Abstract
Estradiol has been shown to interact with the cholinergic system to affect cognition in postmenopausal women. This study further investigated the interaction of estradiol and cholinergic system functioning on verbal memory and attention in two groups of healthy younger (ages 50–62) and older (ages 70–80) postmenopausal women. Twenty-two postmenopausal women were randomly and blindly placed on 1 mg of 17-beta estradiol orally for one month then 2 mg for two months or matching placebo pills after which they participated in three anticholinergic challenge sessions when verbal memory performance was assessed. Subjects were administered either the antimuscarinic drug scopolamine (SCOP), the antinicotinic drug mecamylamine (MECA) or placebo. After the first challenge phase, they were crossed over to the other hormone treatment for another three months and repeated the challenges. Results showed that estradiol pretreatment significantly attenuated the anticholinergic drug-induced impairments on a test of episodic memory (the Buschke Selective Reminding test) for the younger group only, while estradiol treatment impaired performance of the older group. The results suggest that younger subjects may experience more cholinergic benefit from estradiol treatment than older subjects, supporting the concept of a critical period for postmenopausal estrogen use.
Keywords: estradiol, cholinergic system, verbal memory, critical period
Introduction
Cognitive changes in women after menopause have been widely reported (Halbreich et al 1995). The ability of estrogen therapy to reverse or prevent this decline is controversial. The evidence for the beneficial effect of estrogen therapy is robust in studies of women who have undergone surgical menopause (Phillips and Sherwin 1992; Sherwin 1988). These studies show that women who took estrogen therapy (ET) showed less cognitive decline and even some improvement relative to those who received placebo. Additionally, a number of epidemiological studies have shown that women who take hormones during and/or after the menopause transition perform better on cognitive tests than women who do not take hormone therapy (e.g. Duka et al 2000; Jacobs et al 1998; Maki 2005; Resnick et al 1997; Smith et al 2001).
While many studies have shown positive effects of ET on cognition after menopause, there are a number of studies that showed no benefit (Barrett-Connor and Kritz-Silverstein 1993; Binder et al 2001; Ditkoff et al 1991; Grady et al 2002; Polo-Kantola et al 1998). Recent findings from the Women’s Health Initiative (WHI) and Women’s Health Initiative Memory Study (WHIMS) have shown that the risk of diagnosis of dementia in women taking conjugated equine estrogen (CEE) alone and CEE and medroxyprogesterone actate (MPA) was twice that of women in the placebo group (Shumaker et al 2004; Shumaker et al 2003). However, the WHI study was not designed as a study of cognition and was primarily a study of cardiovascular prevention, thus the women were older than is typical for postmenopausal hormone therapy. Thus, the data are at best conflicting regarding the usefulness for estrogen to positively affect cognition among older women.
In the WHI/WHIMS study the women were older, an average age of 65, when the study began. One hypothesis for the lack of a beneficial effect of estrogen and even the finding of negative effects is that the subject group was too old to benefit from the hormone therapy. In prior studies where the subjects were younger than 65 when they started estrogen therapy, beneficial effects were seen (Joffe et al 2006; Phillips and Sherwin 1992; Shaywitz et al 2003; Sherwin 1996; Woo et al 2003). In addition to the WHI/WHIMS study, other studies with women over 65 have shown no benefit of estrogen treatment (Almeida et al 2006; Viscoli et al 2005; Yaffe et al 2006). These findings have led to the proposal of a critical period for an estrogen benefit on cognition (Maki 2006; Resnick and Henderson 2002; Sherwin 2007).
The current study examined the influence of age on the ability of 17-beta estradiol (E2) to affect cholinergic system functioning. A number of studies from the animal literature show that an intact cholinergic system is necessary to see a beneficial effect of estrogen treatment (e.g. Gibbs and Aggarwal 1998; Tinkler and Voytko 2005). For example, the detrimental effects of SCOP on memory for passive avoidance were attenuated when ovariectomized rats were given E2 (Gibbs et al 1998). Voytko and colleagues (Tinkler and Voytko 2005; Voytko 2002) have shown that E2 modulates attentional performance in primates through its interaction with the cholinergic system. After ovariectomy, monkeys were impaired during invalid cues in an attention task. This impairment was improved after E2 and not after placebo (Voytko 2002). Thus, E2 uses the muscarinic cholinergic system to influence visuospatial attention (Tinkler and Voytko 2005). We have also demonstrated the E2-cholinergic interaction in postmenopausal women (Dumas et al 2006). We found that three months of 1 mg of E2 per day attenuated the impairment seen during anticholinergic drug challenge on tests of attention and tests with a speed component.
Further evidence examining the estrogen-cholinergic interaction in older women has been shown in a single photon emission tomography (SPET) study by Norbury and colleagues (Norbury et al 2007). They examined the relationship between muscarinic receptor density and estrogen therapy in younger premenopausal women and two groups of older postmenopausal women who were either long-term users of estrogen therapy or never-users. Younger women had more muscarinic receptors than either group of older women. Additionally, their data showed that ET users had a higher density of muscarinic receptors than never-users (Norbury et al 2007). Thus, these data contribute to the accumulating evidence showing an important relationship between estrogen and the cholinergic system in postmenopausal women.
In the current study, we have continued to utilize both a muscarinic antagonist, scopolamine (SCOP) and a nicotinic antagonist, mecamylamine (MECA) in our E2-cholinergic model. Our prior study (Dumas et al 2006) found interactions of E2 with both the muscarinic and nicotinic antagonists and have extended the investigation of these interactions in the current study. While the prior animal studies (e.g. (Gibbs and Aggarwal 1998; Tinkler and Voytko 2005) and the imaging study by Norbury et al. (2007) showed specific evidence of the estrogen-muscarinic interaction, these studies did not assess the estrogen-nicotinic interaction. In addition, there was some indication that E2 also attenuated the effects of the anticholinergic drugs on a measure of verbal memory but this effect did not reach significance in our prior study(Dumas et al. 2006). However, only one dose of E2 was tested and the possibility exists that brain systems and/or cognitive operations may be differentially sensitive to E2 dose. In the current study we examined the effect of 2 mg per day of E2 in our cholinergic challenge model to further examine the E2-cholinergic interaction in younger and older postmenopausal women.
Methods
Subjects
Subjects were 22 cognitively normal women, ages 50–81, M = 65 (SD = 10.2). Four additional subjects passed the screening but withdrew before beginning hormone treatment because of the time commitment of the study. Subjects were stratified during recruitment by age into two groups. The younger group was ages 50–62. (M = 55.8, SD = 4.3, N = 11). The older group was ages 70–81 (M = 74.3, SD = 3.7, N = 11). See Table 1 for demographic characteristics.
Table 1.
Demographic characteristics for younger and older subjects.
| Young | Old | |
|---|---|---|
| Age *** | 55.8 (4.3) | 74.3 (3.7) |
| Education | 14.6 (1.4) | 14.5 (2.6) |
| Years Since Menopause *** | 5.8 (8.6) | 22.0 (5.3) |
| Years on Estrogen | .85 (1.9) | .83 (1.8) |
| Years Since Estrogen ** | .80 (1.32) | 12.7 (12.0) |
| BMI | 24.8 (4.1) | 27.0 (4.5) |
p < .01
p < .0001
Subjects were recruited through notices and advertisements in local newspapers and direct mailings. Subjects were required to be postmenopausal, without menses for one year and without surgically-induced menopause. Exclusion criteria included smoking, a history of breast cancer, and use of hormone therapy during the last year. Twelve subjects had previously taken hormone or estrogen therapy after menopause. The length of time of prior hormone use ranged from one week to six years (See Table 1). Medical exclusion criteria for E2 treatment included: contraindications for hormone therapy, estrogen-dependent neoplasia, untreated blood pressure greater than 160/100, history of deep vein thrombosis or other thromboembolic disease, hepatoma, severe migraines or stroke on oral contraceptives, current use of barbiturates, rifampin, insulin, carbamezepine, oral hypoglycemics, antidepressants, or lipid-lowering drugs, known intolerance to conjugated estrogens, diabetes, untreated thyroid disease, clinical osteoporosis, and a history or presence of severe menopausal symptoms. In addition, the following exclusions applied to the challenge drugs: heavy alcohol (more than an average of 1 drink per day) or coffee use (more than three cups per day), significant cardiovascular disease, asthma, active peptic ulcer, hyperthyroidism, pyloric stenosis, narrow angle glaucoma, epilepsy, or current Axis I psychiatric disorders. The alcohol criterion was used to ensure subjects were not alcohol dependent, and the caffeine criterion was used to ensure subjects would not experience caffeine withdrawal on testing days.
Upon meeting these criteria, subjects were approved for further screening at the University of Vermont (UVM) General Clinical Research Center (GCRC). After signing informed consent documents, subjects gave a medical history, underwent a physical and laboratory tests assessing hematopoietic, renal, hepatic and hormonal function. Subjects were cognitively evaluated using the Mini Mental State Exam (MMSE; (Folstein et al 1975), Brief Cognitive Rating Scale (Reisberg et al 1988), and the Mattis Dementia Rating Scale (DRS, (Jurica et al 2001) to establish a Global Deterioration Scale score (GDS) which rated the degree of cognitive impairment (Reisberg and Ferris 1988). Subjects were required to have an MMSE score greater than or equal to 27, a DRS score of 123 or greater, and a GDS score of 1 or 2.
Behavioral screening consisted of a partial Structured Clinical Interview for DSM-IV-TR (SCID; (First et al 2001) to establish the presence/absence of Axis I psychiatric disorders. In addition, subjects completed the Beck Depression Inventory (BDI). A cut off score of 10 was used for the BDI, and subjects scoring over this criterion were discontinued from further participation. All subjects met these criteria for the cognitive and behavioral screening.
Study Design
The overall study design was a 2 (age group: younger and older) by 2 (E2 treatment: E2 and placebo) by 3 (cholinergic challenge drug: 2.5 µg/kg SCOP, 20 mg MECA, and placebo) design. E2 treatment and cholinergic challenge were completely within-subjects manipulations.
Estradiol Administration
After meeting all inclusion criteria, subjects were randomly and blindly assigned to the E2 or placebo condition for three months (Phase 1). In the E2 condition, subjects took 1 mg of 17-beta E2 per day for one month and then 2 mg per day for two months. This was done because early pilot trials revealed that typical estrogen-related side effects (e.g. breast tenderness or spotting) tended to be troublesome if a subject was begun on 2 mg of E2 from the beginning. Using 1 mg of E2 for the first 30 days minimized these problems. In the placebo condition, subjects took similar appearing placebo pills for three months. After three months, subjects completed three challenge days (described below). They were then crossed over to the other condition, either placebo or E2, for another three months (Phase 2) and completed three more challenge days after those three months. After completion of the second phase of the study, all subjects took 10 mg per day of medroxyprogesterone acetate for 12 days to produce sloughing of any endometrium that developed.
Cholinergic Challenge Procedure
After three months of E2 or placebo treatment, subjects came to the UVM GCRC for three cholinergic challenge days. On each challenge day, subjects reported to the UVM GCRC by 0900. Each participant performed a baseline motor skill sobriety test to have as a comparison to a second test before discharge in the afternoon. An intravenous line (IV) was inserted and blood was drawn for an E2 assay. A double-blind, double placebo method of administration of the challenge drugs was followed. Subjects received one of the following medications: 2.5 µg/kg SCOP IV, 20 mg MECA orally, or placebo (IV and oral). At time 0, a pill was administered containing the MECA dose or placebo. Thirty minutes later, an injection of the SCOP dose or placebo was administered through the IV. On each day only one of the drugs was active or both were placebo. The order of the drug administration across the three days was determined randomly but balanced for the two age groups. Ninety minutes after the injection and two hours after oral pill administration, cognitive testing began at a running time of 120 minutes. After the cognitive testing, that took approximately 70 minutes, subjects were given lunch. Vitals signs and pupil diameter were assessed at seven time points throughout the session, running times of 0, 30, 60, 120, 180, 210, and 240 minutes. At the end of the study day, after passing the sobriety test to the satisfaction of the research nurse and covering physician, subjects were discharged.
Cognitive Battery
The cognitive testing battery was constructed to evaluate a number of cognitive domains potentially sensitive to cholinergic manipulation as well as those domains affected by loss of and subsequent treatment with E2 similar to our prior study (Dumas et al. 2006). These cognitive domains included tests of verbal episodic memory and attention. Before each of the challenge sessions, subjects were trained on the cognitive battery until they reached asymptotic performance to minimize learning or practice effects across sessions during each challenge phase. Equivalent forms were created for all testing and training days and these forms were counterbalanced evenly across study days for the younger and older subjects.
Verbal Episodic Memory
The Buschke Selective Reminding Task (SRT; (Buschke 1973) and the NYU Immediate Paragraph Recall test (Kluger et al 1999) were used as measures of verbal episodic memory. In the SRT, subjects were read a list of 16 unrelated words balanced for word frequency and imagery, followed by an immediate recall trial. On subsequent trials (up to eight), subjects were immediately reminded of words they had failed to recall on the prior trial. Three measures were obtained from the SRT task: the total number of words recalled across all lists, recall consistency, and recall failure.
For the NYU Immediate Paragraph Recall (PR) test subjects were read a short paragraph and then asked to recall the story. The dependent measure for the PR task was the number of information units recalled verbatim from memory.
Attention
The Critical Flicker Fusion (CFF) task (Kupke and Lewis 1989) and the Choice Reaction Time (CRT) task (Hindmarch 1984) from the Milford Test Battery and the Connors Continuous Performance Test (CPT; (Conners 1995) were used as the measures of attention. During the CFF task there were two different types of trials. In an ascending trial, the subject pressed a button that indicated when the frequency of flashing lights had increased to the point that the lights appear to be no longer flashing but rather appear continuously on (“fused”). The lights began flashing at a rate of 12 Hz and the frequency was increased to 50 Hz. In a descending trial, the participant pressed a button when the frequency of apparently fused lights was decreased such that lights began to appear to be flashing. The lights began flashing at 50 Hz and decreased to 12 Hz. The participant needed to respond before the frequency hit the upper or lower limit in each trial. The participant was presented with three of each trial type. Dependent measures for this task were the median detection frequency across all trials, as well as the median detection frequency on the ascending and descending trials separately.
The CRT task was a reaction time task in which subjects kept their index finger on a “home” light sensitive diode (LSD) until one of six LCD lights arrayed in a semicircle, approximately 25 cm from the “home” key, was lit on the response box. The subject lifted her index finger and moved it to cover the LSD corresponding to the illuminated LCD. She then returned her finger to the “home” LSD. Three performance measures were obtained from the CRT. The first was the median total reaction time (RT) per trial. The second was the median recognition RT, the amount of time it took the subject to lift her finger off of the home LSD once the signal to respond appeared. The third measure was the median motor RT, the time it took the participant to move her finger and to cover the LSD corresponding to the illuminated LCD.
In the computerized CPT task, individual letters appeared on the computer screen for 300 ms with a response period of two seconds for 120 trials for a total time of 4.6 minutes. Subjects were instructed to press a button when they saw an A followed by an X. The dependent measures were hits, errors of omission and commission, and hit reaction time.
Behavioral Measures
After the cognitive battery was finished, subjects completed the behavioral assessment measures, the Profile of Mood States (POMS; (McNair et al 1971) and a Physical Symptom Checklist (PSCL).
Data Analysis
Our main analysis of interest was whether three months of E2 treatment would attenuate the negative effects of the antimuscarinic and antinicotinic cholinergic drugs on memory performance differentially for the younger and older groups. First, we investigated the effect of E2 treatment on cognitive performance by examining cognitive performance on the placebo challenge day (see Table 2). A 2 (E2 treatment: E2 and PLC) X 2 (age group: young and old) mixed model repeated measures ANOVA was run for each dependent measure. Second, we examined the effect of the anticholinergic drug challenge on cognitive performance (see Table 3). We calculated difference scores for the dependent measures from each task. Performance on the placebo challenge day was subtracted from performance on each of the other cholinergic challenge days (within a phase) in an effort to examine the specific effects of the cholinergic drugs on cognitive performance. This was done to control for anticipated baseline differences in performance for subjects in different phases of the study. Then we used one sample t-tests to test for effects of each of the anticholinergic drugs separately for each age group. A significant finding indicated that a cholinergic drug affected performance on a specific task. Finally, we examined the ability of E2 to attenuate the anticholinergic induced impairment for both age groups on our cognitive tasks, behavioral measures, and vital signs. The effects of SCOP and MECA were analyzed in separate models in order to examine the ability of E2 to attenuate anti-muscarinic and anti-nicotinic induced cognitive impairment separately. For the SCOP challenge day, 2 (E2 treatment: E2 and PLC) X 2 (age group: young and old) mixed model repeated measures ANOVAs were run for each dependent measure. For the MECA challenge day, 2 (E2 treatment: E2 and PLC) X 2 (age group: young and old) mixed model repeated measures ANOVAs were run for each dependent measure. If E2 attenuated the negative effects of the anticholinergic drugs on memory or attention, then main effects of E2 treatment would be seen in these analyses.
Table 2.
Performance on the cognitive tasks for estradiol and placebo treatments on the cholinergic placebo challenge day with standard errors. A significant effect of age is indicated.
| Cognitive Construct | Task | Dependent Variable | Age Group | E2 | PLC |
|---|---|---|---|---|---|
| Attention | |||||
| CFF | |||||
| Total (Hz) | Young | 28.69 (.9) | 29.13 (.7) | ||
| Old | 27.70 (.9) | 28.20 (.7) | |||
| Ascending (Hz) | Young | 28.79 (1.2) | 29.12 (.6) | ||
| Old | 26.77 (1.2) | 27.80 (.6) | |||
| Descending (Hz) | Young | 28.75 (.8) | 28.80 (.8) | ||
| Old | 28.52 (.8) | 28.64 (.8) | |||
| CRT | |||||
| Total (ms) | Young | 734.86 (26.9) | 742.12 (26.9) | ||
| Old | 811.62 (26.7) | 832.92 (26.9) | |||
| Recognition RT (ms) | Young | 423.63 (19.7) | 424.85 (19.7) | ||
| Old | 455.25 (19.7) | 447.73 (19.7) | |||
| Motor RT (ms)1 | Young | 301.30 (19.8) | 310.07 (19.8) | ||
| Old | 368.33 (19.8) | 378.46 (19.8) | |||
| CPT | |||||
| Hits (proportion correct) | Young | 35.94 (2.3) | 39.64 (.3) | ||
| Old | 38.00 (2.2) | 39.18 (.3) | |||
| Errors of Omission (number of errors) | Young | 4.06 (2.3) | .36 (.3) | ||
| Old | 2.00 (2.2) | .73 (.3) | |||
| Errors of Comission (number of errors) | Young | .09 (.2) | .45 (.4) | ||
| Old | .82 (.2) | .82 (.4) | |||
| Hit RT (ms) | Young | 463.27 (28.5) | 468.94 (28.1) | ||
| Old | 476.49 (28.1) | 502.76 (28.1) | |||
| Verbal Memory | |||||
| SRT | |||||
| Total Recall (number correct) | Young | 78.36 (3.5) | 81.18 (3.5) | ||
| Old | 72.45 (3.5) | 68.64 (3.5) | |||
| Recall Consistency (number correct) | Young | 39.82 (4.2) | 44.36 (4.2) | ||
| Old | 34.36 (4.2) | 30.55 (4.2) | |||
| Recall Failure (number of failures) | Young | 12.64 (2.7) | 12.09 (2.7) | ||
| Old | 18.00 (2.73) | 20.64 (2.73) | |||
| PR | |||||
| NYU Scoring (percent correct) | Young | .40 (.05) | .39 (.05) | ||
| Old | .32 (.05) | .28 (.05) |
Main effect of Age for Motor RT: F(1,20) = 5.88, p < .05
Table 3.
Performance differences for cognitive tasks for each drug challenge by treatment type and age group with standard errors. Numbers represent difference scores (cholinergic drug minus placebo). Significant effects of estradiol treatment and age are indicated. See text for cholinergic challenge effects.
| Cognitive Construct | Task | Dependent Variable | Age Group | 2.5 µg/kg SCOP + E2 | 2.5 µg/kg SCOP + PLC | 20 mg MECA + E2 | 20 mg MECA + PLC |
|---|---|---|---|---|---|---|---|
| Attention | |||||||
| CFF | |||||||
| Total (Hz) | Young | −.76 (.73) | −1.45 (.52) | 1.77 (1.05) | .81 (.51) | ||
| Old | −.72 (.76) | −.62 (.52) | 1.39 (1.05) | 1.11 (.54) | |||
| Ascending (Hz) | Young | −.55 (.96) | −1.03 (.60) | 1.37 (.60) | .28 (.60) | ||
| Old | .31 (1.0) | −.42 (.60) | .33 (.60) | .88 (.63) | |||
| Descending (Hz) | Young | −1.35 (.62) | −1.54 (.62) | .44 (.55) | .26 (.55) | ||
| Old | −1.47 (.62) | −1.04 (.62) | .35 (.55) | .47 (.58) | |||
| CRT | |||||||
| Total (ms) | Young | 43.73 (26.1) | 22.95 (26.1) | 11.31 (25.9) | 39.23 (25.5) | ||
| Old | 39.56 (26.1) | 65.51 (26.1) | 86.51 (25.9) | 30.32 (25.8) | |||
| Recognition RT (ms) | Young | 11.83 (15.5) | 19.64 (15.5) | 2.53 (12.0) | −1.75 (14.2) | ||
| Old | 16.37 (15.5) | 41.24 (15.5) | −15.56 (13.4) | −1.44 (12.6) | |||
| Motor RT (ms) | Young | 18.24 (20.2) | −8.77 (20.2) | 14.38 (20.1) | 25.07 (20.1) | ||
| Old | −5.35 (20.2) | 16.81 (20.2) | 61.03 (20.1) | 29.56 (20.1) | |||
| CPT | |||||||
| Hits (proportion correct) | Young | −2.89 (2.4) | −5.18 (1.5) | −.01 (1.38) | −.55 (.4) | ||
| Old | .86 (2.4) | −1.49 (1.5) | 0.82 (1.26) | .18 (0.4) | |||
| Errors of Omission (number of errors) | Young | 2.89 (2.4) | 4.91 (1.5) | −.21 (1.4) | .55 (.4) | ||
| Old | −.89 (2.4) | 1.58(1.5) | −.91 (1.3) | −.18 (.4) | |||
| Errors of Comission (number of errors) | Young | 1.3 (0.5) | .91 (.5) | .85 (.4) | −.27 (.3) | ||
| Old | .10 (0.5) | 1.10 (.5) | −.27 (.3) | .18 (.3) | |||
| Hit RT (ms) | Young | 52.49 (18.2) | 12.29 (17.3) | 22.46 (19.3) | 18.99 (17.4) | ||
| Old | −14.49 (18.2) | −43.90 (18.2) | 7.78 (17.4) | −8.48 (17.4) | |||
| Verbal Memory | |||||||
| SRT | |||||||
| Total Recall (number correct)1 | Young | −13.55 (3.6) | −20.00 (3.6) | −2.64 (2.7) | −7.18 (2.7) | ||
| Old | −16.00 (3.6) | −7.55 (3.6) | −5.73 (2.7) | −2.55 (2.7) | |||
| Recall Consistency (number correct)2 | Young | −11.91 (3.6) | −19.9 (3.6) | −1.45 (3.1) | −8.36 (3.1) | ||
| Old | −15.73 (3.6) | −5.73 (3.6) | −6.54 (3.1) | −2.18 (3.1) | |||
| Recall Failure (number of failures)3 | Young | 11.08 (2.9) | 16.82 (4.1) | 3.01 (2.4) | 3.73 (2.4) | ||
| Old | 13.00 (2.9) | 7.00 (4.1) | 3.36 (2.4) | 3.00 (2.4) | |||
| PR | |||||||
| NYU Scoring (number correct) | Young | −.15 (0.04) | −.05 (.07) | −.05 (0.1) | .01 (.1) | ||
| Old | −.02 (0.04) | .05 (.07) | .003 (.1) | .07 (0.1) |
SCOP analysis age and treatment interaction for total recall: F(1,20) = 10.73, p < .01
SCOP analysis age and treatment interaction for recall consistency: F(1,20) = 17.44, p < .001
SCOP analysis age and treatment interaction for recall failure: F(1,20) = 5.57, p < .05
In the behavioral and vital signs analyses, when significant effects of E2 treatment were found, we used the behavioral or vitals signs measure as a covariate in any cognitive analysis that found significant effects of E2. This allowed us to examine the contribution of behavioral and vital signs factors to the E2 effects on cognition in this study.
Results
For the analyses that follow, an alpha level of p < .05 was considered statistically significant.
The Effects of Age on the Estradiol-Cholinergic Interaction Verbal Episodic Memory
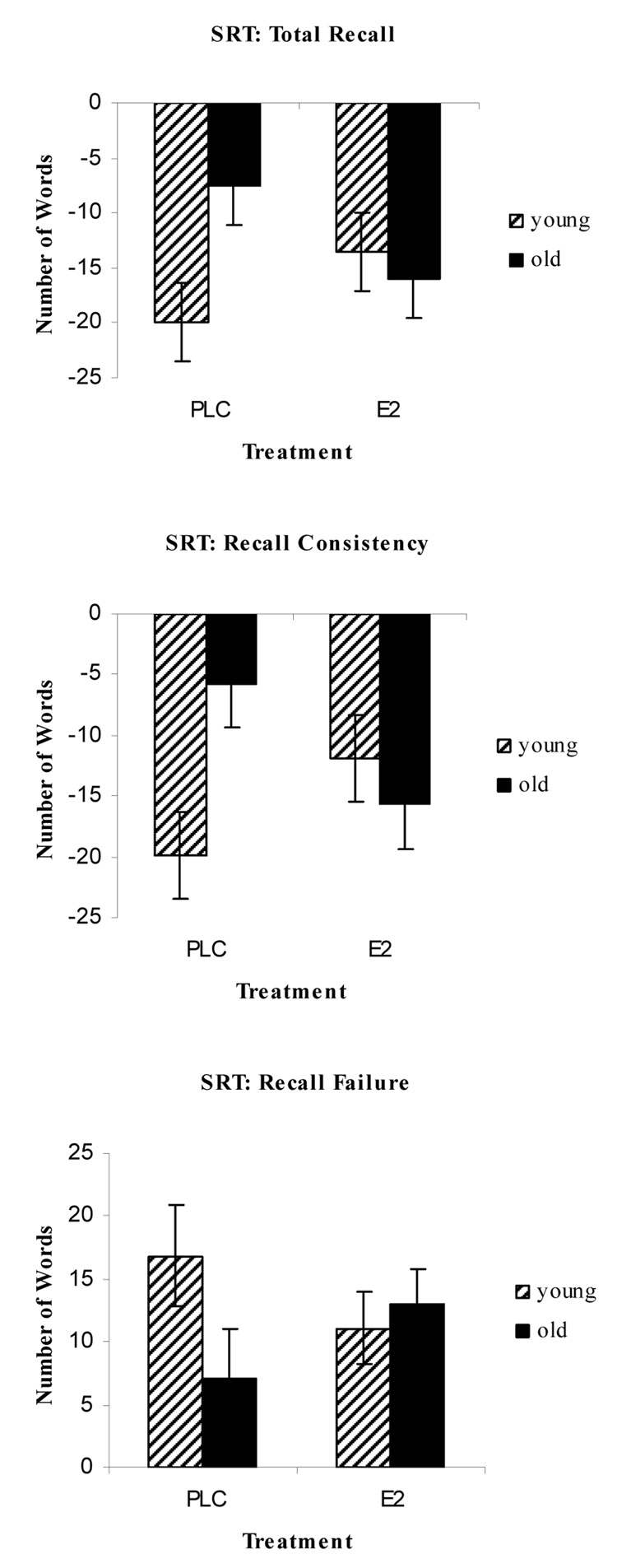
For the Buschke SRT on the cholinergic placebo challenge day, there was a trend for an age effect for the total recall (F(1,20) = 4.14, p > .05), recall consistency (F(1.20) = 3.12, p > .09), and recall failure (F(1,20) = 3.97, p > .06) measures. For these three measures the pattern of means showed that younger subjects recalled more words, were more consistent and had fewer recall failures relative to the older subjects (see Table 2). There were no effects of E2 treatment or interactions of E2 treatment and age. Next we examined the effects of SCOP and MECA on Buschke SRT performance separately for younger and older subjects. For the younger adults, there were impairments for the SCOP analysis on total recall (t(10) = 4.76, p < .001), recall consistency (t(10) = 5.66, p < .001) and recall failure (t(10) = 3.89, p < .01). For the MECA analysis, younger subjects were impaired on total recall (t(10) = 2.53, p < .05) and recall consistency (t(10) = 2.44, p < .05). The older subjects were impaired on the total recall during the SCOP challenge (t(10) = 2.34, p < .05), while there were no impairments from the MECA challenge. Finally, we examined the ability of E2 treatment to attenuate the impairment during the anticholinergic challenge and found interactions of age and treatment for all three dependent measures during the SCOP challenge (see Table 3). There were interactions of E2 and age on total recall (F(1,20) = 10.73, p = .01), recall consistency (F(1,20) = 17.44, p < .001), and recall failure (F(1,20) = 5.57, p < .05; see Figure 1). When this interaction was probed, we found that in the placebo hormone condition, the younger subjects performed worse than the older subjects in the total recall (t(21) = 2.48, p < .05) and recall consistency (t(21) = 2.97, p < .01) when we controlled for baseline placebo challenge day performance. After E2 treatment this age difference disappeared for the total recall (t(21) = .45, p > .66) and recall consistency measures (t(21) = .71, p > .48). The pattern of means was similar for the recall failure measure in the placebo and E2 conditions, but the t-tests did not reach significance for the placebo comparison. There was also a trend for older adults to have less recall consistency after the E2 phase compared to the placebo phase (t(21) = 2.04, p > .05). The pattern of means were similar for the total recall and recall failure measures showing the E2 impaired performance for the older subjects on this task. In all cases for the SCOP challenge, these interactions showed that E2 relative to placebo improved performance for the younger group and impaired performance for the older group on the SRT measures.
Figure 1.
Difference scores on the Buschke SRT on the SCOP challenge day. Difference scores were calculated by subtracting placebo challenge day performance from SCOP challenge day performance. For all three measures there was a significant age by treatment interaction that showed the younger group improved with estrogen treatment while the older group is impaired.
For the MECA challenge, there was a trend towards a significant interaction on recall consistency (F(1,20) = 3.37, p = .08; see Table 3). The pattern of means was similar to the SCOP challenge where the younger group showed improvements after E2 treatment while the older group showed impairments after E2 treatment.
For the Paragraph Recall on the cholinergic challenge day, there was a trend towards an age effect on the total units recalled (F(1,10) = 4.07, p > .05) and the pattern or means showed that younger adults recalled more units than the older adults (see Table 2). There was no effect of E2 treatment nor an interaction of age and E2 treatment. Next we examined the effects of SCOP and MECA on Paragraph Recall performance separately for younger and older subjects. For both the younger and older subjects, neither the SCOP nor MECA challenges impaired performance. Finally, we examined the interaction of E2 and age on Paragraph Recall performance for the SCOP and MECA challenges separately. There were no main effects or interactions that involved E2 during SCOP or MECA challenge. During the SCOP challenge there was a main effect of age for number of units recalled (F(1,20) = 4.37, p < .05) that showed older group recalled more units than the younger group. There were no effects under MECA challenge (see Table 3).
Attention
On the CFF, there were no effects of E2 treatment or age on the dependent measures from this task: rising, falling, or total median frequency (largest F: F(1,20) = 2.06, p > .17) during the cholinergic placebo day. Next we examined the effects of SCOP and MECA on CFF performance separately for younger and older subjects (see Table 2). For the younger subjects, SCOP impaired CFF performance as measured by the median total frequency (t(9) = 3.05, p < .05) and the median falling frequency (t(9) = 2.53, p < .05). The younger subjects were not impaired during the MECA challenge day. The older subjects showed a different pattern of anticholinergic effects with no impairments during the SCOP challenge and impairments after MECA challenge on the median falling frequency (t(9) = 2.89, p < .05) and trend towards impairment on the median total frequency (t(9) = 1.98, p > .08). Finally, there were no main effects or interactions of age and E2 treatment for either the SCOP or MECA analysis (largest F for the SCOP analysis: F(1,20) = .71, p > .4; MECA analysis: F(1,20) = 1.81, p > .2; see Table 3).
For the CRT, the analysis of the E2 effect during the placebo cholinergic challenge day showed only an effect of age group on the median motor RT (F(1,20) = 5.88, p < .05) that showed older subjects had longer motor RTs than younger subjects (see Table 2). Next we examined the effects of SCOP and MECA on CRT performance separately for younger and older subjects. For there younger subjects, neither SCOP nor MECA impaired RT on any of the CRT measures (largest t: t(10) = 1.27, p > .23). For the older subjects, SCOP impaired recognition RT (t(10) = 3.10, p < .05) and total RT (t(10) = 2.25, p < .05) on the CFF. There were no main effects or interactions of age and E2 treatment for either the SCOP or MECA analysis (largest F for the MECA analysis: F(1.20) = 2.37, p > .15; SCOP analysis: F(1,20) = 1.46, p > .25; see Table 3).
For the CPT, there were no effects of E2 treatment or age on performance (largest F: F(1,20) = 2.27, p > .15) during the cholinergic placebo day (see Table 2). Next we examined the effects of SCOP and MECA on CPT performance separately for younger and older subjects. Younger subjects were impaired during the SCOP challenge on measures of hits (t(10) = 3.15, p < .01) and errors of omission (t(10) = 3.01, p < .05), and during the MECA challenge there were trends towards impairment on hits (t(10) = 1.94, p > .08) and errors of omissions (t(10) = 1.94, p > .08). Older subjects only showed trends towards impairments during SCOP challenge on measures of errors of commission (t(9) = 2.09, p > .06) and hit RT (t(9) = 1.94, p > .08), and there were no impairments during MECA challenge. Finally, we examined whether E2 attenuated these anticholinergic-induced impairments on CPT performance and there were no interactions of age and E2 treatment for either the SCOP analysis, but there were main effects of age for the hits (F(1,20) = 4.48, p < .05) and hit RT (F(1,20) = 11.20, p <.01). The pattern of means showed that younger adults had fewer hits and longer RTs relative to older adults under SCOP challenge. For the MECA analysis, there was a trend towards an interaction of E2 treatment and age for the hit RT measure (F(1,18) = 3.55, p > .08), but no other main effects or interactions were found (see Table 3).
Behavioral Measures
Questionnaires assessing mood and physical symptoms were administered after cognitive testing was completed to examine whether there were any negative mood or physical symptoms as a result of the E2 treatment and challenge drugs. Difference scores were calculated for each of the SCOP and MECA challenge days relative to the placebo challenge day similar to the cognitive data analysis. To preview the results from the behavioral assessments, E2 treatment did little to influence subjective or objective behavioral ratings. However, there were some effects of age that in general showed that the younger group reported more behavioral symptoms than the older group (see Table 4).
Table 4.
Behavioral ratings difference scores with standard errors. Ratings from the cholinergic placebo challenge day were subtracted from each study drug day rating. Significant effects of estradiol treatment and age are indicated.
| Task | Dependent Variable | Age Group | 2.5 µg/kg SCOP + E2 | 2.5 µg/kg SCOP + PLC | 20 mg MECA + E2 | 20 mg MECA + PLC |
|---|---|---|---|---|---|---|
| Physical Symptom Checklist | ||||||
| Total 1,2 | Young | 10.18 (1.2) | 4.77 (.7) | 7.95 (.9) | 4.77 (.9) | |
| Old | 10.81 (1.0) | 6.09 (.8) | 8.22 (.9) | 6.09 (.9) | ||
| POMS | ||||||
| Tension | Young | 2.18 (1.1) | 1.17 (1.2) | 2.18 (1.3) | 1.64 (1.3) | |
| Old | 1.20 (1.2) | 1.20 (1.2) | 1.40 (1.4) | 2.10 (1.4) | ||
| Depression | Young | .91 (1.1) | −1.50 (1.2) | 1.09 (.75) | −.27 (1.4) | |
| Old | .40 (1.2) | 2.90 (1.2) | .90 (.8) | 2.20 (1.4) | ||
| Anger/Hostility | Young | 1.55 (1.1) | 1.26 (.6) | 1.00 (.9) | .55 (.9) | |
| Old | .10 (1.1) | .60 (.6) | .10 (.9) | 1.20 (.9) | ||
| Vigor 3,4 | Young | −7.37 (.8) | −6.87 (1.4) | −5.36 (.9) | −5.64 (1.7) | |
| Old | −2.30 (.9) | .50 (1.4) | −2.4 (1.0) | −1.5 (1.8) | ||
| Fatigue5,6 | Young | 4.82 (1.4) | 6.83 (1.9) | 4.64 (.8) | 5.18 (2.1) | |
| Old | .20 (1.4) | .40 (1.9) | 1.60 (.9) | .90 (2.2) | ||
| Confusion/Bewilderment7,8 | Young | 2.45 (.7) | 3.30 (1.2) | 2.27 (.9) | 2.82 (.9) | |
| Old | .80 (.8) | −.60 (1.2) | −1.60 (.9) | −1.60 (.9) | ||
| Total Mood Disturbance | Young | 19.27 (3.7) | 21.54 (6.2) | 16.55 (3.4) | 15.55 (5.6) | |
| Old | 5.00 (3.9) | 4.00 (6.4) | 8.00 (3.5) | 6.30 (5.8) |
SCOP analysis age main effect for Total Physical Symptoms: F(1,20) = 6.58, p < .05
MECA analysis age main effect for Total Physical Symptoms: F(1,20) = 10.31, p < .01
SCOP analysis age main effect for Vigor Scale: F(1,19) = 4.56, p < .05
MECA analysis age main effect for Vigor Scale: F(1,19) = 19.90, p < .01
SCOP analysis age main effect for Fatigue Scale: F(1,19) = 6.32, p < .05
MECA analysis age main effect for Fatigue Scale: F(1,19) = 4.63, p < .05
SCOP analysis age main effect for Confusion/Bewilderment Scale: F(1,19) = 5.56, p < .05
MECA analysis age main effect for Confusion/Bewilderment Scale: F(1,19) = 8.00, p < .05
On the POMS, main effects of age were found for some of the subscales on MECA and SCOP challenge days. During the SCOP challenge days, main effects of age were found for the vigor (F(1,19) = 19.9, p = .0003), fatigue (F(1,19) = 6.32, p = .02), confusion (F(1,19) = 5.56, p = .03), and total mood disturbance (F(1,19) = 5.37, p = .03) scores. On the MECA challenge day, there were effects of age on the vigor (F(1,19) = 6.56, p = .02), fatigue (F(1,19) = 4.63, p = .04), and confusion (F(1,19) = 8.00, p = .01) subscales. For both the MECA and SCOP challenges the younger group showed less vigor, more fatigue, and more confusion than the older group.
Age main effects were also found on the PSCL for SCOP (F(1,20) = 6.58, p = .02) and MECA (F(1,20) = 10.38, p = .004). The younger subjects reported more physical symptoms during the anticholinergic challenges than the older group.
Vital Signs
Blood pressure, pulse, and pupil diameter were monitored at seven time points throughout the challenge day. Analyses were conducted on the maximum change score from the baseline measurement for each variable (see Table 5). Importantly, no effects involving E2 were significant for any of the dependent measures. Main effects of age were found on the pupil diameter measure during SCOP (F(1,20) = 6.32, p = .02) and MECA (F(1,20) = 4.75, p = .04) challenge days. In both cases, the younger subjects had larger pupil diameter changes than the older group.
Table 5.
Vital signs difference scores with standard errors. The maximum change from baseline for each measure was calculated. Next measures from the placebo cholinergic challenge day were subtracted from each study drug day rating. Significant effects of estradiol treatment and age are indicated
| Vital Signs | Age Group | 2.5 µg/kg SCOP + E2 | 2.5 µg/kg SCOP + PLC | 20 mg MECA + E2 | 20 mg MECA + PLC |
|---|---|---|---|---|---|
| Systolic Blood Pressure (mmHg) | |||||
| Young | 4.09 (11.6) | 9.55 (11.1) | 5.18 (10.6) | 18.18 (10.6) | |
| Old | 17.45 (11.3) | 4.82 (11.1) | 28.55 (10.6) | 29.27 (10.6) | |
| Diastolic Blood Pressure (mmHg) | |||||
| Young | 9.4 (8.9) | 3.36 (5.1) | 7.54 (6.1) | 6.45 (6.1) | |
| Old | 6.36 (8.4) | 5.27 (5.1) | 3.64 (6.1) | 6.18 (6.1) | |
| Pulse (bpm) | Young | 5.6 (3.9) | 16.09 (3.7) | −4.18 (4.2) | −4.45 (4.2) |
| Old | 7.9 (3.7) | 3.5 (3.9) | 1.55 (4.2) | −7.27 (4.2) | |
| Pupil Diameter (mm) 1,2 | Young | −.75 (.2) | −.55 (.2) | −1.68 (.3) | −1.27 (.3) |
| Old | −.14 (.2) | −.10 (.2) | −1.0 (.3) | −.45 (.3) |
SCOP analysis age main effect for pupil diameter: F(1,20) = 6.32, p < .05
MECA analysis age main effect for pupil diameter: F(1,20) = 4.75, p < .05
Relationship between Verbal Memory and Behavioral, Vitals Signs, and Demographic Measures
In an effort to determine whether the E2 and age interactions observed during SCOP challenge on the SRT were the result of changes in mood or physical symptoms covariate analyses were conducted. We used 2 (E2 treatment: E2 and PLC) X 2 (age: young and old) mixed model ANCOVAs during the SCOP challenge day using POMS total, PSCL total, and pupil diameter as covariates in each model. In all cases for the SRT, the E2 and age interactions remained significant (p = .04 for POMS total, p = .03 for PSCL total, and p = .01 for pupil diameter). Thus, changes in mood and physical symptoms as a result of the challenge drugs did not alter the relationship between age group and E2 treatment found on the SRT.
In addition, we examined the potential relationship between cognitive performance and demographic variables such as education, BMI, and years since menopause. There were no age differences or correlations with cognitive performance for education and BMI. There was an expected age difference in years since menopause (t(21) = 5.31, p < .001). However, only one correlation was found between the hit RT measure from the CPT and years since menopause (for the younger subjects only, r = −.69, p < .05). When we used years since menopause as a covariate in the analysis of the CPT hit RT for the SCOP challenge, the statistics and data patterns did not change. There were no effects of age or E2 treatment and no interaction. Thus, these demographic factors did not explain the data patterns found in this study.
Discussion
The major finding of this study is that the beneficial effects of E2 on verbal memory were seen for a younger group (mean age 55.8) of postmenopausal women and not for an older group (mean age 74.3) of subjects. More specifically these beneficial effects of E2 were only seen during the anticholinergic challenge with the muscarinic antagonist SCOP, although trends were in this direction for the nicotinic antagonist MECA. Thus, this study provides experimental evidence for the epidemiologic finding that younger postmenopausal women may benefit from E2 therapy while older women may not.
The existence of a critical period for the beneficial effects of estrogen has been proposed by a number of researchers after review of the prior epidemiological, clinical trial, and animal literature examining the effects of estrogen on cognition (Resnick & Henderson 2002; Maki 2006; Sherwin 2007). This study was specifically designed to examine the effects of age on the ability of E2 to attenuate the impairment seen from anticholinergic challenge. Importantly, support for the critical period hypothesis was not seen when we examined the effect of E2 therapy on memory performance alone without the cholinergic challenge. This finding may help to the further specify the hypothesis of age-specific benefits to include a role for the cholinergic system with regards to the E2 effect. Based on the results of the current study, beneficial effects of E2 will be seen when the cholinergic system is temporarily impaired in younger women.
These data also showed different patterns than the data from our prior study examining the E2-cholinergic interaction (Dumas et al. 2006). In that study, 1 mg of E2 per day attenuated the anticholinergic impairment on tests of attention and tests with a speed component. The pattern of means on the SRT was in the direction that indicated E2 may attenuate the anticholinergic impairment with additional manipulations. In the current study, we increased the E2 dose to 2 mg per day. This increase in E2 dose revealed the age by E2 interaction on a verbal memory measure and showed that E2 was beneficial only for the younger subjects. We found no effects of the E2 treatment on the measures of attention did show a significant E2-cholinergic interaction in our prior study. However, this the current study, the anticholinergic drugs did not cause impairments on the attention tests. One explanation for this finding is that we used only single doses each of SCOP and MECA in the current study while we used two doses each in Dumas et al. (2006). These doses represented the low and high doses of SCOP and MECA, respectively, used in Dumas et al. (2006). The reason for this choice was in part logistical to successfully run 22 subjects through a very long and intense study in a reasonable amount of time. However, this choice limited our ability to see a full range of E2-cholinergic interactions. Thus, the lack of E2-cholinergic interactions on the attention measures may have been limited by the dosage restrictions.
The results suggest that additional factors may play a role in the presence or absence of this interaction which were not assessed here, including individual genetics (e.g. apolipoprotein E), history of cognitive complaints and brain morphology, all of which have been shown individually to be related to the degree of cognitive responsivity to either E2 treatment, cholinergic drug challenge, or risk of memory impairment (Kozauer et al 2007; Saykin et al 2006; Wishart et al 2006). These factors will be assessed proactively in future studies. A second issue related to the dose of E2 chosen. In our prior study (Dumas et al. 2006) we used 1 mg of E2 while here we used 2 mg per day. Differences in effects of the E2-cholinergic interaction on verbal episodic memory in this study and the prior study may be explained by this difference. Why effects on attention and speed measures were less prominent in the present study is less clear. Little work has been done on dose-response effects of E2 on cognitive functioning in humans, largely because of the logistical difficulties inherent in these studies. It may be that different doses of E2 have differential effects on particular neurochemical systems, e,g, basal forebrain cholinergic systems, hippocampus, etc.
In addition, our data showed effects of the E2-cholinergic interaction on the Buschke SRT but not on the Paragraph Recall test. While both tasks are verbal episodic recall tasks, they differ on the amount of organization in the to-be-remembered information. On the Paragraph Recall test subjects had to recall a short story and on the SRT subjects recalled a list of 16 unrelated words. Prior research shows that subjects remember related words better than unrelated words (Hunt and Einstein 1981) and that participants remember a series of statements better if they are given an organizational framework for understanding the statements than if they are not (Bransford and Johnson 1972). As a result, relative to the SRT, Paragraph Recall may be an easier task. There is much evidence from the animal literature that describes the increasing involvement of the cholinergic system with increasing task difficulty (Sarter et al 2003). Thus, the differential task difficulty in the SRT and Paragraph Recall tests may explain their differential sensitivity to the E2-cholinergic interaction.
Another interesting result from this study is the finding that younger postmenopausal women were more reactive to the anti-cholinergic challenges and showed greater changes on the memory, behavioral and vital signs measures than the older women. The mechanism for this finding may be related to the aging hippocampal memory system. Cholinergic input to the hippocampus may be important for determining the efficiency of the hippocampal mechanisms in older adults (Decker 1987). Prior research shows aging impairs hippocampally mediated cognitive processes like episodic memory (Landfield 1988; Light et al 1986). With increased age, cholinergic inputs to the hippocampus may deteriorate and result in a break down of these memory processes. Wink and colleagues (Wink et al 2006) have shown that the processes of aging affect hippocampal integrity in a similar manner as SCOP challenge thus implying that aging may be a similar process to loss of cholinergic input. In younger postmenopausal women, these cholinergic inputs may be relatively intact and may be more sensitive to cholinergic manipulation while in older women these cholinergic inputs to the hippocampus may have already deteriorated. Prior studies with muscarinic blockade have shown that elderly subjects tend to be more sensitive than younger subjects to cholinergic blockade (Newhouse et al 1988; Sunderland et al 1988), however these studies were small, included both sexes, and did not examine explicitly middle-aged versus older females not on hormone therapy. Thus, the question of whether age interacts with hormone status to change cholinergic sensitivity is not settled. Estrogen status or time since menopause may be a critical determinant of cholinergic system integrity and thus may play a role in determining sensitivity to cholinergic system challenge.
There are strong links suggesting that estrogen status is important to cholinergic functioning. A series of investigations in animal models has shown that long-term loss of brain estrogen produces a decrease in the functional status of basal forebrain cholinergic neurons, particularly projecting to the hippocampus and cortex (Gibbs 1998). Furthermore, in this investigation, treatment with E2 within six months partially restored choline acetyltransferase (ChAT) mRNA in the medial septum and trkA mRNA in the nucleus basalis. In addition, Gibbs (2000) and Markowska and Savonenko (2002) have shown that if E2 treatment in rats is delayed 10 or 9 months, respectively, after ovariectomy, beneficial effects of E2 treatment are not seen and is further evidence for a “window of opportunity” to see a beneficial effect of E2 treatment (Gibbs and Gabor 2003).
The importance of the estrogen-cholinergic interaction has also been shown in animals performing learning and memory tasks. E2 administration up to 72 hours prior to testing to ovariectomized rats completely counteracted the negative learning effects of the cholinergic antagonist scopolamine on alternation learning (Dohanich et al 1994). E2 replacement in ovariectomized rats enhanced acquisition of spatial memory tasks and partially blunted the effects of hippocampal administration of the anticholinergic scopolamine (Gibbs 1999). In a radial arm maze task, 30 days of E2 administration to ovariectomized rats improved working memory and prevented the amnestic effects of scopolamine (Fader et al 1999). E2 administration attenuated the negative effects of scopolamine and lorazepam on multiple trial passive avoidance acquisition and retention; interestingly, the effect appeared to be specific to low and intermediate but not high serum levels of E2 (Gibbs et al 1998). A single subthreshold dose of E2 was able to potentiate the effects of the cholinergic or glutamatergic agonist on avoidance learning when administered directly to the hippocampus (Farr et al 2000). Studies in primates have also shown that E2 effects on visual spatial attention appear to be modulated by cholinergic muscarinic receptors (Tinkler and Voytko 2005).
However, the ability of E2 replacement to enhance cholinergic function may be age-related. Savonenko and Markowska (Savonenko and Markowska 2003) found that the ability of E2 to decrease behavioral vulnerability to scopolamine was limited to younger ovarectomized animals and was not seen in older rats. The ability of E2 to enhance basal forebrain cholinergic function appears to decline with age and with the time since loss of endogenous hormonal support. E2 alone or E2 plus progesterone up to three months after ovarectomy produce mutually more improvement on spatial memory performance than in animals for whom a 10 month delay occurred in E2 administration. Daniel and colleagues (Daniel et al 2006) showed that if E2 is administered to rats immediately following ovarectomy, improvements are seen in acquisition and delay trials of a working memory task, but not seen if E2 administration is delayed for up to five months. However some primate studies have shown that E2 benefits cognitive performance even when administration is delayed after ovarectomy or natural menopause even for several years (Lacreuse 2006; Lacreuse et al 2002; Rapp et al 2003). The reason for this discrepancy is not clear, but the length of the “window” or critical period may be quite different depending on the natural lifespan of the animal. For humans, it may extend for several years, potentially even up to a decade.
These data suggest that part of the reason that several prior studies have not seen significant effects on cognitive performance in the early postmenopausal period with estrogen therapy may be the lack of significant cholinergic impairment at this age. Thus, if a significant part of E2 effects on cognitive performance are mediated through cholinergic system stimulation or maintenance, the lack of significant cholinergic dysfunction at the age that most women go through menopause means that it will be difficult to demonstrate cognitive improvement. However, in the model described in this paper and in our prior work (Dumas et al. 2006) cholinergic dysfunction is produced artificially, much as it is in animal models, allowing the E2 effect to be expressed. Thus the primary motivation for estrogen therapy after menopause in terms of preserving cognitive function may be through its actions to sustain and/or preserve cholinergic system integrity which may tend to counteract effects of aging without hormones and/or the addition weight of pathologic conditions that are detrimental to cholinergic system functioning such as Alzheimer's disease. Loss of this support of the basal forebrain cholinergic system may be a primary reason why women are at higher risk for developing Alzheimer's disease and the magnitude of the sex difference in risk tends to increase with age. As the cholinergic system ages, the ability of E2 to provide sustained benefit may decline and thus not only may there be critical period for initiation of administration of E2 therapy, but there may also be an optimal length of time. How long that period may be will have to be the subject of future research.
Acknowledgements
This work was supported by NIA F32 AG023430 to JD, AA IIRG 99-1811 and NIA R01 AG021476 to PN, and NCRR-00109. The authors wish to thank the research nursing staff of the University of Vermont GCRC for their hard work and support of this study and to our volunteers for their dedication to clinical research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Almeida OP, Lautenschlager NT, Vasikaran S, Leedman P, Gelavis A, Flicker L. A 20-week randomized controlled trial of estradiol replacement therapy for women aged 70 years and older: effect on mood, cognition, and quality of life. Neurobiology of Aging. 2006;27:141–149. doi: 10.1016/j.neurobiolaging.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Barrett-Connor E, Kritz-Silverstein D. Estrogen replacement therapy and cognitive function in older women. Journal of the American Medical Association. 1993;269:2637–2641. [PubMed] [Google Scholar]
- Binder EF, Schechtman KB, Birge SJ, Williams DB, Kohrt WM. Effects of hormone replacement therapy on cognitive performance in elderly women. Maturitas. 2001;38:137–146. doi: 10.1016/s0378-5122(00)00214-0. [DOI] [PubMed] [Google Scholar]
- Bransford JD, Johnson MK. Contextual prerequisites for understanding: Some investigations of comprehension and recall. Journal of Verbal Learning and Verbal Behavior. 1972;11:717–726. [Google Scholar]
- Buschke H. Selective reminding for analysis of memory and learning. Journal of Verbal Learning and Verbal Behavior. 1973;12:543–550. [Google Scholar]
- Conners CK. The Continuous Performance Test. 3.0 ed. Toronto: Multi-Health Systems; 1995. [Google Scholar]
- Daniel JM, Hulst JL, Berbling JL. Estradiol replacement enhances working memory in middle-aged rats whien initiated immediately after ovariectomy but not after a lone-term period of ovarian hormone deprivation. Endocrinology. 2006;147:607–614. doi: 10.1210/en.2005-0998. [DOI] [PubMed] [Google Scholar]
- Ditkoff EC, Crary WG, Cristo M, Lobo RA. Estrogen improves psychological function in asymptomatic postmenopausal women. Obstetrics and Gynecology. 1991;78:991–995. [PubMed] [Google Scholar]
- Dohanich GP, Fader AJ, Javorsky DJ. Estrogen and estrogen-progesterone treatments counteract the effect of scopolamine on reinforced T-maze alternation in female rats. Behavioral Neuroscience. 1994;108:988–992. doi: 10.1037//0735-7044.108.5.988. [DOI] [PubMed] [Google Scholar]
- Duka T, Tasker R, McGowan JF. The effects of 3-week estrogen hormone replacement on cognition in elderly healthy females. Psychopharmacology. 2000;149:129–139. doi: 10.1007/s002139900324. [DOI] [PubMed] [Google Scholar]
- Dumas JA, Hancur-Bucci C, Naylor M, Sites C, Newhouse PA. Estrogen treatment effects on anticholinergic-induced cognitive dysfunction in normal post-menopausal women. Neuropsychopharmacology. 2006;31:2065–2078. doi: 10.1038/sj.npp.1301042. [DOI] [PubMed] [Google Scholar]
- Fader AJ, Johnson PEM, Dohanich GP. Estrogen improves working but not reference memory and prevents amnestic effects of scopolamine on a radial-arm maze. Pharmacology Biochemistry and Behavior. 1999;62:711–717. doi: 10.1016/s0091-3057(98)00219-6. [DOI] [PubMed] [Google Scholar]
- Farr SA, Banks WA, Morley JE. Estradiol potentiates acetylcholine and glutamate-mediated post-trial memory processing in the hippocampus. Brain Research. 2000;864:263–269. doi: 10.1016/s0006-8993(00)02184-3. [DOI] [PubMed] [Google Scholar]
- First MB, RL S, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IVTR Axis I Disorders-Patient Edition. SCID-I/P, 2/2001 ed. Washington, D.C.: American Psychiatric Press Inc; 2001. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. "Mini-mental state": a practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Impairment of basal forebrain cholinergic neurons associated with aging and long-term loss of ovarian function. Experimental Neurology. 1998;151:289–302. doi: 10.1006/exnr.1998.6789. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Estrogen replacement enhances acquisition of a spatial memory task and reduces deficits associated with hippocampal muscarinic receptor inhibition. Hormones & Behavior. 1999;36:222–233. doi: 10.1006/hbeh.1999.1541. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Effects of gonadal hormone replacement on measures of basal forebrain cholinergic function. Neuroscience. 2000;101:931–938. doi: 10.1016/s0306-4522(00)00433-4. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Aggarwal P. Estrogen and basal forebrain cholinergic neurons: implications for brain aging and Alzheimer's disease-related cognitive decline. Hormones & Behavior. 1998;34:98–111. doi: 10.1006/hbeh.1998.1451. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Burke AM, Johnson DA. Estrogen replacement attenuates effects of scopolamine and lorazepam on memory acquisition and retention. Hormones and Behavior. 1998;34:112–125. doi: 10.1006/hbeh.1998.1452. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Gabor R. Estrogen and Cognition: Applying Preclinical Findings to Clinical Perspectives. Journal of Neuroscience Research. 2003;74:637–643. doi: 10.1002/jnr.10811. [DOI] [PubMed] [Google Scholar]
- Grady D, Yaffe K, Kristof M, Lin F, Richards C, Barrett-Connor E. Effect of postmenopausal hormone therapy on cognitive function: the heart and estrogen/progestinreplacement study. American Journal of Medicine. 2002;113:543–548. doi: 10.1016/s0002-9343(02)01270-6. [DOI] [PubMed] [Google Scholar]
- Halbreich U, Lumley LA, Palter S, Manning C, Gengo F, Joe S-h. Possible acceleration of age effects on cognition following menopause. Journal of Psychiatric Research. 1995;29:153–163. doi: 10.1016/0022-3956(95)00005-p. [DOI] [PubMed] [Google Scholar]
- Hindmarch I. Psychological performance models as indicators of the effects of hypnotic drugs on sleep. Psychopharmacology. 1984;S1:58–68. doi: 10.1007/978-3-642-69659-6_4. [DOI] [PubMed] [Google Scholar]
- Hunt RR, Einstein GO. Relational and item-specific information in memory. Journal of Verbal Learning and Verbal Behavior. 1981;20:497–514. [Google Scholar]
- Jacobs DM, Tang MX, Stern Y, Sano M, Marder K, Bell KL, et al. Cognitive function in nondemented older women who took estrogen after menopause. Neurology. 1998;50:368–373. doi: 10.1212/wnl.50.2.368. [DOI] [PubMed] [Google Scholar]
- Joffe H, Hall JE, Gruber S, Sarmiento IA, Cohen LS, Yurgelun-Todd D, Martin KA. Estrogen therapy selectively enhances prefrontal cognitive processes: a randomized, double-blind, placebo-controlled study with functional magnetic resonance imaging in perimenopausal and recently postmenopausal women. Menopause. 2006;13:411–422. doi: 10.1097/01.gme.0000189618.48774.7b. [DOI] [PubMed] [Google Scholar]
- Jurica PJ, Leitten CL, Mattis S. Dementia Rating Scale-2. Lutz, FL: Psychological Assessment Resources Inc.; 2001. [Google Scholar]
- Kluger A, Ferris SH, Golomb J, Miettleman M, Reisberg B. Neuropsychological prediction of decline to dementia in nondemented elderly. Journal of Geriatric Psychiatry and Neurology. 1999;12:168–179. doi: 10.1177/089198879901200402. [DOI] [PubMed] [Google Scholar]
- Kozauer NA, Mielke MM, Chuen Chan GK, Rebok GW, Lyketsos CG. Apolipoprotein E genotype and lifetime cognitive decline. International Psychogeriatrics. 2007;22:1–15. doi: 10.1017/S104161020700587X. [DOI] [PubMed] [Google Scholar]
- Kupke T, Lewis R. Relative influence of subject variables and neurological parameters on neuropsychological performance of adult seizure patients. Archive of Clinical Neuropsychology. 1989;4:351–363. [PubMed] [Google Scholar]
- Lacreuse A. Effects of ovarian hormones on cognitive function in nonhuman primates. Neuroscience. 2006;138:859–867. doi: 10.1016/j.neuroscience.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Lacreuse A, Wilson ME, Herndon JG. Estradiol, but not raloxifene, improves aspects of spatial working memory in aged ovariectomized rhesus monkeys. Neurobiology of Aging. 2002;23:589–600. doi: 10.1016/s0197-4580(02)00002-7. [DOI] [PubMed] [Google Scholar]
- Landfield PW. Hippocampal neurobiological mechanisms of age-related memory dysfunction. Neurobiology of Aging. 1988;9:571–579. doi: 10.1016/s0197-4580(88)80116-7. [DOI] [PubMed] [Google Scholar]
- Light LL, Singh A, Capps JL. Dissociation of memory and awareness in young and older adults. Journal of Clinical and Experimental Neuropsychology. 1986;8:62–74. doi: 10.1080/01688638608401297. [DOI] [PubMed] [Google Scholar]
- Maki PM. A Systematic Review of Clinical Trials of Hormone Therapy on Cognitive Function: Effects of Age at Initiation and Progestin Use. Annals of the New York Academy of Science. 2005;1052:182–197. doi: 10.1196/annals.1347.012. [DOI] [PubMed] [Google Scholar]
- Maki PM. Hormone therapy and cognitive function: is there a critical period for benefit? Neuroscience. 2006;138:1027–1030. doi: 10.1016/j.neuroscience.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Markowska AL, Savonenko AV. Effectiveness of Estrogen Replacement in Restoration of Cognitive Function after Long-Term Estrogen Withdrawal in Aging Rats. Journal of Neuroscience. 2002;22:10985–10995. doi: 10.1523/JNEUROSCI.22-24-10985.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppleman LF. Profile of Mood States. San Diego, CA: Educational and Industrial Testing Service; 1971. [Google Scholar]
- Newhouse PA, Sunderland T, Tariot PN, Weingartner H, Thomason K, Mellow AM, et al. The effects of acute scopolamine in geriatric depression. Archives of General Psychiatry. 1988;45:906–912. doi: 10.1001/archpsyc.1988.01800340028004. [DOI] [PubMed] [Google Scholar]
- Norbury R, Travis MJ, Erlandsson K, Waddington W, Ell PJ, Murphy DGM. Estrogen therapy and brain muscarinic receptor density in healthy females: A SPET study. Hormones & Behavior. 2007;51:249–257. doi: 10.1016/j.yhbeh.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Phillips SM, Sherwin BB. Effects of estrogen on memory function in surgically menopausal women. Psychoneuroendocrinology. 1992;17:485–495. doi: 10.1016/0306-4530(92)90007-t. [DOI] [PubMed] [Google Scholar]
- Polo-Kantola P, Portin R, Polo O, Helenius H, Irjala K, Erkkola R. The effect of short-term estrogen replacement therapy on cognition: a randomized, double-blind, cross-over trial in postmenopausal women. Obstetrics & Gynecology. 1998;91:459–466. doi: 10.1016/s0029-7844(97)00700-x. [DOI] [PubMed] [Google Scholar]
- Rapp PR, Morrison JH, Roberts JA. Cyclic Estrogen Replacement Improves Cognitive Function in Aged Overiectomized Rhesus Monkeys. The Journal of Neuroscience. 2003;23:5708–5714. doi: 10.1523/JNEUROSCI.23-13-05708.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisberg B, Ferris SH. Brief cognitive rating scale (BCRS) Psychopharmacology Bulleting. 1988;24:629–635. [PubMed] [Google Scholar]
- Reisberg B, Ferris SH, de Leon MJ, Crook T. Global Deterioration Scale (GDS) Psychopharmacol Bull. 1988;24:661–663. [PubMed] [Google Scholar]
- Resnick SM, Henderson VW. Hormone therapy and risk of Alzheimer disease: A critical time. Journal of the American Medical Association. 2002;288:2170–2172. doi: 10.1001/jama.288.17.2170. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Metter EJ, Zonderman AB. Estrogen replacement therapy and longitudinal decline in visual memory: a possible protective effect. Neurology. 1997;49:1491–1497. doi: 10.1212/wnl.49.6.1491. [DOI] [PubMed] [Google Scholar]
- Sarter M, Bruno JP, Givens B. Attentional functions of cortical cholinergic inputs: What does it mean for learning and memory? Neurobiology of Learning and Memory. 2003;80:245–256. doi: 10.1016/s1074-7427(03)00070-4. [DOI] [PubMed] [Google Scholar]
- Savonenko AV, Markowska AL. The cognitive effects of ovariectomy and estrogen replacement are modulated by aging. Neuroscience. 2003:821–830. doi: 10.1016/s0306-4522(03)00213-6. [DOI] [PubMed] [Google Scholar]
- Saykin AJ, Wishart HA, Rabin LA, Santulli RB, Flashman LA, West JD, et al. Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology. 2006;67:834–842. doi: 10.1212/01.wnl.0000234032.77541.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaywitz SE, Naftolin F, Zelterman D, Marchione KE, Holahan JM, Palter SF, Shaywitz BA. Better oral reading and short-term memory inmidlife, postmenopausal women taking estrogen. Menopause. 2003;10:420–426. doi: 10.1097/01.GME.0000060241.02837.29. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen and/or androgen replacement therapy and cognitive functioning in surgically menopausal women. Psychoneuroendocrinology. 1988;13:345–357. doi: 10.1016/0306-4530(88)90060-1. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. Hormones, mood, and cognitive functioning in postmenopausal women. Obstetrics & Gynecology. 1996;87:20S–26S. doi: 10.1016/0029-7844(95)00431-9. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. The critical period hypothesis: can it explain discrepancies in the oestrogen-cognition literature. Journal of Neuroendocrinology. 2007;19:88–81. doi: 10.1111/j.1365-2826.2006.01508.x. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS, et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women. Journal of the Americam Medical Association. 2004;291:2947–2957. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, et al. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women. Journal of the American Medical Association. 2003;289:2651–2662. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- Smith YR, Giordani B, Lajiness-O'Neill R, Zubieta J. Long-term estrogen replacement is associated with improved nonverbal memory and attentional measures in postmenopausal women. Fertility and Sterility. 2001;76:1101–1107. doi: 10.1016/s0015-0282(01)02902-8. [DOI] [PubMed] [Google Scholar]
- Sunderland T, Tariot PN, Newhouse PA. Differential responsivity of mood, behavior, and cognition to cholinergic agents in elderly neuropsychiatric populations. Brain Research Reviews. 1988;13:371–389. doi: 10.1016/0006-8993(88)91227-9. [DOI] [PubMed] [Google Scholar]
- Tinkler GP, Voytko ML. Estrogen modulates cognitive and cholinergic processes in surgically menopausal monkeys. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2005;29:423–431. doi: 10.1016/j.pnpbp.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Viscoli CM, Brass LM, Kernan WN, Sarrel PM, Suissa S, Horowitz RI. Estrogen therapy and risk of cognitive decline: results form the Women's Estrogen for Stroke Trial (WEST) American Journal of Obstetrics & Gynecology. 2005;192:387–393. doi: 10.1016/j.ajog.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Voytko ML. Estrogen and the cholinergic system modulate visuospatial attention in monkeys (Macaca fascicularis) Behavioral Neuroscience. 2002;116:187–197. doi: 10.1037//0735-7044.116.2.187. [DOI] [PubMed] [Google Scholar]
- Wink AM, Bernard F, Salvador R, Bullmore ET, Suckling J. Age and cholinergic effects on hemodynamic and functional coherence of the human hippocampus. Neurobiology of Aging. 2006;27:1395–1404. doi: 10.1016/j.neurobiolaging.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Wishart HA, Saykin AJ, Rabin LA, Santulli RB, Flashman LA, Guerin SJ, et al. Increased Brain Activation During Working Memory in Cognitively Intact Adults With the APOE {epsilon}4 Allele. Am J Psychiatry. 2006;163:1603–1610. doi: 10.1176/ajp.2006.163.9.1603. [DOI] [PubMed] [Google Scholar]
- Woo J, Lau E, Ho SC, /chan C, Chan AS, Haines CJ, et al. Comparison of Pueraria lobata with hormone replacement therapy in treating the adverse health consequences of menopause. Menopause. 2003;10:352–361. doi: 10.1097/01.GME.0000054764.94658.33. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Vittinghoff E, Ensrud KE, Johnson KC, Diem S, Hanes V, Grady D. Effects of ultra-low dose transdermal estradiol on cognition and health-related quality of life. Archives of Neurology. 2006;63:945–950. doi: 10.1001/archneur.63.7.945. [DOI] [PubMed] [Google Scholar]