Abstract
Our purpose was to evaluate the safety and effectiveness of purified isoflavones in producing an increase in plasma isoflavones and a corresponding change in serum sex hormone binding globulin (SHBG) and steroid hormone levels in men diagnosed with early stage prostate cancer. In this Phase II randomized, double-blinded, placebo-controlled trial, 53 prostate cancer patients with a Gleason score of 6 or below were supplemented with 80 mg purified isoflavones or placebo for 12 weeks. Changes in plasma isoflavones, serum steroid hormones, and safety markers were analyzed from baseline to 12 wk. A total of 50 subjects completed the study. Although significant increases in plasma isoflavones (P < 0.001) was observed with no clinical toxicity, the corresponding modulation of serum SHBG, total estradiol, and testosterone in the isoflavone-treated group compared to men receiving placebo was nonsignificant. Increasing plasma isoflavones failed to produce a corresponding modulation of serum steroid hormone levels in men with localized prostate cancer. The study establishes the need to explore other potential mechanisms by which prolonged and consistent purified isoflavone consumption may modulate prostate cancer risk.
Introduction
The American Cancer Society estimates that there will be about 234460 new cases of prostate cancer in the United States in 2006, and about 27350 men will die of this disease (1). The initiation and progression of prostate cancer is a multistep process including several intermediate steps and may involve a complex series of both exogenous and endogenous factors (2–5). Although it is clear that clinical prostate cancer incidence and mortality vary greatly between populations, the frequency of latent prostate cancer is evenly distributed among populations, suggesting that external factors such as diet and other lifestyle factors are important in the transformation from latent into more aggressive, clinical cancer (4–6). These specific features of prostate cancer, namely, high prevalence, long latency between lesions and clinically evident cancer, treatment related morbidity, significant mortality, and morbidity provide the most opportunistic and promising approach for evaluating agents for chemoprevention in men with early stage disease or those who are at high risk for prostate cancer to prevent disease progression and shifting the time of diagnosis to invasive disease.
Epidemiological and laboratory studies have demonstrated that several nutrients, including isoflavones, could induce apoptosis and suppress the formation and growth of human cancers including prostate cancer (7–14). Population studies have consistently reported lower incidence of clinically evident disease in populations consuming isoflavones. An inverse relationship between dietary intake, plasma (8–13), and prostatic fluid (14) concentrations of isoflavones and the incidence of prostate cancer and benign prostatic hyperplasia (BPH) has been observed in these populations, demonstrating the potential role of isoflavones in mediating epigenetic effects. In vitro data have consistently shown that genistein modulates cell proliferation, (15–19), angiogenesis (20–21), tumor cell invasion and tumor metastasis (17,22,23) cell cycle regulation (26), antioxidant (22,25), and induction of apoptotic cell death (26), demonstrating that isoflavones have several cellular effects that are both genomic and nongenomic. Although the mechanism of action of isoflavones is not clear, it has been observed that steroid hormones play an important role in increasing or decreasing the risk of prostate cancer. Patients with prostate cancer have been observed to have higher free testosterone (unbound) levels and lower levels of sex hormone binding globulin (SHBG), estrone (8), and estradiol (27). Androgens are essential for the function and growth of the prostate and are known to stimulate the proliferation of human prostatic cells (28–30). Administration of hormonal therapies has been shown to produce prostate cancer in rodents, whereas castration and estrogen therapy can reduce the risk of prostate cancer (27–30,32). It is clear from recent studies that testosterone and estradiol are important contributors of androgenic and estrogenic activity (34–36). Furthermore, SHBG, because it binds to and sequesters testosterone and estradiol, controls the bioavailability of these sex hormones to target cells as well as their mutual balance. This effect on the active estrogen/testosterone balance may be another potential autoregulatory mechanism for the protective effect of SHBG in prostate cancer (28). Recent evidence suggests that SHBG can function as a hormone with a direct interaction with prostate cells (29). Because the biological activity of testosterone is determined in part by the extent to which it is bound to plasma SHBG, the next logical step would be to look for ways to increase this binding protein levels and reduce serum testosterone levels to reduce the risk of prostate cancer and prevent progression of disease.
To our knowledge, although early pilot trials (33–35) have demonstrated the pharmacokinetics and safety of whole soy and purified isoflavones with single and multiple-dose administration in healthy, early stage, or treated cancer patient cohorts using varying doses of soy and other isoflavones formulations, to date, there have been no randomized, placebo-controlled clinical trials of purified isoflavones on steroid hormones in men with localized prostate cancer. In addition, although there may be multiple pathways by which isoflavones can impact prostate cancer progression, our central hypothesis is that the effect of supplementation with a constant dose of purified isoflavones (vs. a placebo) will produce a corresponding increase in plasma levels of isoflavones, resulting in changes in the serum steroid hormone levels, indicated by an increase in serum SHBG and estradiol and decrease in free testosterone, thereby potentially contributing to a decreased or stabilization of disease progression in men diagnosed with localized prostate cancer. To test this hypothesis, the specific aim of the Phase II randomized, double-blinded, placebo-controlled trial was to recruit and randomize men with early stage prostate cancer to receive purified isoflavones (Prevastein HC® 80mgs/day, IND #61,949 Kumar) vs a placebo and observe the effectiveness of the study agent in producing an increase in plasma levels of isoflavones (daidzein, glycitein, and genistein) and a corresponding decrease in steroid hormones such as free testosterone and increase in SHBG and total estradiol. In addition, our aim was to evaluate compliance to study agents and related toxicity.
Materials and Methods
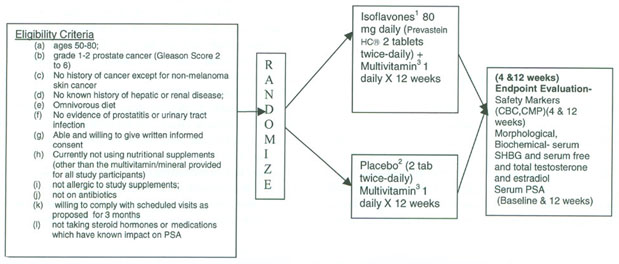
The placebo-controlled, randomized, double-blinded clinical trials study was conducted in a cohort of men recruited from member institutions of the Moffitt Community Clinical Oncology Program (CCOP) Research Base, approved by the institutional review boards at these institutions. Men between the ages of 50 and 80, diagnosed with localized prostate cancer [Gleason Score 2–6; patients with a Gleason primary pattern 4; (4+1 or 4+2) were not eligible] based on pathological assessment from biopsy specimens with no prior or current therapy for prostate cancer or history of cancer except nonmelanoma skin cancer, were eligible. Patients who received neoadjuvant hormonal therapy, vegans, and/or soy users, as ascertained by baseline food records, were excluded from the study. Subjects with a known history of hepatic and/or renal disease, prostatitis, BPH, urinary tract infection, on antibiotics within 30 days of registration, or with a body mass index (BMI) greater than 32 kg/m2 were excluded from the study (Fig. 1, Study Schema).
Figure 1.
Purifled isoflavones in prostate cancer progression: study schema.
On determination of eligibility and the granting of informed consent, patients were registered using a telephone-based registration and randomization system (36) determined by a preset algorithm. These assignments were stratified by Gleason Score (2, 3 vs. 4, 5, and 6), To preserve the double blind, starter supplies of study agents were labeled with numeric codes by the CCOP Research Pharmacy, which housed and dispensed the coded supplements to the investigators. Subjects were assigned to 1 of 2 arms: the intervention arm will be provided supplementation with isoflavones in the form of Prevastein HC, which will deliver 80 mg (40 mg/dose to provide a consistent dose) biologically active isoflavones, and those in the control group will receive an identical placebo providing inert ingredients. Prevastein HC (Archer Daniels Midland Corporation, Decatur, IL) is a botanical test compound containing the glycoside forms of isoflavones (genistein, daidzein, glycerin) with a similar ratio as those found in soy foods. Each tablet contained 40% soy isoflavones in the aglycone form. To avoid possible confounding due to vitamin/mineral deficiencies and prevent the use of other nonstandardized supplements, which may contain isoflavones or other large doses of antioxidants, a standard formulation containing 100% U.S. recommended daily allowance for vitamins (Pan American Lab Company [Miami, FL] brand name PBA Multi Vitaformula) was provided by the investigators to subjects in both groups during the entire study period. The duration of the intervention was 12 wk, with scheduled follow-up at 4 wk and with the study ending at 12 weeks. Subjects provided baseline demographic, anthropometric, medical, and family history of cancer, alcohol, tobacco use, and nutritional history including nutritional supplement use. Subjects completed a weekly 2-day food record, verified by interview to ensure compliance to diet and instruction to avoid isoflavone-rich foods, and records were analyzed using the University of Minnesota Nutrition Data System Research version for analysis of nutrient composition at the Arizona Diet and Behavioral Assessment Center (www.azdiet-behavior.azcc.arizona.edu). Compliance to study agent was monitored by requiring subjects to complete a daily Study Agent Intake log and pill count in addition to analysis of plasma isoflavones (daidzein, glycitein, and genistein) at baseline, 4 wk, and 12 wk using high-performance liquid chromatography and extraction procedure developed by Craft Technologies, Inc. (Wilson, NC). Nonfasting blood samples were drawn for analysis of serum free testosterone, total estradiol, and SHBG at baseline and 12 wk. Safety was monitored by using a daily symptom log and measurement of safety markers at the baseline enrollment visit, 4 wk, and at 12 wk by analysis of a comprehensive metabolic panel (spectrophotometry, ion selective electrode hexokinase) and a complete blood count (electronic cell sizing sorting cytometry/microscopy) at LabCorp Diagnostic Laboratories in Tampa, Florida. In addition, to evaluate and ensure subject safety, any change in medical condition and use of concomitant medications were monitored throughout the study period. All safety and compliance data were collected at baseline, 4, and 12 wk. All adverse events (AEs) were reported on the Adverse Event Case Report Forms regardless of whether they were related to study drug. The severity of the events was determined using the National Cancer Institute Common Terminology Criteria (CTC) for Adverse Events version 2.0 (http://ctep.info.nih.gov). All AEs, including laboratory abnormalities that in the opinion of the study physicians are clinically significant, were followed according to good medical practices and documented. AEs were reported to the Moffitt CCOP Research Base Operations Center, the Food and Drug Administration.
Statistical Method
At the conclusion of the intervention, a pooled t-test was used to compare the 2 group mean change of plasma isoflavone levels, nutrient intake, and serum steroid hormones; or if the equal variances between 2 groups did not meet, a Satterthwaite t-test was used. Paired t-tests were, in addition, used to compare posttreatment vs pretreatment changes for each group in intake of the nutrients and plasma isoflavone concentrations. Paired t-tests were justified in this case. Even if the data are only approximately normally distributed, the test is robust with respect to the normality assumption. These tests were 2-sided at 0.05 significance levels.
Although compliance was monitored, we employed “intent to treat principle” in all group comparisons. Subjects were analyzed according to the group into which they were randomized without regard to compliance or actual diet. Additional analyses, taking compliance into consideration, have been done to compare the plasma isoflavone level change of 2 groups at Weeks 4 and 12 by using t-test. The 2 groups were also compared for the mean change in all steroid hormone levels using the pooled t-test. A 95% confidence interval for the differences of these group means was formed. Finally, the correlation between treatment-associated change in plasma isoflavones and change in steroid hormones were analyzed using Spearman's correlation coefficient. Prior to unblinding, the incidence of toxicity was evaluated. All the analysis was implemented in SAS version 9.
Results
Of a total of 242 men with Grade I-2 prostate cancer screened for the study between 2003–2005, only 53 men met the eligibility criteria and were consecutively admitted to the study. Men who were otherwise eligible, although recommended for active surveillance, opted for the more aggressive and evolving treatment options such as cryosurgery or seed implants in place of active surveillance. A total of 50 men completed the intervention and were able to provide complete data pretreatment and posttreatment, including serum and plasma for analysis. Of the subjects, 3 dropped out of the study, including 1 from the placebo group and 2 from the isoflavone-treated group. Reasons for dropping out of the study included noncompliance to study agent (1 subject) and Grade I to II AEs that resulted in 2 subjects not willing to continue and dropping out of the study. Thus, a 94.3% subject retention rate was achieved in the subjects recruited.
Initial comparison of baseline demographic variables such as age, race, anthropometrics measurements such as height, weight BMI, smoking history, family history of cancer, and personal history of BPH is displayed in Table 1. Although no significant differences were observed in the 2 groups on these variables, notably, over 50% of prostate cancer patients in both groups were former or current smokers and had a mean BMI >25. An increased number of subjects in the placebo group (78.6%) reported current or history of alcohol consumption compared to the placebo group (56.00%; P = 0.08).
Table 1.
Demographic Characteristics of Subjects at Baseline
| Characteristics | Treatment Group | Placebo Group | P Value | |
|---|---|---|---|---|
| N | 25 | 28 | ||
| Age (yr) | 71.75 ± 6.39 | 71.92(±5.59) | 0.92 | |
| Weight (lb) | 180.34 ± 24.51 | 186.57 ± 19.81) | 0.31 | |
| Height (cm) | 176.15 ± 8.55 | 176.43 ± 5.90) | 0.89 | |
| Body mass index (kg/m2) | 26.31 ± 2.49 | 27.18 ± 3.35) | 0.21 | |
| Race, No. (%) | ||||
| White | 23 (92.00) | 27 (96.43) | 0.56 | |
| Black | 1 (4.00) | 1 (3.57) | ||
| Unknown | 1 (4.00) | 0 (0.00) | ||
| Former or current smoker, No. (%) |
||||
| Yes | 16 (64.00) | 15 (53.57) | 0.44 | |
| No | 9 (36.00) | 13 (46.43) | ||
| Former or current alcohol, No. (%) |
0.08 | |||
| Yes | 14 (56.00) | 22 (78.57) | ||
| No | 11 (44.00) | 6 (21.43) | ||
| Family history of cancer, No. (%) |
0.53 | |||
| Yes | 5 (20.83) | 4 (14.29) | ||
| No | 19 (79.17) | 24 (85.71) | ||
| History of benign prostatic hyperplasia, No. (%) |
0.96 | |||
| Yes | 7 (28.00) | 8 (28.57) | ||
| No | 18 (72.00) | 20 (71.43) | ||
Subjects in both groups reported similar average intake of macronutrients and micronutrients at baseline (Table 2). Although protein, fats, cholesterol, and percent fat intake increased slightly in the treatment group during the intervention, this was not reflected as significant changes in weight, as caloric intake remained stable. No significant changes in anthropometric variables such as weight and BMI were observed during the study period.
Table 2.
Change in Plasma Isoflavones at Month 1 and Month 3 by Group
| Treatment Group |
Placebo Group |
P Valuea |
||||
|---|---|---|---|---|---|---|
| Value: Mean Change (SD) (mcg/ml) |
Month 1 Change (mcg/ml) |
Month 3 Change (mcg/ml) |
Month 1 Change (mcg/ml) |
Month 3 Change (mcg/ml) |
Month 1 Change |
Month 3 Change |
| Daidzein | 0.32 (0.23) | 0.189 (0.136) | −0.004 (0.025) | −0.006 (0.0273) | <0.001 | <0.001 |
| Glycitein | 0.005 (0.004) | 0.0035 (0.0052) | −0.00026 (0.0018) | −0.00016 (0.0021) | <0.001 | 0.0126 |
| Genistein | 0.52 (0.36) | 0.378 (0.228) | −0.005 (0.0199) | −0.004 (0.022) | <0.001 | <0.001 |
P compared the change between 2 groups by Satterthwaite t-test.
Changes in plasma isoflavone levels (daidzein, glycitein, and genistein; Table 2) at baseline, 4, and 12 wk were analyzed. Significant increases in plasma daidzein (P < 0.0001), glycitein (P < 0.0001), and genistein (P < 0.0001) were observed from baseline to 4 wk in the treatment group compared to the placebo group. Similarly, significant increases in plasma daidzein (P < 0.0001), glycitein (P = 0.01), and genistein (P < 0.0001) were observed from baseline to 12 wk in the treatment group compared to the placebo group. Compared to plasma isoflavones daidzein and genistein, changes in plasma glycitein were observed to be much lower, although significantly higher in the isoflavone-treated group compared to the placebo.
The baseline and final concentrations of serum free testosterone, SHBG, and total estradiol for the 2 groups are displayed in Table 3. Although greater mean reduction of serum-free testosterone was observed in subjects in the isoflavone-treated group compared to the placebo group, these changes between the 2 groups were not statistically significant for this duration of intervention (P = 0.3). We failed to observe an increase in serum SHBG levels in the treatment group, as hypothesized. Total estradiol decreased in both groups.
Table 3.
Change in Serum Steroid Hormone and SHBG Concentrations From Baseline to Postintervention by Groupa
| Treatment Group (N = 22) |
Placebo Group (N = 27) |
||||||
|---|---|---|---|---|---|---|---|
| Value: Mean (SD) | Baseline | Last Week | Pb | Baseline | Last Week | Pb | P Valuec |
| Total estradiol (pmol/L) | 33.14 (14.77) | 31.72 (15.93) | 0.73 | 32.19 (13.38) | 29.74 (14.11) | 0.43 | 0.37 |
| SHBG (nmol/L) | 45.81 (16.99) | 45.14 (17.04) | 0.6 | 38.37 (14.71) | 37.63 (13.00) | 0.51 | 0.97 |
| Free testosterone (pg/ml) | 11.26 (4.87) | 10.00 (3.18) | 0.15 | 9.38 (2.28) | 8.98 (2.91) | 0.4 | 0.37 |
Abbreviation is as follows: SHBG, sex hormone binding globulin.
P, paired t-test comparing mean at baseline with at week 12 by group.
P, 2-sided P values calculated for testing the difference in changes between treatment groupand the placebo group by pooled t-test or by Satterthwaite t-test if equal variance does not meet.
Related and unrelated or potentially related gastrointestinal, metabolic, and constitutional categories of AEs were all Grades I to II events and were similar in the 2 groups and did not produce clinical toxicity, thus not requiring early stopping or discontinuation of the study agent. Previous clinical trials in men with prostate cancer have reported relatively minor side effects of chronic isoflavone treatment including symptoms related to estrogenic effects such as breast changes, lowered libido, and increased frequency of hot flashes (2). Unlike earlier reports, no symptoms related to estrogenic effects at this dose and period of intervention were observed.
Discussion
In this Phase II trial, evaluation of the effectiveness of intervention was based on the magnitude of change in plasma levels of isoflavones in the isoflavone-supplemented group compared to the placebo group and a corresponding modulation in steroid hormonal levels implicated in prostate cancer that could be achieved. The increased plasma isoflavone levels in subjects given the isoflavone preparation is an important finding, as there have been several reports of significant variation in absorption of isoflavones in the literature and thus bioavailability (37–40). Based on these controversial reports, we paid special attention to dose, multidose regimen to provide a consistent dose per day and the aglycone formulation of the purified isoflavones in this study. In addition, compliance to multidose regimens and formulations of isoflavones has been an issue in other clinical trials. We were able to demonstrate that compliance could be achieved as indicated by a significant increase in plasma diadzein, glycitein, and genistein that was achieved in the isoflavone-treated group throughout the treatment period compared to men in the placebo arm. These observations have implications, in addition, in planning future long-term chemoprevention trials with this agent and in this patient population. In addition, we observed a greater increase in plasma isoflavones at 4 wk relative to the increase observed at 12 wk. This trend may be attributed to a potential stabilization of serum levels of isoflavones or to declining compliance in subject intake of agent with time, although reported compliance measures based on daily agent intake logs and pill counts indicated continued compliance. Future clinical trials of longer duration of intervention in a larger, well-powered sample size may be needed to elucidate the etiology of this observation.
Similar to earlier pilot trials in other prostate cancer patient cohorts, increase in serum levels of isoflavones failed to produce a corresponding, significant modulation of steroid hormone levels in men with localized prostate cancer. This may be attributed to the fact that these, similar to our study, were pilot studies with an intervention of short duration in a disease of significantly long latency. In addition, several potential chemopreventive agents have multiple chemoprevention-associated activities. Some of these activities may be interrelated. Also, a single activity, even if it is the agent's predominant pharmacological activity, may not be the most important or the only one effecting chemoprevention. In addition to hormonal effects, purified isoflavones have been shown to have several cellular effects. We have previously reported that purified isoflavone genistein induces apoptosis and inhibits growth in both androgen-sensitive and androgen-independent prostate cancer cells in vitro (15). Based on these observations, it is clear that there may be multiple pathways contributing to the prostate cancer prevention effects of purified isoflavones. Future clinical trials must address fundamental molecular pathways of isoflavones including those substrates of apoptosis, angiogenesis, and proliferation that have emerged in prior in vitro studies, in addition to changes in steroid hormone levels, that may contribute to the stabilization or inhibiting the progression of prostate cancer.
Acknowledgments and Notes
The research study was funded by the National Institute of Health—National Cancer Institute U10 CA81920.
References
- 1.American Cancer Society . Cancer Facts and Figures, 2006. Atlanta, GA: American Cancer Society; 2006. [Google Scholar]
- 2.Kumar NB, Cantor A, Allen K, Riccardi D, Besterman-Dahan K, et al. The specific role of isoflavones in reducing prostate cancer risk. Prostate. 2004;59:141–147. doi: 10.1002/pros.10362. [DOI] [PubMed] [Google Scholar]
- 3.Bostwick DG, Qian J. High-grade prostatic intraepithelial neoplasia. Mod Pathol. 2004;17:360–379. doi: 10.1038/modpathol.3800053. [DOI] [PubMed] [Google Scholar]
- 4.Bostwick DG, Burke HB, Djakiew D, Euling S, Ho SM, et al. Human prostate cancer risk factors. Cancer Rev. 2004;1(Suppl 10):2371–2490. doi: 10.1002/cncr.20408. [DOI] [PubMed] [Google Scholar]
- 5.Kelloff GJ, Leiberman R, Steele VE, Boone CW, Lubet RA, et al. Chemoprevention of prostate cancer. Eur Urol. 1999;35:342–350. doi: 10.1159/000019906. [DOI] [PubMed] [Google Scholar]
- 6.Klotz L. Active surveillance versus radical treatment for favorable-risk localized prostate cancer. Curr Treat Options Oncol. 2006;7:355–362. doi: 10.1007/s11864-006-0003-z. [DOI] [PubMed] [Google Scholar]
- 7.Miller DC, Gruber SB, Hollenbeck BK, Montie JE, Wei JT. Incidence of initial local therapy among men with lower-risk prostate cancer in the United States. J Natl Cancer Inst. 2006;98:1134–1141. doi: 10.1093/jnci/djj308. [DOI] [PubMed] [Google Scholar]
- 8.Adlercreutz H, Houjo H, Higashi A, Fotsis T, Hämäläinen E, et al. Urinary excretion of lignans and isoflavonoid phytoestrogens in Japanese men and women consuming a traditional Japanese diet. Am J Clin Nutr. 1991;54:1093–1100. doi: 10.1093/ajcn/54.6.1093. [DOI] [PubMed] [Google Scholar]
- 9.Jarred RA, Keikha M, Dowling C, McPherson SJ, Clare AM, et al. Induction of apoptosis in low to moderate-grade human prostate carcinoma by red clover-derived dietary isoflavones. Cancer Epidemiol Biomarkers Prev. 2002;11:1689–1696. [PubMed] [Google Scholar]
- 10.Chan JM, Gann PH, Giovannucci EL. Role of diet in prostate cancer development and progression. J Clin Oncol. 2005;23:8152–8160. doi: 10.1200/JCO.2005.03.1492. [DOI] [PubMed] [Google Scholar]
- 11.Blumenfeld AJ, Fleshner N, Casselman B, Trachtenberg J. Nutritional aspects of prostate cancer: a review. Can J Urol. 2000;7:927–935. [PubMed] [Google Scholar]
- 12.Brossner C, Petritsch K, Fink K, Auprich M, Madersbacher S, et al. Phytoestrogen tissue levels in benign prostatic hyperplasia and prostate cancer and their association with prostatic diseases. Urology. 2004;64:707–711. doi: 10.1016/j.urology.2004.04.046. [DOI] [PubMed] [Google Scholar]
- 13.Shimizu H, Ross RK, Bernstein L, Yatani R, Henderson BE, et al. Cancers of the prostate and breast among Japanese and white immigrants in Los Angeles County. Br J Cancer. 1991;63:963–966. doi: 10.1038/bjc.1991.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morton MS, Chan PS, Cheng C, Blacklock N, Matos-Ferreira A, et al. Lignans and isoflavonoids in plasma and prostatic fluid in men: samples from Portugal, Hong Kong, and the United Kingdom. Prostate. 1997;32:122–128. doi: 10.1002/(sici)1097-0045(19970701)32:2<122::aid-pros7>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 15.Fotsis T, Pepper M, Adlecruetz H, Hase T, Monttesano R, et al. Genistein, a dietary ingested isoflavonoid, inhibits cell proliferation and in Vitro angiogenesis. J Nutr. 1995;125:790–797. doi: 10.1093/jn/125.suppl_3.790S. [DOI] [PubMed] [Google Scholar]
- 16.Messina M, Barnes S. The role of soy products in reducing risk of cancer. J Natl Cancer Inst. 1991;83:541–546. doi: 10.1093/jnci/83.8.541. [DOI] [PubMed] [Google Scholar]
- 17.Bemis DL, Capodice JL, Desai M, Buttyan R, Katz AE. A concentrated aglycone isoflavone preparation (GCP) that demonstrates potent anti-prostate cancer activity in vitro and in vivo. Clin Cancer Res. 2001;10:5282–5292. doi: 10.1158/1078-0432.CCR-03-0828. [DOI] [PubMed] [Google Scholar]
- 18.Jagadeesh S, Kyo S, Banerjee PP. Genistein represses telomerase activity via both transcriptional and posttranslational mechanisms in human prostate cancer cells. Cancer Res. 2006;66:2107–2115. doi: 10.1158/0008-5472.CAN-05-2494. [DOI] [PubMed] [Google Scholar]
- 19.Ouchi H, Ishiguro H, Ikeda N, Hori M, Kubota Y, et al. Genistein induces cell growth inhibition in prostate cancer through the suppression of telomerase activity. Int J Urol. 2005;12:73–80. doi: 10.1111/j.1442-2042.2004.00973.x. [DOI] [PubMed] [Google Scholar]
- 20.Adlercreutz H, Mousavi Y, Clark J, Höckerstedt K, Hamalainen E, et al. Dietary phytoestrogens and cancer: in vitro and in vivo studies. J Steroid Biochtm Mol Biol. 1992;41:331–337. doi: 10.1016/0960-0760(92)90359-q. [DOI] [PubMed] [Google Scholar]
- 21.Skogseth H, Larsson E, Halgunset J. The invasive behaviour of prostatic cancer cells is suppressed by inhibitors of tyrosine kinase. APMIS. 2006;114:61–66. doi: 10.1111/j.1600-0463.2006.apm_230.x. [DOI] [PubMed] [Google Scholar]
- 22.Huang X, Chen S, Xu L, Liu Y, Deb DK, et al. Genistein inhibits p38 map kinase activation, matrix metalloproteinase type 2, and cell invasion in human prostate epithelial cells. Cancer Res. 2005;65:3470–3478. doi: 10.1158/0008-5472.CAN-04-2807. [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Kucuk O, Hussain M, Abrams J, Cher ML, et al. Antitumor and antimetastatic activities of docetaxel are enhanced by genistein through regulation of osteoprotegerin/receptor activator of nuclear factor-kappaB (RANK)/RANK ligand/MMP-9 signaling in prostate cancer. Cancer Res. 2006;66:4816–4825. doi: 10.1158/0008-5472.CAN-05-3752. [DOI] [PubMed] [Google Scholar]
- 24.Handayani R, Rice L, Cui Y, Medrano TA, Samedi VG, et al. Soy isoflavones alter expression of genes associated with cancer progression, including interleukin-8, in androgen-independent PC-3 human prostate cancer cells. J Nutr. 2006;136:75–82. doi: 10.1093/jn/136.1.75. [DOI] [PubMed] [Google Scholar]
- 25.Raschke M, Rowland IR, Magee PJ, Pool-Zobel BL. Genistein protects prostate cells against hydrogen peroxide-induced DNA damage and induces expression of genes involved in the defense against oxidative stress. Carcinogenesis. 2006;27:2322–2330. doi: 10.1093/carcin/bgl082. [DOI] [PubMed] [Google Scholar]
- 26.Kazi A, Daniel KG, Smith DM, Kumar NB, Dou QP. Inhibition of the proteasome activity, a novel mechanism associated with the tumor cell apoptosis-inducing ability of genistein. Biochem Pharmacol. 2003;66:965–976. doi: 10.1016/s0006-2952(03)00414-3. [DOI] [PubMed] [Google Scholar]
- 27.Barnes S. Effect of genistein on in vitro and in vivo models of cancer. J Nutr. 1995;125:777s–783s. doi: 10.1093/jn/125.3_Suppl.777S. [DOI] [PubMed] [Google Scholar]
- 28.Adlercreutz H, Mazur W, Bartels P, Elomaa V, Watanabe S, et al. Phytoestrogens and prostate disease. J Nutr. 2000;130:658S–659S. doi: 10.1093/jn/130.3.658S. [DOI] [PubMed] [Google Scholar]
- 29.Parsons JK, Carter HB, Platz EA, Wright EJ, Landis P, et al. Serum testosterone and the risk of prostate cancer: potential implications for testosterone therapy. Cancer Epidemiol Biomarkers Prev. 2005;14:2257–2260. doi: 10.1158/1055-9965.EPI-04-0715. [DOI] [PubMed] [Google Scholar]
- 30.Carruba G. Estrogens and mechanisms of prostate cancer progression. Ann NY Acad Sci. 2006;1089:201–217. doi: 10.1196/annals.1386.027. [DOI] [PubMed] [Google Scholar]
- 31.Hempstock J, Kavanagh JP, George NJR. Growth inhibition of prostate cell lines in vitro by phytoestrogens. Br J Urol. 1998;82:560–563. doi: 10.1046/j.1464-410x.1998.00769.x. [DOI] [PubMed] [Google Scholar]
- 32.Schleicher R, Zheng M, Zhang M, Lamartiniere CA. Genistein inhibition in prostate cancer cell growth and metastasis in vivo; Proceedings of the 2nd International Symposium on the Role of Soy in Preventing and Treating Chronic Disease; Belgium. 1996. [Google Scholar]
- 33.Fischer L, Mahoney C, Jeffcoat AR, Koch MA, Thomas BE, et al. Clinical characteristics and pharmacokinetics of purified soy isofiavones: multiple-dose administration to men with prostate neoplasia. Nutr Cancer. 2004;48:160–170. doi: 10.1207/s15327914nc4802_5. [DOI] [PubMed] [Google Scholar]
- 34.Busby MG, Jeffcoat AR, Bloedon LT, Koch MA, Black T, et al. Clinical characteristics and pharmacokinetics of purified soy isoflavones: single-dose administration to healthy men. Am J Clin Nutr. 2002;75:126–136. doi: 10.1093/ajcn/75.1.126. [DOI] [PubMed] [Google Scholar]
- 35.Hussain M, Banerjee M, Sarkar FH, Djuric Z, Pollak MN, et al. Soy isoflavones in the treatment of prostate cancer. Nutr Cancer. 2003;47:111–117. doi: 10.1207/s15327914nc4702_1. [DOI] [PubMed] [Google Scholar]
- 36.Papaconstantinou C, Krischer JP. An automated patient registration and treatment randomization system. J Med Syst. 1995;19:445–456. doi: 10.1007/BF02260848. [DOI] [PubMed] [Google Scholar]
- 37.Kano M, Takayanagi T, Harada K, Sawada S, Ishikawa F. Bioavailability of isoflavones after ingestion of soy beverages in healthy adults. J Nutr. 2006;136:2291–2296. doi: 10.1093/jn/136.9.2291. [DOI] [PubMed] [Google Scholar]
- 38.Cassidy A. Factors affecting the bioavailability of soy isoflavones in humans. J AOAC Int. 2006;89:1182–1188. [PubMed] [Google Scholar]
- 39.Anupongsanugool E, Teekachunhatean S, Rojanasthien N, Pongsatha S, Sangdee C. Pharmacokinetics of isoflavones, daidzein and genistein, after ingestion of soy beverage compared with soy extract capsules in postmenopausal Thai women. BMC Clin Pharmacol. 5:2. doi: 10.1186/1472-6904-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Setchell KD, Brown NM, Zimmer-Nechemias L, Brashear WT, Wolfe BE, et al. Evidence for lack of absorption of soy isoflavone glycosides in humans, supporting the crucial role of intestinal metabolism for bioavailability. Am J Clin Nutr. 2002;76:447–453. doi: 10.1093/ajcn/76.2.447. [DOI] [PubMed] [Google Scholar]