Abstract
Obesity-linked diseases are associated with suppressed endothelial progenitor cell (EPC) function. Adiponectin is an adipose-derived protein that is downregulated in obese and diabetic subjects. Here, we investigated the effects of adiponectin on EPCs. EPC levels did not increase in adiponectin deficient (APN-KO) in response to hindlimb ischemia. Adenovirus-mediated delivery of adiponectin increased EPC levels in both WT and APN-KO mice. Incubation of human peripheral blood mononuclear cells with adiponectin led to an increase of the number of EPCs. Adiponectin induced EPC differentiation into network structures and served as a chemoattractant in EPC migration assays. These data suggest that hypoadiponectinemia may contribute to the depression of EPC levels that are observed in patients with obesity-related cardiovascular disorders.
Keywords: adiponectin, angiogenesis, endothelial progenitor cells
1. Introduction
Angiogenesis plays a crucial role in the tissue damage following ischemia. Circulating endothelial progenitor cells (EPCs) exist in blood stream and migrate to sites of ischemia where they contribute to blood vessel formation [1]. Obesity-linked diseases including type 2 diabetes, dyslipidemia and hypertension are associated with impaired EPC function [2–4]. EPC mobilization in response to wounding is impaired in diabetic mice [5], and glucose-intolerant individuals exhibit lower levels of circulating EPCs [6]. However, the molecular links between metabolic dysfunction and EPCs have not been clarified at the molecular level.
Adiponectin is an adipose-derived protein, which plays a protective role in the development of obesity-linked diseases [7]. A number of experimental studies show that adiponectin deficiency contributes to diet-induced insulin resistance, salt-sensitive hypertension, various pathological heart conditions and impaired endothelium-dependent vasodilation (reviewed in [7]). It has also been reported that adiponectin exerts favorable effects on angiogenesis. We have shown that adiponectin-deficiency contributes to impaired angiogenic responses in ischemic hindlimb or heart, whereas adenovirus-mediated delivery of adiponectin promotes ischemia-mediated neovascularization [8,9]. Adiponectin supplementation has also been shown to stimulate blood vessel growth in both mouse Matrigel plug implantation and rabbit corneal angiogenesis models [10]. Here, we examined the effect of adiponectin on EPC level and functions that are associated with angiogenesis.
2. Materials and Methods
2.1. Materials
Bacterially-produced mouse adiponectin was prepared as described previously [10]. The adenoviral vectors expressing β-galactosidase (Ad-βgal) or murine adiponectin (Ad-APN) from the cytomegalovirus promoter/enhancer have been described previously [11]. DiI-Ac-LDL and FITC-conjugated Lectin were obtained from Biomedical Technologies (Waltham, MA). Human recombinant VEGF was purchased from Roche (Indianapolis, IN).
2.2. Animals
Adiponectin-KO (APN-KO) and wild-type (WT) mice in a C57/BL6 background were used for this study [12]. Study protocols were approved by the Institutional Animal Care and Use Committee at Boston University School of Medicine and Nagoya University. Mice at the ages of 8–11 weeks were subjected to unilateral hind limb surgery under anesthesia with sodium pentobarbital (50 mg/kg intraperitoneally). The entire left femoral artery and vein were excised surgically in this model [9]. In some experiments, 2 × 108 plaque-forming units (pfu) of Ad-APN or Ad-βgal were injected into the jugular vein of APN-KO mice 3 d prior to the hindlimb ischemia surgery. Mouse peripheral blood and bone marrow were obtained on postoperative day 7.
2.3. Cell culture of endothelial progenitor cells
Human peripheral blood mononuclear cells (PBMC) were isolated employing a Histopaque density gradient procedure as described previously [13]. Cells were seeded on fibronectin-coated dishes and cultured in endothelial cell growth medium-2. Some experiments were performed by the addition of the incubated 30μg/ml of recombinant adiponectin protein or Bovine serum albumin (BSA) as control.
2.4. Cell mobilization assay in mice hindlimb ischemia model
Mouse MNCs were seeded on fibronectin-coated dishes and cultured in endothelial cell growth medium-2 for five days. EPCs were assessed by co-staining with DiI-Ac-LDL and FITC-conjugated Lectin. After staining, cells were quantified by examining 15 random microscopic fields and double-positive cells as EPCs were counted. The expression of Sca-1 and Flk-1 antigens in MNCs from peripheral blood and bone marrow were assessed by FACS analysis as described previously [14]. MNCs were incubated with Sca-1 FITC and Flk-1 PE for 30 min. FACS analysis was performed using a FACS Calibur instrument and Cell Quest software (BD Biosciences).
2.5. Human EPC culture assay and flow cytometry
Five days after culture, human EPCs were assayed by co-staining with DiI-Ac-LDL and FITC-conjugated Lectin. After staining, cells were quantified by examining 15 random microscopic fields and double-positive cells as EPCs were counted. FACS was used to detect the expression of the VEGF receptor, Flk-1, CD31 and CD45.
2.6. Tube formation assay and EPC incorporation
The formation of vascular-like structures by EPC on growth factor-reduced Matrigel (BD Biosciences, Bedford, MA) was performed according to the manufacturer’s instructions. EPCs or HUVEC were seeded on coated plates at 3×104 cells/cm2 in EGM-2 medium containing adiponectin or BSA (30 μg/ml) and incubated at 37 °C for 24 h. Network formation was assessed using an inverted phase contrast microscope (Nikon, Tokyo, Japan). In some experiments, EPC incorporation was assessed by seeding HUVEC and DiI-Ac-LDL labeled EPC at a ratio of 1:1 on Matrigel [15]. Network formation was assessed using a fluorescence microscope to assess the frequency of labeled cell incorporation. The degree of tube formation was quantified by measuring the length of tubes in five randomly low power fields.
2.7. Migration assay
EPC migration assay were measured using a modified Boyden chamber assay (ChemoTx® plate, Neuroprobe, Inc., Gaithersburg, MD) as described previously [16]. EPC were cultured with Adiponectin or BSA (30 μg/ml) for 4 days and then labeled with Dil-Ac-LDL (10 μg/ml) for 3 hours. Cells were resuspended, and the labeled cell suspension was applied to the top of the filter. Adiponectin (30 μg/ml), VEGF (20 ng/ml) or bovine serum albumin (BSA) (30 μg/ml) in phenol red free EBM were added into the lower chamber and incubated for 12 h. EPCs migrating through the filter into the chambers were determined with the microplate fluorometer.
2.8. Statistical Analysis
Data are presented as mean ± SD. The mean value was compared between 2 groups using an unpaired t test. The comparison among more than 3 groups was performed by analysis of variance (ANOVA) with Scheffe’s F test. A value of P<0.05 was accepted as statistically significant. All analyses were performed using Stat View-J (version 5.0; SAS Institute Inc).
Results
Genetic manipulation of APN influences EPC levels in mice
We have shown that APN-KO mice are impaired in hindlimb perfusion after ischemia [9]. Thus we investigated EPC levels in WT and APN-KO mice using the colony formation assay. EPCs in PBMCs were initially identified by staining with DiI-Ac-LDL and lectin [1]. As shown in Figure 1a, no differences were detected in EPC levels between WT and APN-KO mice before ischemia surgery. However, seven days after the induction of ischemia, the number of EPCs derived from WT mice increased significantly, whereas APN-KO mice showed little increase or no increase in EPC levels in response to injury. To corroborate these findings, we analyzed EPC levels in peripheral blood and bone marrow by flow cytometry. In this assay, EPCs were identified as Sca-1/Flk-1 positive cells. Sham-treated WT and APN-KO mice displayed similar levels of circulating Sca-1/Flk-1 positive cells (Figure 1b). However, the number of Sca-1/Flk-1 positive cells were significantly lower in APN-KO mice than in WT mice on day 7 after hindlimb ischemia.
Figure 1. Effect of adiponectin on EPC levels in mice.
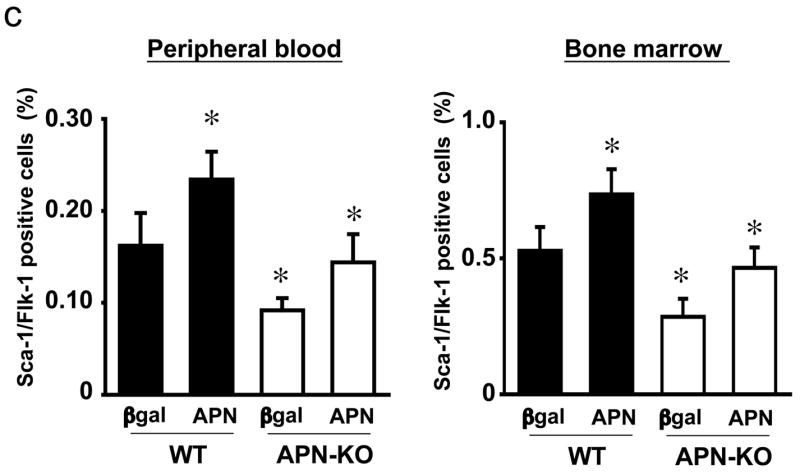
A) Quantitative analysis of the number of DiI-Ac-LDL/lectin double-positive cells in cultured PBMC from WT (n=5) and APN-KO mice (n=5) on day 7 after hindlimb ischemia or sham operation. B) Quantitative analysis of the number of Sca-1/Flk-1 positive cells in peripheral blood and bone marrow from WT and APN-KO mice on day 7 after hindlimb ischemia surgery or sham operation. C) Quantitative analysis of the number of Sca-1/Flk-1 positive cells in peripheral blood and bone marrow from WT and APN-KO mice administered Ad-βgal or Ad-APN on post-operative day 7. Results are presented as mean ± SD. *P<0.05, **P<0.01 vs. sham-operated WT mice, respectively (n=5).
To confirm that the impaired EPC response to ischemia was a result of adiponectin deficiency, WT and APN-KO mice were transduced with an adenoviral vector expressing adiponectin (Ad-APN) or with a control adenovirus (Ad-βgal) delivered through the jugular vein 3d before hindlimb surgery. Adenovirus-mediated delivery of adiponectin via this method results in a 3.2-fold increase in circulating in WT mice (data not shown). Adiponectin treated WT mice showed a significant increase in the number of Sca-1/Flk-1 positive cells on 7 days after hindlimb surgery compared with control mice that were treated with Ad-βgal. Treatment with Ad-APN also increased the number of Sca-1/Flk-1 positive cells both in peripheral blood and bone marrow in APN-KO mice to levels similar to those to control mice (Figure 1c). In addition, adiponectin-deficiency did not affect the frequency of Sca1+ cells in bone marrow that were positive for markers of cell proliferation or apoptosis (data not shown). Collectively, these data are consistent with the hypothesis that adiponectin increases EPC level, perhaps through mobilization from the bone marrow.
Effect of Adiponectin on EPC differentiation in vitro
To investigate the effect of adiponectin on EPC differentiation, human PBMCs were cultured in the presence or absence of physiological levels of recombinant adiponectin protein. As shown in Figure 2a and 2b, the number of EPCs, as determined by DiI-Ac-LDL/lectin double-positive cells, was significantly increased by treatment with adiponectin after 5 days in culture. Treatment of cells with an equivalent amount of BSA protein had no effect on the number of DiI-Ac-LDL/lectin double-positive cells.
Figure 2. Adiponectin promotes EPC differentiation.
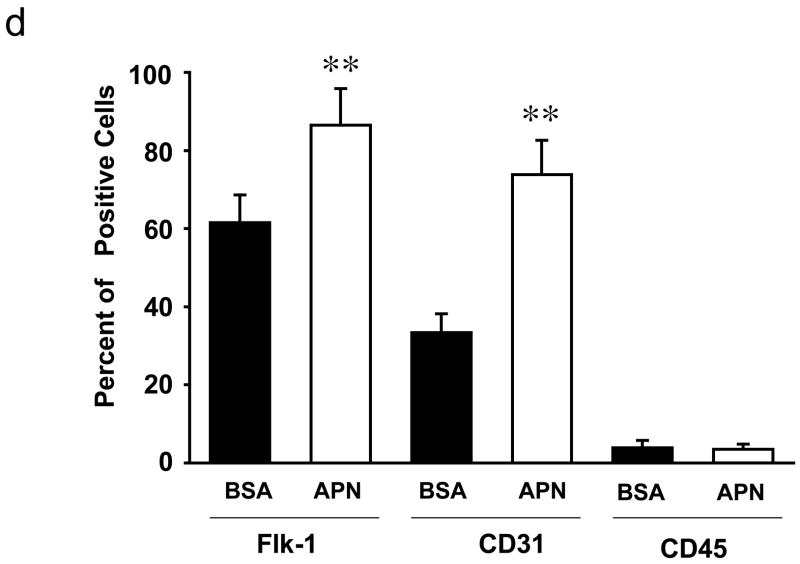
Representative photographs of human EPCs, as determined by DiI-Ac-LDL/lectin double-positive cell staining, in the presence or absence of 30 μg/ml recombinant adiponectin protein. Cells were analyzed on day 5 of ex vivo culture. B) Quantitative analysis of the number of DiI-Ac-LDL/lectin double-positive cells on day 5 of ex vivo culture. C) Expressions of Flk-1 and CD31 assessed by fluorescence FACS. EPCs were isolated from human MNCs on day 5 of ex vivo culture. D) Quantitative analysis of Flk-1 and CD31 expressions assessed by fluorescence FACS. Results are presented as mean ± SD. **P<0.01 vs. BSA, respectively (n=3–5).
To corroborate these findings, cells cultured in the presence of adiponectin or BSA were quantified for Flk-1 or CD31 cell surface expression by flow cytometric procedures (Figure 2c). Adiponectin-treatment led to a significant increase in the expression of these endothelial cell marker proteins compared with PBMCs that were treated with BSA (Figure 2d). Freshly isolated mononuclear fractions express CD45, a common leukocyte antigen, in addition to endothelial cell markers, but CD45 expression is lost when cells are cultured in the presence of VEGF [13,17]. Accordingly, after 5 days in culture few cells expressed CD45, and the expression of this marker did not increase in the presence of adiponectin.
Effect of Adiponectin on EPC function in vitro
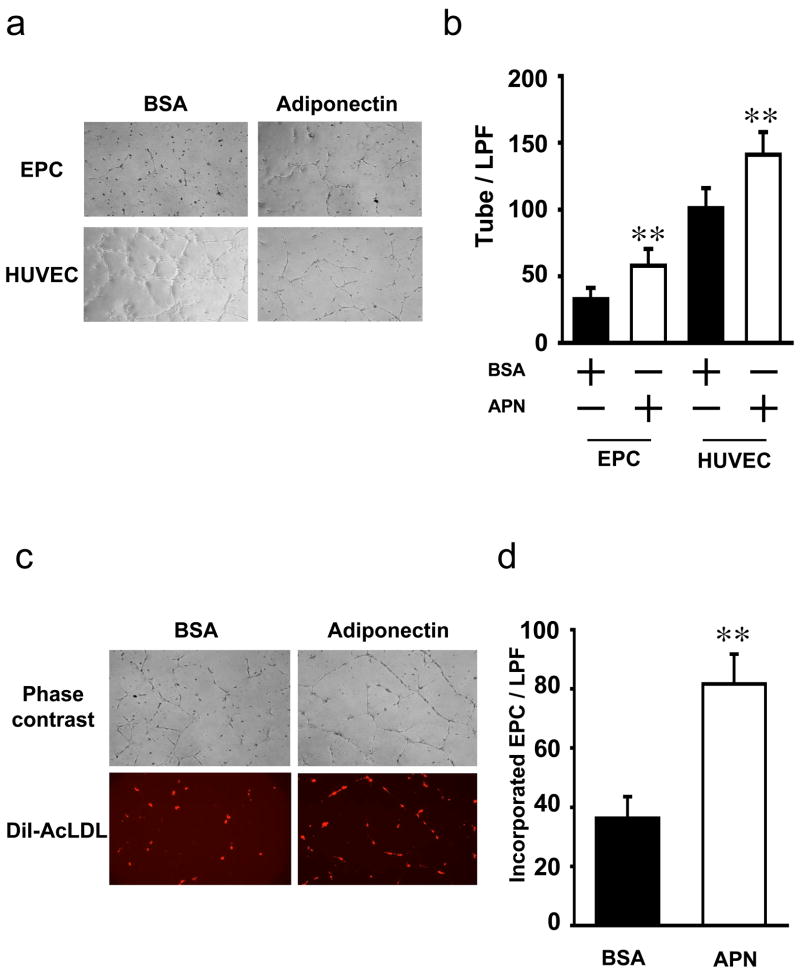
The effects of adiponectin on EPC function were investigated using the Matrigel network-formation assay. Treatment with adiponectin promoted the formation of network structures when EPCs or HUVECs were plated on Matrigel matrix (Figure 3a and b). Figure 3c shows representative photographs of co-cultured HUVECs and DiI-Ac-LDL–labeled EPCs on a Matrigel matrix. Incorporation of EPC into network structures was significantly enhanced in the presence of adiponectin (Figure 3d). Furthermore, the total number of network projections per microscopic field was significantly higher when EPCs were co-culture with HUVECs in the presence of adiponectin (Figure 3e).
Figure 3. Adiponectin promotes EPC function.
A) Representative phase contrast images are shown of cultured EPCs or HUVECs in the presence of adiponectin or BSA. B) Quantitative analysis of network projections formed on Matrigel for each experimental group. C) Representative phase contrast and fluorescent images are shown of HUVECs co-cultured for 24 h with EPCs tagged with DiI-Ac-LDL isolated from human MNCs. D) Quantitative analysis of the number of EPCs incorporation into HUVEC on Matrigel. E) Quantitative analysis of network projections formed on Matrigel for each experimental group. F) EPC migration assay was performed using a modified Boyden chamber using BSA (control) adiponectin or VEGF in the lower chamber. Results are presented as mean ± SD. **P<0.01 vs. BSA, respectively (n=3–5).
Cellular migration is required for vascular network formation. Therefore, we examined the effect of adiponectin on unidirectional migration of DiI-Ac-LDL-labeled EPCs using a modified Boyden chamber assay. As shown in Figure 3f, inclusion of adiponectin in the lower chamber led to a significant increase in the migratory activity. The stimulation of migratory activity by adiponectin was similar to that seen with recombinant VEGF.
Discussion
The present study demonstrates that adiponectin regulates the level of circulating EPC, defined as Sca-1+/Flk-1+ cells, in response to ischemic injury. The number of EPCs derived from APN-KO mice was significantly reduced compared to WT mice under conditions of hindlimb ischemia. Conversely, delivery of Ad-APN increased EPCs levels in response to ischemia in both WT and APN-KO mice. Adiponectin deficiency or overexpression also modulated levels of Sca-1+/Flk-1+ cells in bone marrow in a negative or positive manner, respectively. Because increasing evidence suggests that bone marrow derived EPC significantly contribute to cardiovascular function, these data indicate that hypo-adiponectinemia might contribute to vascular-deficiency through impaired EPC mobilization. Consistent with this hypothesis, Matsuo et al. have shown that plasma adiponectin levels are associated with EPC number in patients with coronary artery disease [18].
The present study shows that adiponectin can stimulate the expression of the endothelial cell marker proteins Flk-1 and CD31 in cultured progenitor cell populations. Adiponectin also increased the number of DiI-Ac-LDL/lectin positive cells in cultures of PBMCs. It should be noted that although EPCs are typically defined by their ability to express Flk-1 and CD31, or the ability of DiI-Ac-LDL and lectin uptake, these markers or features are not necessarily unique for EPCs [19]. In addition, adiponectin stimulated PBMC incorporation into developing endothelial cell networks in vitro, and served as a chemoattractant in a Boyden chamber migration assay. Thus, it appears that adiponectin can directly act on the EPC fraction of PBMC to promote differentiation and function.
While it is clear that adiponectin increases circulating EPC levels in mice, we cannot conclude from our studies that this increase results from the mobilization of progenitor cells from bone marrow. In our experiments, ischemia led to an increase in Sca-1/Flk-1-positive cells in both bone marrow and peripheral blood. It is possible that serial measurements in the bone marrow and peripheral blood would reveal different peak points of progenitor cell accumulation in these two compartments, which could be indicative of a mobilization mechanism. Our experiments did not detect differences in the low rates of proliferation or apoptosis in Sca1+ cells in bone marrow between wild-type and adiponectin-deficient mice. Thus, the adiponectin-mediated increase in circularing EPC levels may occur through its ability to promote progenitor cell differentiation. While this hypothesis is supported by our in vitro data, further studies will be required to ascertain the mechanism by which adiponectin increases circulating EPC levels in vivo.
Consistent with the findings reported here, it has been shown that adiponectin stimulates nitric oxide production in endothelial cells through the AMPK-dependent and AMPK-independent phosphorylation of endothelial nitric oxide synthase eNOS [10,20]. Because eNOS is required for the mobilization of EPCs [5,21], adiponectin may affect EPC action through its ability to stimulate eNOS activity in these cells. In addition, PPARγ agonists are reported to increase the number and function of EPCs in patients with type II diabetes and coronary artery disease [22,23]. Plasma adiponectin levels have been shown to be increased by PPARγ agonist treatment, and this upregulation contributes to improved insulin sensitivity [22,24]. Thus, in light of the data reported here, it is tempting to speculate that the favorable effects of PPARγ agonists on EPC number and function may be mediated in part by the action of these drugs on adiponectin expression.
Taken together, these data provide evidence for a novel adipocyte-bone marrow-signaling axis in EPC regulation that could influence vascular remodeling. These findings provide a plausible molecular mechanism linking dysregulated angiogenesis to diabetes and other obesity-related diseases that are associated with hypoadiponectinemia. Thus, therapeutic approaches aimed at increasing adiponectin production could be useful for treating diseases associated with vascular insufficiency.
Acknowledgments
This work was supported by grants from the National Institutes of Health (HL86785, AG15052 and HL77774 and by NHLBI grant N01-HV-28178 from the National Institutes of Health (USA)) to K. Walsh. This work was also supported by a grant from the Japanese Ministry of Education, the Nakashima foundation and the Aichi D.R.G foundation to R. Shibata. N. Ouchi was supported from an American Heart Association Scientist Development Grant, Northeast Affiliate. S. Kihara was supported from a Grant-in-Aid for Scientific Research on Priority Areas. We gratefully acknowledge the technical assistance of Megumi Kondo and Rie Miura.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Asahara T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 2.Tepper OM, Galiano RD, Capla JM, Kalka C, Gagne PJ, Jacobowitz GR, Levine JP, Gurtner GC. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation. 2002;106:2781–2786. doi: 10.1161/01.cir.0000039526.42991.93. [DOI] [PubMed] [Google Scholar]
- 3.Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 4.Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, Zeiher AM, Dimmeler S. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89:E1–E7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 5.Gallagher KA, et al. Diabetic impairments in NO-mediated endothelial progenitor cell mobilization and homing are reversed by hyperoxia and SDF-1 alpha. J Clin Invest. 2007;117:1249–1259. doi: 10.1172/JCI29710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fadini GP, Sartore S, Schiavon M, Albiero M, Baesso I, Cabrelle A, Agostini C, Avogaro A. Diabetes impairs progenitor cell mobilisation after hindlimb ischaemia-reperfusion injury in rats. Diabetologia. 2006;49:3075–3084. doi: 10.1007/s00125-006-0401-6. [DOI] [PubMed] [Google Scholar]
- 7.Hopkins T, Ouchi N, Shibata R, Walsh K. Adiponectin actions in the cardiovascular system. Cardiovasc Res. 2007;74:11–18. doi: 10.1016/j.cardiores.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shibata R, et al. Adiponectin protects against the development of systolic dysfunction following myocardial infarction. J Mol Cell Cardiol. 2007;42:1065–74. doi: 10.1016/j.yjmcc.2007.03.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shibata R, Ouchi N, Kihara S, Sato K, Funahashi T, Walsh K. Adiponectin stimulates angiogenesis in response to tissue ischemia through stimulation of amp-activated protein kinase signaling. J Biol Chem. 2004;279:28670–28674. doi: 10.1074/jbc.M402558200. [DOI] [PubMed] [Google Scholar]
- 10.Ouchi N, Kobayashi H, Kihara S, Kumada M, Sato K, Inoue T, Funahashi T, Walsh K. Adiponectin stimulates angiogenesis by promoting cross-talk between AMP-activated protein kinase and Akt signaling in endothelial cells. J Biol Chem. 2004;279:1304–1309. doi: 10.1074/jbc.M310389200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsuda M, et al. Role of adiponectin in preventing vascular stenosis. The missing link of adipo-vascular axis. J Biol Chem. 2002;277:37487–37491. doi: 10.1074/jbc.M206083200. [DOI] [PubMed] [Google Scholar]
- 12.Maeda N, et al. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med. 2002;8:731–737. doi: 10.1038/nm724. [DOI] [PubMed] [Google Scholar]
- 13.Hur J, et al. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol. 2004;24:288–293. doi: 10.1161/01.ATV.0000114236.77009.06. [DOI] [PubMed] [Google Scholar]
- 14.Shimada T, et al. Angiogenesis and vasculogenesis are impaired in the precocious-aging klotho mouse. Circulation. 2004;110:1148–1155. doi: 10.1161/01.CIR.0000139854.74847.99. [DOI] [PubMed] [Google Scholar]
- 15.Galasso G, et al. Impaired angiogenesis in glutathione peroxidase-1-deficient mice is associated with endothelial progenitor cell dysfunction. Circ Res. 2006;98:254–261. doi: 10.1161/01.RES.0000200740.57764.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rocnik EF, Liu P, Sato K, Walsh K, Vaziri C. The novel SPARC family member SMOC-2 potentiates angiogenic growth factor activity. J Biol Chem. 2006;281:22855–64. doi: 10.1074/jbc.M513463200. [DOI] [PubMed] [Google Scholar]
- 17.Yoder MC, et al. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801–9. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsuo Y, Imanishi T, Kuroi A, Kitabata H, Kubo T, Hayashi Y, Tomobuchi Y, Akasaka T. Effects of plasma adiponectin levels on the number and function of endothelial progenitor cells in patients with coronary artery disease. Circ J. 2007;71:1376–82. doi: 10.1253/circj.71.1376. [DOI] [PubMed] [Google Scholar]
- 19.Rafii S, Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med. 2003;9:702–12. doi: 10.1038/nm0603-702. [DOI] [PubMed] [Google Scholar]
- 20.Chen H, Montagnani M, Funahashi T, Shimomura I, Quon MJ. Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J Biol Chem. 2003;278:45021–45026. doi: 10.1074/jbc.M307878200. [DOI] [PubMed] [Google Scholar]
- 21.Aicher A, Heeschen C, Mildner-Rihm C, Urbich C, Ihling C, Technau-Ihling K, Zeiher AM, Dimmeler S. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat Med. 2003;9:1370–1376. doi: 10.1038/nm948. [DOI] [PubMed] [Google Scholar]
- 22.Werner C, Kamani CH, Gensch C, Bohm M, Laufs U. The peroxisome proliferator-activated receptor-gamma agonist pioglitazone increases number and function of endothelial progenitor cells in patients with coronary artery disease and normal glucose tolerance. Diabetes. 2007;56:2609–2615. doi: 10.2337/db07-0069. [DOI] [PubMed] [Google Scholar]
- 23.Wang CH, et al. Pioglitazone increases the numbers and improves the functional capacity of endothelial progenitor cells in patients with diabetes mellitus. Am Heart J. 2006;152:1051 e1–8. doi: 10.1016/j.ahj.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 24.Semple RK, Chatterjee VK, O’Rahilly S. PPAR gamma and human metabolic disease. J Clin Invest. 2006;116:581–589. doi: 10.1172/JCI28003. [DOI] [PMC free article] [PubMed] [Google Scholar]