SUMMARY
Microsomal triglyceride transfer protein (MTP) is needed to assemble chylomicrons in the endoplasmic reticulum (ER) of enterocytes. We explored the role of an ER stress protein, inositol-requiring enzyme 1β (IRE1β), in regulating this process. High-cholesterol and high-fat diets decreased intestinal IRE1β mRNA in wild-type mice. Ire1b−/− mice fed high-cholesterol and high-fat diets developed more pronounced hyperlipidemia because these mice secreted more chylomicrons and expressed more intestinal MTP, though not hepatic MTP, than wild-type mice did. Chylomicron secretion and MTP expression also were increased in primary enterocytes isolated from cholesterol-fed Ire1b−/− mice. There was no correlation between ER stress and MTP expression. Instead, cell culture studies revealed that IRE1β, but not its ubiquitous homolog IRE1α, decreased MTP mRNA through increased posttranscriptional degradation. Conversely, knockdown of IRE1β enhanced MTP expression. These studies show that IRE1β plays a role in regulating MTP and in chylomicron production.
INTRODUCTION
Chylomicrons are specialized vehicles synthesized by the intestine to transport large quantities of dietary fat and fat-soluble vitamins (Hussain et al., 1996; Hussain, 2000). Increased absorption of lipids is associated with metabolic disorders such as obesity, atherosclerosis, and diabetes. Chylomicron biosynthesis depends on apolipoprotein B (apoB), a structural protein, and microsomal triglyceride transfer protein (MTP), an endoplasmic reticulum (ER)-resident chaperone (Hussain et al., 2003a, 2003b). MTP transfers several lipids and helps form primordial apoB lipoproteins (Hussain et al., 2003a, 2003b). The phospholipid transfer activity of MTP is sufficient for lipoprotein assembly. Evolutionarily, it is the oldest activity (Sellers et al., 2003; Rava et al., 2006; Rava and Hussain, 2007).
The central role of the ER in chylomicron assembly prompted us to examine the inositol-requiring enzyme 1β (IRE1β) in the intestinal epithelial cells of vertebrates (Bertolotti et al., 2000, 2001; Iwawaki et al., 2001). IRE1β is closely related to the ubiquitously expressed ER stress-response protein IRE1 α (Bernales et al., 2006). IRE1 proteins anchored in the ER membrane contain a sensory domain that is exposed to the lumen and an effector domain facing the cytosol. The conserved effector domain of IRE1 is homologous to the viral-induced RNA-degrading enzyme RNase L. IRE1 proteins mediate the splicing of the X-box-binding protein 1 (XBP-1) mRNA precursor (Zhang et al., 2005; Niwa et al., 1999; Calfon et al., 2002). The spliced mRNA is translated more efficiently, and the synthesized protein acts as a transcription factor for several genes involved in the unfolded protein response (Yoshida et al., 2001). Although IRE1α acts mainly via XBP-1 splicing, there are exceptions. Glucose enhances IRE1α phosphorylation in pancreatic β cells and augments insulin biosynthesis without increasing XBP-1 splicing (Lipson et al., 2006). In Drosophila, IRE1 can degrade specific mRNAs undergoing translation at the ER membrane and halt protein synthesis (Hollien and Weissman, 2006).
Ire1b−/− mice develop normally and are fertile, but they are resistant to toxin-induced colitis (Bertolotti et al., 2001). In this study, we show that Ire1b−/− mice respond to cholesterol-rich and fat-rich diets with enhanced intestinal MTP expression and chylomicron production.
RESULTS
Plasma Non-HDL Lipids Are Elevated in Ire1b−/− Mice Fed High-Cholesterol and High-Fat Diets
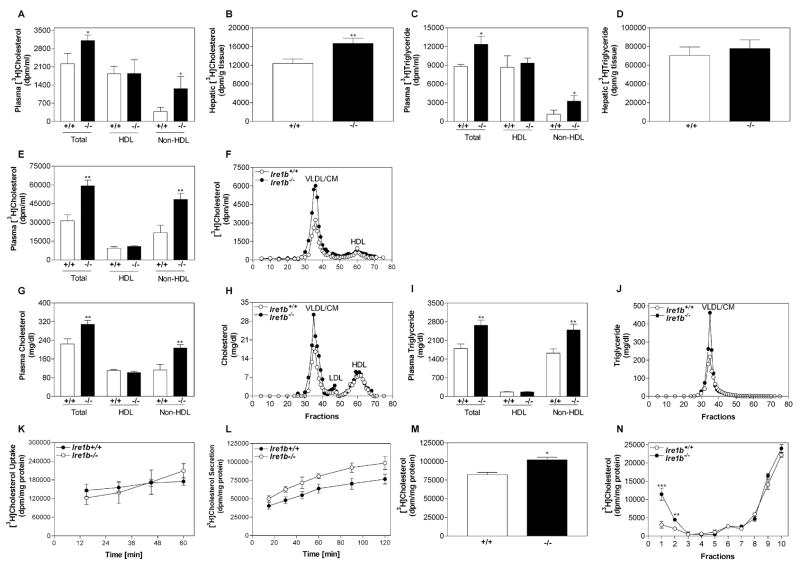
To test whether IRE1β plays a role in lipid metabolism, Ire1b+/+ (WT) and Ire1b−/− (KO) mice were placed on a high-cholesterol diet or a high-fat western diet for 2 weeks (Figure 1). Compared with standard chow, a western diet significantly increased plasma levels of cholesterol and triglyceride in WT mice (Figures 1A and 1B, +/+), similar to the results of other studies (Napoli et al., 2003). A high-cholesterol diet did not increase plasma levels of cholesterol and triglyceride in WT SV129 mice (Figures 1A and 1B, +/+), which agrees with the results of Schwarz et al. (2001). In contrast, because of a 2- to 4-fold increase in non-high-density lipoprotein (non-HDL) apoB lipoproteins (Figures 1C and 1D), both of these diets significantly increased plasma levels of cholesterol (Figure 1A) and triglycerides (Figure 1B) in KO mice. HDL cholesterol and triglyceride levels were similar in WT and KO mice (Figures 1E and 1F). Gel filtration showed that lipid levels were increased in all apoB lipoproteins (very low-density lipoproteins/chylomicrons [VLDL/CM] and low-density lipoproteins [LDL]) but not in HDL lipids (Figures 1G–1J) of KO mice. Thus, on high-cholesterol or high-fat diets, Ire1b−/− mice develop more pronounced hyperlipidemia than WT mice do.
Figure 1. Plasma Non-HDL Lipids Are Elevated and Intestinal Lipids Are Decreased in Ire1b−/− Mice Fed High-Cholesterol and High-Fat Diets.
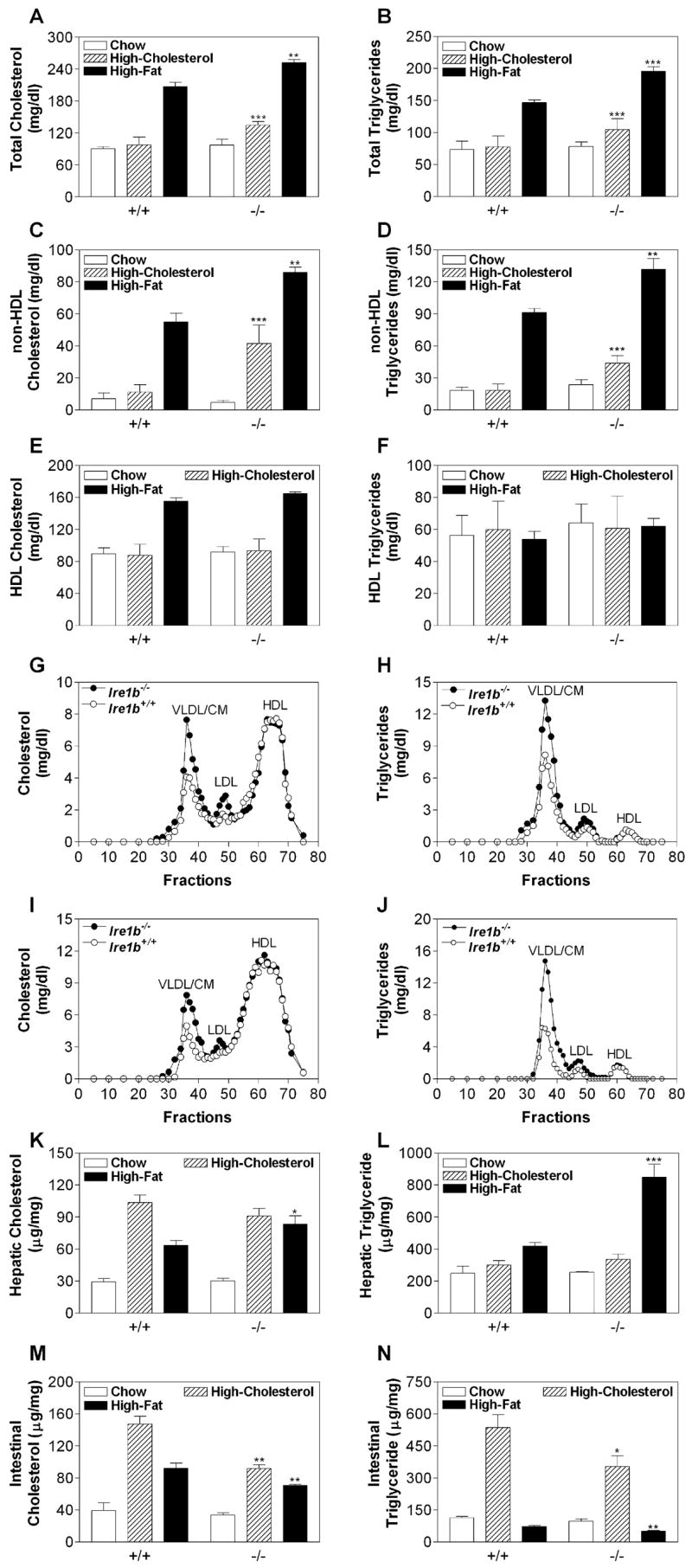
Wild-type Ire1b+/+ (+/+) and Ire1b−/− (−/−) male mice were fed normal chow, chow supplemented with 2% w/w cholesterol (average of five experiments with n = 3–5 animals per group), or a high-fat diet (n = 3 per group) for 2 weeks. (A–F) Plasma samples were used to measure total, non-HDL, and HDL cholesterol as well as triglyceride.
(G–J) Plasma samples from high-cholesterol (G and H) and high-fat (I and J) fed animals were separated by gel filtration to measure lipids in different lipoproteins.
(K–N) Liver and intestinal samples (n = 3) were used to measure cholesterol and triglyceride mass.
Values are mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001 versus Ire1b+/+.
Intestinal Lipid Levels Are Reduced in Ire1b−/− Mice fed High-Cholesterol and High-Fat Diets
WT mice on high-fat and high-cholesterol diets accumulated significantly higher amounts of cholesterol in their livers compared with chow-fed animals (Figure 1K, +/+), as reported by Schwarz et al. (2001). Similar increases were seen in KO mice (Figure 1K, −/−). There were no significant differences in hepatic cholesterol and triglyceride levels in cholesterol-fed WT and KO mice (Figures 1K and 1L, hatched bars). However, hepatic cholesterol and triglyceride levels were higher in KO mice than they were in WT mice fed a high-fat diet (Figures 1K and 1L, black bars). KO mice accumulated less intestinal cholesterol and triglyceride on both diets compared with WT mice (Figures 1M and 1N). Thus, on high-cholesterol and high-fat diets, Ire1b−/− mice show lower intestinal lipid accumulation than WT mice do.
Cholesterol-Fed Ire1b−/− Mice Absorb More Lipids
Increases in plasma apoB lipoprotein levels and decreases in intestinal lipid levels could be explained by similar uptake in WT and KO mice and enhanced secretion of intestinal lipoproteins in KO mice. Long-term cholesterol absorption studies using a dual-label fecal excretion method failed to show any significant differences (see Figure S1 available online), indicating similar uptake. Next, we performed short-term absorption studies. Radioactive cholesterol and triolein (Figures 2A–2D) were fed to cholesterol-fed WT and KO mice. After 2 hr, there was an approximately 50% increase in plasma [3H]cholesterol of KO mice compared with WT mice because of a 3.5-fold increase in non-HDL apoB lipoproteins, with no change in HDL levels (Figure 2A). The KO mice also showed an approximately 30% increase in hepatic [3H]cholesterol (Figure 2B). These studies show increased absorption of radiolabeled cholesterol by KO mice. Compared with WT mice, plasma [3H]triolein-derived counts were approximately 60% higher in KO mice because of a 2-fold increase in non-HDL apoB lipoproteins (Figure 2C), but hepatic [3H]triolein counts were similar in these mice (Figure 2D). These observations are consistent with the understanding that absorbed dietary cholesterol and fatty acids are primarily delivered to the liver and the peripheral tissues, respectively. These results suggest that IRE1β deficiency increases lipid absorption via apoB lipoproteins.
Figure 2. Intestines from Ire1b−/− Mice Secrete More Lipids with Chylomicrons.
Ire1b+/+ (+/+; white bars) and Ire1b−/− (−/−; black bars) mice were fed a high-cholesterol diet for 2 weeks and fed 1 μCi of [3H]cholesterol or [3H]triolein as well as 0.1 mg of cholesterol in 15 μl of olive oil.
(A and C) After 2 hr, plasma was used to measure radioactivity.
(B and D) Liver slices were digested with OptiSolv and used for radioactivity determinations.
(E–J) In another experiment, Ire1b+/+ and Ire1b−/− mice fasted overnight were injected intraperitoneally with poloxamer 407 (30 μg/mouse). After 1 hr, mice were fed 1 μCi [3H]cholesterol and 0.1 mg unlabeled cholesterol. Plasma was collected after 2 hr and used to measure radioactivity (E) as well as cholesterol (G) and triglyceride (I) mass in total plasma, HDL, and non-HDL lipoproteins. Plasma was separated by gel filtration to determine lipids in different lipoproteins (F, H, and J).
(K) Enterocytes were isolated from cholesterol-fed male Ire1b+/+ and Ire1b−/− mice and incubated with 1 μCi/ml of [3H]cholesterol for different lengths of time to study uptake. At each time point, cellular lipids were extracted with isopropanol and counted.
(L) To study secretion, cells were labeled with [3H]cholesterol for 1 hr, washed, and chased for various lengths of time in the presence of 1.2 mM oleic acid. Radioactivity was measured in conditioned media.
(M and N) To identify lipoproteins carrying cholesterol, enterocytes from cholesterol-fed male Ire1b+/+ and Ire1b−/− mice were labeled for 1 hr with 1 μCi/ml of [3H]cholesterol, washed, and chased for 2 hr in the presence of 1.2 mM oleic acid-containing micelles. Conditioned media were used to measure radioactivity (M) or for density gradient ultracentrifugation. Fractions were collected from the top and assayed for radioactivity (N).
Each measurement was performed in triplicate with n = 3 mice per group. Values are mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001 versus Ire1b+/+.
To confirm increased lipid absorption in KO mice, mice fed high-cholesterol diets were injected intraperitoneally with the lipoprotein lipase inhibitor poloxamer 407 (Millar et al., 2005; Anwar et al., 2007) and fed 1 hr later with [3H]cholesterol in olive oil. There was a 90% increase in the appearance of [3H]cholesterol in the plasma of KO versus WT mice, which was associated with a 2.2-fold increase in non-HDL (Figure 2E). These counts were 20 times higher in poloxamer 407-injected animals than those seen in mice not injected with the lipase inhibitor (compare to Figure 2A), indicating significant inhibition of lipoprotein clearance. Gel filtration analysis showed increased counts in the apoB lipoproteins (VLDL/CM) of KO mice (Figure 2F). Mass measurements showed that total plasma cholesterol and triglyceride levels were greater in apoB lipoproteins (Figures 2G–2J). These studies indicate increased lipid absorption via apoB lipoprotein production in Ire1b−/− mice.
Despite the decreased intestinal cholesterol level in KO mice (Figures 1M and 1N), isolated primary enterocytes showed no differences in the uptake of radiolabeled cholesterol (Figure 2K), and NPC1L1 mRNA levels were similar in WT and KO mice (Figures S2A and S2C). However, cholesterol secretion was higher in KO enterocytes (Figure 2L). Therefore, KO enterocytes take up cholesterol as efficiently as WT enterocytes but secrete more cholesterol than WT, explaining the lower levels of intestinal cholesterol in KO mice.
Primary Enterocytes from Cholesterol-Fed Ire1b−/− Mice Secrete More Chylomicrons
The gel filtration and lipoprotein precipitation methods used above (Figures 2A–2J) do not distinguish between different triglyceride-rich lipoproteins. To identify the types of intestinal lipoproteins increased in IRE1β deficiency, we studied lipoprotein production in primary enterocytes isolated from cholesterol-fed KO and WT mice. [3H]cholesterol secretion was approximately 25% greater in KO enterocytes than it was in WT enterocytes (Figure 2M), which could reflect a more substantial increase in chylomicrons with no increase in other lipoproteins. Therefore, we subjected the conditioned media to potassium bromide density gradient ultracentrifugation to separate chylomicrons from other lipoproteins. The WT enterocytes secreted [3H]cholesterol into chylomicrons at the top of the gradient (Luchoomun and Hussain, 1999) and into HDL particles at the bottom of the gradient (Iqbal and Hussain, 2005), consistent with the secretion of cholesterol via both apoB-dependent and apoB-independent pathways (Iqbal and Hussain, 2005; Iqbal et al., 2003; Hussain et al., 2005). The secretion of [3H]cholesterol into chylomicron-like particles was approximately 3.7-fold greater in KO versus WT enterocytes (Figure 2N, fraction 1), while secretion into HDL lipoproteins was similar (Figure 2N, fractions 8–10). Thus, IRE1β deficiency selectively increases chylomicron secretion.
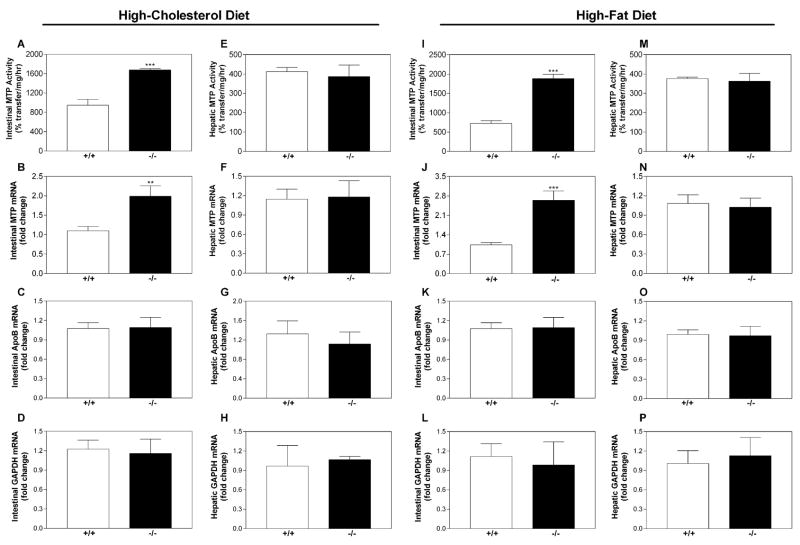
Intestinal MTP mRNA and Activity Are Elevated in Cholesterol- and Fat-Fed Ire1b−/− Mice
MTP is an essential chaperone and a rate-limiting factor for chylomicron assembly (Hussain et al., 2003a, 2003b). In fat-fed animals, the intestines (Figures S3A and S3C), but not the livers (Figures S3B and S3D), of KO mice had 2- to 3-fold higher amounts of MTP protein. The triglyceride transfer activity of MTP was 2- to 2.5-fold higher in KO intestines (Figures 3A and 3I), but hepatic MTP activity was not different (Figures 3E and 3M). Levels of MTP mRNA were 2- to 2.7-fold higher in the intestines of cholesterol-fed and fat-fed Ire1b−/− mice (Figures 3B and 3J), but there were no differences in apoB (Figures 3C and 3K) or GAPDH (Figures 3D and 3L) mRNA. Hepatic MTP mRNA levels were similar in KO and WT mice (Figures 3F and 3N), as were levels of apoB and GAPDH (Figures 3G, 3H, 3O, and 3P). MTP activity and mRNA levels were not changed in chow-fed KO or WT mice, and we did not find differences between cholesterol-fed KO and WT animals in the intestinal and hepatic mRNA levels of several ER proteins involved in lipid metabolism and ER stress responses (Figures S2A–S2D). Thus, IRE1β deficiency in cholesterol-fed and fat-fed mice leads to a selective increase in intestinal MTP.
Figure 3. MTP mRNA, Protein, and Activity Are Increased in the Intestines of Ire1b−/− Mice.
Intestinal and liver samples from Ire1b+/+ (+/+; white bars) and Ire1b−/− (−/−; black bars) mice (n = 3 per group) fed a cholesterol-enriched or fat-enriched diet were used for MTP activity assays (A, E, I, and M). RNA was used to measure MTP (B, F, J, and N), apoB (C, G, K, and O), GAPDH (D, H, L, and P), and ARPp0 mRNA in triplicate. The ratio between the gene of interest and ARPp0 in Ire1b+/+ mice was used to normalize mRNA levels in all samples. Values are mean ± SD. **p < 0.01, ***p < 0.001 compared to Ire1b+/+. Data are representative of two experiments.
To test whether increases in MTP lead to increased apoB lipoprotein secretion, we transfected COS cells, which do not express apoB or MTP, with an apoB48 expression plasmid and different amounts of a human MTP-FLAG expression plasmid (Figure S4). No apoB was secreted in the absence of MTP, but apoB secretion increased as MTP expression increased, consistent with other studies (Gordon et al., 1994; Leiper et al., 1994).
Changes in MTP Expression Are Not Correlated with ER Stress
We next sought to identify how IRE1β deficiency leads to increased MTP. Although IRE1β-mediated XBP-1 mRNA splicing in cholesterol-fed WT mice could theoretically suppress MTP mRNA, intestinal samples from chow-fed and cholesterol-fed animals did not induce XBP-1 mRNA splicing (Figure 4A) or IRE1α, BiP, or CHOP mRNA levels, which increase during ER stress (Figures 4B–4D). To increase sensitivity, we used enterocytes isolated from cholesterol-fed WT and KO mice and found an increase in MTP mRNA levels but no changes in IRE1α, BiP, or CHOP (Figure 4E) or in XBP-1 mRNA splicing (Figure 4F). As a control, XBP-1 mRNA splicing was evident in tunicamycin-exposed human hepatoma Huh7 cells (Figure 4F). These data indicate that a high-cholesterol diet does not induce intestinal ER stress. In contrast, the high-fat diet induced similar levels of ER stress in the intestines of both WT and KO mice, as evidenced by XBP-1 mRNA splicing (Figure 4A), and increases in IRE1α, BiP, and CHOP mRNA (Figures 4B–4D). Thus, induction of ER stress is independent of genotype and is a consequence of a high-fat diet.
Figure 4. XBP-1 Splicing Does Not Regulate MTP.
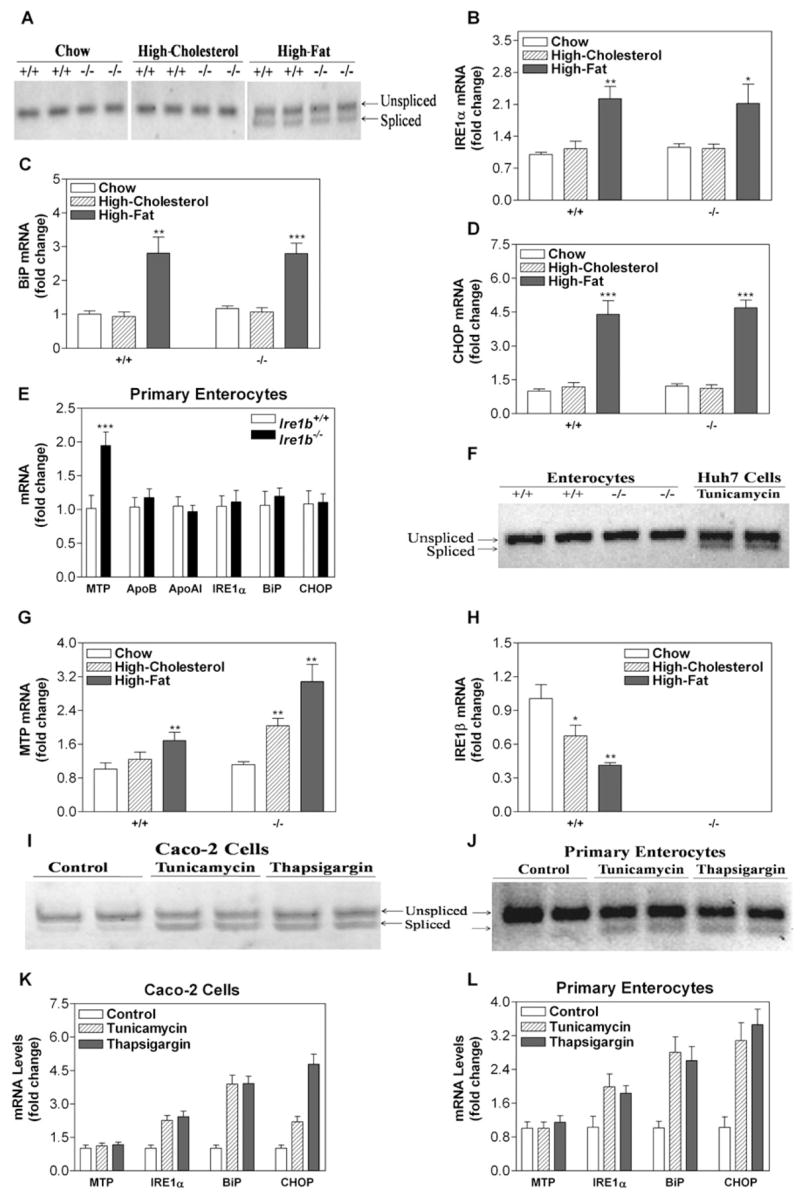
RNA was isolated from the intestinal tissues of Ire1b+/+ and Ire1b−/− mice (n = 3) fed with or without high-cholesterol or high-fat diet for 2 weeks.
(A) Spliced and unspliced XBP-1 mRNA levels.
(B–D, G, and H) Intestinal RNA was used to determine in triplicate the levels of IRE1α (B), BiP (C), CHOP (D), MTP (G), and IRE1β (H) mRNAs. Values are mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001 versus chow-fed animals.
(E and F) RNA also was isolated from the primary enterocytes of Ire1b+/+ and Ire1b−/− mice fed a high-cholesterol diet and used to quantify mRNA levels of ER stress and lipoprotein genes (E) and XBP-1 splicing (F). RNA from Huh7 cells treated with tunicamycin (5 μg/ml) was used as a control (F). Values are mean ± SD. ***p < 0.001 versus Ire1b+/+.
(I and K) Differentiated Caco-2 cells were treated with tunicamycin (5 μg/ml) or thapsigargin (1 μM) for 17 hr and used to quantify spliced as well as unspliced XBP-1 mRNA (I) and to measure MTP, IRE1α, BiP, CHOP, and ARPp0 mRNA (K).
(J and L) Enterocytes isolated from Ire1b+/+ mice were treated with tunicamycin (10 μg/ml) or thapsigargin (5 μM) for 3 hr and used to measure XBP-1 splicing (J) and MTP, IRE1α, BiP, CHOP, and ARPp0 mRNA (L).
In WT mice, a high-fat diet increased intestinal MTP mRNA by 68% (Figure 4G, +/+). High-cholesterol diet and high-fat diet increased intestinal MTP mRNA in KO mice 2-fold and 3-fold (Figure 4G, −/−), respectively, compared with chow-fed WT. The high-cholesterol and high-fat diets decreased IRE1β mRNA levels by 33% and 59%, respectively, in WT mice (Figure 4H, +/+). Thus, both diets reduce intestinal IRE1β in WT mice but increase MTP mRNA in Ire1b−/− mice. Direct cell stress and activation of XBP-1 splicing in Caco-2 human colon carcinoma cells (Figure 4I) and primary enterocytes (Figure 4J) by tunicamycin or thapsigargin did not affect MTP mRNA (Figures 4K and 4L) or activity (data not shown) but did increase IRE1α, BiP, and CHOP mRNA levels.
In summary, a high-cholesterol diet does not induce ER stress but increases intestinal MTP mRNA in KO mice. A high-fat diet induces similar ER stress in both WT and KO mice but significantly induces MTP mRNA only in the intestines of KO mice. Furthermore, induction of ER stress and XBP-1 in intestinal cells does not affect MTP expression.
MTP mRNA Changes Proportionally to IRE1β Expression
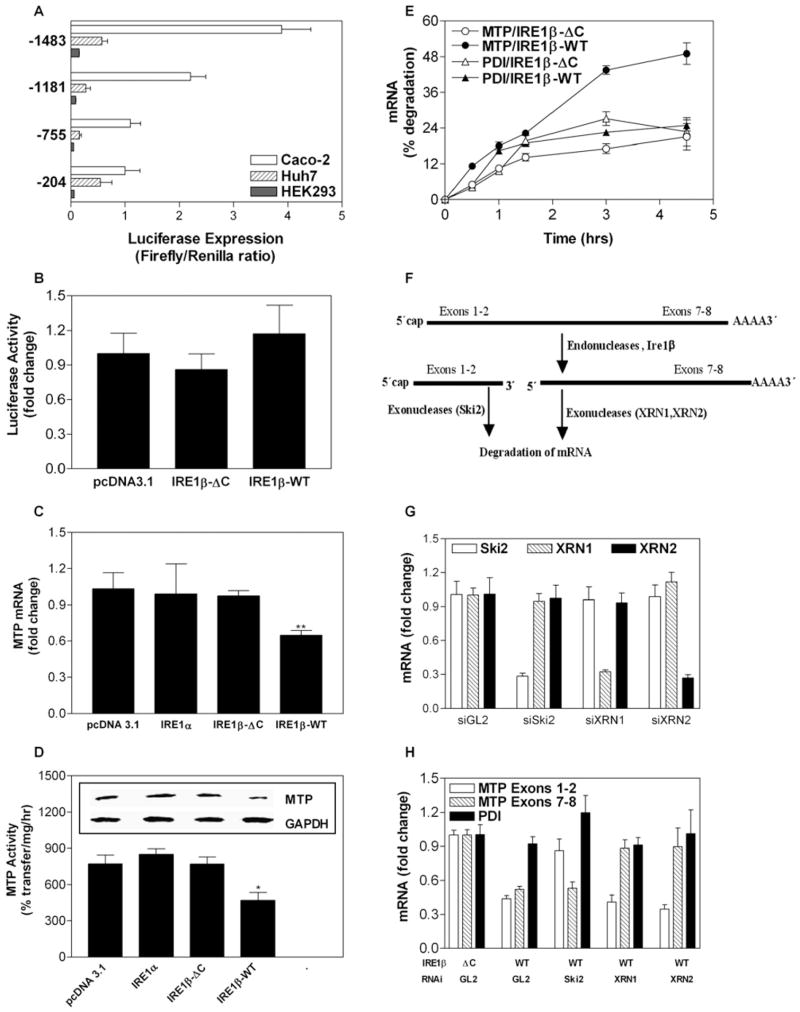
To test whether MTP is directly suppressed by IRE1β, we transfected human hepatoma Huh7 cells, which express MTP but not IRE1β, with c-Myc-tagged plasmids expressing full-length IRE1β (IRE1β-WT) and C-terminally truncated IRE1β (IRE1β-ΔC) lacking the kinase and endoribonuclease domains (Figure 5A) (Urano et al., 2000; Wang et al., 1998). Expression of IRE1β-ΔC did not affect MTP mRNA (Figure 5C), apoB secretion (Figure 5D), or protein disulfide isomerase (PDI) mRNA (Figure 5E). IRE1β-WT expression reduced 28S rRNA (Figure 5B) consistent with Iwawaki et al. (2001), suppressed MTP mRNA (Figure 5C), and decreased apoB lipoprotein secretion (Figure 5D) but did not affect PDI (Figure 5E) or apoB, apoAI, ACAT1, ACAT2, MGAT2, MGAT3, DGAT1, DGAT2, or NPC1L1 mRNA (Figure S2E), suggesting no general effects on cell health.
Figure 5. Overexpression of IRE1β Decreases MTP mRNA Levels.
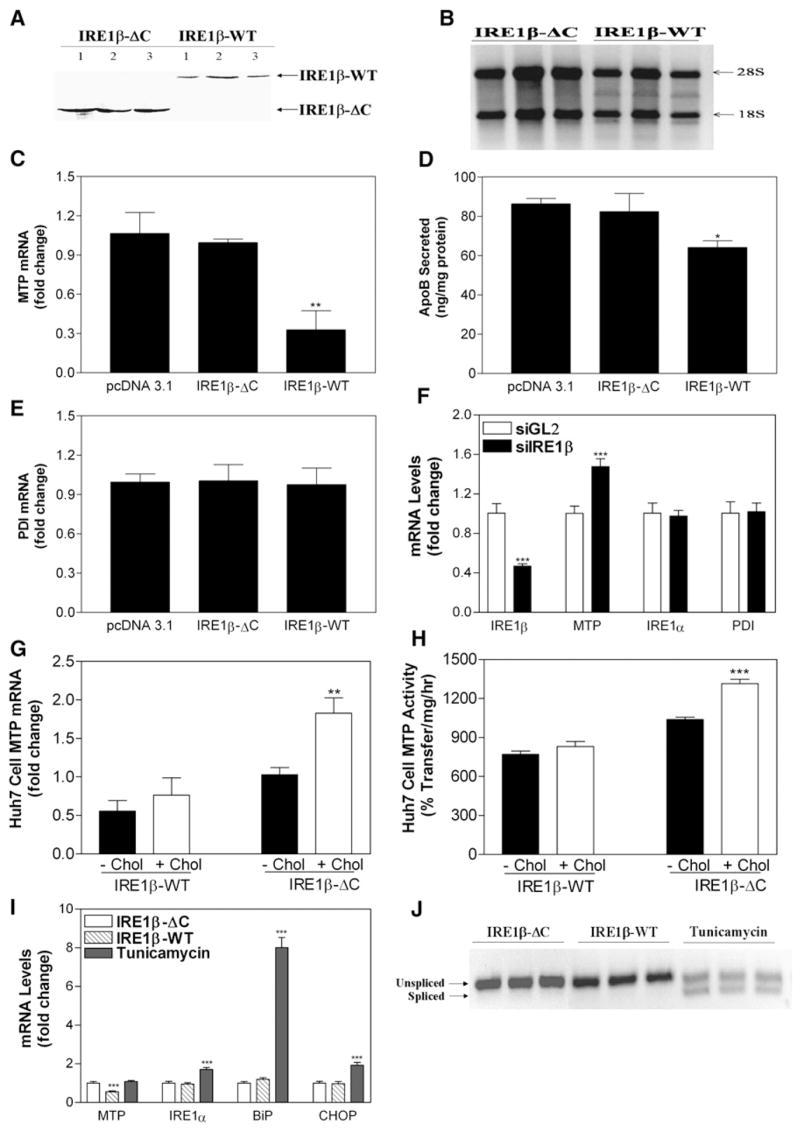
(A) Huh7 cells transfected with different c-Myc-tagged IRE1β expression plasmids were used to determine protein expression using c-Myc antibodies.
(B) Total RNA from IRE1β-ΔC- and IRE1β-WT-transfected Huh7 cells was loaded on 1% agarose gels to visualize changes in 18S and 28S rRNA. (C–E) Cells from three wells were used to measure MTP (C) and PDI (E) mRNA in triplicate. Media were used to measure apoB secretion (D).*p < 0.05, **p < 0.01 compared to pcDNA3.1-treated cells.
(F) RNA interference was used to knock down IRE1β in undifferentiated Caco-2 cells. siGL2 served as a control. Cells were treated with siRNA targeting IRE1β and used to measure IRE1β, MTP, IRE1α, and PDI mRNAs. ***p < 0.001 compared to siGL2-treated cells.
(G and H) To study the effect of cholesterol on MTP expression, Huh7 cells were transfected with either IRE1β-WT or IRE1β-ΔC expression plasmids. After 12 hr, cells were supplemented with or without cholesterol (40 μg/ml), and MTP and ARPp0 mRNA were quantitated (G). Cells treated in parallel were used for MTP activity assays (H). **p < 0.01, ***p < 0.001 compared to IRE1β-ΔC-overexpressing cells without cholesterol treatment.
(I and J) Huh7 cells were transfected with IRE1β expression plasmids or treated with 5 μg/ml tunicamycin. Total RNA was isolated and used to measure MTP, IRE1α, BiP, and CHOP mRNA (I). ***p < 0.001 compared to IRE1β-ΔC-overexpressing cells. In addition, RNA was used to determine XBP-1 splicing (J).
Data are representative of two experiments performed in triplicate. Values are mean ± SD.
We next determined the consequences of decreasing IRE1β on MTP expression in undifferentiated Caco-2 cells through RNA interference. IRE1β siRNA reduced its target mRNA by 60% with no effect on IRE1α mRNA (Figure 5F). More importantly, IRE1β knockdown increased MTP mRNA by 50% (Figure 5F) and did not affect PDI mRNA. These data show that expression and suppression of IRE1β lead to specific changes in MTP mRNA through a mechanism that requires the kinase and ribonuclease domains.
To investigate why cholesterol feeding was necessary to observe differences in plasma lipid levels in WT and KO mice, Huh7 cells were transfected with IRE1β-WT or IRE1β-ΔC and cultured in the presence or absence of cholesterol. Cholesterol treatment of cells expressing IRE1β-ΔC significantly increased MTP mRNA (Figure 5G) and activity (Figure 5H), as occurred in KO mice fed a high-cholesterol diet (Figures 3A and 3B). In contrast, cholesterol supplementation of cells expressing IRE1β-WT did not affect MTP mRNA (Figure 5G) or activity (Figure 5H). Therefore, IRE1β counteracts cholesterol-induced increases in MTP expression. Similar studies were not performed with high fatty acid concentrations because they are toxic to cells.
Next, we asked whether overexpression of IRE1β in Huh7 cells increases ER stress and decreases MTP mRNA. Expression of IRE1β-ΔC and IRE1β-WT had no effect on IRE1α, BiP, or CHOP mRNA (Figure 5I), and no splicing of XBP-1 occurred in these cells (Figure 5J). On the other hand, treatment of Huh7 cells with tunicamycin increased ER stress indicators, IRE1α, BiP, CHOP, and XBP-1 splicing (Figures 5I and 5J). Thus, Huh7 cells respond to ER stress transducers but do not upregulate these genes when transfected with IRE1β. We conclude that IRE1β decreases MTP levels without inducing an ER stress response.
IRE1β Decreases MTP mRNA by Promoting Posttranscriptional Degradation
To determine whether IRE1β-mediated suppression of MTP mRNA occurs at the transcriptional level, we determined the promoter activity of a 1.5 kb sequence upstream of the transcription initiation site. Luciferase activity expressed under the control of the MTP (mttp) promoter could be measured in Huh7 and Caco-2 cells but not HEK293 cells (Figure 6A), indicating that the promoter is sufficient for expression in the intestine and liver; this is consistent with other studies showing that 204 bases are sufficient for MTP expression (Hagan et al., 1994; Hirokane et al., 2004; Kang et al., 2003). To evaluate whether IRE1β affects mttp promoter activity, we cotransfected Huh7 cells with a plasmid expressing luciferase under the control of the 1.5 kb mttp promoter as well as pcDNA3.1, IRE1β-WT, or IRE1β-ΔC expression plasmids. IRE1β-WT did not suppress mttp promoter activity compared with vector control or IRE1β-ΔC (Figure 6B), indicating that IRE1β does not reduce MTP levels by altering mttp promoter activity.
Figure 6. IRE1β Enhances Posttranscriptional Degradation of MTP mRNA.
(A) Caco-2, Huh7, and HEK293 cells in 12-well plates were cotransfected with 40 ng of control plasmid (pCMV-RL) and 1 μg of pGL2 plasmid DNAs with luciferase under the control of various lengths (−204 to −1483 bp) of 5′ sequences upstream of the human MTTP gene. Cells were collected 48 hr later and analyzed with a Dual-Luciferase Reporter kit (Promega).
(B) Promoter activity assays were performed using the dual-luciferase assay system. pCMV-Renilla luciferase served as a control for transfection efficiency. Huh7 cells were transfected with 20 ng pCMV-Renilla luciferase and 1 μg pMTP-1483-luciferase (expressing luciferase activity under the control of a 1.5 kb MTTP promoter) with pcDNA3.1, IRE1β-ΔC (100 ng), or IRE1β-WT (100 ng). After 48 hr, luciferase activity was measured.
(C and D) Mouse fibroblasts L cells stably expressing mouse MTP under the control of the cytomegalovirus promoter were transfected with different plasmids. After 48 hr, MTP mRNA (C) and activity (D) were measured in triplicate. Inset in (D) shows western blot of a representative sample. *p < 0.05, **p < 0.01 versus pcDNA3.1-treated cells.
(E) Huh7 cells were transfected in triplicate with either IRE1β-WT or IRE1β-ΔC expression plasmid DNA. After overnight incubation, cells were cultured in the presence of actinomycin D (1 μg/ml) for different lengths of time in triplicate. RNA was used to quantify MTP, PDI, and ARPp0 mRNA. The MTP/ARPp0 or PDI/ARPp0 ratio at time 0 was normalized to 100%, and loss of MTP was plotted versus time.
(F) Schematic representation of possible internal cleavage of MTP mRNA by IRE1β and its subsequent degradation by different exonucleases.
(G) Huh7 cells were transfected with IRE1β-WT. After 24 hr, cells were transfected with siRNAs against Ski2, XRN1, or XRN2. RNA was isolated after 48 hr and used to quantify Ski2, XRN1, or XRN2 mRNA in control (siGL2) and RNAi-treated cells to determine the efficacy and specificity of RNA interference.
(H) Huh7 cells were transfected in triplicate with IRE1β-ΔC or IRE1β-WT expression plasmids. After 24 hr, cells were transfected with indicated siRNAs. Total RNA from these cells was used to amplify different regions (exons 1–2 and exons 7–8) of MTP mRNA using primers listed in Table S1.
Data are representative of two experiments performed in triplicate. Values are mean ± SD.
To explore posttranscriptional mechanisms, we used mouse fibroblast L cells stably transfected with mouse MTP cDNA under the control of the cytomegalovirus promoter (Dougan et al., 2007). Expression of IRE1β-WT in these cells reduced endogenous MTP mRNA (Figure 6C), activity (Figure 6D), and protein (Figure 6D inset). In contrast, IRE1β-ΔC and IRE1α had no effect on MTP mRNA (Figure 6C), activity (Figure 6D), or protein (Figure 6D inset). To explore whether IRE1β decreases the stability of MTP mRNA, Huh7 cells expressing IRE1β-WT or IRE1β-ΔC were treated with actinomycin D to block new mRNA transcription. MTP mRNA, but not PDI mRNA, decayed at a greater rate in cells expressing IRE1β-WT than in cells expressing IRE1β-ΔC (Figure 6E), indicating that IRE1β-WT promotes MTP mRNA degradation.
A plausible scenario for MTP mRNA degradation would involve its cleavage by IRE1β endonuclease activity (Figure 6F), followed by exonuclease digestion of the cleaved products. In this context, the exonucleases Ski2 and XRN1/2 may perform 3′ → 5′ and 5′ → 3′ exonuclease digestion, respectively, of endonuclease-cleaved mRNA (Newbury, 2006; Houseley et al., 2006; Meyer et al., 2004). Thus, knockdown of these exonucleases should preserve the putative 5′ or 3′ cleavage products of MTP mRNA. To test this, Huh7 cells were transfected with IRE1β-WT or IRE1β-ΔC and treated with Ski2, XRN1, XRN2, or control GL2 siRNA, which specifically reduced 70%–75% of the target mRNAs (Figure 6G). Expression of IRE1β-WT decreased MTP mRNA sequences corresponding to exons 1–2 (5′) and exons 7–8 (3′) when treated with control GL2 siRNA but did not affect PDI mRNA levels (Figure 6H, WT+GL2). Treatment with Ski2 siRNA prevented the loss of sequences corresponding to MTP exons 1–2 but not exons 7–8 (Figure 6H, WT+Ski2). Similarly, XRN1 and XRN2 siRNA selectively limited the loss of exons 7–8. These data are consistent with IRE1β-dependent endonuclease cleavage between exons 2 and 7, followed by 3′ → 5′ exonuclease cleavage of the 5′ fragment(s) by Ski2 and 5′ → 3′ exonuclease cleavage of the 3′ fragment(s) by XRN1 and XRN2.
DISCUSSION
IRE1β deficiency in mice enhances intestinal MTP expression without affecting hepatic MTP and increases lipid absorption and chylomicron secretion in response to high-cholesterol and high-fat diets. IRE1β reduces MTP mRNA levels by augmenting posttranscriptional degradation. Thus, the present study identifies an intestine-specific mechanism controlling the assembly and secretion of apoB lipoproteins, wherein IRE1β regulates chylomicron production by degrading MTP mRNA.
Eating high-cholesterol and high-fat diets enhances MTP expression in the liver, and to a lesser extent in the intestine, of WT mice. In contrast, diet-induced increases in intestinal and hepatic MTP are similar in KO mice. These observations indicate that MTP expression is more responsive to dietary manipulations in the absence of IRE1β and that IRE1β blocks diet-induced upregulation of intestinal MTP in WT mice.
Ire1b−/− mice fed high-cholesterol or high-fat diets produce more intestinal apoB lipoproteins both in vivo (Figures 2A–2J) and in isolated enterocytes (Figures 2M and 2N), which eliminates generalized intestinal damage, permeability, and other pathological mechanisms as potential explanations for this increase. This increased apoB lipoprotein production is probably secondary to increased MTP activity because (1) IRE1β deficiency did not affect apoB mRNA, (2) changes in MTP expression significantly altered the secretion of apoB lipoproteins (Figure S4), and (3) changes in MTP levels affect plasma lipid and apoB lipoprotein levels (Tietge et al., 1999; Ameen et al., 2005; Wolfrum and Stoffel, 2006; Lin et al., 1994). Conversely, repression of MTP was associated with decreased apoB secretion. Chenodeoxycholate reduces MTP expression and apoB secretion in HepG2 cells (Hirokane et al., 2004). MTP antagonists reduce plasma lipids (Cuchel et al., 2007; Wetterau et al., 1998). Thus, changes in MTP activity significantly affect plasma apoB lipoprotein levels. Therefore, increases in MTP due to IRE1β deficiency are sufficient to augment apoB lipoprotein production.
mttp−/− mice are not viable (Raabe et al., 1998). Chow-fed heterozygous mttp+/− mice have plasma lipid levels similar to WT mice but significantly lower intermediate-density lipoprotein (IDL)/LDL cholesterol. A high-fat diet significantly lowers plasma cholesterol in mttp+/− mice owing to a marked reduction in VLDL and LDL cholesterol, and hepatocytes isolated from mttp+/− mice secrete less apoB lipoprotein (Raabe et al., 1998). Half-normal MTP levels reduce apoB lipoprotein secretion to a similar extent in apob+/− and apoB transgenic mice (Leung et al., 2000). Therefore, MTP deficiency reduces apoB lipoprotein production and decreases plasma cholesterol when challenged with a high-fat diet. In Ire1b−/− mice, increases in intestinal MTP increase apoB lipoprotein production and enhance plasma cholesterol levels.
In abetalipoproteinemia, MTP deficiency significantly reduces total and LDL cholesterol. In general, obligate MTTP heterozygotes present with normal plasma cholesterol, but several parents of abetalipoproteinemia patients have been found to have low cholesterol (Raabe et al., 1998; Narcisi et al., 1995; Berriot-Varoqueaux et al., 2000; Di Leo et al., 2005). The variations in plasma cholesterol in heterozygotes might result from an adaptation involving increased synthesis of MTP by the normal allele (Berriot-Varoqueaux et al., 2000). Moreover, plasma apoB lipoproteins have not been characterized, and high-fat feeding studies have not been performed in heterozygotes. Therefore, there are insufficient data to draw conclusions about the effect of half-normal MTP levels on plasma apoB lipoproteins.
Although IRE1 functions primarily via splicing XBP-1 mRNA, it represses ER-targeted mRNA independently of the XBP-1 pathway (Hollien and Weissman, 2006). IRE1β may reduce protein synthesis by cleaving 28S ribosomal RNA (Iwawaki et al., 2001). IRE1α regulates proinsulin synthesis without enhancing XBP-1 splicing (Lipson et al., 2006). We did not observe spliced XBP-1 mRNA in the intestines of cholesterol-fed WT mice, and induction of XBP-1 splicing by tunicamycin and thapsigargin did not alter MTP mRNA levels (Figure 4). Thus, intestinal IRE1β regulates MTP independently of the XBP-1 pathway.
Despite the sequence homology between IRE1α and IRE1β, the effect on MTP mRNA regulation was specific to IRE1β (Figure 6C). The cytosolic domains that contain the kinase and endoribonuclease activities in mouse IRE1β and IRE1α exhibit 62%–63% identity, but the identity of the luminal domains is only 41%. Therefore, IRE1β may have evolved to detect a specific luminal signal—for example, fat status.
The posttranscriptional degradation of MTP mRNA could involve direct endolytic cleavage. IRE1β and MTP mRNA translation are associated with the ER membrane, and overexpression of MTP may therefore facilitate its degradation by IRE1β. Alternatively, increased chylomicron assembly may temporarily stall the protein translational machinery, leading to the recognition of the MTP mRNA by IRE1β directly or via recruitment/activation of another endonuclease. If so, all components are present in the liver, and IRE1β may be the limiting factor for MTP downregulation.
We can only speculate as to why the enterocytes of vertebrates have this regulatory pathway to control chylomicron assembly. One hypothesis is that enterocytes optimize fat absorption by activating the ER stress response and downregulating IRE1β. By upregulating the ER stress response, the ER is protected from the increased load of synthesizing and packaging chylomicrons. By downregulating IRE1β, chylomicron assembly is maximized by enhancing MTP. In the fasting state, it may be beneficial to limit chylomicron secretion by degrading MTP mRNA to conserve critical cellular lipids and fat-soluble vitamins and to avoid rapid fluctuations in membrane phospholipids, neutral lipids, and vitamin E. Alternatively, IRE1β may mediate downregulation of chylomicron secretion in response to satiety signals or respond to endocrine factors from the liver and adipose tissue under conditions of excess whole-body lipid accumulation. In the absence of IRE1β, this postprandial mechanism of optimizing fat absorption is derailed, leading to a sustained hyperlipidemia.
In summary, these studies highlight an intestine-specific mechanism controlling lipid absorption. In this mechanism, IRE1β regulates MTP mRNA levels involving posttranscriptional degradation. IRE1β restricts MTP induction in the intestine by high-fat and high-cholesterol diets. These findings raise the possibility that upregulation of IRE1β may be beneficial in avoiding diet-induced hyperlipidemia.
EXPERIMENTAL PROCEDURES
Materials
[1,2-3H]cholesterol and [3H]triolein were from NEN Life Science Products. Oleic acid, cholate, deoxycholate, taurocholate, and monoacylglycerol were from Sigma. Phosphatidylcholine was from Avanti Polar Lipids. Other chemicals and solvents were from Fisher Scientific.
Animals and Diets
Ire1b−/− (KO) and Ire1b+/+ (WT) mice on an SV129 background were used (Bertolotti et al., 2001). To study the effects of a high-cholesterol diet, 10- to 16-week-old male mice were fed a chow diet containing 2% w/w cholesterol for 14 days, with no significant effect on body weight. Histologic analyses revealed no overt differences in epithelial cell morphology or crypt cell mitotic figures. To study the effects of a high-fat diet, male WT and KO mice were fed a high-fat western diet containing 17%, 48.5%, 21.2%, and 0.2% by weight of protein, carbohydrate, fat, and cholesterol, respectively (TD88137, Harlan Teklad). Food was withdrawn the night before the experiments.
Plasma Lipid Measurements
Total cholesterol and triglyceride levels were measured using kits (Thermo Trace Ltd.). HDL lipid levels were measured after precipitating apoB lipoproteins (Iqbal and Hussain, 2005). Lipid levels in apoB lipoproteins were determined by subtracting HDL lipid levels from total lipid levels. Plasma lipoproteins were separated by gel filtration (flow rate of 0.2 ml/min) using a Superose 6 column, and 200 μl fractions were collected.
Short-Term Lipid Absorption Studies
Age-matched male mice (n = 3 per group) fed a high-cholesterol diet for 2 weeks were fasted overnight and gavaged with 1 μCi of either [3H]triolein or [3H]cholesterol and 0.1 mg of unlabeled cholesterol in 15 μl of olive oil (Iqbal and Hussain, 2005). After 2 hr, plasma was used to measure radioactivity. Tissues (0.1 g) were rinsed and dissolved in 1 ml OptiSolv, and radioactivity was counted.
Secretion of [3H]Cholesterol by Primary Enterocytes
Primary enterocytes from WT and KO mice (Iqbal et al., 2003; Iqbal and Hussain, 2005) were suspended in 4 ml of DMEM containing 1 μCi/ml of [3H]cholesterol and incubated at 37°C. Enterocytes were incubated for 1 hr with micelles containing 1.2 mM oleic acid (Iqbal et al., 2003; Iqbal and Hussain, 2005). After 2 hr, enterocytes were centrifuged and supernatants were subjected to density gradient ultracentrifugation. Fractions were collected and radioactivity was counted. Cell pellets were incubated overnight at 4°C with 1 ml isopropanol to extract lipids. After extraction, proteins were dissolved in 1 ml of 0.1 N NaOH and quantified using Coomassie reagent (Pierce Chemical Company).
Determination of MTP Activity
Small pieces (0.1 g) of liver and proximal small intestine (~1 cm) were homogenized in low-salt buffer and centrifuged, and supernatants were used for protein determination and MTP assay (Athar et al., 2004; Rava et al., 2005) using a kit (Chylos Inc.).
RNA Isolation and Two-Step Quantitative RT-PCR
Total RNA from tissues and cells was isolated using TRIzol (Invitrogen). Purity and integrity were assessed by the A260/A280 ratio and 1% agarose gel electrophoresis, respectively. RNAs with ratios greater than 1.7 were used for cDNA synthesis. The first-strand cDNA was synthesized with an Omniscript RT kit (QIAGEN) and used for quantitative RT-PCR (qPCR Core Kit for SYBR Green I, Eurogentec). Data were analyzed using the ΔΔCT method and are presented as arbitrary units. The primers used were designed using PrimerExpress 3.0 (Applied Biosystems) and are listed in Table S1. A two-step RT-PCR method was used to determine XBP-1 RNA splicing. The primers (Table S1) were designed to differentiate the two forms of XBP-1, which differ by 26 nucleotides. The PCR products were separated by 3% agarose or 12% polyacrylamide gels.
Plasmid Expression
The expression plasmids pIRE1β-WT and pIRE1β-ΔC have been described previously (Urano et al., 2000). Plasmid pMTP1483 was generated by cloning a 1.4 kb mttp promoter into a pGL2 basic vector. Huh7 cells with endogenous MTP and mouse fibroblast L cells expressing mouse MTP under the control of the cytomegalovirus promoter (Dougan et al., 2007) were plated in 100 mm dishes. ExGen 500 (Fermentas) and PolyFect (QIAGEN) were used to transfect cells. After 24–48 hr transfection, cells were used to measure MTP protein and activity (Athar et al., 2004; Rava et al., 2005) as well for RNA extraction.
Small RNA Interference
Huh7 cells transfected with IRE1β-WT or IRE1β–ΔC plasmids for 8 hr were transfected with siRNA against the exoribonucleases Ski2, XRN1, and XRN2 or control siGL2 by the siPORT NeoFX agent (Ambion). After 48 hr, RNA was extracted and used for quantitative RT-PCR.
Western Blot Analyses
Tissue and cell samples were homogenized in PBS containing 1% Triton X-100. Proteins (40 μg) were resolved on 4%–20% gradient gels (Bio-Rad). A monoclonal MTP antibody (BD Biosciences), rabbit anti-PDI (Santa Cruz), and mouse anti-GAPDH (Santa Cruz) were used to detect endogenous proteins. Overexpressed tagged mouse IRE1β proteins were detected using anti-c-Myc (9E10, Santa Cruz) antibodies. Secreted apoB was immunoprecipitated with goat anti-human apoB100 antibody and immunoblotted with 1D1 monoclonal antibody (Academy Bio-Medical Company, Inc.). MTP-FLAG was detected using anti-FLAG (Sigma) antibody. The resulting blots were developed using an ECL kit (Amersham). To quantify mass, in some western blots, we used Alexa Fluor 633 (Invitrogen) anti-mouse or anti-rabbit secondary antibodies and evaluated the blots using Storm 860 (Amersham). Bands were quantified using Scion Image (Scion Corporation).
Statistics
Data are presented as mean ± SD. Unless noted otherwise, n = 3 for each group or condition. Statistical significance (p < 0.05) was determined using Student’s t test (GraphPad Prism).
Supplementary Material
SUPPLEMENTAL DATA
Supplemental Data include four figures, one table, and Supplemental References and can be found with this article online at http://www.cellmetabolism.org/cgi/content/full/7/5/▪▪▪/DC1/.
Acknowledgments
This work was supported in part by NIH grants HL64272 (M.M.H.), HL75662 (I.T.), and DK47119 (D.R.).
References
- Ameen C, Edvardsson U, Ljungberg A, Asp L, Akerblad P, Tuneld A, Olofsson SO, Linden D, Oscarsson J. Activation of peroxisome proliferator-activated receptor alpha increases the expression and activity of microsomal triglyceride transfer protein in the liver. J Biol Chem. 2005;280:1224–1229. doi: 10.1074/jbc.M412107200. [DOI] [PubMed] [Google Scholar]
- Anwar K, Iqbal J, Hussain MM. Mechanisms involved in vitamin E transport by primary enterocytes and in vivo absorption. J Lipid Res. 2007;48:2028–2038. doi: 10.1194/jlr.M700207-JLR200. [DOI] [PubMed] [Google Scholar]
- Athar H, Iqbal J, Jiang XC, Hussain MM. A simple, rapid, and sensitive fluorescence assay for microsomal triglyceride transfer protein. J Lipid Res. 2004;45:764–772. doi: 10.1194/jlr.D300026-JLR200. [DOI] [PubMed] [Google Scholar]
- Bernales S, Papa FR, Walter P. Intracellular signaling by the unfolded protein response. Annu Rev Cell Dev Biol. 2006;22:487–508. doi: 10.1146/annurev.cellbio.21.122303.120200. [DOI] [PubMed] [Google Scholar]
- Berriot-Varoqueaux N, Aggerbeck LP, Samson-Bouma M, Wetterau JR. The role of the microsomal triglyceride transfer protein in abetali-poproteinemia. Annu Rev Nutr. 2000;20:663–697. doi: 10.1146/annurev.nutr.20.1.663. [DOI] [PubMed] [Google Scholar]
- Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol. 2000;2:326–332. doi: 10.1038/35014014. [DOI] [PubMed] [Google Scholar]
- Bertolotti A, Wang X, Novoa I, Jungreis R, Schlessinger K, Cho JH, West AB, Ron D. Increased sensitivity to dextran sodium sulfate colitis in IRE1beta-deficient mice. J Clin Invest. 2001;107:585–593. doi: 10.1172/JCI11476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP, Clark SG, Ron D. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature. 2002;415:92–96. doi: 10.1038/415092a. [DOI] [PubMed] [Google Scholar]
- Cuchel M, Bloedon LT, Szapary PO, Kolansky DM, Wolfe ML, Sarkis A, Millar JS, Ikewaki K, Siegelman ES, Gregg RE, Rader DJ. Inhibition of microsomal triglyceride transfer protein in familial hypercholesterolemia. N Engl J Med. 2007;356:148–156. doi: 10.1056/NEJMoa061189. [DOI] [PubMed] [Google Scholar]
- Di Leo E, Lancellotti S, Penacchioni JY, Cefalu AB, Averna M, Pisciotta L, Bertolini S, Calandra S, Gabelli C, Tarugi P. Mutations in MTP gene in abeta- and hypobeta-lipoproteinemia. Atherosclerosis. 2005;180:311–318. doi: 10.1016/j.atherosclerosis.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Dougan SK, Rava P, Hussain MM, Blumberg RS. MTP regulated by an alternate promoter is essential for NKT cell development. J Exp Med. 2007;204:533–545. doi: 10.1084/jem.20062006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon DA, Jamil H, Sharp D, Mullaney D, Yao Z, Gregg RE, Wetterau J. Secretion of apolipoprotein B-containing lipoproteins from HeLa cells is dependent on expression of the microsomal triglyceride transfer protein and is regulated by lipid availability. Proc Natl Acad Sci USA. 1994;91:7628–7632. doi: 10.1073/pnas.91.16.7628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan DL, Kienzle B, Jamil H, Hariharan N. Transcriptional regulation of human and hamster microsomal triglyceride transfer protein genes. Cell type-specific expression and response to metabolic regulators. J Biol Chem. 1994;269:28737–28744. [PubMed] [Google Scholar]
- Hirokane H, Nakahara M, Tachibana S, Shimizu M, Sato R. Bile acid reduces the secretion of very low density lipoprotein by repressing microsomal triglyceride transfer protein gene expression mediated by hepatocyte nuclear factor-4. J Biol Chem. 2004;279:45685–45692. doi: 10.1074/jbc.M404255200. [DOI] [PubMed] [Google Scholar]
- Hollien J, Weissman JS. Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science. 2006;313:104–107. doi: 10.1126/science.1129631. [DOI] [PubMed] [Google Scholar]
- Houseley J, LaCava J, Tollervey D. RNA-quality control by the exosome. Nat Rev Mol Cell Biol. 2006;7:529–539. doi: 10.1038/nrm1964. [DOI] [PubMed] [Google Scholar]
- Hussain MM. A proposed model for the assembly of chylomicrons. Atherosclerosis. 2000;148:1–15. doi: 10.1016/s0021-9150(99)00397-4. [DOI] [PubMed] [Google Scholar]
- Hussain MM, Kancha RK, Zhou Z, Luchoomun J, Zu H, Bakillah A. Chylomicron assembly and catabolism: role of apolipoproteins and receptors. Biochim Biophys Acta. 1996;1300:151–170. doi: 10.1016/0005-2760(96)00041-0. [DOI] [PubMed] [Google Scholar]
- Hussain MM, Iqbal J, Anwar K, Rava P, Dai K. Microsomal triglyceride transfer protein: a multifunctional protein. Front Biosci. 2003a;8:S500–S506. doi: 10.2741/1071. [DOI] [PubMed] [Google Scholar]
- Hussain MM, Shi J, Dreizen P. Microsomal triglyceride transfer protein and its role in apolipoprotein B-lipoprotein assembly. J Lipid Res. 2003b;44:22–32. doi: 10.1194/jlr.r200014-jlr200. [DOI] [PubMed] [Google Scholar]
- Hussain MM, Fatma S, Pan X, Iqbal J. Intestinal lipoprotein assembly. Curr Opin Lipidol. 2005;16:281–285. doi: 10.1097/01.mol.0000169347.53568.5a. [DOI] [PubMed] [Google Scholar]
- Iqbal J, Hussain MM. Evidence for multiple complementary pathways for efficient cholesterol absorption in mice. J Lipid Res. 2005;46:1491–1501. doi: 10.1194/jlr.M500023-JLR200. [DOI] [PubMed] [Google Scholar]
- Iqbal J, Anwar K, Hussain MM. Multiple, independently regulated pathways of cholesterol transport across the intestinal epithelial cells. J Biol Chem. 2003;278:31610–31620. doi: 10.1074/jbc.M301177200. [DOI] [PubMed] [Google Scholar]
- Iwawaki T, Hosoda A, Okuda T, Kamigori Y, Nomura-Furuwatari C, Kimata Y, Tsuru A, Kohno K. Translational control by the ER transmembrane kinase/ribonuclease IRE1 under ER stress. Nat Cell Biol. 2001;3:158–164. doi: 10.1038/35055065. [DOI] [PubMed] [Google Scholar]
- Kang S, Spann NJ, Hui TY, Davis RA. ARP-1/COUP-TF II determines hepatoma phenotype by acting as both a transcriptional repressor of microsomal triglyceride transfer protein and an inducer of CYP7A1. J Biol Chem. 2003;278:30478–30486. doi: 10.1074/jbc.M304201200. [DOI] [PubMed] [Google Scholar]
- Leiper JM, Bayliss JD, Pease RJ, Brett DJ, Scott J, Shoulders CC. Microsomal triglyceride transfer protein, the abetalipoproteinemia gene product, mediates the secretion of apolipoprotein B-containing lipoproteins from heterologous cells. J Biol Chem. 1994;269:21951–21954. [PubMed] [Google Scholar]
- Leung GK, Veniant MM, Kim SK, Zlot CH, Raabe M, Bjorkegren J, Neese RA, Hellerstein MK, Young SG. A deficiency of microsomal triglyceride transfer protein reduces apolipoprotein B secretion. J Biol Chem. 2000;275:7515–7520. doi: 10.1074/jbc.275.11.7515. [DOI] [PubMed] [Google Scholar]
- Lin MCM, Arbeeny C, Bergquist K, Kienzle B, Gordon DA, Wetterau JR. Cloning and regulation of hamster microsomal triglyceride transfer protein. The regulation is independent from that of other hepatic and intestinal proteins which participate in the transport of fatty acids and triglycerides. J Biol Chem. 1994;269:29138–29145. [PubMed] [Google Scholar]
- Lipson KL, Fonseca SG, Ishigaki S, Nguyen LX, Foss E, Bortell R, Rossini AA, Urano F. Regulation of insulin biosynthesis in pancreatic beta cells by an endoplasmic reticulum-resident protein kinase IRE1. Cell Metab. 2006;4:245–254. doi: 10.1016/j.cmet.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Luchoomun J, Hussain MM. Assembly and secretion of chylomicrons by differentiated Caco-2 cells. Nascent triglycerides and preformed phospholipids are preferentially used for lipoprotein assembly. J Biol Chem. 1999;274:19565–19572. doi: 10.1074/jbc.274.28.19565. [DOI] [PubMed] [Google Scholar]
- Meyer S, Temme C, Wahle E. Messenger RNA turnover in eukaryotes: pathways and enzymes. Crit Rev Biochem Mol Biol. 2004;39:197–216. doi: 10.1080/10409230490513991. [DOI] [PubMed] [Google Scholar]
- Millar JS, Cromley DA, McCoy MG, Rader DJ, Billheimer JT. Determining hepatic triglyceride production in mice: comparison of poloxamer 407 with Triton WR-1339. J Lipid Res. 2005;46:2023–2028. doi: 10.1194/jlr.D500019-JLR200. [DOI] [PubMed] [Google Scholar]
- Napoli C, Martin-Padura I, de Nigris F, Giorgio M, Mansueto G, Somma P, Condorelli M, Sica G, De Rosa G, Pelicci P. Deletion of the p66Shc longevity gene reduces systemic and tissue oxidative stress, vascular cell apoptosis, and early atherogenesis in mice fed a high-fat diet. Proc Natl Acad Sci USA. 2003;100:2112–2116. doi: 10.1073/pnas.0336359100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narcisi TM, Shoulders CC, Chester SA, Read J, Brett DJ, Harrison GB, Grantham TT, Fox MF, Povey S, de Bruin TW, et al. Mutations of the microsomal triglyceride-transfer-protein gene in abetalipo-proteinemia. Am J Hum Genet. 1995;57:1298–1310. [PMC free article] [PubMed] [Google Scholar]
- Newbury SF. Control of mRNA stability in eukaryotes. Biochem Soc Trans. 2006;34:30–34. doi: 10.1042/BST20060030. [DOI] [PubMed] [Google Scholar]
- Niwa M, Sidrauski C, Kaufman RJ, Walter P. A role for presenilin-1 in nuclear accumulation of Ire1 fragments and induction of the mammalian unfolded protein response. Cell. 1999;99:691–702. doi: 10.1016/s0092-8674(00)81667-0. [DOI] [PubMed] [Google Scholar]
- Raabe M, Flynn LM, Zlot CH, Wong JS, Véniant MM, Hamilton RL, Young SG. Knockout of the abetalipoproteinemia gene in mice: reduced lipoprotein secretion in heterozygotes and embryonic lethality in homozygotes. Proc Natl Acad Sci USA. 1998;95:8686–8691. doi: 10.1073/pnas.95.15.8686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rava P, Hussain MM. Acquisition of triacylglycerol transfer activity by microsomal triglyceride transfer protein during evolution. Biochemistry. 2007;46:12263–12274. doi: 10.1021/bi700762z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rava P, Athar H, Johnson C, Hussain MM. Transfer of cholesteryl esters and phospholipids as well as net deposition by microsomal triglyceride transfer protein. J Lipid Res. 2005;46:1779–1785. doi: 10.1194/jlr.D400043-JLR200. [DOI] [PubMed] [Google Scholar]
- Rava P, Ojakian GK, Shelness GS, Hussain MM. Phospholipid transfer activity of microsomal triacylglycerol transfer protein is sufficient for the assembly and secretion of apolipoprotein B lipoproteins. J Biol Chem. 2006;281:11019–11027. doi: 10.1074/jbc.M512823200. [DOI] [PubMed] [Google Scholar]
- Schwarz M, Davis DL, Vick BR, Russell DW. Genetic analysis of cholesterol accumulation in inbred mice. J Lipid Res. 2001;42:1812–1819. [PubMed] [Google Scholar]
- Sellers JA, Hou L, Athar H, Hussain MM, Shelness GS. A Drosophila microsomal triglyceride transfer protein homolog promotes the assembly and secretion of human apolipoprotein B. Implications for human and insect lipid transport and metabolism. J Biol Chem. 2003;278:20367–20373. doi: 10.1074/jbc.M300271200. [DOI] [PubMed] [Google Scholar]
- Tietge UJF, Bakillah A, Maugeais C, Tsukamoto K, Hussain MM, Rader DJ. Hepatic overexpression of microsomal triglyceride transfer protein (MTP) results in increased in vivo secretion of VLDL triglycerides and apolipoprotein B. J Lipid Res. 1999;40:2134–2139. [PubMed] [Google Scholar]
- Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, Ron D. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–666. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- Wang XZ, Harding HP, Zhang Y, Jolicoeur EM, Kuroda M, Ron D. Cloning of mammalian Ire1 reveals diversity in the ER stress responses. EMBO J. 1998;17:5708–5717. doi: 10.1093/emboj/17.19.5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetterau JR, Gregg RE, Harrity TW, Arbeeny C, Cap M, Conolly F, Chu CH, George RJ, Gordon DA, Jamil H, et al. An MTP inhibitor that normalizes atherogenic lipoprotein levels in WHHL rabbits. Science. 1998;282:751–754. doi: 10.1126/science.282.5389.751. [DOI] [PubMed] [Google Scholar]
- Wolfrum C, Stoffel M. Coactivation of Foxa2 through Pgc-1beta promotes liver fatty acid oxidation and triglyceride/VLDL secretion. Cell Metab. 2006;3:99–110. doi: 10.1016/j.cmet.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107:881–891. doi: 10.1016/s0092-8674(01)00611-0. [DOI] [PubMed] [Google Scholar]
- Zhang K, Wong HN, Song B, Miller CN, Scheuner D, Kaufman RJ. The unfolded protein response sensor IRE1alpha is required at 2 distinct steps in B cell lymphopoiesis. J Clin Invest. 2005;115:268–281. doi: 10.1172/JCI21848. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPLEMENTAL DATA
Supplemental Data include four figures, one table, and Supplemental References and can be found with this article online at http://www.cellmetabolism.org/cgi/content/full/7/5/▪▪▪/DC1/.