or fourth closest approximation, and remains at floor for the remaining test stimuli (e.g., Thompson, 1962). Similarly, current results demonstrate significant transfer of fear-potentiated startle to the closest approximation of the CS+ (Class 4), a trend for transfer of potentiation to the second closest approximation (Class 3) and no difference in startle magnitudes among the three farthest approximations of the CS+ (CS- and Classes 1-2).
4.1. Inhibitory Versus Excitatory Fear Generalization
Current results, taken together with past findings by Vervliet et al. (2004, 2005), point to strong and weak tendencies toward excitatory and inhibitory fear generalization, respectively, in healthy humans. Whereas present data demonstrate robust generalization of fear excitation to stimuli resembling the CS+, results from Vervliet and colleagues reveal that fear extinction—an inhibitory process— does not generalize from CS+ approximates to the CS+ itself. The presence and absence of generalization across excitatory and inhibitory fear processes is consistent with findings in rodents in which learned fear-excitation (acquisition) but not learned fear-inhibition (extinction) generalizes across experimental contexts (Bouton, 2004). This pattern of findings may result from the high response-cost associated with overgeneralization of inhibition learning resulting in the miss of a “true” danger.
4.2. Predictions for Anxiety Patients Generated from Current Results
Though the shape of the generalization gradient in healthy humans resembles that of animals, the elevated tendency of anxiety patients to transfer conditioned fear to a CS- with strong resemblance to the CS+ (Grillon and Morgan, 1999; Lissek et al., 2005) supports the prediction of less steep generalization gradients among those with clinical anxiety, whereby startle magnitudes would remain elevated during presentation of Classes 3, 2, and perhaps 1 before dropping to CS- levels. Additionally, given that anxious individuals are characterized by a heightened tendency to appraise ambiguous stimuli as threatening (for a review, see Richards, 2004), those with clinical anxiety relative to healthy controls would be expected to display elevated risk ratings for shock when presented with classes of rings containing ambiguous threat information (i.e., Classes 4, 3, and 2), but would display approximately equal risk ratings for rings with more certain signal-value (i.e., CS+, CS-). A final prediction derives from current reaction-time results demonstrating longer latencies to assess risk when presented with more uncertain threat information (i.e., Classes 4, 3, and 2). Whereas healthy controls in the current study displayed a marked increase in response latency to the closest approximation of the CS+ (i.e., Class 4: see Figure 2C), indicating greater threat uncertainty to C4 versus CS+, anxiety patients would be expected to be more certain of their risk for shock during presentation of Class 4, resulting in equally fast reaction times to CS+ and Class 4. Furthermore, healthy controls showed equally fast reaction times during presentation of the closest approximation of the CS- (i.e., Class 1: see Figure 2C), whereas patients would be expected to experience less certainty of their safety during C1, resulting in significantly longer reaction times to C1 relative to CS- among anxiety patients.
5. Conclusion
Though continuous generalization gradients of conditioned fear are a particularly powerful method for assessing rates of fear generalization that have been applied extensively in animal research, to our knowledge, no psychobiological studies over the past 40 years have applied this method in humans. Given the strong relevance of fear generalization to anxiety disorders such as PTSD, an updated paradigm incorporating fear-potentiated startle (FPS) methods was warranted. Present results provide psychophysiological and behavioral validation of a conditioned FPS paradigm capable of eliciting continuous gradients of generalization. This paradigm represents a novel tool with which to study the pharmacology, genetics, and psychobiology of a central, yet understudied, symptom of pathological anxiety.
Acknowledgements
This work was supported by the Intramural Research Program of the National Institute of Mental Health.
Footnotes
There is small literature from the 1950’s and 1960’s testing the influence of anxiety states and traits on fear-irrelevant generalization tasks such as reaction times to target stimuli and stimuli parametrically varying in similarity to the target stimulus (e.g., Mednick, 1957; Rosenbaum, 1953). Because such generalization tasks do not assess the degree to which conditioned fear is transferred from the CS+ to approximations of the CS+, this literature is not considered relevant to the current paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders 4th ed., text revision (DSM-IV-TR) American Psychiatric Press; Washington, DC: 2000. [Google Scholar]
- Armony JL, Servan-Schreiber D, Romanski LM, Cohen JD, LeDoux JE. Stimulus generalization of fear responses: effects of auditory cortex lesions in a computational model and in rats. Cereb Cortex. 1997;7(2):157–165. doi: 10.1093/cercor/7.2.157. [DOI] [PubMed] [Google Scholar]
- Bass MJ, Hull CL. The irradiation of a tactile conditioned reflex in man. Journal of Comparative Psychology. 1934;17:47–65. [Google Scholar]
- Bocker KBE, Baas JMP, Leon Kenemans J, Verbaten MN. Differences in startle modulation during instructed threat and selective attention. Biological Psychology. 2004;67(3):343–358. doi: 10.1016/j.biopsycho.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context and Behavioral Processes in Extinction. Learn. Mem. 2004;11(5):485–494. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- Davis M, Astrachan DI. Conditioned fear and startle magnitude: Effects of different footshock or backshock intensities used in training. J Exp Psychol Anim Behav Process. 1978;4(2):95–103. doi: 10.1037//0097-7403.4.2.95. [DOI] [PubMed] [Google Scholar]
- Filion DL, Dawson ME, Schell AM, Hazlett EA. The relationship between skin conductance orienting and the allocation of processing resources. Psychophysiology. 1991;28:410–424. doi: 10.1111/j.1469-8986.1991.tb00725.x. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) Research version. New York State Psychiatric Institute; New York: 2001. [Google Scholar]
- Foa EB, Steketee G, Rothbaum BO. Behavioral/cognitive conceptualizations of post-traumatic stress disorder. Behavior Therapy. 1989;20(2):155–176. [Google Scholar]
- Foster C, Evans DG, Eeles R, Eccles D, Ashley S, Brooks L, et al. Predictive testing for BRCA1/2: attributes, risk perception and management in a multi-centre clinical cohort. Br J Cancer. 2002;86(8):1209–1216. doi: 10.1038/sj.bjc.6600253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant DA, Schiller JJ. Generalization of the conditioned galvanic skin response to visual stimuli. J Exp Psychol. 1953;46(5):309–313. doi: 10.1037/h0056698. [DOI] [PubMed] [Google Scholar]
- Grillon C. Models and mechanisms of anxiety: Evidence from startle studies. Psychopharmacology. doi: 10.1007/s00213-007-1019-1. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Morgan CA. Fear-potentiated startle conditioning to explicit and contextual cues in Gulf war veterans with posttraumatic stress disorder. Journal of Abnormal Psychology. 1999;108:134–142. doi: 10.1037//0021-843x.108.1.134. [DOI] [PubMed] [Google Scholar]
- Hedges LV, Olkin I. Statistical methods for meta-analysis. Academic Press; San Diego: 1985. [Google Scholar]
- Hitchcock JM, Davis M. Lesions of the amygdala, but not of the cerebellum or red nucleus, block conditioned fear as measured with the potentiated startle paradigm. Behavioral Neuroscience. 1986;100:11–22. doi: 10.1037//0735-7044.100.1.11. [DOI] [PubMed] [Google Scholar]
- Honig WK, Urcuioli PJ. The legacy of Guttman and Kalish (1956): Twenty-five years of research on stimulus generalization. J Exp Anal Behav. 1981;36(3):405–445. doi: 10.1901/jeab.1981.36-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovland CI. The generalization of conditioned responses. IV. The effect of varying amounts of reinforcement upon the degree of generalization of conditioned responses. Journal of Experimental Psychology. 1937;21(3):261–276. [Google Scholar]
- Jackson TD, Hobfoll SE, Jackson AP, Lavin J. Life stressors, mastery, and perceived partner engagement in HIV-Risk behavior. Journal of Community Psychology. 2001;29(1):1–17. [Google Scholar]
- Kalish HI. Stimulus Generalization. In: Marx MH, editor. Learning: Processes. Macmillan; Oxford, England: 1969. [Google Scholar]
- Keane TM, Zimmering RT, Caddell JM. A behavioral formulation of post traumatic stress disorder in Vietnam veterans. Behavior Therapist. 1985;8:9–12. [Google Scholar]
- Kopp B, Schlimm M, Hermann C. Memory-emotional interactions as revealed by fear generalization in animal-fearful individuals. J Behav Ther Exp Psychiatry. 2005;36(2):145–166. doi: 10.1016/j.jbtep.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Emotion and Motivation: Measuring Affective Perception. Journal of Clinical Neurophysiology. 1998;15:397–408. doi: 10.1097/00004691-199809000-00004. [DOI] [PubMed] [Google Scholar]
- Lipp OV, Siddle DAT, Dall PJ. The effect of warning stimulus modality on blink startle modification in reaction time tasks. Psychophysiology. 2000;37(1):55–64. [PubMed] [Google Scholar]
- Lissek S, Powers AS, McClure EB, Phelps EA, Woldehawariat G, Grillon C, et al. Classical fear conditioning in the anxiety disorders: A meta-analysis. Behaviour Research and Therapy. 2005;43(11):1391–1424. doi: 10.1016/j.brat.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Mackintosh NJ. The psychology of animal learning. Academic Press; New York: 1974. [Google Scholar]
- Mednick MT. Mediated generalization and the incubation effect as a function of manifest anxiety. J Abnorm Psychol. 1957;55(3):315–321. doi: 10.1037/h0044440. [DOI] [PubMed] [Google Scholar]
- Mednick SA. Generalization as a function of manifest anxiety and adaptation to psychological experiments. J Consult Psychol. 1957;21(6):491–494. doi: 10.1037/h0045843. [DOI] [PubMed] [Google Scholar]
- Mednick SA, Wild C. Reciprocal augmentation of generalization and anxiety. J Exp Psychol. 1962;63:621–626. doi: 10.1037/h0048772. [DOI] [PubMed] [Google Scholar]
- Mineka S, editor. Evolutionary memories, emotional processing, and the emotional disorders. Vol. 28 1992. [Google Scholar]
- Mineka S, Zinbarg R. A contemporary learning theory perspective on the etiology of anxiety disorders: it’s not what you thought it was. American Psychologist. 2006;61(1):10–26. doi: 10.1037/0003-066X.61.1.10. [DOI] [PubMed] [Google Scholar]
- Pissiota A, Frans O, Michelgard A, Appel L, Langstrom B, Flaten MA, et al. Amygdala and anterior cingulate cortex activation during affective startle modulation: A PET study of fear. European Journal of Neuroscience. 2003;18:1325–1331. doi: 10.1046/j.1460-9568.2003.02855.x. [DOI] [PubMed] [Google Scholar]
- Ratcliff R. Methods for dealing with reaction time outliers. Psychol Bull. 1993;114(3):510–532. doi: 10.1037/0033-2909.114.3.510. [DOI] [PubMed] [Google Scholar]
- Richards A. Anxiety and the resolution of ambiguity. In: Yiend J, editor. Cognition, emotion, and psychopathology: Theoretical, empirical, and clinical directions. Cambridge University Press; Cambridge: 2004. pp. 130–148. [Google Scholar]
- Rosenbaum G. Stimulus generalization as a function of level of experimentally induced anxiety. Journal of Experimental Anxiety. 1953;45:35–43. doi: 10.1037/h0054120. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg P, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Consulting Psychologist Press; Palo Alto, CA: 1983. [Google Scholar]
- Thompson RF. Role of the cerebral cortex in stimulus generalization. J Comp Physiol Psychol. 1962;55:279–287. doi: 10.1037/h0047856. [DOI] [PubMed] [Google Scholar]
- Valls-Sole J, Valldeoriola F, Tolosa E, Nobbe F. Habituation of the auditory startle reaction is reduced during preparation for execution of a motor task in normal human subjects. Brain Research. 1997;751(1):155–159. doi: 10.1016/s0006-8993(97)00027-9. [DOI] [PubMed] [Google Scholar]
- Vervliet B, Vansteenwegen D, Baeyens F, Hermans D, Eelen P. Return of fear in a human differential conditioning paradigm caused by a stimulus change after extinction. Behav Res Ther. 2005;43(3):357–371. doi: 10.1016/j.brat.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Vervliet B, Vansteenwegen D, Eelen P. Generalization of Extinguished Skin Conductance Responding in Human Fear Conditioning. Learn. Mem. 2004;11(5):555–558. doi: 10.1101/lm.77404. [DOI] [PubMed] [Google Scholar]