Abstract
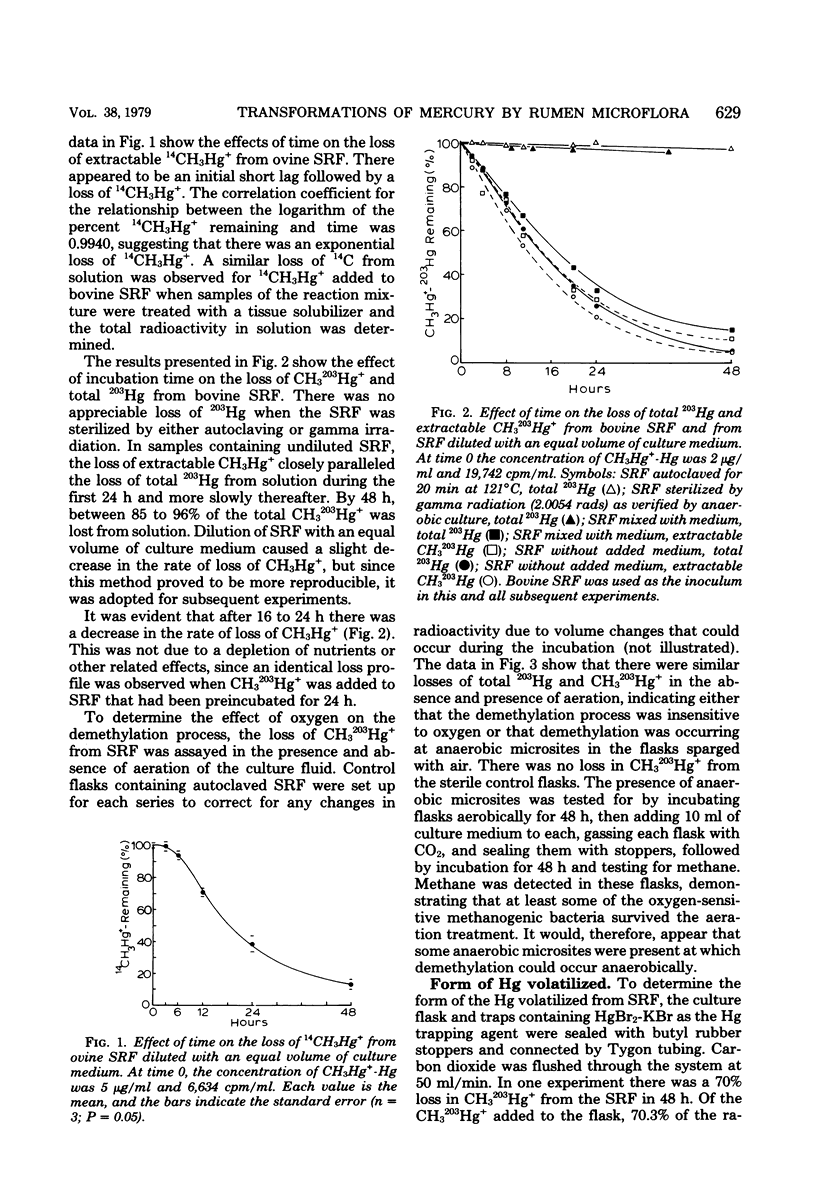
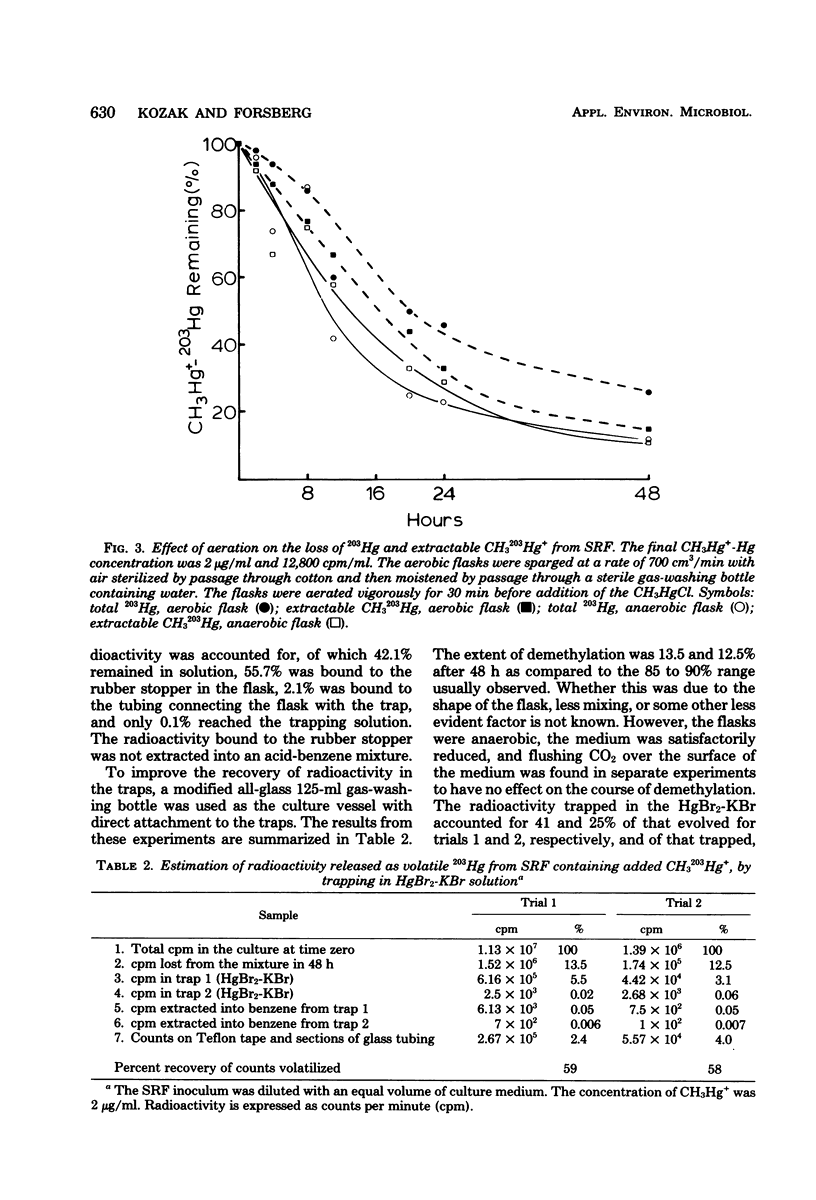
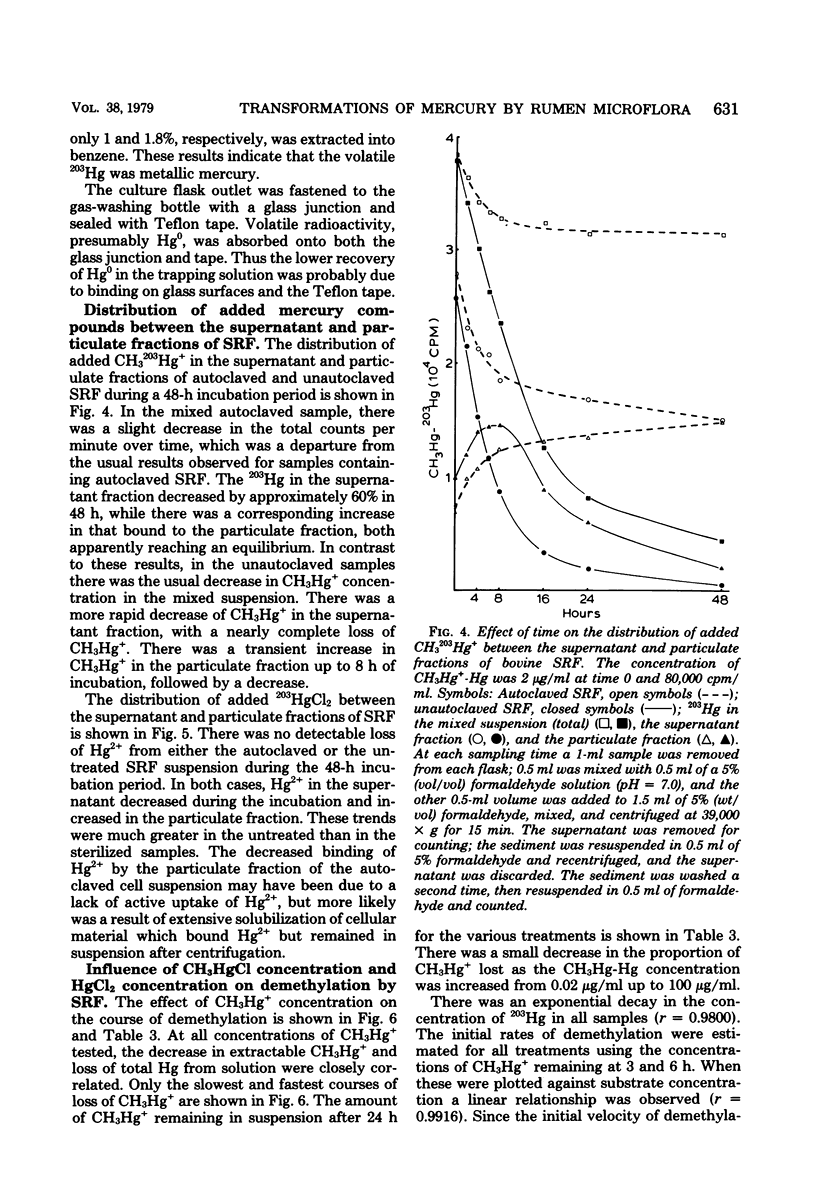
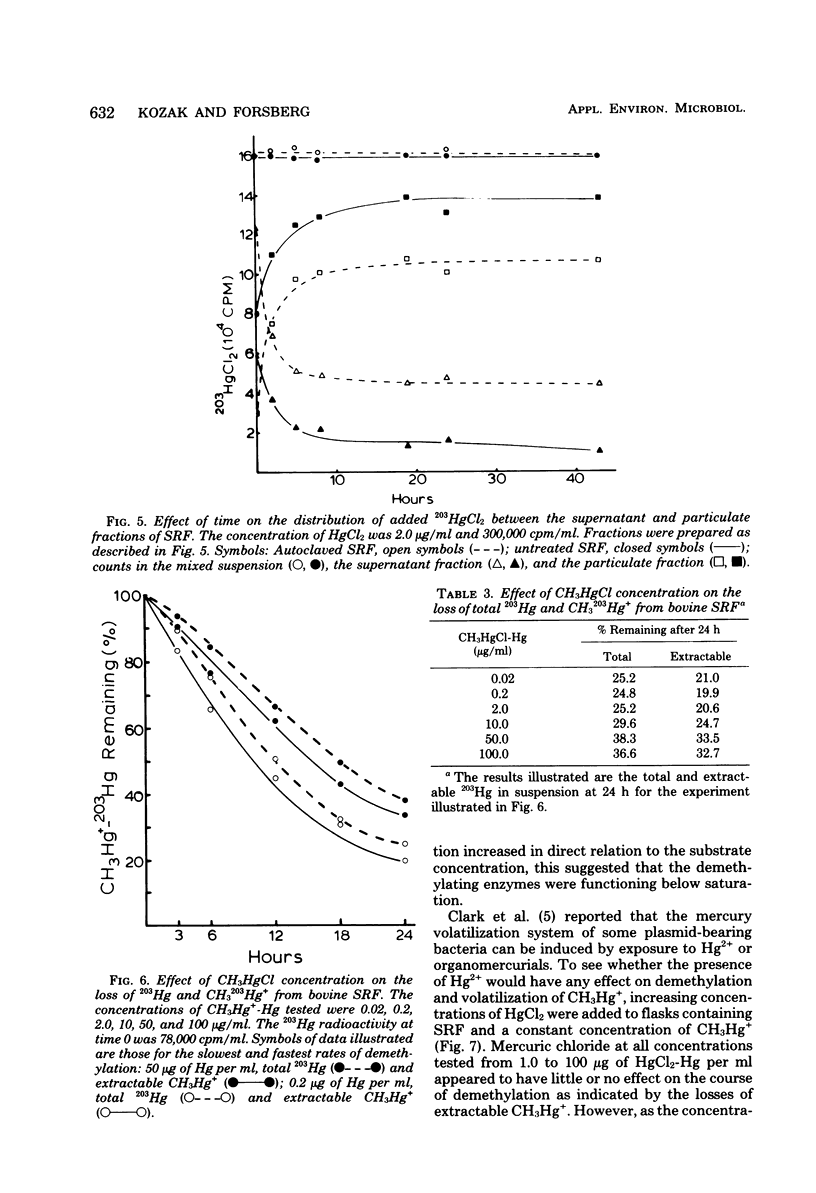
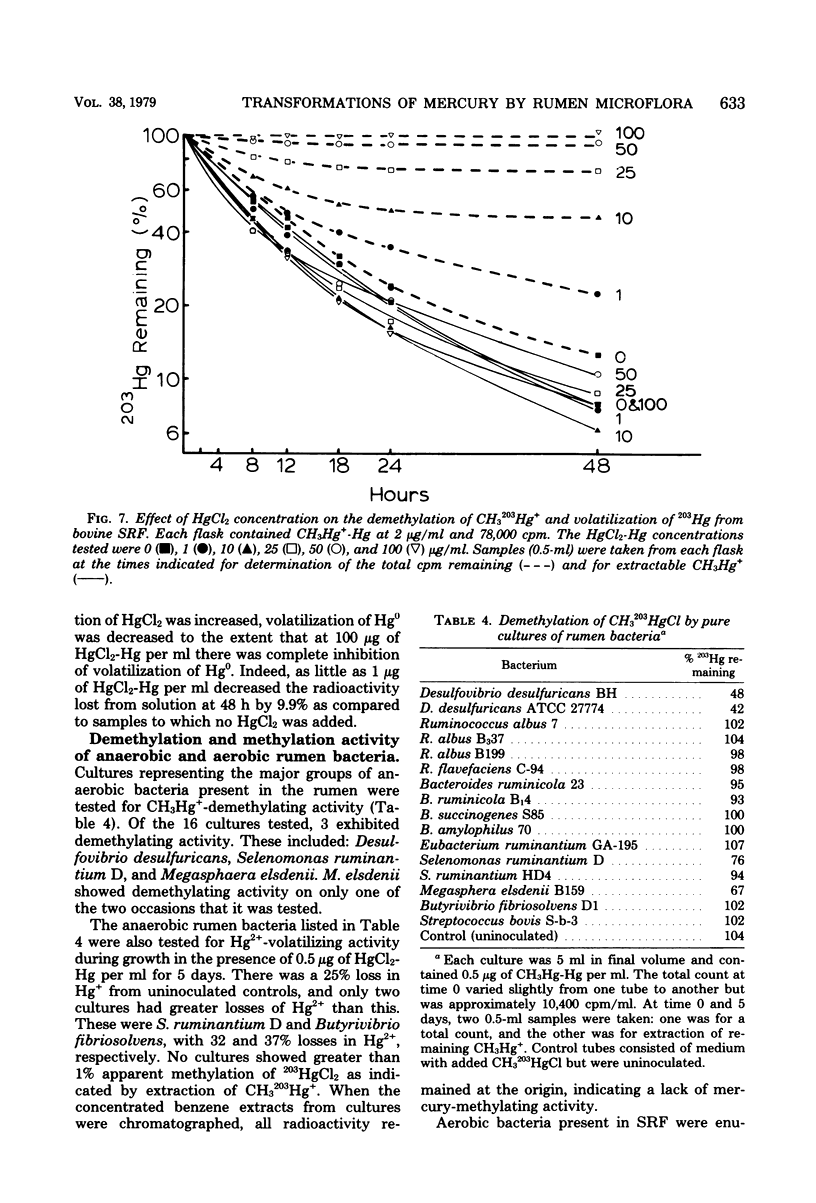
The microflora in strained rumen fluid did not methylate or volatilize 203Hg2+ at detectable rates. However, there was an exponential decay in the concentration of added CH3Hg+, which was attributed to demethylation. The major product of demethylation was metallic mercury (Hg0), and it was released as a volatile product from the reaction mixture. Demethylation occurred under both anaerobic and aerobic conditions. The rate of demethylation was proportional to the concentration of added CH3Hg+-Hg from 0.02 to 100 microgram of Hg per ml. The presence of HgCl2 had almost no inhibitory effect on the rate of cleavage of the carbon-mercury bond of CH2HgCl, but it completely inhibited volatilization of the Hg formed, when the concentration of HgCl2-Hg reached 100 micrograms/ml. Three of 11 species of anaerobic rumen bacteria catalyzed demethylation. These were Desulfovibrio desulfuricans, Selenomonas ruminantium, and Megasphaera elsdenii. None of the 11 species caused detectable methylation, and only two caused limited volatilization of Hg2+. Three species of bacteria out of 90 fresh aerobic isolates from rumen contents were demethylators: two were identified as Pseudomonas sp., and the third was a Micrococcus sp. Demethylation by the rumen microflora appeared to be carried out by both aerobic and anaerobic bacteria and, on the basis of Hg2+ sensitivity, probably resulted from the activity of two enzymes, a CH3-Hg+ hydrolase and a Hg2+ reductase.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ammerman C. B., Miller S. M., Fick K. R., Hansard S. L., 2nd Contaminating elements in mineral supplements and thier potential toxicity: a review. J Anim Sci. 1977 Mar;44(3):485–508. doi: 10.2527/jas1977.443485x. [DOI] [PubMed] [Google Scholar]
- Anderson L. E., McClure W. O. An improved scintillation cocktail of high-solubilizing power. Anal Biochem. 1973 Jan;51(1):173–179. doi: 10.1016/0003-2697(73)90465-x. [DOI] [PubMed] [Google Scholar]
- Beckert W. F., Moghissi A. A., Au F. H., Bretthauer E. W., McFarlane J. C. Formation of methylmercury in a terrestrial environment. Nature. 1974 Jun 14;249(458):674–675. doi: 10.1038/249674a0. [DOI] [PubMed] [Google Scholar]
- Bryant M. P. Commentary on the Hungate technique for culture of anaerobic bacteria. Am J Clin Nutr. 1972 Dec;25(12):1324–1328. doi: 10.1093/ajcn/25.12.1324. [DOI] [PubMed] [Google Scholar]
- Clark D. L., Weiss A. A., Silver S. Mercury and organomercurial resistances determined by plasmids in Pseudomonas. J Bacteriol. 1977 Oct;132(1):186–196. doi: 10.1128/jb.132.1.186-196.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards T., McBride B. C. Biosynthesis and degradation of methylmercury in human faeces. Nature. 1975 Feb 6;253(5491):463–464. doi: 10.1038/253462a0. [DOI] [PubMed] [Google Scholar]
- Fontenot J. P., Webb K. E., Jr Health aspects of recycling animal wastes by feeding. J Anim Sci. 1975 Jun;40(6):1267–1277. doi: 10.2527/jas1975.4061267x. [DOI] [PubMed] [Google Scholar]
- Forsberg C. W. Effects of heavy metals and other trace elements on the fermentative activity of the rumen microflora and growth of functionally important rumen bacteria. Can J Microbiol. 1978 Mar;24(3):298–306. doi: 10.1139/m78-050. [DOI] [PubMed] [Google Scholar]
- Forsberg C. W. Nutritional characteristics of Megasphaera elsdenii. Can J Microbiol. 1978 Aug;24(8):981–985. doi: 10.1139/m78-161. [DOI] [PubMed] [Google Scholar]
- Howard B. H., Hungate R. E. Desulfovibrio of the sheep rumen. Appl Environ Microbiol. 1976 Oct;32(4):598–602. doi: 10.1128/aem.32.4.598-602.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe S. M., McGee J., Lengemann F. W. Transfer of inorganic mercury to milk of goats. Nature. 1972 Jun 30;237(5357):516–518. doi: 10.1038/237516a0. [DOI] [PubMed] [Google Scholar]
- Jensen S., Jernelöv A. Biological methylation of mercury in aquatic organisms. Nature. 1969 Aug 16;223(5207):753–754. doi: 10.1038/223753a0. [DOI] [PubMed] [Google Scholar]
- Nader C. J., Walker D. J. Metabolic fate of cysteine and methionine in rumen digesta. Appl Microbiol. 1970 Nov;20(5):677–681. doi: 10.1128/am.20.5.677-681.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neathery M. W., Miller W. J., Gentry R. P., Stake P. E., Blackmon D. M. Cadmium-109 and methyl mercury-203 metabolism, tissue distribution, and secretion into milk of cows. J Dairy Sci. 1974 Oct;57(10):1177–1183. doi: 10.3168/jds.S0022-0302(74)85033-2. [DOI] [PubMed] [Google Scholar]
- Neathery M. W., Miller W. J. Metabolism and toxicity of cadmium, mercury, and lead in animals: a review. J Dairy Sci. 1975 Dec;58(12):1767–1781. doi: 10.3168/jds.S0022-0302(75)84785-0. [DOI] [PubMed] [Google Scholar]
- Rowland I. R., Davies M. J., Grasso P. Metabolism of methylmercuric chloride by the gastro-intestinal flora of the rat. Xenobiotica. 1978 Jan;8(1):37–43. doi: 10.3109/00498257809060381. [DOI] [PubMed] [Google Scholar]
- Rowland I. R., Davies M. J., Grasso P. The methylation of mercury by the gastro-intestinal contents of the rat. Biochem Soc Trans. 1975;3(4):502–504. doi: 10.1042/bst0030502. [DOI] [PubMed] [Google Scholar]
- Rowland I. R., Davies M. J., Grasso P. Volatilisation of methylmercuric chloride by hydrogen sulphide. Nature. 1977 Feb 24;265(5596):718–719. doi: 10.1038/265718a0. [DOI] [PubMed] [Google Scholar]
- Rowland I. R., Grasso P., Davies M. J. The methylation of mercuric chloride by human intestinal bacteria. Experientia. 1975 Sep 15;31(9):1064–1065. doi: 10.1007/BF02326961. [DOI] [PubMed] [Google Scholar]
- SCOTT H. W., DEHORITY B. A. VITAMIN REQUIREMENTS OF SEVERAL CELLULOLYTIC RUMEN BACTERIA. J Bacteriol. 1965 May;89:1169–1175. doi: 10.1128/jb.89.5.1169-1175.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell J. L., Davison K. L. Metabolism of mercury, administered as methylmercuric chloride or mercuric chloride, by lactating ruminants. J Agric Food Chem. 1975 Jul-Aug;23(4):803–808. doi: 10.1021/jf60200a013. [DOI] [PubMed] [Google Scholar]
- Spangler W. J., Spigarelli J. L., Rose J. M., Flippin R. S., Miller H. H. Degradation of methylmercury by bacteria isolated from environmental samples. Appl Microbiol. 1973 Apr;25(4):488–493. doi: 10.1128/am.25.4.488-493.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangler W. J., Spigarelli J. L., Rose J. M., Miller H. M. Methylmercury: bacterial degradation in lake sediments. Science. 1973 Apr 13;180(4082):192–193. doi: 10.1126/science.180.4082.192. [DOI] [PubMed] [Google Scholar]
- Tezuka T., Tonomura K. Purification and properties of an enzyme catalyzing the splitting of carbon-mercury linkages from mercury-resistant Pseudomonas K-62 strain. I. Splitting enzyme 1. J Biochem. 1976 Jul;80(1):79–87. doi: 10.1093/oxfordjournals.jbchem.a131261. [DOI] [PubMed] [Google Scholar]
- Thauer R. K., Jungermann K., Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977 Mar;41(1):100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A. A., Murphy S. D., Silver S. Mercury and organomercurial resistances determined by plasmids in Staphylococcus aureus. J Bacteriol. 1977 Oct;132(1):197–208. doi: 10.1128/jb.132.1.197-208.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westö G. Determination of methylmercury salts in various kinds of biological material. Acta Chem Scand. 1968;22(7):2277–2280. doi: 10.3891/acta.chem.scand.22-2277. [DOI] [PubMed] [Google Scholar]
- Wood J. M., Kennedy F. S., Rosen C. G. Synthesis of methyl-mercury compounds by extracts of a methanogenic bacterium. Nature. 1968 Oct 12;220(5163):173–174. doi: 10.1038/220173a0. [DOI] [PubMed] [Google Scholar]
- Zehnder A. J., Brock T. D. Methane formation and methane oxidation by methanogenic bacteria. J Bacteriol. 1979 Jan;137(1):420–432. doi: 10.1128/jb.137.1.420-432.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]


