Abstract
Background & Aims
Maintaining the integrity of networks of interstitial cells of Cajal (ICC) is essential to preserve orderly contractile activity and neuroregulation in the gastrointestinal tract and to restore these functions after tissue damage or surgeries. Maintenance of ICC requires insulin- or insulin-like growth factor-I (IGF-I)-dependent production of membrane-bound stem cell factor (SCF) and may involve regeneration from local progenitors. Our goal was to identify ICC precursors in postnatal murine gastric muscles.
Methods
We used flow cytometry and immunohistochemistry to examine freshly dissected and cultured muscles for cells expressing CD34, an adhesion molecule expressed by stromal tumors; CD44, which occurs on mesenchymal stem cells; and receptors for SCF (Kit), insulin (Insr) and IGF-I (Igf1r). Slow waves were studied by intracellular recording.
Results
In gastric muscles we identified rare, KitlowCD44+CD34+Insr+Igf1r+ cells resembling common embryonic precursors of ICC and smooth muscle. These putative progenitors were absent from organotypic cultures lacking mature ICC (Kit+CD44+CD34−Insr−Igf1r−) due to prolonged insulin/IGF-I deprivation but were rescued by IGF-I that also prevented ICC loss. Soluble SCF failed to prevent the loss of mature ICC but dramatically expanded the putative progenitors, which supported robust slow wave activity despite retaining an immature, Kit+CD44+CD34+Insr+Igf1r+ phenotype. Differentiation of these cells into mature, network-forming ICC required IGF-I. Conversely, restoration of ICC networks by IGF-I after prolonged insulin and IGF-I deprivation required the survival of the presumed progenitors.
Conclusions
KitlowCD44+CD34+Insr+Igf1r+ cells may be local progenitors for gastric ICC and stromal tumors. Loss of these cells may contribute to gastrointestinal dysmotilities.
INTRODUCTION
Interstitial cells of Cajal (ICC) play critical roles in gastrointestinal motility. Multipolar myenteric ICC (ICC-MY) in phasic muscles generate and actively propagate electrical slow waves required for orderly segmenting and peristaltic contractions1,2. Elongated intramuscular ICC (ICC-IM) mediate efferent inputs to smooth muscle cells and the pacemaker apparatus3 and relay afferent mechanical signals4.
ICC are reduced or otherwise affected in several dysmotilities including achalasia, diabetic and idiopathic gastroparesis, mechanical ileus, intestinal pseudo-obstructions, slow-transit constipation, inflammations and malformations5–10. ICC depletion is probably central to the pathogenesis of these disorders and patients would likely benefit from its reversal. The mechanisms of ICC loss remain unclear. ICC depend on stem cell factor (SCF) signaling via Kit for development and maintenance11–17. Therefore, disparate pathological conditions could lead to ICC loss by affecting the SCF/Kit pathway. For example, we have reported that ICC depletion in murine diabetic gastroparesis7 may be the consequence of decreased SCF production by the smooth muscle17, which becomes dystrophic due to reduced insulin/insulin-like growth factor-I (IGF-I) signaling18.
Depletion of ICC could result from increased attrition of mature cells and/or impaired regeneration. ICC have considerable regenerative capacity, which could restore their networks after partial mechanical obstruction8, inflammation9,10, surgical transection and anastomosis10,19, or correction of pyloric hypertrophy5. It is, however, unclear if regeneration is from mature ICC or from local reserve/progenitor cells. Progenitors for ICC have not been positively identified in adult tissues. Prenatally, ICC develop from Kit+ mesodermal mesenchymal precursors11,12,14. ICC-IM of the foregut may also develop from ventrally emigrating neural tube cells20. Progenitors committed to ICC have also been described during the early postnatal period21,22. ICC precursors may persist in adulthood although their numbers are likely very small. It has been suggested that gastrointestinal stromal tumors (GIST) may originate from ICC precursors due to activating mutations of Kit23,24. Because most GIST also express CD3423,24, an adhesion molecule also reported in some ICC23, it has been proposed that a CD34+ subset of ICC may give rise to GIST25 and represent ICC progenitors. Although widespread expression of CD34 by ICC has been questioned26, the existence of very small populations of CD34+ cells expressing low levels of Kit and, possibly, not resembling ICC, cannot be discounted.
In this study we attempted to identify ICC progenitors in murine gastric muscles by flow cytometry and immunohistochemistry using antibodies against Kit, CD34, insulin receptors (Insr), IGF-I receptors (Igf1r), and CD44, a mesenchymal stem cell marker27. We report that in both juvenile and adult tissues, clusters of rare cells with KitlowCD44+CD34+Insr+Igf1r+ phenotype and resembling embryonic ICC precursors11,14 could be expanded with soluble SCF and converted into mature, slow wave-producing ICC with IGF-I. These cells may, therefore, represent adult ICC progenitors.
METHODS
Adult and 9–18-day-old BALB/c mice were maintained and the experiments performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals. Protocols were approved by the Institutional Animal Use and Care Committee at the University of Nevada, Reno. Detailed description of the methods listed below can be found in the Supplement.
Organotypic cultures of corpus+antrum muscles13,17,18 were maintained with normoglycemic, serum-free Medium 199 (Sigma, St. Louis, MO), which was used either alone (“basal media”) or supplemented with murine SCF (50 or 100 ng/mL), IGF-I (100 ng/mL) and interferon-γ (IFNγ; 100 U/mL) as indicated (all from Sigma). Electrical slow waves were recorded by intracellular techniques13.
Freshly dissected or cultured gastric tissues were immunostained using established techniques17. Confocal microscopy was performed using a Zeiss (Thornwood, NY) LSM 510 META system. Post-acquisition modification was limited to maximum-transparency projection, noise reduction, assignment of pseudocolor, and adjustment of brightness and contrast. Quantification of cells expressing Kit, CD34 and CD44 in dissociated corpus+antrum muscles was by flow cytometry according to previously published protocols17 with modifications using a Beckman Coulter (Fullerton, CA) XL/MCL flow cytometer. Listmode data files were analyzed with FlowJo (Tree Star, Ashland, OR).
Data are expressed as mean±SEM. One-way ANOVA or Kruskal–Wallis one-way ANOVA on ranks followed by all-pairwise multiple comparisons (Tukey or Dunn’s test, respectively) were used for statistical comparisons. A probability value of P<0.05 was used as a cut-off for statistical significance.
RESULTS
We first attempted to identify ICC progenitors by analyzing the expression of Kit, CD44 and CD34 in enzymatically dispersed corpus+antrum muscles of 7–14-day-old BALB/c mice by flow cytometry, a technique that can reliably detect small cell populations (Figure 1). ICC were identified as strongly Kit+ cells lacking the myeloid markers F4/80 and CD11b and the hematopoietic marker CD45. This gating scheme excluded macrophages, dendritic and mast cells17 (Figure 1B). In 10 tissues, ICC (Kit+ subsets in Figure 1C–D) represented 3.37±0.28% of events with light scatter properties characteristic of live cells. Surprisingly, all ICC expressed CD44 (Figure 1C). Only a very small fraction of the Kit− cells was CD44+. CD34 was mainly present in Kit− cells26 and only a very small proportion of Kit+ ICC expressed any CD34 (Kit+CD34low cells; 0.36±0.17% of Kit+ ICC representing 0.01±0.004% of all cells; Figure 1D). A small but clearly recognizable population with low levels of Kit was positive for CD34 (KitlowCD34+) and represented 0.62±0.14% of all cells. KitlowCD34+ cells also expressed CD44 (Figure 1E–F).
Figure 1.
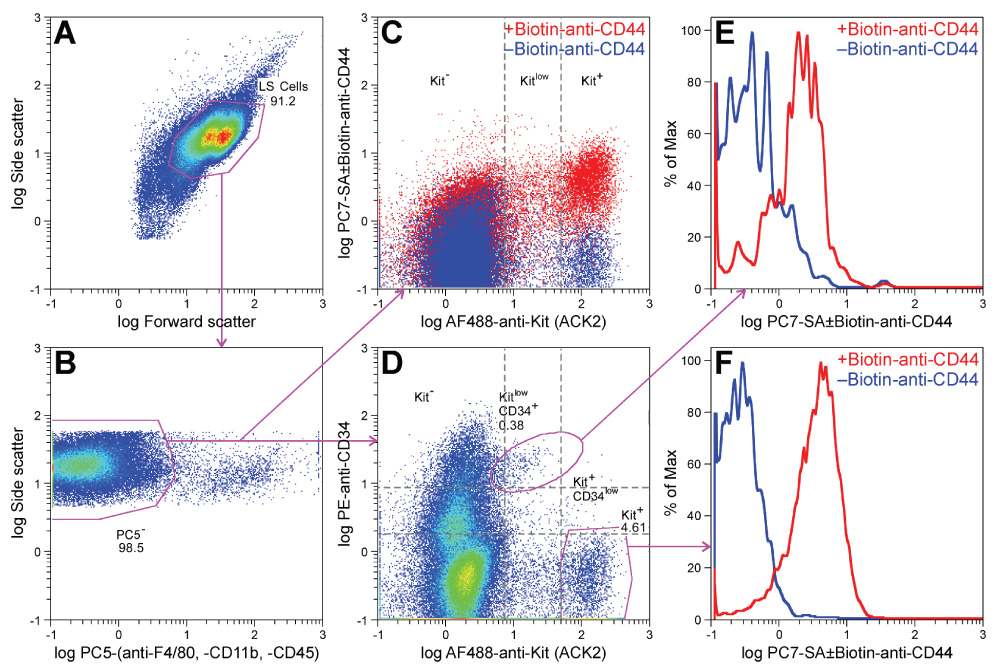
Identification of KitlowCD44+CD34+ presumed ICC progenitors in dispersed postnatal murine gastric corpus+antrum muscles by flow cytometry. A representative of 10 experiments is shown. Numbers indicate cell frequencies (%). (A) Gates used for selecting cells with light scatter properties characteristic of live cells (LS cells). (B) Gates used for detecting cells that do not express macrophage markers (F4/80, CD11b) and the general hematopoietic marker CD45 (phycoerythrine-cyanine 5− (PC5−) cells). (C) Expression of CD44 in the PC5− population shown by an overlay of two aliquots of the same sample labeled either with biotin-anti-CD44 plus phycoerythrine-cyanine 7 (PC7)-streptavidin (SA) (red) or with PC7-SA only (control; blue). Kit was detected with Alexa Fluor (AF) 488-anti-Kit antibodies. (D) Relationship between Kit and CD34 expression (detected with phycoerythrine (PE)-anti-CD34 antibodies) in the PC5− population. (E, F) Histogram representation of CD44 expression in the KitlowCD34+ subset and in the Kit+ ICC, respectively.
We studied the morphology of these cells by immunohistochemistry (n=8). Consistent with the flow cytometry results, all mature, Kit+ ICC expressed moderate levels of CD44 (Figure 2A–C; Figure S1A–D). Most CD34 immunofluorescence was unrelated to ICC. However, we occasionally found weak CD34 expression in some ICC-IM of the circular muscle layer (arrows in Figure S1A–D). These cells probably corresponded to the Kit+CD34low population detected by flow cytometry. A very small population of strongly CD34+ cells that did not resemble ICC also expressed Kit and CD44. These cells were round or oval-shaped, had no or only short processes, and formed small clusters in the myenteric region (Figure 2D–F), on the submucosal surface of the circular muscle layer (Figure S1E–H), and on the serosal surface of the longitudinal muscle. CD34+ cells with the same morphology also expressed Insr and Igf1r α subunits (Figure S2), which we never detected in mature ICC17. These rare clusters did not occur preferentially in any particular region of the tissues and could only be found by systematically surveying all whole-mounts. Because of their KitlowCD44+CD34+ phenotype, low frequency, expression of Insr and Igf1r, and undifferentiated morphology resembling that of embryonic ICC progenitors11,14, we designated these cells as “presumed ICC precursors”.
Figure 2.
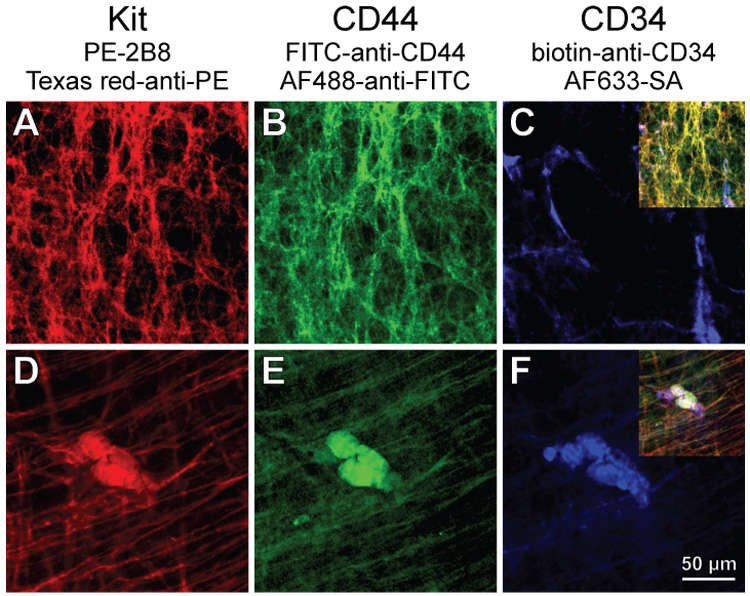
Identification of cells co-expressing Kit (A,D), CD44 (B,E) and CD34 (C,F) in the myenteric region of the gastric antrum of juvenile mice by immunohistochemistry. FITC, fluorescein isothiocyanate. Insets in C and F show digital overlays. (A–C) Mature ICC-MY expressing Kit and CD44 but not CD34. (D–F) Co-localization of CD34 with variable levels of Kit and CD44 in a rare cluster of small, round cells.
To establish the KitlowCD44+CD34+ cells’ relationship with ICC, we tried to manipulate them in their niche by exposing organotypic cultures of intact gastric corpus+antrum muscles to conditions known to affect ICC13,17,18. In agreement with our previous findings17,18, culturing juvenile (day 14–16; n=6) tissues for 85 days with unsupplemented, serum-free basal media resulted in a near-complete loss of Kit+ ICC, and we also found no evidence of survival of the presumed ICC precursors (Figure 3A, C). Relative to freshly dissected tissues, a significant decrease in the Kit+/low population was detectable by flow cytometry as early as 50 days of culturing (Figure 4A, E). In adult tissues (n=3), these changes occurred even earlier and became evident after 32 days, when most areas were completely devoid of Kit+ cells (Figure 3E) and the few remaining ICC displayed faint Kit immunofluorescence and formed small, disrupted networks (Figure 3F). Surprisingly, in some of the tissues cultured for 85 days and lacking Kit+ ICC, we found disrupted networks of Kit−CD44+ cells with morphology resembling ICC networks, primarily in the myenteric region (Figure 3B, D). Kit−CD44+ cells were also recognizable as a weakly stained but well-defined cluster by flow cytometry (Figure S3) and, unlike in freshly dissociated tissues where they were very few, represented 2.91±0.17% of all cells (n=9). Despite the lingering of Kit−CD44+ cells in some of these cultures, and in agreement with previous results17,18, we were unable to detect any oscillatory activity in 3 juvenile tissues cultured for 70 days under identical conditions. Similarly, in 70-day growth factor-deprived adult tissues we only recorded sporadic slow waves in 2 of 22 impalements and the muscles were depolarized (Figure 5 “70d Basal media”). Thus, the loss of Kit+ ICC was paralleled by the apparent loss of the presumed precursors and lack of slow waves. This and the depolarization of the smooth muscle cells13 also suggest that the residual Kit−CD44+ cells probably represented nonfunctional, possibly senescent, ICC.
Figure 3.
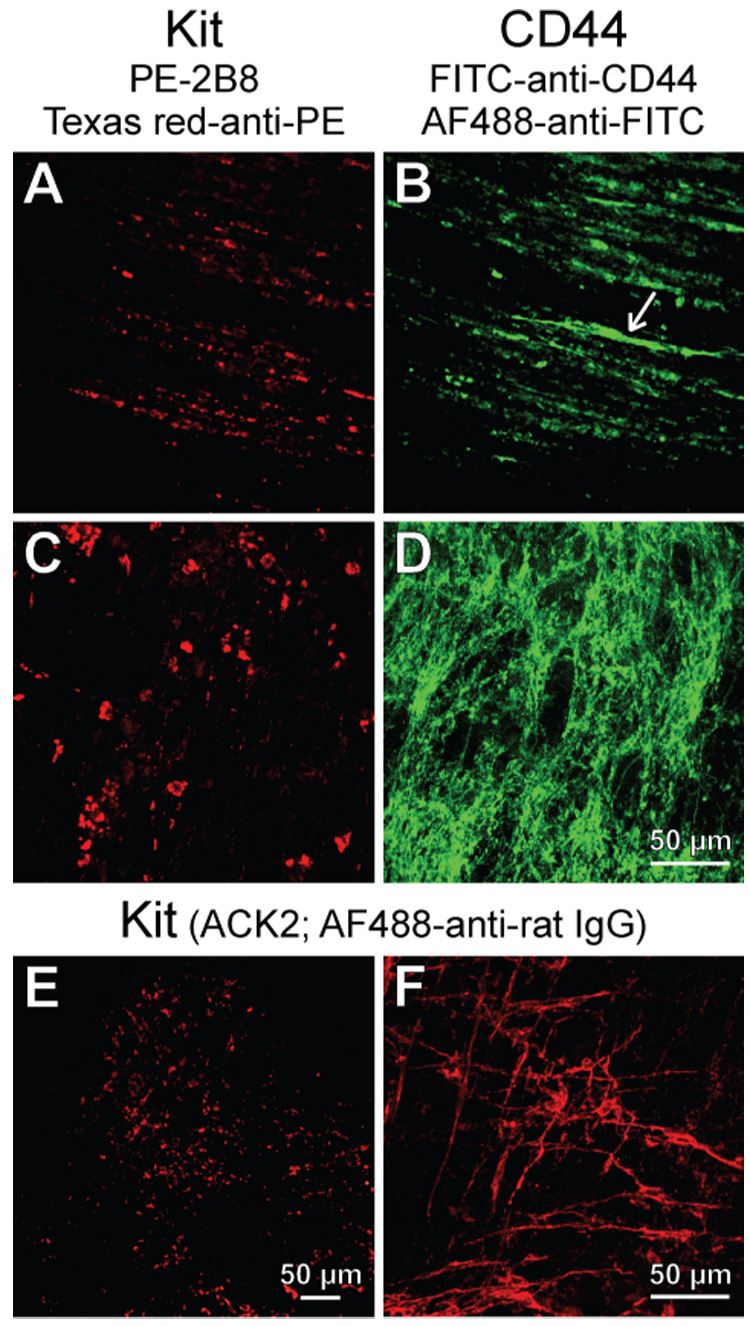
Loss of Kit+ cells in long-term growth factor-deprived cultures. (A–D) Juvenile gastric corpus+antrum tissues maintained with unsupplemented basal media for 85 days. Kit (A,C) and CD44 (B,D) immunofluorescence in the same fields-of-view. Note Kit−CD44+ cells in the circular muscle (arrow in B) and networks of Kit−CD44+ cells in the myenteric region (D). (E,F) Kit immunofluorescence from adult gastric corpus+antrum tissues cultured with unsupplemented, normoglycemic basal media for 32 days. Residual Kit+ ICC-MY were only found in the mid-corpus (F).
Figure 4.
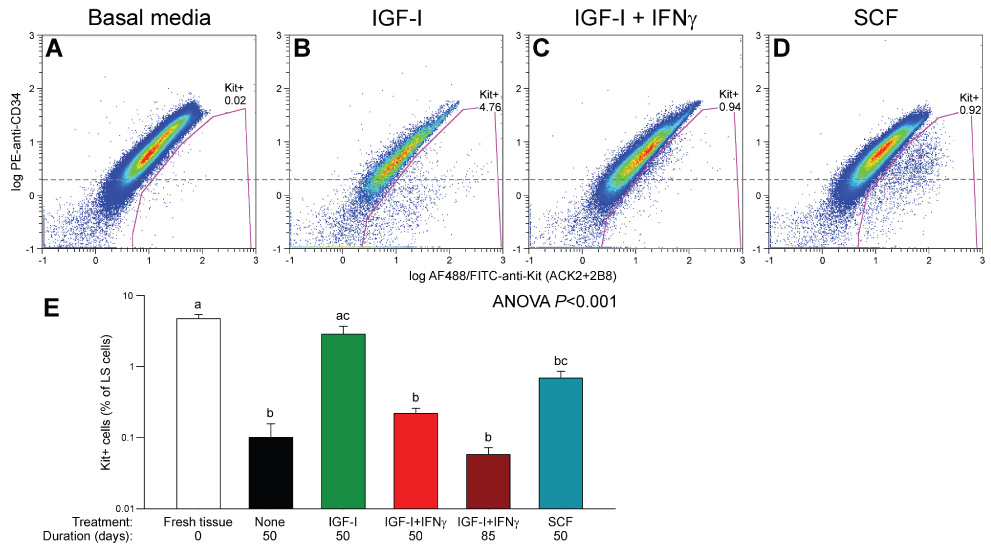
Flow cytometry analysis of Kit and CD34 in organotypic cultures of juvenile gastric corpus+antrum muscles. Tissues were maintained with unsupplemented basal media (A; n=5), 100 ng/ml IGF-I (B; n=5), 100 ng/ml IGF-I+100 U/ml IFNγ (C; n=3) or 50 ng/ml SCF (D; n=3) for 50 days. Gating was performed as in Figure 1A,B; only LS+PC5− cells are shown. Numbers indicate cell frequencies (%). Dashed line represents the approximate boundary between CD34+ and CD34− cells. Most Kit+ cells were CD34− in the IGF-I-treated tissues (B) and CD34+ in the SCF-treated muscles (D). (E) Statistical comparison of Kit+ cells in the above groups, freshly dissected juvenile muscles (n=5) and tissues treated with IGF-I+IFNγ for 85 days (n=3) by ANOVA following arcsin square root transformation. Groups not sharing the same superscript were different by all-pairwise Tukey test.
Figure 5.
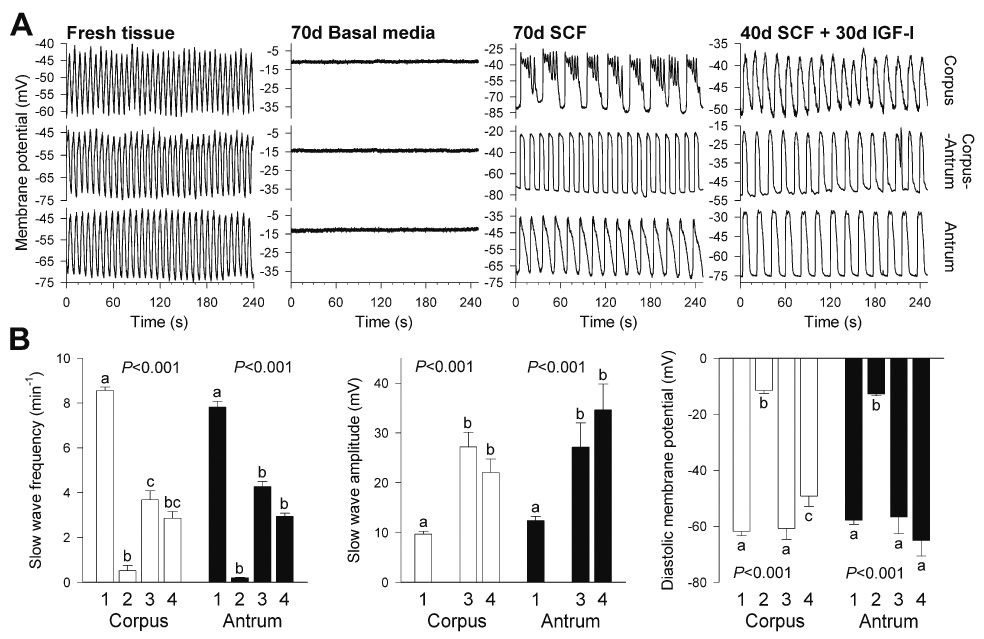
Effects of in vitro treatments on gastric electrical slow wave activity in organotypic cultures of adult gastric corpus+antrum muscles. (A) Representative recordings from freshly dissected (n=5) and cultured tissues (n=3 each) maintained for 70 days with basal media, 100 ng/mL SCF or sequential application of 100 ng/mL SCF (40 days) and 100 ng/mL IGF-I (30 days). (B) Quantitative analysis of slow wave parameters. Bars are labeled as follows: (1) fresh tissue (corpus: n=31 recordings; antrum: n=46); (2) basal media (corpus: n=13; antrum: n=9); (3) SCF (corpus: n=16; antrum: n=12); (4) SCF followed by IGF-I (corpus: n=14; antrum: n=9). Growth factor-deprived cultures, which only displayed sporadic, irregular slow waves in 2 out of 22 recordings, were excluded from the analysis of slow wave amplitudes. P values are from one-way ANOVA or Kruskal–Wallis one-way ANOVA on ranks. Groups not sharing the same superscripts were different by post-hoc multiple comparisons.
In agreement with our previous reports17,18, this loss of ICC could be completely prevented by 100 ng/mL IGF-I (n=3) and the rescued ICC had a mature, Kit+CD44+CD34− phenotype (Figure 4B, E; Figure S4A–D). Rare clusters of presumed ICC precursors were also preserved (Figure S4E–H). The IGF-I effects were probably mediated by SCF17 because they were blocked by IFNγ, an inhibitor of Kit expression28 (Figure 4C, E; Figure S4I–L) (n=4).
Next we stimulated the presumed ICC progenitors with SCF, the natural ligand for Kit. As expected15,16, high concentrations of soluble SCF for 32 days (adult tissues; 100 ng/mL) or 50 days (juvenile tissues; 50 ng/mL) failed to support mature ICC and most areas were almost completely devoid of these cells. However, in all 6 tissues studied, soluble SCF increased the number of the presumed precursors and caused their focal expansion into chords (densely packed cells occurring in one or two parallel rows) extending between smooth muscle fibers (Figure 6A) or clusters in the myenteric region (Figure 6B) and on the submucosal and serosal surfaces. Clusters on the submucosal surface (asterisk in Figure 6C) resembling those found in freshly dissected muscles (Figure S1E–H) or in organotypic cultures maintained with IGF-I (Figure S4E–H) gave rise to chords extending along the smooth muscle fibers and deeper into the circular muscle (arrows in Figure 6C). These clusters and chords appeared randomly throughout the corpus and antrum. The SCF-stimulated cells retained their immature, oval-shaped morphology; had only short or no processes and remained tightly packed, showing strong resemblance to embryonic ICC progenitors11,14. Although these cells were now strongly Kit+, they also continued to express CD34 (Figure 4D) and CD44 (not shown) and occurred at significantly lower frequency than Kit+ ICC in normal tissues (Figure 4E). In 3 adult tissues, increasing the duration of treatment to 61 to 76 days caused these cells’ further growth into nearly continuous sheets (Figure 6D–F). However, they still failed to differentiate into mature ICC and retained an immature phenotype characterized by lack of long processes and expression of Insr/Igf1r and CD34. Surprisingly, and despite the apparent lack of mature ICC networks, these tissues displayed robust slow wave activity (Figure 5A “70d SCF”). Whereas the frequency of the slow waves remained at relatively low levels characteristic of cultured tissues18, their amplitude was significantly higher than in freshly dissected muscles (Figure 5B). Smooth muscle depolarization was also prevented. Since these cells also became phenotypically more similar to mature ICC by virtue of strong expression of Kit and generation of slow waves and somewhat reduced expression of CD34 and Insr/Igf1r, we designated them as “committed ICC progenitors”.
Figure 6.
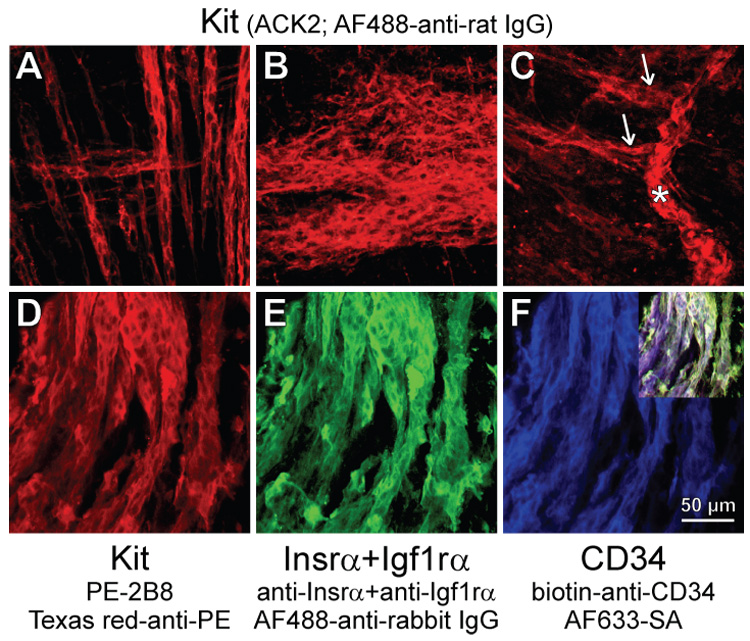
Loss of mature ICC and expansion of clusters of presumed ICC progenitors in organotypic cultures maintained with soluble SCF. (A,B) Presumed ICC precursors in adult gastric muscles cultured for 36 days with 100 ng/ml SCF. (A) Precursors forming chords within the circular and longitudinal muscle layers in the orad corpus. (B) Cluster of presumed precursors in the myenteric region of the antrum. (C) Presumed precursors in a day 14 distal antrum cultured with 50 ng/ml SCF for 50 days. Note cluster on the submucosal surface (asterisk) giving rise to chords extending along muscle fibers (arrows) and the lack of mature ICC. (D–F) Immature phenotype of presumed subserosal ICC progenitors in an adult tissue (corpus) maintained with 100 ng/ml SCF for 61 days. Note dominance of the the presumed precursors, the lack of processes and the continuing co-expression of Kit (D), Insr/Igf1r (E), and CD34 (F). Inset in F shows digital overlay.
Finally, we investigated whether the cells that responded to soluble SCF could be converted into mature ICC by IGF-I and whether the efficacy of IGF-I treatments was dependent on the survival of presumed precursors. After ~30 days of culturing with IGF-I following a 40-day pretreatment with SCF, mature, interconnected ICC networks dominated in all 4 adult gastric muscles studied (Figure 7A). Although none of the mature networks stained for Insr/Igf1r, in two tissues ICC continued to express CD34 (Figure 7B–C) and one culture contained some residual Kit+CD34+Insr+Igf1r+ clusters. These tissues also displayed robust slow wave activity (Figure 5 “40d SCF+30d IGF-I”). In contrast, IGF-I treatment initiated after 40 days of culturing with unsupplemented, serum-free basal media (n=4) failed to maintain or rescue the small number of ICC that usually remain after this duration of growth factor deprivation13,17 (compare Figure 7E and Figure 3F), indicating that most mature ICC in the tissues that received successive soluble SCF and IGF-I treatments probably originated from the presumed progenitors.
Figure 7.
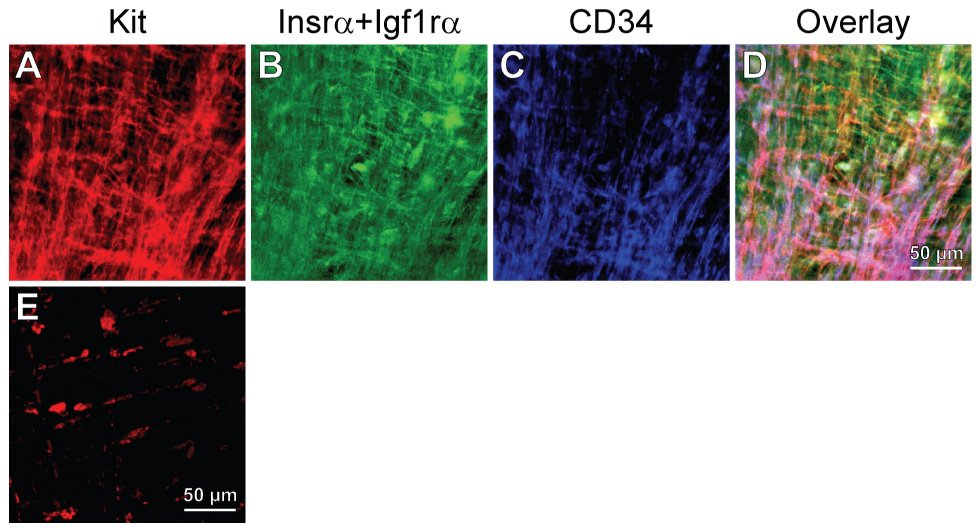
IGF-I promotes the differentiation of ICC from its precursors. (A–D) IGF-I-induced conversion into mature ICC of presumed ICC progenitors following stimulation with soluble SCF. Kit (A), Insr/Igf1rα (B), and CD34 (C) immunofluorescence and their digital overlay (D) in an adult gastric corpus maintained with 100 ng/mL IGF-I for 23 days following a 41-day pretreatment with 100 ng/mL SCF and stained as in Figure 6D–F. The mature, interconnected Kit+ ICC networks lacked Insrα and Igf1rα although some cells continued to express CD34. (E) Failure of IGF-I treatment to maintain or rescue Kit+ ICC when applied after a period of growth factor deprivation. Adult tissue cultured with unsupplemented basal media for 42 days before replacing IGF-I for 28 days.
DISCUSSION
Cell turnover is not usually considered in the gastrointestinal tunica muscularis, even though the neuromuscular apparatus is capable of morphological and functional regeneration19,29,30. In postnatal muscles, only precursors for neural crest-derived cells (neurons, glia) have been identified, and the source of regenerating mesodermal cells (smooth muscle and ICC) remains obscure. In this study we have identified a rare cell type with KitlowCD44+CD34+Insr+Igf1r+ phenotype and investigated whether these cells could contribute to the regeneration and maintenance of mature ICC networks. We have found that these putative progenitors were absent from organotypic cultures with depleted ICC populations but were rescued by IGF-I that also prevented ICC loss. They increased in number in response to soluble SCF and supported robust slow wave activity despite retaining immature gene expression and phenotype. Their differentiation into mature, network-forming ICC required IGF-I. Conversely, restoration of previously depleted ICC networks by IGF-I required the survival of the presumed progenitors. Thus, the KitlowCD44+CD34+Insr+Igf1r+ cells may be local ICC precursors. Recently we have also obtained more direct evidence in this regard by isolating and culturing cells with comparable phenotypes and detecting their differentiation into ICC31.
We hypothesized that ICC progenitors should co-express Kit, the only established cell surface marker for ICC11–19, and CD34, a presumed adhesion molecule detectable in 60–70% of GIST23,24, most of which develop as a result of activating Kit mutations23 and are believed to originate from a CD34-expressing subset of ICC23–25. Although expression of CD34 by ICC remains controversial 26, the presence of CD34 in GIST could reflect the abnormal proliferation of Kit+CD34+ precursors, which could have been overlooked in immunohistochemistry experiments because of low Kit expression, unexpected localization, or low frequency26. Indeed, by flow cytometry, a technique particularly suitable for the detection of rare cells, we consistently found a small population of cells with KitlowCD34+ phenotype. By immunohistochemistry, they were identified as rare, small, oval-shaped cells, which had only short or no processes and formed small clusters on the surfaces of the smooth muscle layers. Because of their KitlowCD34+ phenotype, very low frequency and undifferentiated morphology, we designated these cells as potential ICC progenitors. In contrast, only a negligible proportion of mature Kit+ ICC were CD34+. However, in organotypic cultures where differentiation of network-forming ICC was induced after stimulating the proliferation of the presumed progenitors with soluble SCF, considerable amounts of CD34 protein remained detectable in mature ICC. This finding suggests that ICC in normal tissues may also express CD34 when their rate of turnover is accelerated, and may explain the conflicting reports on this subject23–26.
The KitlowCD34+ cells also expressed Insr, Igf1r, and CD44, a hyaluronan-binding protein32 found in mesenchymal stem cells27. Surprisingly, CD44 was also detected in all gastric ICC-IM and ICC-MY. CD44 mRNA is also expressed by isolated small intestinal ICC33. Since no other major cell type in these tissues appeared to express CD44, it can be considered a novel ancillary marker for ICC. Thus, its presence on the KitlowCD34+ cells also suggests a developmental relationship between them and ICC.
The significance of CD34 and CD44 expression by ICC and their presumed progenitors remains unclear but it is remarkable that both have been linked to cell adhesion and migration25,32. CD34, an 11-kDa glycosylated transmembrane protein, has been described in hematopoietic stem cells, endothelium, fibroblasts, and various tumors25,26. Thus, high levels of CD34 in the KitlowCD44+CD34+Insr+Igf1r+ presumed ICC progenitors and a decrease and eventual loss of expression during their differentiation into ICC could reflect a decline of migratory phenotype. However, no overt gastrointestinal abnormalities were reported in CD34 knock-out mice34, suggesting that the functions of this protein are probably redundant.
CD44 is a transmembrane glycoprotein expressed by mesenchymal stem cells and tumors27,35. It typically occurs in lipid rafts, where it binds to extracellular matrix components including hyaluronan32. The intracellular portion interacts with the cytoskeleton32 and various signaling molecules35 and mediates matrix degradation, cell growth, and migration32,35. Thus, CD44 and CD34 may play similar roles in the presumed ICC progenitors. However, CD44 is expressed in ICC throughout their differentiation and, in some cases, even after the loss of Kit expression and pacemaker activity. Thus, CD44 may be utilized as a surrogate ICC marker that may allow better monitoring the fate of these cells in conditions involving a reduction in Kit expression. Through the activation of matrix metalloproteinase 932, an enzyme responsible for the liberation of soluble SCF36, CD44 may also regulate the ratio between soluble and membrane-bound SCF and, thereby, contribute to the regulation of ICC turnover. However, CD44 functions also appear to be highly redundant as CD44 knock-out mice show no overt abnormalities37. We also couldn’t detect any abnormality in ICC or electrical pacemaking in CD44 knock-out mice (unpublished).
A developmental relationship between KitlowCD44+CD34+ cells and gastric ICC was suggested by (1) the ICC’s continuing expression of CD44; (2) the occasional presence of CD34 on the surface of network-forming ICC when their turnover was stimulated; and, most convincingly, (3) by our serendipitous finding that the small clusters of KitlowCD44+CD34+ cells in the gastric musculature could be selectively expanded with soluble SCF. The soluble SCF-stimulated cells grew into densely packed clusters producing robust, rhythmic, slow wave-like electrical activity (a feature of ICC-MY) and into chords extending along the longitudinal axes of smooth muscle fibers within the muscle layers (the location of ICC-IM in the stomach). Moreover, these enlarged populations of KitlowCD44+CD34+ cells could be converted into normal networks containing both main ICC classes with IGF-I. In response to soluble SCF, cells that appeared indistinguishable from the rare presumed progenitors became the dominant Kit+ cell type while maintaining an immature surface marker expression pattern (Kit+CD44+CD34+Insr+Igf1r+). These cells also remained tightly packed and apparently lacked long processes although some processes may have been masked by the high density of their clusters. In contrast, the mature, interconnected ICC networks underwent a progressive and profound depletion. These observations strongly indicate that the KitlowCD44+CD34+ cells, rather than the mature ICC, were the target of soluble SCF. This is consistent with earlier reports that soluble SCF, unlike its membrane-anchored isoform, is virtually ineffective at maintaining mature ICC15,16. Stimulation of the proliferation of murine embryonic or neonatal ICC precursors by soluble SCF was also described by Nakahara et al.15; and Wu et al.14 reported a selective growth of oval, Kit+ cells with virtually no processes in response to 100 ng/mL soluble SCF in primary cultures made from fetal mouse bowel. The morphology of these cells and their clusters (see e.g. Figures 8C, 10C and 10F in ref.14) bore a close resemblance to the presumed ICC progenitors and their clusters we detected both in unstimulated juvenile tissues and following stimulation with 50–100 ng/mL SCF. The KitlowCD44+CD34+ presumed ICC progenitors also appeared strikingly similar to some rare, Kit+ oval cells and their densely packed networks encircling the fetal foregut11,14 and small intestine11 (see e.g. Figure 3A–C in ref.14), which have been identified as the developmental origin of ICC-MY and the longitudinal smooth muscle layer11,12. Thus, KitlowCD44+CD34+ cells may represent reserve progenitors “left over” from normal development, which may be activated by signals to replace senescent or damaged ICC5,8–10,19.
The consistent finding of robust, regular slow wave-like activity in the SCF-treated tissues was unexpected because they virtually lacked mature ICC and were dominated by morphologically undifferentiated KitlowCD44+CD34+Insr+Igf1r+ cells. This finding suggests that at least some of these cells may have been developmentally committed to become ICC-MY. The underlying electrical activity closely resembled slow waves occurring in normal tissues, except that their amplitude was higher and their frequency slower. However, these differences cannot be ascribed to the immaturity of the pacemaker cells, since the parameters did not change significantly after converting the precursors into mature ICC by IGF-I. For reasons unclear, slow waves in stomach organotypic cultures typically occur at reduced frequencies13,18. The high amplitude of the oscillations may have simply reflected the high density and close coupling of the cells producing them. Although the appearance of these events strongly suggested that they represented bona fide slow waves, further studies are required to characterize their pharmacological characteristics to clearly distinguish them from oscillations produced by smooth muscle cells in the absence of ICC1,2.
The final evidence supporting this developmental relationship came from experiments with sequential application of soluble SCF (to increase the number of the presumed progenitors) and IGF-I (to differentiate them into mature ICC). We used IGF-I because soluble SCF alone for up to 76 days was unable to induce full differentiation and because IGF-I had been consistently found to support normal ICC networks and electrical slow waves in long-term organotypic cultures17,18. Indeed, IGF-I caused the differentiation of the KitlowCD44+CD34+Insr+Igf1r+ cells into normal, slow wave-producing ICC, which, similarly to ICC in postnatal tissues, no longer expressed Insr/Igf1r17, although still expressed some CD34. However, IGF-I was totally ineffective when applied after a 40-day hiatus in growth factor supplementation but before the complete loss of mature ICC, suggesting that most of IGF-I’s effects on gastric ICC17,18 may have reflected its support of ICC precursors and the stimulation of their differentiation. In view of the decline and eventual loss of Insr/Igf1r expression by the differentiating precursors, some of these actions may have been indirect. Indeed, we previously reported that the effects of IGF-I and insulin on ICC were mediated by soluble and membrane-bound SCF produced by gastric smooth muscle cells17 and in the present study we found that the effects of IGF-I could be inhibited by IFNγ, an inhibitor of Kit transcription28. Thus, IGF-I (and insulin) may in part support the maintenance of ICC via soluble and membrane-associated SCF, which appear to play key but different roles in ICC turnover: While soluble SCF is sufficient to support the KitlowCD44+CD34+Insr+Igf1r+ cells, membrane-bound SCF is necessary for their differentiation into ICC. The same dichotomy between the roles of SCF isoforms in the differentiation of ICC from their embryonic precursors has also been proposed by Wu et al.14 The SCF-mediated actions may be complemented by some direct effects of IGF-I on the precursors before they lose expression of Insr/Igf1r; and these effects may be important for the partial preservation of ICC in mutant mice that lack membrane-bound SCF (Sl/Sld) or have reduced Kit tyrosine kinase activity (W/Wν)17.
In summary, we propose that maintenance of ICC networks may involve constant remodeling from local precursors orchestrated by the following autocrine/paracrine cascade (Figure 8): Under the influence of insulin, gastrointestinal muscles produce IGF-I, which, in concert with insulin, supports the maintenance of KitlowCD44+CD34+Insr+Igf1r+ local progenitors. This effect is largely mediated by soluble SCF produced by smooth muscle cells17. In case of damage to ICC, the same mechanisms can also increase the number of the precursors and induce their partial differentiation involving a reduction in CD34 and the generation of electrical slow waves. Full differentiation of these committed progenitors into network-forming, Kit+CD44+CD34−Insr−Igf1r− ICC requires membrane-bound SCF, which is also produced by the smooth muscle in response to IGF-I (and insulin). IGF-I and insulin may also have direct effects on ICC precursors until they lose expression of Insr and Igf1r. Mature ICC may lose Kit and their ability to produce slow waves before losing CD44. It remains to be established whether the KitlowCD44+CD34+Insr+Igf1r+ cells are indeed surviving embryonic ICC precursors.
Figure 8.
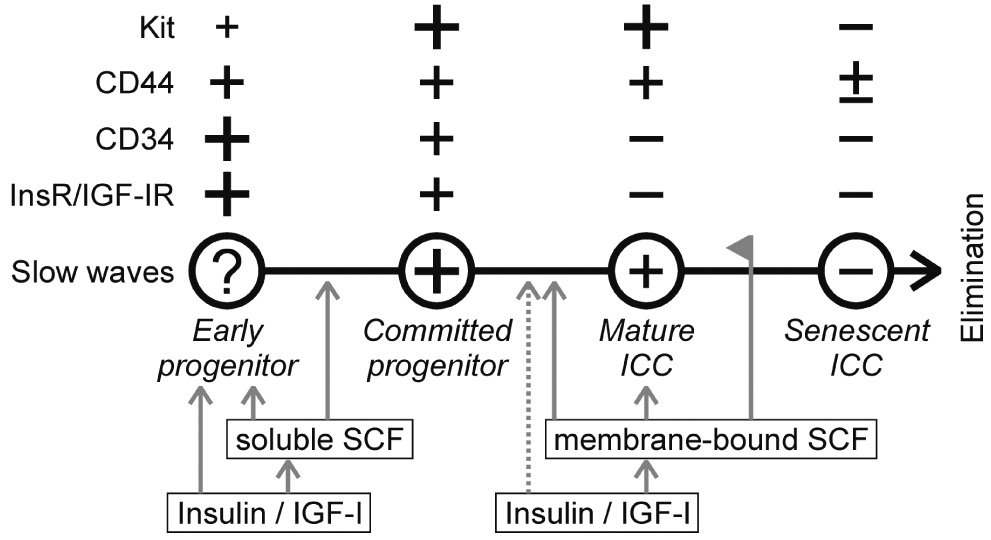
Proposed model for ICC turnover in the postnatal murine gastric corpus and antrum. +, present; −, absent. The size of the + signs is proportional to the approximate degree of expression. Gray arrows indicate maintenance or stimulation; dashed arrow, possible stimulation. Line with flag indicates inhibition.
Supplementary Material
Acknowledgements
The authors thank Dr. Grant W. Hennig for the slow wave analysis software.
Grant support: Supported in part by National Institutes of Health (NIH) grant DK58185. The Tissue Culture, Molecular and Morphology Core Laboratories were supported by NIH grant DK41315. The University of Nevada, Reno Cytometry Center was supported in part by the Nevada Biomedical Research Infrastructure Network grant RR16464 from the NIH.
Abbreviations
- AF
Alexa Fluor
- FITC
fluorescein isothiocyanate
- GIST
gastrointestinal stromal tumors
- ICC
interstitial cell(s) of Cajal
- ICC-IM
intramuscular ICC
- ICC-MY
myenteric ICC
- IGF-I
insulin-like growth factor-I
- Igf1r
insulin-like growth factor-I receptor
- INFγ
interferon-γ
- Insr
insulin receptor
- LS
light scatter
- PC5
phycoerythrin-cyanine 5 tandem conjugate
- PC7
phycoerythrin-cyanine 7 tandem conjugate
- PE
R-phycoerythrin
- SA
streptavidin
- SCF
stem cell factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosures: No conflicts-of-interest exist.
Note on Authorship: Andrea Lőrincz and Doug Redelman share first authorship on this paper.
REFERENCES
- 1.Sanders KM, Koh SD, Ward SM. Interstitial cells of cajal as pacemakers in the gastrointestinal tract. Annu Rev Physiol. 2006;68:307–343. doi: 10.1146/annurev.physiol.68.040504.094718. [DOI] [PubMed] [Google Scholar]
- 2.Huizinga JD. Gastrointestinal peristalsis: joint action of enteric nerves, smooth muscle, and interstitial cells of Cajal. Microsc Res Tech. 1999;47:239–247. doi: 10.1002/(SICI)1097-0029(19991115)47:4<239::AID-JEMT3>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 3.Ward SM, Sanders KM. Interstitial cells of Cajal: primary targets of enteric motor innervation. Anat Rec. 2001;262:125–135. doi: 10.1002/1097-0185(20010101)262:1<125::AID-AR1017>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 4.Fox EA, Phillips RJ, Martinson FA, et al. C-Kit mutant mice have a selective loss of vagal intramuscular mechanoreceptors in the forestomach. Anat Embryol (Berl) 2001;204:11–26. doi: 10.1007/s004290100184. [DOI] [PubMed] [Google Scholar]
- 5.Vanderwinden JM, Rumessen JJ. Interstitial cells of Cajal in human gut and gastrointestinal disease. Microsc Res Tech. 1999;47:344–360. doi: 10.1002/(SICI)1097-0029(19991201)47:5<344::AID-JEMT6>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 6.Streutker CJ, Huizinga JD, Driman DK, et al. Interstitial cells of Cajal in health and disease. Part I: normal ICC structure and function with associated motility disorders. Histopathology. 2007;50:176–189. doi: 10.1111/j.1365-2559.2006.02493.x. [DOI] [PubMed] [Google Scholar]
- 7.Ördög T, Takayama I, Cheung WK, et al. Remodeling of networks of interstitial cells of Cajal in a murine model of diabetic gastroparesis. Diabetes. 2000;49:1731–1739. doi: 10.2337/diabetes.49.10.1731. [DOI] [PubMed] [Google Scholar]
- 8.Chang IY, Glasgow NJ, Takayama I, et al. Loss of interstitial cells of Cajal and development of electrical dysfunction in murine small bowel obstruction. J Physiol. 2001;536:555–568. doi: 10.1111/j.1469-7793.2001.0555c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang XY, Vannucchi MG, Nieuwmeyer F, et al. Changes in interstitial cells of Cajal at the deep muscular plexus are associated with loss of distention-induced burst-type muscle activity in mice infected by Trichinella spiralis. Am J Pathol. 2005;167:437–453. doi: 10.1016/S0002-9440(10)62988-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yanagida H, Yanase H, Sanders KM, et al. Intestinal surgical resection disrupts electrical rhythmicity, neural responses, and interstitial cell networks. Gastroenterology. 2004;127:1748–1759. doi: 10.1053/j.gastro.2004.09.053. [DOI] [PubMed] [Google Scholar]
- 11.Torihashi S, Ward SM, Sanders KM. Development of c-Kit-positive cells and the onset of electrical rhythmicity in murine small intestine. Gastroenterology. 1997;112:144–155. doi: 10.1016/s0016-5085(97)70229-4. [DOI] [PubMed] [Google Scholar]
- 12.Klüppel M, Huizinga JD, Malysz J, et al. Developmental origin and Kit-dependent development of the interstitial cells of cajal in the mammalian small intestine. Dev Dyn. 1998;211:60–71. doi: 10.1002/(SICI)1097-0177(199801)211:1<60::AID-AJA6>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 13.Ördög T, Ward SM, Sanders KM. Interstitial cells of cajal generate electrical slow waves in the murine stomach. J Physiol. 1999;518(Pt 1):257–269. doi: 10.1111/j.1469-7793.1999.0257r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu JJ, Rothman TP, Gershon MD. Development of the interstitial cell of Cajal: origin, kit dependence and neuronal and nonneuronal sources of kit ligand. J Neurosci Res. 2000;59:384–401. doi: 10.1002/(SICI)1097-4547(20000201)59:3<384::AID-JNR13>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 15.Nakahara M, Isozaki K, Vanderwinden JM, et al. Dose-dependent and time-limited proliferation of cultured murine interstitial cells of Cajal in response to stem cell factor. Life Sci. 2002;70:2367–2376. doi: 10.1016/s0024-3205(02)01517-5. [DOI] [PubMed] [Google Scholar]
- 16.Rich A, Miller SM, Gibbons SJ, et al. Local presentation of Steel factor increases expression of c-kit immunoreactive interstitial cells of Cajal in culture. Am J Physiol Gastrointest Liver Physiol. 2003;284:G313–G320. doi: 10.1152/ajpgi.00093.2002. [DOI] [PubMed] [Google Scholar]
- 17.Horváth VJ, Vittal H, Lőrincz A, et al. Reduced stem cell factor links smooth myopathy and loss of interstitial cells of cajal in murine diabetic gastroparesis. Gastroenterology. 2006;130:759–770. doi: 10.1053/j.gastro.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 18.Horváth VJ, Vittal H, Ördög T. Reduced insulin and IGF-I signaling, not hyperglycemia, underlies the diabetes-associated depletion of interstitial cells of Cajal in the murine stomach. Diabetes. 2005;54:1528–1533. doi: 10.2337/diabetes.54.5.1528. [DOI] [PubMed] [Google Scholar]
- 19.Mei F, Yu B, Ma H, et al. Interstitial cells of Cajal could regenerate and restore their normal distribution after disrupted by intestinal transection and anastomosis in the adult guinea pigs. Virchows Arch. 2006;449:348–357. doi: 10.1007/s00428-006-0258-6. [DOI] [PubMed] [Google Scholar]
- 20.Sohal GS, Ali MM, Farooqui FA. A second source of precursor cells for the developing enteric nervous system and interstitial cells of Cajal. Int J Dev Neurosci. 2002;20:619–626. doi: 10.1016/s0736-5748(02)00103-x. [DOI] [PubMed] [Google Scholar]
- 21.Faussone-Pellegrini MS. Cytodifferentiation of the interstitial cells of Cajal related to the myenteric plexus of mouse intestinal muscle coat. An E.M. study from foetal to adult life. Anat Embryol (Berl) 1985;171:163–169. doi: 10.1007/BF00341410. [DOI] [PubMed] [Google Scholar]
- 22.Liu LW, Thuneberg L, Huizinga JD. Development of pacemaker activity and interstitial cells of Cajal in the neonatal mouse small intestine. Dev Dyn. 1998;213:271–282. doi: 10.1002/(SICI)1097-0177(199811)213:3<271::AID-AJA4>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 23.Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577–580. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- 24.Streutker CJ, Huizinga JD, Driman DK, et al. Interstitial cells of Cajal in health and disease. Part II: ICC and gastrointestinal stromal tumours. Histopathology. 2007;50:190–202. doi: 10.1111/j.1365-2559.2006.02497.x. [DOI] [PubMed] [Google Scholar]
- 25.Robinson TL, Sircar K, Hewlett BR, et al. Gastrointestinal stromal tumors may originate from a subset of CD34-positive interstitial cells of Cajal. Am J Pathol. 2000;156:1157–1163. doi: 10.1016/S0002-9440(10)64984-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vanderwinden JM, Rumessen JJ, De Laet MH, et al. CD34 immunoreactivity and interstitial cells of Cajal in the human and mouse gastrointestinal tract. Cell Tissue Res. 2000;302:145–153. doi: 10.1007/s004410000264. [DOI] [PubMed] [Google Scholar]
- 27.Blondheim NR, Levy YS, Ben-Zur T, et al. Human mesenchymal stem cells express neural genes, suggesting a neural predisposition. Stem Cells Dev. 2006;15:141–164. doi: 10.1089/scd.2006.15.141. [DOI] [PubMed] [Google Scholar]
- 28.Taniguchi S, Dai CH, Price JO, et al. Interferon gamma downregulates stem cell factor and erythropoietin receptors but not insulin-like growth factor-I receptors in human erythroid colony-forming cells. Blood. 1997;90:2244–2252. [PubMed] [Google Scholar]
- 29.Kelly KA, Code CF. Canine gastric pacemaker. Am J Physiol. 1971;220:112–118. doi: 10.1152/ajplegacy.1971.220.1.112. [DOI] [PubMed] [Google Scholar]
- 30.Faussone-Pellegrini MS, Vannucchi MG, Ledder O, et al. Plasticity of interstitial cells of Cajal: a study of mouse colon. Cell Tissue Res. 2006;325:211–217. doi: 10.1007/s00441-006-0174-8. [DOI] [PubMed] [Google Scholar]
- 31.Lőrincz A, Horváth VJ, Bardsley MR, et al. Survival and differentiation into interstitial cells of Cajal (ICC) of conditionally immortalized KitlowCD44+CD34+ cells with stem cell-like characteristics following allogeneic transplantation into diabetic NOD mice. Neurogastroenterol Motil. 2007;19:24. [Google Scholar]
- 32.Isacke CM, Yarwood H. The hyaluronan receptor, CD44. Int J Biochem Cell Biol. 2002;34:718–721. doi: 10.1016/s1357-2725(01)00166-2. [DOI] [PubMed] [Google Scholar]
- 33.Chen H, Ördög T, Chen J, et al. Differential gene expression in functional classes of interstitial cells of Cajal in murine small intestine. Physiol Genomics. 2007 doi: 10.1152/physiolgenomics.00113.2007. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki A, Andrew DP, Gonzalo JA, et al. CD34-deficient mice have reduced eosinophil accumulation after allergen exposure and show a novel crossreactive 90-kD protein. Blood. 1996;87:3550–3562. [PubMed] [Google Scholar]
- 35.Bourguignon LY, Singleton PA, Zhu H, et al. Hyaluronan-mediated CD44 interaction with RhoGEF and Rho kinase promotes Grb2-associated binder-1 phosphorylation and phosphatidylinositol 3-kinase signaling leading to cytokine (macrophage-colony stimulating factor) production and breast tumor progression. J Biol Chem. 2003;278:29420–29434. doi: 10.1074/jbc.M301885200. [DOI] [PubMed] [Google Scholar]
- 36.Roskoski R., Jr Signaling by Kit protein-tyrosine kinase--the stem cell factor receptor. Biochem Biophys Res Commun. 2005;337:1–13. doi: 10.1016/j.bbrc.2005.08.055. [DOI] [PubMed] [Google Scholar]
- 37.Protin U, Schweighoffer T, Jochum W, et al. CD44-deficient mice develop normally with changes in subpopulations and recirculation of lymphocyte subsets. J Immunol. 1999;163:4917–4923. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


