Abstract
We have initiated studies to evaluate the suitability of performing therapeutic conditioning trials in experimental autoimmune encephalomyelitis (EAE) mice treated with alpha lipoic acid (ALA). EAE was induced in SJL mice by active immunization with myelin antigen. Once daily subcutaneous injection of ALA served as the unconditional stimulus (US) administered with the conditional stimulus (CS) saccharin-flavored drinking water under a regimen of restricted water access. In the first study, we found that water restriction and saccharin administration were compatible with disease development and effective ALA treatment of EAE mice. In the second study, mice were conditioned to once daily administration of ALA paired with administration of saccharin flavored water (US + CS) on days 7 – 16. Test trials spanned experimental days 17 – 32 in groups receiving either saccharin-flavored water (CS, in the experimental group) versus unflavored water (CSo, in the control group) and compared several measures of EAE severity using multivariate ANOVA (MANOVA). Reduced disease severity in the experimental group (US+CS:CS) compared to the control group (US+CS:CSo) suggested that conditioning had occurred. These results demonstrate an approach for conducting therapeutic conditioning trials in EAE mice and suggest considerations for future investigations.
Keywords: Animal model, experimental autoimmune encephalomyelitis, EAE, behavioral conditioning, therapy, alpha lipoic acid, thioctic acid, mouse, SJL
1. Introduction
Pharmacological effects of a treatment agent are typically understood in the context of underlying pathophysiology, biology and pharmacology separate from psychobiological phenomena (Benedetti et al., 2005). However, the full pharmacotherapeutic response is often accompanied by (or includes) placebo effects (Pacheco-Lopez et al., 2006). It has been suggested that placebo effects involve associative learning, verbally-mediated expectancy and/or conditioning. A conditioned therapeutic response utilizing the pharmacological response to treatment as the unconditional stimulus (US) and a novel taste or odor as the conditional stimulus (CS) in an animal disease model may provide a useful experimental approach in which to focus upon the conditioning components of placebo effects (Ader, 1997).
Conditioned suppression of immune responses has been demonstrated following paired administration of the immunosuppressive drug cyclophosphamide (the US) and flavored drinking water (the CS). Following paired administration of this US and CS, the CS alone was capable of suppressing the antibody response to injected sheep red blood cells in rats (Ader and Cohen, 1975). Clinically relevant immunosuppression has also been demonstrated following conditioning in autoimmune antibody-mediated lupus-prone mice (Ader et al., 2001), in T cell-mediated allograft transplantation (mice) (Exton et al., 1999) and in adjuvant-induced arthritis (rats) (Klosterhalfen and Klosterhalfen, 1983). These results have demonstrated that conditioned immunosuppression can occur in rodents, thereby raising the possibility that a conditioned therapeutic response will be possible in mice with T cell-mediated autoimmune disease.
CNS structures necessary for acquiring (e.g. during conditioning) versus expression (e.g. during the testing period) of conditioned responses have been examined in rats with lesions placed precisely in the CNS prior to or following conditioned immunosuppression of a response to experimental antigen challenge (Hucklebridge, 2002). Lesions in the insular cortex (but not parietal cortex) interfered with acquisition of the conditioned immune response and interfered with conditioned taste aversion but did not interfere directly with unconditioned responses (Ramirez-Amaya and Bermudez-Rattoni, 1999). Thus, there appeared to be a common requirement for insular cortex integrity during associative learning of the conditioned behavioral response (taste aversion) and the conditioned immune response (Hucklebridge, 2002). In contrast, lesions in the amygdala interfered with conditioning of the immune response but did not interfere with conditioned taste aversion (Ghanta et al., 1987). Moreover, such lesions interfered with integration between immune status perception and downstream immune function (Hucklebridge, 2002). Thus, the discrete outcomes associated with behavioral versus immune conditioning involve both overlapping (e.g. utilizing common brain regions) and non-overlapping structural pathways (e.g. utilization of distinct brain regions).
Experimental autoimmune encephalomyelitis (EAE) is an experimental rodent T lymphocyte-mediated autoimmune disease with clinical and immunological similarities to the human paralytic inflammatory central nervous system (CNS) autoimmune disease, multiple sclerosis (MS). Alpha lipoic acid (ALA) is highly effective at suppressing or treating murine EAE in a dose dependent fashion (Marracci et al., 2002; Morini et al., 2004). We propose that ALA might serve as a suitable US with which to apply a behavioral conditioning approach for examining a conditioned pharmacotherapeutic response.
We hypothesized that a suitable murine disease model in which to investigate the conditioned pharmacotherapeutic response may be expected to possess the following attributes: 1) For obvious reasons, an unconditional stimulus (US) capable of reducing or treating disease must be available. This requires that a means of avoiding induction of overly severe or overly mild disease and a means of quantifying disease severity are available; 2) It must be possible to achieve a limited duration or limited magnitude of the unconditional therapeutic response (UR) (e.g. treatment, not cure) during conditioning in order to permit an observable response (recall) to the CS (e.g. the conditional response, CR) following discontinuation of the US; 3) The nature, severity and course of the disease must be compatible with planned exposure to the US, CS and/or other control conditions expected in a conditioning study (Ader, 2003). For example, if using flavored water as the CS, the disease model must permit water consumption on a limited schedule to ensure paired (contingent) exposures to the US and CS. Here we report the development of a protocol for examining a pharmacotherapeutic response in EAE mice treated with ALA and associative conditioning.
2. Materials and methods
2.1 Animals
Inbred, female SJL mice 10 – 12 weeks old at experiment start were obtained from Jackson Laboratory (Bar Harbor, ME). Experimentation was performed at the Veterinary Medical Unit, Portland VA Medical Center, and was approved by the Institutional Animal Care and Use Committee.
2.2. Disease induction and treatment
2.2.1. Immunization
Anesthetized mice were immunized (on experimental day 0) subcutaneously at four sites across their flanks with 0.2 ml sterile aqueous-in-oil emulsion of Complete Freund's Adjuvant (CFA) containing 200μg of heat killed strain H37Ra Mycobacterium tuberculosis and 150μg peptide corresponding to myelin proteolipid protein (PLP) residues 139-151 (HSLGKWLGHPDKF).
2.2.2. ALA administration
ALA was given subcutaneously at a single neck nape site. Fifty mg/Kg body weight (1 mg/20 gram mouse in 0.4 ml saline) ALA or vehicle (control) was injected on the indicated days immediately prior to the morning administration of water.
2.3. Conditioned stimulus
2.3.1. Hydration
All mice were placed on controlled water access seven days prior to disease induction. Drinking water was available twice/day at the same times/day to ensure adequate and relatively uniform consumption of water or saccharin-flavored water (Risinger and Cunningham, 1992): Two hours each morning and one-half hour each afternoon, 6.5 hours apart. Saccharin (0.15 % w/v)-flavored drinking water was used as the conditioned stimulus (CS) and unflavored water was the conditioned control stimulus (CSo). Body weight in all mice was monitored daily as was water intake in singly housed mice to ensure adequate hydration. Mice displaying signs of dehydration during EAE were administered supplemental nutritional fluids by intraperitoneal injection and were provided a high-water-content soft mix rodent chow.
2.4. Clinical evaluation
Active immunization of SJL mice with PLP 139-151 peptide in CFA induced T cell-mediated inflammation of the spinal cord and ascending paralysis. The severity of paralytic disease was rated daily on a scale of 0-9: 0, no deficit; 1, limp tail; 2, limp tail+mild hind limb weakness; 3, limp tail+moderate hind limb weakness; 4, limp tail+moderately severe hind limb weakness; 5, limp tail+severe hind limb weakness; 6, limp tail+hind limb paralysis; 7, limp tail+hind limb paralysis+mild fore limb weakness; 8, limp tail+hind limb paralysis+moderate fore limb weakness; 9, limp tail+hind limb paralysis+severe fore limb weakness or paralysis (Jones et al., 2003).
2.5. Experimental protocol
2.5.1. Experiment 1: Water only and ALA groups
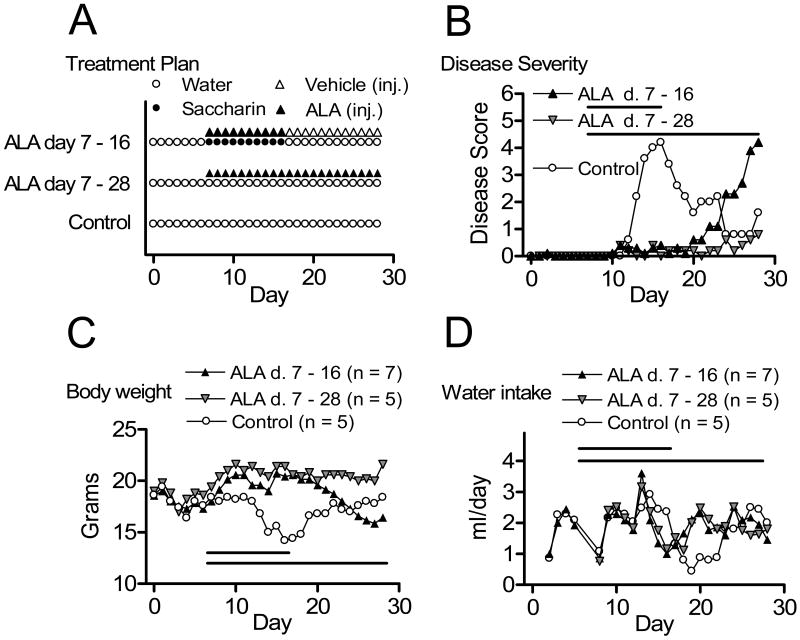
In Experiment 1 we simply wanted to determine whether moderately severe EAE could be induced in water restricted mice and whether ALA would reduce symptoms of EAE when utilizing a protocol including both restricted access to water and administration of saccharin-flavored water. The schedules of ALA and saccharin treatments were selected to simulate future conditioning trials and to determine the duration of ALA effect (Figure 1, A). Mice were assigned randomly to one of three groups, and EAE was induced. One group (Control, n = 5) received no additional manipulations other than disease induction. Two groups received ALA: One group (ALA days 7-16, n = 7) received injections of ALA paired with saccharin-flavored water on days 7-16 followed by daily vehicle injection days 17 - 28; and one group (ALA days 7-28, n = 5) received ALA injections paired with unflavored water on days 7-28. Disease was monitored daily for 28 days following disease induction in all three groups (Figure 1, B).
Figure 1.
ALA suppressed development of clinical paralysis in water restricted mice. Mice were immunized on day 0, with ALA treatment on days 7 – 16, days 7 – 28 or no ALA treatment (Control) (A). Mean daily disease severity (B), Mean daily body weight (C) and mean water intake (D) for each group are shown.
2.5.2. Experiment 2: Saccharin and ALA pairing
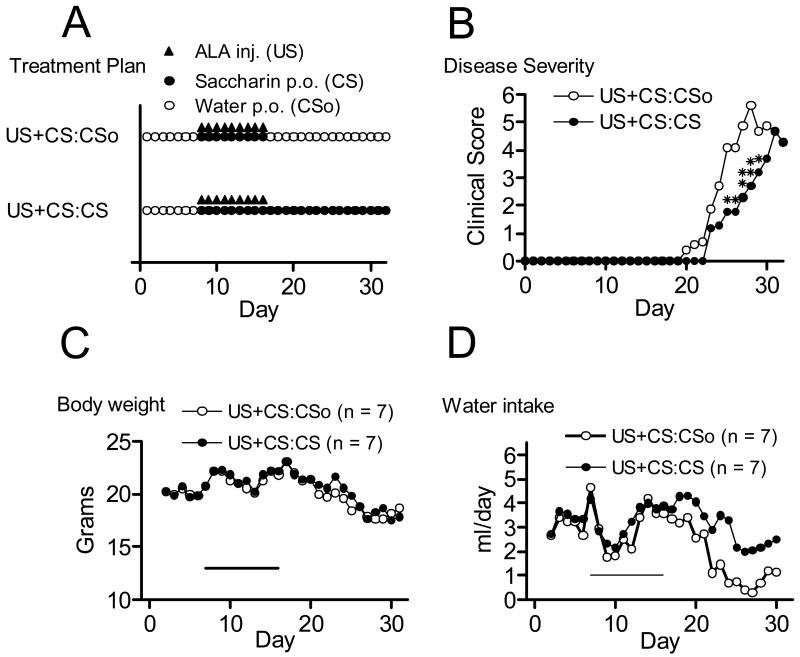
In experiment 2, disease was induced in two groups of mice (n = 7/group) on day 0 and both groups were conditioned by co-administration of ALA (injection) and saccharin flavored drinking water (US+CS) during the conditioning period (10 daily trials on days 7 to 16, Fig. 2, A). The control group received unflavored drinking water on days 17 to 32 (US+CS:CSo during the test period) while the experimental group received saccharin-flavored drinking water during this time (US+CS:CS). We compared disease course and severity in these two conditioned groups during the testing period.
Figure 2.
Comparison of conditioned control (UCS/CS:CSo) and conditioned recall (UCS/CS:CS) groups (n=7). Both groups were conditioned by paired administration of ALA and saccharin flavored drinking water on days 7 – 16 (A). Administration of the CSo versus CS occurred during the testing (recall) period, days 17 – 32. Mean daily disease severity (B), Mean daily body weight (C) and mean daily water intake (D) are shown for each group. Single asterisk, p < 0.05; double asterisk, p < 0.01, by Mann-Whitney U test.
2.5.3. Outcome measures and alphawise error
Typically, EAE studies assess some or all of the following four disease severity measures (Fleming et al., 2005): 1) day of onset, calculated by counting the number of days from immunization to the day that the first sign of disease is displayed by an animal; 2) day of maximum (peak) disease severity, calculated by counting the number of days from immunization to the day that an animal is rated at its maximum disease severity; 3) maximum disease severity, calculated by determining the maximum disease severity score for a given animal; and 4) cumulative disease index (CDI), calculated by summing the disease severity score for each day for an animal.
We recognized that performing multiple comparisons increases the alphawise error rate. Setting alpha to .05, and testing all four variables (assuming that the measures are orthogonal to one another), the true alpha would be .19 in both experiments. If the measures were intercorrelated, then the alphawise error would be even higher.
3. Results
3.1. Experiment 1 results: Intercorrelations and method of analysis
In Experiment 1, the average intercorrelation among the disease severity measures was .33, ranging from .14 to .79. Each measure was significantly correlated with at least one other measure. Thus, the true alpha would be inflated substantially above .20 were we to have performed statistical analyses on each of the four disease severity measures.
To control for alphawise error, we used multivariate analysis of variance (MANOVA) to compare the groups on all four dependent variables. There were no missing data points which would have necessitated the use of a hierarchical modeling procedure. MANOVA yields an exact solution.
3.2. Experiment 1 results: Water restriction, disease induction and ALA administration
Figure 1 displays the treatment schedules for each group (panel A), the average severity per day for each group (panel B), daily body weights (panel C) and daily water consumption (panel D). Water restricted mice (Control) developed moderately severe disease followed by spontaneous recovery. ALA treatment was effective in water restricted mice with or without saccharin. Discontinuation of ALA treatment was followed by moderately severe disease.
Table 1 provides the means for each treatment group for each of the four disease severity measures in Experiment 1. These treatment groups varied significantly by MANOVA, F(8,24) = 13.7 (by Pillai's Trace), p < .0001, η2 = .82, observed power = 1.00. Multivariate comparisons (comparable to the univariate Tukey's Least Significant Difference test) revealed that the ALA 7-28 group overall had less severe disease than the ALA 7-16 group (p < .0001, η2 = .86), which in turn had less severe disease scores than the untreated control group (p < .0001, η2 = .90). Thus, under these experimental conditions, daily ALA treatment initiated prior to disease onset was effective at suppressing development of paralytic EAE. This therapeutic response was transient, depending on continued administration of ALA.
Table 1. Group Means and Standard Deviations For Outcome Measures.
| Day of Onset | Day of Peak Severity | Maximum Disease Severity | Cumulative Disease Index | ||
|---|---|---|---|---|---|
| Experiment 1 | |||||
|
| |||||
| Control | (n = 5) | ||||
| (No ALA or saccharin) | 13.2 (1.1) | 14.6 (0.9) | 4.6 (1.1) | 35.4 (16.3) | |
|
| |||||
| ALA 7-16 | (n = 7) | ||||
| (ALA+saccharin days 7-16 only) | 12.6 (6.3) | 26.0 (2.2) | 4.6 (1.4) | 21.4 (8.7) | |
|
| |||||
| ALA 7-28 | (n = 5) | ||||
| (ALA only days 7-28) | 17.2 (8.2) | 20.2 (5.7) | 1.4 (0.9) | 4.8 (3.6) | |
|
| |||||
| Experiment 2 | |||||
|
| |||||
| US+CS:CSo | (n = 7) | ||||
| (ALA+saccharin days 7-16 only) | 27.3 (2.5) | 23.9 (2.2) | 6.3 (1.5) | 43.6 (8.5) | |
|
| |||||
| US+CS:CS | (n = 7) | ||||
| (ALA+saccharin days 7-16, saccharin only days 17-28) | 29.3 (2.4) | 26.0 (2.5) | 4.6 (2.0) | 23.9 (13.8) | |
3.3. Experiment 1 results: Effects of disease and saccharin consumption
Induction of EAE using the procedures of Experiment 1 had a transient effect on lowering of body weights (Figure 1, C) in concert with disease progression, as shown previously (Sanna et al., 2003), and increases in disease severity were accompanied by decreased water consumption (Figure 1, D). There was not an obvious link between these changes (body weight and fluid intake) and the presence versus absence of saccharin in the drinking water on days 7 – 16.
3.4. Experiment 2 results: Intercorrelations among measures
As was the case in Experiment 1, in Experiment 2 each outcome measure was correlated significantly with at least one other measure. The average intercorrelation among the four measures was .45, ranging from .04 to .82.
3.5. Experiment 2 results: Effects of ALA-saccharin pairing on disease
Figure 2 displays the treatment schedules for each conditioned group (panel A), the average severity per day for each group (panel B), daily body weights and daily water consumption (panels C and D, respectively). We again used MANOVA to compare the groups in order to control for alphawise error. The result was statistically significant, F(4,9) = 3.6, p < .05, η2 = .62, observed power = .65. Table 1 (lower section) provides the means for each group for each of the four outcome measures in Experiment 2. Overall, the conditioned group receiving saccharin-flavored water during the testing period (US+CS:CS) had reduced disease severity compared to the group receiving unflavored water (US+CS:CSo). While body weights were very similar comparing the two groups over the duration of the experiment (Figure 2), water intake in the control group (US+CS:CSo) decreased to a greater extent in concert with the greater rise in disease severity observed in that group, in agreement with Experiment 1. The decreased voluntary water consumption due to paralysis in the US+CS:CSo group was not accompanied by extra weight loss due to supplemental perenteral injections of nutritional fluid.
4. Discussion
Restricted access to unflavored or saccharin-flavored drinking water has been used extensively in rodent associative conditioning studies but the influences of these experimental manipulations in EAE studies have not been reported previously. Our results demonstrated that water restriction and administration of saccharin flavored water did not cause development of overly mild or overly severe disease, and the previously demonstrated temporal link between clinical response and administration of ALA (Marracci et al., 2002) was retained in water-restricted, saccharin-treated mice. Thus, effects of restricted water access and administration of saccharin-flavored water were minimal in this EAE model and would not be expected to interfere with detection of a conditioned therapeutic response during the testing period.
The experimental and control groups selected for Experiment 2 (US+CS:CS and US+CS:CSo, respectively) were described by Ader as being critical for studies of conditioned immune responses (Ader et al., 2001). These two conditioned groups receive identical amounts of the US+CS during the conditioning period but they differ in the presence versus absence of the CS during the testing period. Thus, differences between these two groups are attributable to administration of the CS during the testing period. These experimental and control groups have also been used previously in various studies demonstrating conditioned immune responses. As examples: Immunization of Balb/c mice with keyhole limpet hemocyanin (the US) paired with the CS, chocolate milk drinking solution generated two groups of conditioned mice in which reexposure to the CS elicited an elevated anti-KLH antibody titer in experimental mice compared to reexposure to the CSo in the controls (Ader et al., 1993); and two groups of rats conditioned with injected LPS administered with saccharin flavored water were used to demonstrate conditioned differences in splenic secretion of interleukin-2 during administration of the CS, saccharin-flavored water compared to recipients of the CSo, unflavored water during the testing period (Janz et al., 1996). In each of these studies and in Experiment 2, differences between the US+CS:CS and US+CS:CSo groups were attributable to the CS, a result consistent with conditioning. Thus, the possibility has been raised that the therapeutic response to ALA can be augmented by associative conditioning in EAE mice.
Some studies have used unconditioned control groups with pharmacological immunosuppression as the US. For example, rats conditioned with injected cyclosporin A administered with saccharin flavored water (US+CS) were compared to unconditioned rats (Cyclosporin A administered with unflavored drinking water during conditioning, US+CSo) to demonstrate a conditioned immunosuppression during subsequent test trials with the CS (Exton et al., 1998). Still other studies have included various groups to control for residual pharmacological effects of the US, effects of the CS, and for associative versus non-associative learning (Niemi et al., 2006). These studies identify useful control groups that should be included in future investigations into treatment of EAE with ALA and conditioning.
A comparison between the effect size in Experiment 1 and the effect size in Experiment 2 was informative. In Experiment 1, the effect size from comparing the ALA 7-16 group to those animals receiving over twice as much cumulative dose of ALA in the ALA 7-28 group was .86 (a large effect size). In Experiment 2, the comparison between the US+CS:CS group and the US+CS:CSo group (that each received an identical amount of ALA treatment as the ALA 7-16 group of Experiment 1), yielded a comparable effect size of .62 (a medium to large effect size). Although slightly different, the observed effect sizes in these two experiments may provide an early indication of the extent to which these two treatment regimens might be similarly efficacious (e.g. ALA at a high cumulative dose without conditioning versus ALA at a lower dose with conditioning, both compared to ALA at the low cumulative dose without conditioning). It should be noted that the current experiments used repeated unreinforced daily exposure to the CS (saccharin) during the testing period, a procedure that may have weakened a conditioned response. Thus, a more suitable means of assessing the true magnitude of the conditioned therapeutic response may exist, involving manipulations of the schedule of test trials with the CS and/or scheduled reinforcement with subtherapeutic ALA during the testing period (Gottlieb, 2004).
The results presented here demonstrate an approach for augmenting the therapeutic response to ALA in EAE mice. The results suggest that it is now appropriate to conduct broader studies which include additional control groups. It will also be important to evaluate (and perhaps avoid) the effects of repeated unreinforced daily exposure to the CS (saccharin) during the testing period. Such refinements in experimental approach should lead toward a delineation of distinct pathways involved in the pharmacotherapeutic response or conditioning aspects of the placebo response. This would have potentially important implications for improving the therapeutic efficacy of ALA and possibly other treatment agents.
Acknowledgments
This work was supported in part by the Office of Research and Development, Medical Research Service, Department of Veterans Affairs; and by the National Center for Complementary and Alternative Medicine, AT002656. Misty White, Meghan Musser (NCNM), Abby Buenafe, PhD (OHSU) and Deb Hickman, DVM (VAMC) are acknowledged for their helpful discussions and/or technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ader R. The role of conditioning in pharmacotherapy. In: Harrington A, editor. The placebo effect : an interdisciplinary exploration. Harvard University Press; Cambridge, Mass.: 1997. pp. 138–165. [Google Scholar]
- Ader R. Conditioned immunomodulation: research needs and directions. Brain Behav Immun. 2003;17 1:S51–57. doi: 10.1016/s0889-1591(02)00067-3. [DOI] [PubMed] [Google Scholar]
- Ader R, Cohen N. Behaviorally conditioned immunosuppression. Psychosom Med. 1975;37:333–340. doi: 10.1097/00006842-197507000-00007. [DOI] [PubMed] [Google Scholar]
- Ader R, Cohen N, Ader R, Felten DL, Cohen N. Conditioning and Immunity, Psychoneuroimmunology. Third. Academic Press; New York: 2001. pp. 3–34. [Google Scholar]
- Ader R, Kelly K, Moynihan JA, Grota LJ, Cohen N. Conditioned enhancement of antibody production using antigen as the unconditioned stimulus. Brain Behav Immun. 1993;7:334–343. doi: 10.1006/brbi.1993.1033. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Mayberg HS, Wager TD, Stohler CS, Zubieta JK. Neurobiological mechanisms of the placebo effect. J Neurosci. 2005;25:10390–10402. doi: 10.1523/JNEUROSCI.3458-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exton MS, Schult M, Donath S, Strubel T, Bode U, del Rey A, Westermann J, Schedlowski M. Conditioned immunosuppression makes subtherapeutic cyclosporin effective via splenic innervation. Am J Physiol. 1999;276:R1710–1717. doi: 10.1152/ajpregu.1999.276.6.R1710. [DOI] [PubMed] [Google Scholar]
- Exton MS, von Horsten S, Schult M, Voge J, Strubel T, Donath S, Steinmuller C, Seeliger H, Nagel E, Westermann J, Schedlowski M. Behaviorally conditioned immunosuppression using cyclosporine A: central nervous system reduces IL-2 production via splenic innervation. J Neuroimmunol. 1998;88:182–191. doi: 10.1016/s0165-5728(98)00122-2. [DOI] [PubMed] [Google Scholar]
- Fleming KK, Bovaird JA, Mosier MC, Emerson MR, LeVine SM, Marquis JG. Statistical analysis of data from studies on experimental autoimmune encephalomyelitis. J Neuroimmunol. 2005;170:71–84. doi: 10.1016/j.jneuroim.2005.08.020. Epub 2005 Sep 2029. [DOI] [PubMed] [Google Scholar]
- Ghanta V, Hiramoto RN, Solvason B, Spector NH. Influence of conditioned natural immunity on tumor growth. Ann N Y Acad Sci. 1987;496:637–46. doi: 10.1111/j.1749-6632.1987.tb35824.x. [DOI] [PubMed] [Google Scholar]
- Gottlieb DA. Acquisition with partial and continuous reinforcement in pigeon autoshaping. Learn Behav. 2004;32:321–334. doi: 10.3758/bf03196031. [DOI] [PubMed] [Google Scholar]
- Hucklebridge F. Behavioral conditioning of the immune system. Int Rev Neurobiol. 2002;52:325–351. doi: 10.1016/s0074-7742(02)52015-8. [DOI] [PubMed] [Google Scholar]
- Janz LJ, Green-Johnson J, Murray L, Vriend CY, Nance DM, Greenberg AH, Dyck DG. Pavlovian conditioning of LPS-induced responses: effects on corticosterone, splenic NE, and IL-2 production. Physiol Behav. 1996;59:1103–1109. doi: 10.1016/0031-9384(95)02171-x. [DOI] [PubMed] [Google Scholar]
- Jones RE, Bourdette D, Moes N, Vandenbark A, Zamora A, Offner H. Epitope spreading is not required for relapses in experimental autoimmune encephalomyelitis. J Immunol. 2003;170:1690–1698. doi: 10.4049/jimmunol.170.4.1690. [DOI] [PubMed] [Google Scholar]
- Klosterhalfen W, Klosterhalfen S. Pavlovian conditioning of immunosuppression modifies adjuvant arthritis in rats. Behav Neurosci. 1983;97:663–666. doi: 10.1037//0735-7044.97.4.663. [DOI] [PubMed] [Google Scholar]
- Marracci GH, Jones RE, McKeon GP, Bourdette DN. Alpha lipoic acid inhibits T cell migration into the spinal cord and suppresses and treats experimental autoimmune encephalomyelitis. J Neuroimmunol. 2002;131:104–114. doi: 10.1016/s0165-5728(02)00269-2. [DOI] [PubMed] [Google Scholar]
- Morini M, Roccatagliata L, Dell'Eva R, Pedemonte E, Furlan R, Minghelli S, Giunti D, Pfeffer U, Marchese M, Noonan D, Mancardi G, Albini A, Uccelli A. Alpha-lipoic acid is effective in prevention and treatment of experimental autoimmune encephalomyelitis. J Neuroimmunol. 2004;148:146–153. doi: 10.1016/j.jneuroim.2003.11.021. [DOI] [PubMed] [Google Scholar]
- Niemi MB, Pacheco-Lopez G, Kou W, Harting M, Rey AD, Besedovsky HO, Schedlowski M. Murine taste-immune associative learning. Brain Behav Immun. 2006;20:527–531. doi: 10.1016/j.bbi.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Pacheco-Lopez G, Engler H, Niemi MB, Schedlowski M. Expectations and associations that heal: Immunomodulatory placebo effects and its neurobiology. Brain Behav Immun. 2006;20:430–446. doi: 10.1016/j.bbi.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Ramirez-Amaya V, Bermudez-Rattoni F. Conditioned enhancement of antibody production is disrupted by insular cortex and amygdala but not hippocampal lesions. Brain Behav Immun. 1999;13:46–60. doi: 10.1006/brbi.1998.0547. [DOI] [PubMed] [Google Scholar]
- Risinger FO, Cunningham CL. Genetic differences in ethanol-induced hyperglycemia and conditioned taste aversion. Life Sci. 1992;50:PL113–118. doi: 10.1016/0024-3205(92)90463-y. [DOI] [PubMed] [Google Scholar]
- Sanna V, Di Giacomo A, La Cava A, Lechler RI, Fontana S, Zappacosta S, Matarese G. Leptin surge precedes onset of autoimmune encephalomyelitis and correlates with development of pathogenic T cell responses. J Clin Invest. 2003;111:241–250. doi: 10.1172/JCI16721. [DOI] [PMC free article] [PubMed] [Google Scholar]