Abstract
To turn a disease from highly fatal to highly curable is extremely difficult, especially when the disease is a type of cancer. However, we can gain some insight into how this can be done by looking back over the 50-year history of taming acute promyelocytic leukaemia (APL). APL is the M3 type of acute myeloid leukaemia characterized by an accumulation of abnormal promyelocytes in bone marrow, a severe bleeding tendency and the presence of the chromosomal translocation t(15;17) or variants. APL was considered the most fatal type of acute leukaemia five decades ago and the treatment of APL was a nightmare for physicians. Great efforts have been made by scientists worldwide to conquer this disease. The first use of chemotherapy (CT) was unsuccessful due to lack of supportive care and cytotoxic-agent-related exacerbated coagulopathy. The first breakthrough came from the use of anthracyclines which improved the complete remission (CR) rate, though the 5-year overall survival could only be attained in a small proportion of patients. A rational and intriguing hypothesis, to induce differentiation of APL cells rather than killing them, was raised in the 1970s. Laudably, the use of all-trans retinoic acid (ATRA) in treating APL resulted in terminal differentiation of APL cells and a 90–95% CR rate of patients, turning differentiation therapy in cancer treatment from hypothesis to practice. The combination of ATRA with CT further improved the 5-year overall survival. When arsenic trioxide (ATO) was used to treat relapsed APL not only the patients but also the ancient drug were revived. ATO exerts dose-dependent dual effects on APL cells: at low concentration, ATO induces partial differentiation, while at relatively high concentration, it triggers apoptosis. Of note, both ATRA and ATO trigger catabolism of the PML–RARα fusion protein which is the key player in APL leukaemogenesis generated from t(15;17), targeting the RARα (retinoic acid receptor α) or promyelocytic leukaemia (PML) moieties, respectively. Hence, in treating APL both ATRA and ATO represent paradigms for molecularly targeted therapy. At molecular level, ATRA and ATO synergistically modulate multiple downstream pathways/cascades. Strikingly, a clearance of PML–RARα transcript in an earlier and more thorough manner, and a higher quality remission and survival in newly diagnosed APL are achieved when ATRA is combined with ATO, as compared to either monotherapy, making APL a curable disease. Thus, the story of APL can serve as a model for the development of curative approaches for disease; it suggests that molecularly synergistic targeted therapies are powerful tools in cancer, and dissection of disease pathogenesis or anatomy of the cancer genome is critical in developing molecular target-based therapies.
Keywords: acute promyelocytic leukaemia, all-trans retinoic acid, differentiation, arsenic trioxide, apoptosis, synergy
1. Introduction
Leukaemia represents a group of haematological malignancies characterized by clonal expansion of haematopoietic cells with uncontrolled proliferation, decreased apoptosis and blocked differentiation. This group of diseases ranked fifth for male mortality and sixth for female among all human cancers, and is the number one cancer killer in young people, with some 300 000 new cases and 222 000 deaths each year worldwide (Ahn et al. 1991; Yang & Zhang 1991; Jemal et al. 2005; Parkin et al. 2005). According to the disease progression and haematopoietic lineages involved (Bennett et al. 1985), leukaemia can be divided into acute or chronic, lymphoid or myeloid types, with a number of subtypes further classified based on distinct stages of differentiation block along with each lineage. Importantly, the responses of leukaemia to therapies differ from one type or subtype to another, rendering the disease pathogenesis-based and individualized therapeutic strategies extremely important. Translational research across bench and bedside may not only shed new light on leukaemogenesis and gain insights into therapeutic mechanisms, but also provide opportunities for designing more sophisticated therapeutic protocols, as highlighted by the development of curative approaches for acute promyelocytic leukaemia (APL).
2. Acute promyelocytic leukaemia as a unique subtype of acute myeloid leukaemia
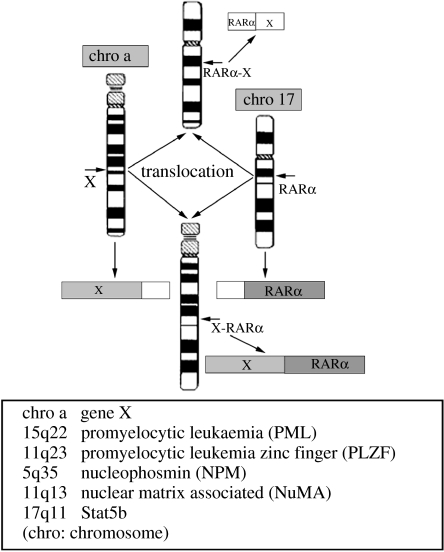
APL as an independent clinical entity was described for the first time by a Swedish physician, Leif Hillestad, after a careful review of a group of his own cases as well as others reported in the literature (Hillestad 1957). More detailed features of APL were then described by Bernard et al. (1959) and Caen et al. (1957; box 1). Owing to the very rapid natural course of the disease and its severe complications such as the bleeding syndrome, APL was considered as ‘the most malignant form of acute leukaemia’. In 1976, the French–American–British Nomenclature Committee assigned APL as the M3 subtype of acute myeloid leukaemia (AML M3), based on the unique morphology of promyelocytes in the bone marrow (Bennett et al. 1976). Thereafter, two variants of APL, the hypogranular variant (Bennett et al. 1980) and the hyperbasophilic microgranular variant (McKenna et al. 1982), were also reported. In the same year, the characteristic phenotype of APL was linked to a specific chromosomal marker, the balanced reciprocal translocation between the long arms of chromosomes 15 and 17 (t(15;17)(q22;q21)) identified by Rowley et al. (1977), which was present in 98% of APL patient samples. Interestingly, 17q21 was subsequently shown to be involved in all variant chromosomal translocations of APL, including t(11;17)(q23;q21) (Chen et al. 1993a), t(5;17)(q35;q21) (Corey et al. 1994), t(11;17)(q13;q21) (Wells et al. 1996) and dup(17)(q11;q21) (Arnould et al. 1999; figure 1), suggesting that a locus in this region was important for normal haemopoiesis and its disruption crucial for disease pathogenesis of APL. To date, APL has been shown to be characterized by three features (Zhou et al. 2005): the accumulation of abnormal promyelocytes in bone marrow; the occurrence of fibrinogenopenia and disseminated intravascular coagulation that is often worsened by chemotherapy (CT); and the presence of the chromosomal translocation t(15;17)(q22;q21) or variants.
Box 1. Important discoveries in dissecting and taming APL.
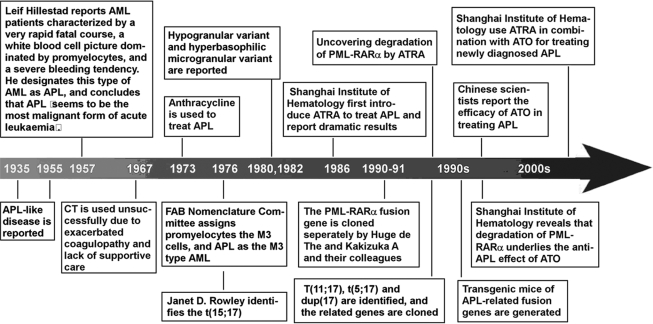
Figure 1.
Chromosomal translocations in APL.
3. Development of curative therapeutic agents for acute promyelocytic leukaemia
The second half of the twentieth century had witnessed the successive emergence of three types of therapeutic agents for APL. From the 1960s to mid-1980s, CT was the only available treatment for APL, with significantly improved clinical response after the introduction of anthracyclines and supportive care in the 1970s. The discovery of the clinical efficacy of all-trans retinoic acid (ATRA) by Chinese haematologists in the mid-1980s turned a new page in the history of leukaemia therapy. ATRA is a derivative of vitamin A and its application has dramatically augmented the complete remission (CR) and long-term survival rates of APL patients (Huang et al. 1988). In the mid-1990s, the first controlled clinical trial of arsenic trioxide (ATO) further improved the clinical outcome of refractory or relapsed APL (Sun et al. 1992; Shen et al. 1997; box 1).
(a) Chemotherapeutic agents
The clinical management of APL in the first decade after its formal recognition was recorded as a nightmare for physicians owing to its unpredictable onset of life-threatening bleeding disorders (Degos 2003). CT was first used against APL in 1967 but its efficacy turned out to be unsatisfactory. At that time, there was a shortage of proper supportive care and the cytotoxic drugs used as induction treatment often exacerbated coagulopathy, with approximately 10–30% of patients dying due to haemorrhage (Drapkin et al. 1978). However, an important observation was made by Bernard and his colleagues in 1973 (Bernard et al. 1973) that APL appeared to be particularly sensitive to anthracyclines. Subsequently, the use of anthracyclines such as daunorubicin and appropriate management of the APL-related coagulopathy were proven to be effective in improving the CR rate (55–80%) during the 1980s (Slack et al. 2002; Tallman et al. 2002; Degos 2003). However, even with consolidation and maintenance therapy, the median duration of CR was no more than 1–2 years, with only 20–35% of patients reaching a 5-year disease-free survival under CT treatment alone. The remaining patients died from haemorrhage, relapse or refractory disease (Chan et al. 1981; Cordonnier et al. 1985; Kantarjian et al. 1986; Sanz et al. 1988).
(b) Treatment of acute promyelocytic leukaemia with all-trans retinoic acid: the first example of differentiation induction therapy of human cancer
For a long time, the central dogma in leukaemia therapy was to inhibit malignant cell proliferation or to kill them by using cytotoxic agents such as CT and/or radiotherapy. However, the fact that differentiation arrest, e.g. the accumulation of APL blasts blocked at the promyelocytic stage of granulocytic differentiation, was one of the major features of human cancer, suggested the possibility of inducing cell differentiation as an alternative way to treat leukaemia. This notion also matched the philosophy of traditional Chinese medicine (TCM): transforming a bad element into a good one was better than simply eliminating the element. Nonetheless, to translate this idea into a treatment strategy represented a great challenge. A breakthrough was made in the 1970s by Sachs et al. (Paran et al. 1970; Fibach et al. 1973) was showed that myeloid leukaemic cells could be induced by cytokines to resume normal differentiation and become non-dividing mature granulocytes or macrophages. Based on this observation, Sachs (1978a,b) hypothesized that treatment with agents which induce leukaemic cells to complete differentiation could be a novel therapeutic strategy. As a continuation of this endeavour, Breitman et al. (1980, 1981) reported that retinoic acid (RA) could induce terminal differentiation of human APL cells in vitro. However, clinical trials of APL patients with 13-cis RA revealed quite unsatisfactory results (Runde et al. 1992; Warrell et al. 1993).
Our team from the Shanghai Institute of Hematology (SIH) started to undertake a series of experiments on differentiation therapy for leukaemia in 1980. Among a number of compounds screened, ATRA appeared to be a strong inducer of terminal differentiation in HL-60, a cell line with promyelocytic features, and in fresh leukaemic cells from APL patients. This intriguing result was the impetus for an in vivo clinical trial which was carried out in 1986. Dramatically, 23 out of 24 (95.8%) APL patients treated with ATRA at a dose of 45–100 mg m−2 d−1 went into CR without developing bone-marrow hypoplasia or abnormalities of clotting. The remaining patient achieved CR when CT was added. The most striking feature of the treatment was the gradual terminal differentiation of malignant cells in the bone marrow, as indicated by the presence of Auer rods in some mature granulocytes, followed by the re-emergence of normal haematopoietic cells when patients achieved remission (Huang et al. 1988). Hypothesis-oriented cancer differentiation therapy was therefore brought into practice for the first time. Subsequently, a great number of randomized studies from haematology/oncology centres around the world not only confirmed these results, but also showed improved rates of CR, decreased severe adverse effects and prolonged duration of remission (Warrell et al. 1993; Tallman et al. 1997; Fenaux et al. 2000; Wang 2003; Zhou et al. 2005). Trials combining ATRA with anthracycline-containing CT were soon initiated, and the results showed that an ATRA/CT combination could achieve CR in 90–95% of patients and 5-year disease-free survival (DFS) in 50–75% of cases (Slack et al. 2002; Tallman et al. 2002; Degos 2003; Wang 2003), an unprecedented result in the treatment of AML. However, one-third to half of patients still relapsed probably due to a selection of clones resistant to ATRA and CT.
(c) Arsenic trioxide: an ancient remedy revived due to highly effective and selective effects in acute promyelocytic leukaemia
To overcome the limitations of ATRA in relapsed or refractory patients, efforts were made by investigators worldwide to search for effective alternative therapies. Fortunately, great benefit was brought to these patients as well as to those newly diagnosed by application of ATO, a breakthrough also first reported in China. Arsenic is a common, natural substance that exists in organic and inorganic forms. The organic arsenicals consist of an arsenic atom in its trivalent or pentavalent state linked covalently to a carbon atom. There are three inorganic forms of arsenic: red arsenic (As4S4, also known as ‘realgar’), yellow arsenic (As2S3, also known as ‘orpiment’) and white arsenic, or arsenic trioxide (As2O3) which is made by burning realgar or orpiment (Zhu et al. 2002a).
For a long time, arsenic compounds were used in TCM as therapeutic agents for some severe diseases with the ancient philosophy of ‘treating an evil with a toxic’ (Li SH, 1578 in the Ming Dynasty of China, in Li 1982). Arsenic was also used to treat chronic myeloid leukaemia (CML) in the eighteenth and nineteenth centuries in Western countries, but was discarded as a treatment in the early twentieth century owing to its toxicity and the advent of radiation and cytotoxic CT. In the 1990s, Sun and his colleagues (Sun et al. 1992) showed that intravenous infusions of Ailing-1, a crude solution composed of 1% ATO with a trace amount of mercury chloride, induced CR in two-thirds of patients with APL with an impressive 10-year survival rate (30%). In 1996–1997, the SIH reported studies of pure ATO being used to treat relapsed APL (Chen et al. 1996, 1997; Shen et al. 1997). In these studies, 15 relapse patients who previously achieved CR by ATRA-containing treatment were intravenously administered with ATO at a dose of 0.16 mg kg−1 d−1 for 28–54 days. Clinical CR was achieved in 9 out of 10 (90%) patients treated with ATO alone and in 5 out of 5 patients treated with ATO combined with low-dose chemotherapeutic drugs or ATRA. During the treatment course, no bone-marrow depression or other severe side effects were encountered. The SIH also conducted the first pharmacokinetics study of ATO and found that the in vivo drug accumulation could be significantly reduced after the treatment courses (Shen et al. 1997). These results showed that ATO is an effective and relatively safe drug for APL patients refractory to ATRA and conventional CT.
Since 1996, a large number of reports have shown that arsenic compounds induce CR in 85–90% of patients with newly diagnosed or relapsed APL (Wang 2003). However, in relapsed patients after a new remission induced by ATO, the 2-year DFS was only 42% (Niu et al. 1999). Tetra-arsenic tetra-sulphide was also reported to be effective in APL treatment (Lu et al. 2002). Furthermore, after CR achieved by arsenic compounds, a molecular remission (i.e. negative for promyelocytic leukaemia–retinoic acid receptor α (PML–RARα) transcript detected by reverse transcriptase polymerase chain reaction (RT-PCR)) can be obtained either with arsenic compounds or with ATRA plus CT as a consolidation treatment. It seems that arsenic compounds appropriately used in post-remission therapy may prevent recurrence and allow a longer survival (Soignet et al. 2001; Lu et al. 2002; Wang 2003).
4. Mechanism of action of all-trans retinoic acid therapy in acute promyelocytic leukaemia
(a) Cellular mechanism of all-trans retinoic acid
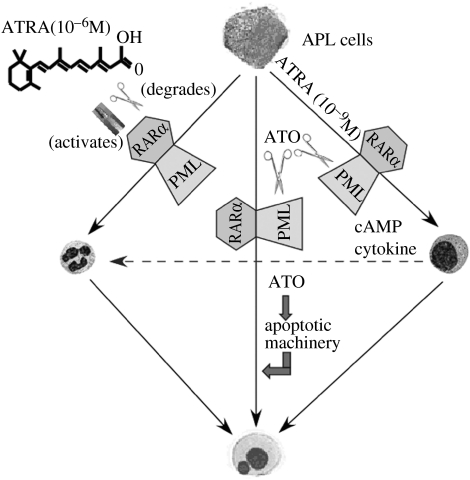
The action mode of ATRA appears to be quite different from that of CT. The persistence of t(15;17) in a large number of morphologically mature granulocytes during in vivo remission induction is a strong indicator that ATRA drives the differentiation of immature neoplastic cells into mature granulocytes (Huang et al. 1988; Castaigne et al. 1990; Warrell et al. 1991; Elliott et al. 1992). It has been assumed that ATRA acts on at least two stages of myeloid cell development: promyelocytes and earlier neoplastic progenitor cells which are capable of self-renewal but are already committed to the myeloid lineage (Warrell et al. 1993). After an irreversible commitment to differentiation is induced by ATRA, the maturing cells originating from the leukaemic clone eventually enter into programmed cell death (PCD; figure 2; Martin et al. 1990; Gianni et al. 2000; Altucci et al. 2001). Refined cell biology work on an ATRA-sensitive APL cell line NB4 and ATRA-resistant NB4 subclone NB4-R1 allowed the establishment of a two-step model for induction of APL cell differentiation (Ruchaud et al. 1994; Zhu et al. 2001). This model suggests that there are two discrete steps in the maturation process: an RA-dependent priming step that maintains proliferation while the cells become competent to undergo maturation in response to retinoids, and a cyclic adenosine monophosphate (cAMP)-dependent second step that triggers RA-primed cells to undergo terminal maturation. Further work showed that even endogenously presented physiological concentrations of ATRA (10−9–10−8 M) were able to induce terminal differentiation of APL cells in the presence of cAMP. The recent finding by the SIH that ATRA induces an immediate increase in the intracellular cAMP level and activation of the protein kinase A (PKA) pathway in APL cells after exposure to the drug, and this effect appears to be abrogated by PKA antagonists, suggests a coordinated activation mechanism between RA nuclear signalling and cell-membrane-associated cAMP/PKA signalling (Zhu et al. 2002b).
Figure 2.
The schematic represents induction of APL cell differentiation and apoptosis by ATRA and ATO. At pharmacological concentration (10−6 M), ATRA can induce degradation of the PML–RARα oncoprotein, leading to activation of repressed target genes. ATRA can also activate PML–RARα to recruit coactivators. A two-step model of promyelocytic differentiation is also shown. The first step is a priming for differentiation, presumably through derepression of PML/RARα repressed genes by ATRA. This can be bypassed by rexinoids. The second is maturation per se and can be induced by high doses of ATRA or differentiating agents such as cytokines or cAMP. ATO at low concentration induces partial differentiation of promyelocytes, while combined use of cAMP or cytokines can trigger terminal differentiation. The mature granulocytes can enter into programmed cell death. ATO can also induce apoptosis by targeting PML–RARα and activation of apoptotic machinery.
(b) Molecular mechanism of all-trans retinoic acid: PML–RARα as a key player in acute promyelocytic leukaemia leukaemogenesis
The molecular characterization of t(15;17) by de The and his colleagues and several other groups including the SIH in the early 1990s provided essential information on both APL pathogenesis and the molecular mechanism of ATRA therapy. It was demonstrated that one of the genes encoding a retinoic acid receptor (RAR), RARα, which is located on 17q21, was juxtaposed to the promyelocytic leukaemia (PML) gene on 15q21, resulting in the PML–RARα fusion gene. Owing to the heterogeneity of the chromosomal breakpoints on 15q22, distinct exon sets of PML can be fused to exon 2 of RARα to form long (PML exons 1–6), short (PML exons 1–3) or variant (PML exon 1 through to truncated exon 6) PML–RARα. The PML–RARα gene was soon considered not only a molecular marker for diagnosis and monitoring the minimal residual disease, but also, and more importantly, a major player in disturbing several pathways indispensable for promyelocytic differentiation. It has been well established that retinoids exert a wide range of physiological effects including those for haemopoiesis via their two families of nuclear receptor, RARs and retinoid X receptors (RXRs), each consisting of three subtypes, α, β and γ (Melnick & Licht 1999). Like other members of the nuclear receptor superfamily, RARs and RXRs function as ligand-inducible transcription factors. The active form of RARα is a protein complex with RXR, and the RAR/RXR heterodimer binds to the RA response element (RARE) located on the regulatory region of target genes. The RAR/RXR heterodimer transactivates gene expression in the presence of physiological concentrations of ligands, i.e. retinoids. It has been demonstrated that the receptor undergoes a configurational change upon retinoid binding, which allows the dissociation of a corepressor complex with histone deacetylase (HDAC) activity and the recruitment of a coactivator complex with histone acetylase activity. The PML–RARα chimeric protein acts as a dominant negative mutant over wild-type RARα in several ways. Unlike the wild-type receptor, the chimeric protein forms a homodimer and prevents activation of key RA target genes by sequestering proteins essential for normal RARα functions, such as RXR and other RARα cofactors. Moreover, the PML–RARα homodimer is able to bind to RARE, either on its own or with RXR, and thus recruit higher amounts of HDAC complexes with a higher affinity to repress transcription (Melnick & Licht 1999). Interestingly, this recruitment of corepressor complex can be mediated through both RARα and PML moieties of the chimeric protein; it was recently reported that the K160 sumoylation site in the PML moiety is responsible for the binding of a potent repressor, Daxx, which seems to be required for PML–RARα transforming activity (Zhu et al. 2005). On the other hand, PML–RARα interferes with the normal function of PML, a protein with an essential role in growth suppression and apoptosis. PML is a tumour suppressor characterised by a multiprotein nuclear structure, the PML oncogenic domain or PML–nuclear body (PML-NB), a doughtnut-shaped macromolecular structure of approximately 0.2–1.0 μm. Cells typically contain 10–30 of these macromolecular structures. Cytoplasmic PML is a critical transforming growth factor β (TGFβ) regulator (Lin et al. 2004). It becomes apparent that PML and the PML–NB act as molecular hubs for controlling apoptosis in a protein level-dependent manner (Jensen et al. 2001; Salomoni & Pandolfi 2002; Bernardi & Pandolfi 2003). At lower levels, PML is essential for the proper function of proapoptotic transcription factors, ultimately leading to caspase activation, while at higher levels PML may trigger apoptosis independent of transcription or caspase activation through protein sequestration into the PML–NB (Quignon et al. 1998; Salomoni & Pandolfi 2002). PML also regulates cell proliferation and senescence. In APL, PML–RARα delocalizes PML into aberrant microspeckled nuclear structures through physical association, leading to disruption of the PML–NB (Melnick & Licht 1999) and inhibition of cytoplasmic PML function (Lin et al. 2004). PML–RARα may affect transcription in other pathways including those in which the transcription factor AP1 and interferon (IFN)-responsive factors are involved. PML–RARα also binds to promyelocytic leukaemia zinc finger (PLZF) protein and potentially affects its functions (e.g. growth suppression, transcription repression and control of developmental programme and differentiation; Melnick & Licht 1999; Zhou et al. 2005). Recently, it was reported that PML–RARα was cleaved in several positions by neutrophil elastase (NE) which was produced at maximal levels in promyelocytes. Interestingly, NE-mediated cleavage of PML–RARα may alter its activity and is important for the development of APL in mice (Lane & Ley 2003). This result, together with the fact that PML is also involved in transcriptional repression by interacting with Daxx at the residue K160 sumoylation site, could explain why PML is the most common fusion partner of RARα in APL. Definite evidence of the leukaemogenic effect of PML–RARα has been provided by studies in transgenic mice in which APL is induced by the fusion gene (Brown et al. 1997; Grisolano et al. 1997; He et al. 1997). Detailed analysis using this platform revealed that dimerization of PML–RARα was essential for the transformation (Kogan et al. 2000). Moreover, it appears that PML–RARα may cooperate with activated mutations in protein tyrosine kinases, such as FLT3 (Shih et al. 2003), which confer proliferative and/or survival advantage to haematopoietic stem/progenitor cells. FLT3 mutations were detected in a sizable portion of patients with APL, particularly those with short-form PML–RARα. While PML–RARα alone induces APL-like disease in mice with a long latency and low penetrance (15–30%), FLT3/PML–RARα coexpression results in a short latency APL-like disease with complete penetrance (Kelly et al. 2002; Sohal et al. 2003).
Similar to PML–RARα, fusion genes resulting from variant chromosomal translocations in APL also encode chimeric proteins capable of dimerization, functioning as double-edged swords to interfere with the signalling pathways of both RA and its partner protein (gene X, figure 1), and induce leukaemia phenotypes in transgenic animals. For example, PLZF–RARα chimeric proteins generated by t(11;17)(q23;q21) (Chen et al. 1993a,b) can bind as homodimers to RAREs (Melnick & Licht 1999) and act in a dominant negative manner to inhibit the activity of wild-type RARα (Melnick & Licht 1999). It is noteworthy that PLZF–RARα homodimers bind to a direct repeat of the GGGTCA sequence separated by 5 bp (DR5G) at similar levels as PML–RARα, but the binding affinity is stronger than that of PML–RARα to the repeat of the GGTTCA sequence (Dr5T; Dong et al. 1996; Melnick & Licht 1999). Although it is possible that PLZF–RARα homodimers display altered target-gene specificity, in the presence of RXR the PLZF–RARα/RXR heterodimer binds to RAREs in vitro with a higher affinity than PLZF–RARα homodimers (Licht et al. 1996). PLZF–RARα interacts with corepressors SMRT, NCoR, Sin3A and HDAC 1, both in vitro and in vivo, and thereby may cause a deeper repression of target gene expression. Of note, APL patients with t(11;17) exhibit resistance to the differentiation-inducing effect of ATRA while PLZF–RARα alone induces an early onset leukaemia with a disease phenotype reminiscent of CML in transgenic mice. Contrary to PLZF–RARα, fusion genes generated by other variant translocations have been shown to be linked to an ATRA-sensitive phenotype (Melnick & Licht 1999).
(c) All-trans retinoic acid-induced catabolism of PML–RARα as the basic mechanism in acute promyelocytic leukaemia cell differentiation
PML–RARα is a ‘drugable’ target of ATRA (Raelson et al. 1996). Under the pharmacological concentration of ATRA (10−7–10−6 M), configurational modulation of the PML–RARα homodimer caused release of corepressors and HDAC, and recruitment of coactivator complex, resulting in relief of transcriptional repression. While this model needs further exploration, particularly with regard to the modulation of the PML-binding site of Daxx, it gets support from the clinical and experimental data of APL with t(11;17) and PLZF–RARα chimeric protein. It has been shown that though the interaction with corepressors and HDAC of the RARα moiety in PLZF–RARα could be modulated by 10−6 M ATRA, binding of the PLZF moiety to the corepressor complex on the N-terminal POZ domain was retained even under a very high ligand concentration (10−5 M). As a result, ATRA alone cannot induce maturation of PLZF–RARα-harbouring cells; HDAC inhibitor is required to cooperate with ATRA to induce differentiation of these cells (Melnick & Licht 1999). More recently, proteolysis of PML–RARα via different pathways has drawn much attention. Although it was reported that ATRA could trigger a caspases-mediated cleavage of the PML–RARα chimeric protein on PML (Nervi et al. 1998), further dissection of the pathways involved in PML–RARα catabolism led to the discovery of a ubiquitin/proteasome system (UPS)-mediated degradation of PML–RARα and RARα, which was dependent on the binding of SUG-1 in the AF2 transactivation domain of RARα with the 19S proteasome (vom Baur et al. 1996; Brown et al. 1997). The degradation of PML–RARα contributes to the restoration of normal retinoid signalling and PML–NB functions, although this event seems to occur relatively late compared to the modulation of chimeric protein's trans-regulatory activity.
ATRA also induces cAMP, a differentiation enhancer that boosts transcriptional activation, reverses the silencing of the transactivating function of RXR, restores ATRA-triggered differentiation in mutant ATRA-resistant cells (Kamashev et al. 2004) and inhibits cell growth by modulating several major players in G(1)/S transition regulation. Although the precise mechanism of this induction is not yet understood, the downstream PKA activity is able to phosphorylate RARα, and probably also PML–RARα, and modulate the receptor's interaction with corepressor and coactivator complexes. Therefore, the final effect of the modulation/degradation of either PML–RARα or wild-type RARα as hormone-inducible transcription factors should be translated into a profound change of the cellular transcriptome. Indeed, systems analysis of transcriptome and proteome in ATRA-induced APL cell differentiation reveals: induction of an array of transcription factors and cofactors, activation of calcium signalling, stimulation of the IFN pathway, activation of the UPS necessary for degradation of PML–RARα and restoration of the PML–NB, cell-cycle arrest, induction of cyclooxygenase 1 (Rocca et al. 2004), inhibition of angiogenesis (Kini et al. 2001), downregulation of tissue factors (Zhu et al. 1999a) and gain of apoptotic potential. At the SIH, a number of novel genes were cloned and designated as RA-induced genes (RIGs, such as RIG-G, E, K and I) with very interesting functional features (Liu et al. 2000). Consequently, the abnormal promyelocytes differentiate terminally and die through PCD.
5. Mechanism of action of arsenic trioxide therapy in acute promyelocytic leukaemia
(a) Cellular mechanism of arsenic trioxide
The fact that ATO is effective in APL resistant to ATRA suggests that the compound may target the same primordial disease mechanism but in a distinct way. Indeed, ATO exerts dose-dependent dual effects on APL cell cultures, triggering apoptosis at relatively high concentrations (0.5–2×10−6 M) and inducing partial differentiation at low concentrations (0.1–0.5×10−6 M; Chen et al. 1997). In line with these in vitro data, clinical response of APL to ATO is associated with incomplete cytodifferentiation and the induction of apoptosis with caspase activation in leukaemic cells (Shen et al. 1997; Soignet et al. 1998). It has been well established that ATO binds to adjacent -SH groups of cysteins in cellular proteins to form a five-member ring structure and many of the effects of ATO depend on the redox status of the cells. In APL cells, some major actions of ATO, such as induction of apoptosis, can be prevented by pre-treatment with -SH-group reducing agents while cotreatment with -SH-group oxidants enhances the effects of ATO (Miller et al. 2002).
(b) Molecular mechanisms of arsenic trioxide
Examination of the PML protein sequence indicated the presence of a cysteine-rich region that could be the principal candidate for interaction with trivalent arsenic. Interestingly, ATO at 0.1–2×10−6 M can induce the modulation and catabolism of PML–RARα proteins, but with a pattern and a kinetics distinguishable from those induced by 10−6 M ATRA. Hence, within 24 hours of ATO treatment, APL cells experience a series of changes, including reaggregation of PML–NB antigens, recruitment of PML–RARα proteins onto NBs and degradation of PML–RARα (Zhu et al. 1997). That ATO targets the PML moiety of PML–RARα is supported by the observation that a similar modulation process of wild-type PML, but not RARα, also occurs in APL or non-APL cells. Through a yet unknown mechanism, ATO causes PML to be located at the nuclear matrix and sumoylated at two important residues with different consequences: sumolyation at K160 is necessary for 11S proteasome recruitment and subsequent ATO-induced degradation and sumolyation at K490 is necessary for nuclear localization (Muller et al. 1998; Lallemand-Breitenbach et al. 2001). Recently, Hayakawa & Privalsky (2004) reported that ATO treatment also induced phosphorylation of the PML protein through a mitogen-activated protein (MAP) kinase pathway.
With regard to the mechanisms underlying ATO-triggered APL cell apoptosis, a number of events can be important: downregulation of Bcl-2 (Chen et al. 1996) which cooperates with PML–RARα to block neutrophil differentiation (Kogan et al. 2001), collapse of mitochondrial transmembrane potentials (MTP) in a thiol-dependent manner (Zhu et al. 1999b; Chen et al. 2001), activation of caspases (Soignet et al. 1998; Huang et al. 1999) and modulation of PML (Zhu et al. 1997). Interestingly, increased PML phosphorylation seems to be associated with increased sumoylation of PML and increased PML-mediated apoptosis. Conversely, MAP-kinase-cascade inhibitors, the introduction of phosphorylation or sumoylation-defective mutations of PML impair ATO-mediated apoptosis. Thus, phosphorylation by MAP kinase cascades and potentiates the antiproliferative functions of PML and helps to mediate the proapoptotic effects of ATO. On the other hand, though ATO is able to promote APL cell differentiation, its mechanism of action is obviously different from that of ATRA. ATO induces a partial differentiation whereas ATRA results in granulocyte maturation. Moreover, ATO has no significant influence, in contrast to ATRA, on the trans-regulatory properties of either PML–RARα or RARα. To this end, a surprising finding was made by the SIH that the differentiation-inducing effect of ATO on APL cells could be greatly potentiated by cAMP and that the treatment of NB4 cells with both the agents achieved terminal differentiation. Hence, a scenario in agreement with the previously mentioned two-step model of APL cell differentiation can be proposed (Ruchaud et al. 1994; Zhu et al. 2001). The effect of ATO may correspond to a priming process when a critical set of target genes repressed by PML–RARα is derepressed owing to rapid degradation of the chimeric protein. However, ATO treatment alone may not be able to provide a situation where a high enough expression level of these genes or a cross-talk with other important pathways could occur, as in the case of ATRA treatment. Instead, the expression of these genes only reaches an intermediate or low level. As a result, the primed cells will only undergo partial differentiation and then quickly enter into apoptosis. Nevertheless, a second-step activation of the essential pathways or networks by cAMP, cytokines or HDAC inhibitors will ensure a full differentiation. Evidence further supporting this scenario came from a recent systems analysis of the transcriptome of ATO-induced APL cell differentiation/apoptosis (Zheng et al. 2005) showing that many ATRA-regulated genes were also regulated by ATO, but at a much lower level.
6. Incorporation of effective therapies: systems-biology-based synergistic targeting that makes acute promyelocytic leukaemia a curable disease
Although the remarkable contribution of ATRA or ATO to the induction and maintenance of CR in APL were well established by the end of the twentieth century, haematologists/oncologists were still facing a great challenge at the beginning of the new millennium: was it possible to reach the goal of making APL a curable disease in the great majority of cases by taking advantage of the ‘triad’ tools? As discussed previously, evidence derived from multi-lines suggests treatment regimen containing agents against different targets, or the same targets but through different mechanisms, may confer a superior outcome. Indeed, ATRA integrated with CT yields a higher CR rate and a longer overall survival (Slack et al. 2002; Tallman et al. 2002; Degos 2003; Wang 2003; Zhou et al. 2005). To this end, exploring the complexity of APL pathogenesis and its mechanisms of differentiation/apoptosis may lead to a workable selection of therapeutic approaches.
(a) Rationale for all-trans retinoic acid/arsenic trioxide combination based on promising pre-clinical studies
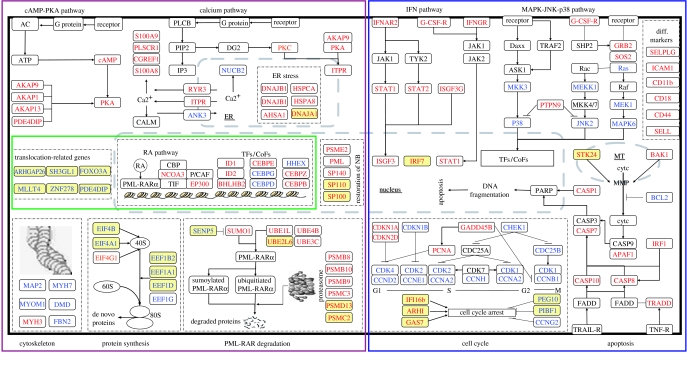
An intriguing question was raised in a discussion at the SIH in 2000: could synergistic effects be attained when ATRA and ATO are combined to treat newly diagnosed APL so as to further increase the 5-year DFS? The striking common feature of the two otherwise unrelated agents is the modulation/degradation of the PML–RARα oncoprotein (Zhu et al. 2001; Zhou et al. 2005). In fact, effects of the ATRA/ATO combination in accelerating differentiation or inducing apoptosis in arsenic-resistant NB4 cells were reported (Gianni et al. 1998). The major concern was that the effects of the in vitro study might not necessarily be reproduced in in vivo settings and also that the drug combination might bring not just enhanced therapeutic effect but also toxicity. Using transplants of a PML–RARα transgenic APL mouse model, Lallemand-Breitenbach et al. (1999) demonstrated that combining arsenic with RA accelerated tumour regression through enhanced differentiation and apoptosis. Although RA or arsenic alone only prolonged survival two- to threefold, associating the two drugs led to tumour clearance after a nine-month relapse-free period. These results were consistent with those reported by Rego et al. (2000) and Jing et al. (2001). To get further insights into the molecular mechanism, the SIH used a systems approach to analyse dynamic changes reflecting therapeutic effects at both the transcriptome and proteomic levels (Zheng et al. 2005), which was largely facilitated by the integration of advanced technologies from multi-fields. At the global scale, component plane presentation–self-organising maps (CPP-SOM; Xiao et al. 2003) allowed comparison of transcriptome/proteome changes within or between the treatment series. At the transcriptome level, ATO-treated NB4 cells revealed a much smaller number of regulated genes (487) than RA-treated cells (1113), though many of those genes overlapped between the ATO and ATRA treatment series. Transcriptome changes of ATO-plus-ATRA treatment series appear to be highly similar to those of ATRA treatment series, but with some synergistically/additively up- or downregulated genes. At the early stage (within 6 hours), ATRA/ATO modulated an array of transcription factors/cofactors associated with myeloid-specific gene expression, nuclear receptor signalling molecules, IFN pathway members and factors involved in calcium homeostasis and the cAMP/PKA pathway. At 12–24 hours, there was an amplification of RA signalling and a strong activation of the UPS system, as indicated by upregulation of genes such as UBE2L6, PSMC2 and PSMD13. Based on the observation that genes encoding components of typical immunoproteasomes were specifically upregulated by RA, while those encoding subunits of the conventional UPS system (e.g. PSMD11) were significantly induced by ATO, it can be deduced that the protein degradation system is probably much more enhanced to degrade futile proteins, including PML–RARα, in cells cotreated with RA and ATO than either of them alone. This seems to be in accordance with previous data that the more degradation of PML–RARα, the better recovery from the disease (Shen et al. 2004). After 48–72 hours of treatment, the expression of differentiation markers and functional molecules reached a maximum, while genes promoting the cell cycle or enhancing cell proliferation were significantly repressed. Restoration of the apoptotic potential appeared to be parallel to the progression of differentiation. In addition to the recovery of the NB, upregulation of caspase genes became obvious at this stage. Interestingly, synergistically/additively downregulated genes include a group of genes which are known to be involved in various chromosome translocations in human malignancies (figure 3); for instance, ARHGAP26, SH3GL1 and MLLT4 are translocation partners of the MLL gene in acute leukaemia. Synergistic/additive downregulation of these genes may implicate a more effective manner to eliminate oncogenic properties or reduce cell survival potentials in APL cells treated by RA and ATO than those treated by RA or ATO alone. Moreover, a group of genes related to cell proliferation, e.g. IFI16b and GAS7, and apoptosis, e.g. STK24, were also found among the synergistically/additively modulated ones. However, an antagonistic effect of RA and ATO on gene regulation was also detected, although impacted genes appeared to be only of a small number. One such example is CYP51, a gene of the cytochrome p450 family involved in the catabolism of RA (Marill et al. 2003). This gene seems to be induced by RA but the induction is abolished under the joint effect of RA/ATO, which may lead to an increased sensitivity of APL cells to RA treatment. Although the impact of ATO on transcriptome changes appeared to be minor, it was more profound than that of ATRA, suggesting that ATO might particularly enhance post-transcriptional/translation modifications (figure 3). From the study, it is clearly shown that ATRA exerts its effects on APL cells mainly through nuclear receptor-mediated transcriptional regulation, whereas ATO exercises its impact through targeting multiple pathways/cascades at the level of proteome, transcriptome and probably metabolome as well.
Figure 3.
Ideogram illustration of dynamic changes underlying RA/ATO-induced differentiation/apoptosis in APL. Upregulated genes/proteins are marked in red whereas those downregulated are marked in blue. Synergistically/additively regulated genes/proteins are highlighted with a yellow background. Molecular events at the early, intermediate and late stages are rimmed by green, brown and blue lines, respectively. Intracellular compartments in which molecular events occur are also indicated in the ideogram.
(b) Clinical trial of all-trans retinoic acid/arsenic trioxide: two hits on one target accelerate and deepen acute promyelocytic leukaemia clearance
Trials had been conducted using ATRA/ATO in treating relapsed APL. A recent report showed that ATRA did not significantly improve the response to ATO in patients relapsing from APL (Raffoux et al. 2003). In contrast, among newly diagnosed APL patients, Shen et al. (2004) at the SIH achieved dramatic results. Sixty-one newly diagnosed APL cases were randomized into three groups and treated with ATRA, ATO or ATRA/ATO in combination, respectively. A sensitive and specific real-time quantitative RT-PCR of the PML–RARα fusion transcripts was used as molecular marker to evaluate tumour burden. Although CR rates in these groups were all high (90% or more), the time to achieve CR appeared to differ significantly, with the combination group having the shortest. An earlier recovery of platelet count was also found in this group. The disease burden as reflected by fold-change of PML–RARα transcripts at CR decreased more significantly in the combined therapy compared with ATRA or ATO monotherapy groups (p<0.01). This difference persisted after consolidation (p<0.05). Importantly, none of the 20 cases in the combination group, but 7 out of 37 cases in monotherapy groups, (p<0.05) relapsed after a follow-up of 8–30 months (median: 18 months). Thus, an ATRA/ATO combination for remission/maintenance therapy of APL brought much better results than either of the two drugs used alone in terms of the quality of CR and the DFS. The latest clinical data from the SIH revealed a 43-month DFS rate of over 92% in a group of 56 newly diagnosed APL patients using the ‘triad’ protocol (Liu et al. 2006).
(c) Opening to other agents with synergistic effects: crosstalk promise
The successful application of ATRA/ATO/CT therapy in APL justifies the systems view and integrative approach in understanding and treating diseases. Nevertheless, in dealing with new challenges, such as the rare cases escaping from even the ‘triad’ targeting, this approach should be kept open to new knowledge and further innovation. One such innovation could be the integration of a cAMP/PKA pathway agonist based on the concept of the two-step induction of differentiation. In RA-sensitive or RA-resistant mouse models of APL, continuous infusions of 8-chloro-cAMP triggers major growth arrest, significantly enhancing both spontaneous and RA-/ATO-induced differentiation, and accelerates the restoration of normal haemopoiesis. Recently, theophylline, also an ancient drug and a well-known phosphodiesterase inhibitor capable of stabilizing endogenous cAMP, appeared to be of interest. This compound was shown to be able to impair APL growth and to enhance spontaneous or ATO-triggered cell differentiation in vivo. Remarkably, in an APL patient resistant to ATRA/ATO therapy, theophylline induced blast clearance and restored normal haemopoiesis (Guillemin et al. 2002; Zhu et al. 2002b). These results suggest that cAMP signalling is essential for the intricate cell differentiation process and the activation of the cAMP pathway is probably an alternative option not only for APL synergistic differentiation therapy but also for other subtypes of myeloid leukaemia. Another group of compounds worthy of particular attention are protein tyrosine kinase (PTK) inhibitors against mutant FLT3 (Sohal et al. 2003), some of them having already entered into clinical trial. The possible beneficial effects of combination therapies targeting both aberrant transcription factors and PTKs in APL and some other AML subtypes remain to be explored.
7. Conclusion and perspectives
A 50-year endeavour by several generations of biomedical scientists and haematologists/oncologists worldwide has turned APL from being once considered ‘the most malignant form’ to currently the most curable form of AML. A few lessons may thus be drawn from this story. It becomes evident that by targeting the molecules critical to the pathogenesis of certain diseases, cells can be induced to return to normal or to die by PCD. A close collaboration between bench and bedside is important not only for unravelling leukaemia pathogenesis, designing targeted therapy and elucidating drug mechanisms, but also for developing systems-biology-based synergistic targeting therapy which may in turn greatly improve the clinical outcome. Benefits gained from ATO also emphasize the importance of integrating TCM with modern medicine. The sequencing of the human genome and ongoing functional genomic research are accelerating the dissection of disease mechanisms and identification of therapeutic targets. This in turn may facilitate the screening of promising treatments. However, the history of APL has not come to an end. By extending the model of APL, there is reason to hope that other leukaemia subtypes can eventually be cured by specifically tailored cell-modifying treatments.
Footnotes
One contribution of 14 to a Theme Issue ‘Biological science in China’.
References
- Ahn Y.O, Koo H.H, Park B.J, Yoo K.Y, Lee M.S. Incidence estimation of leukemia among Koreans. J. Korean Med. Sci. 1991;6:299–307. doi: 10.3346/jkms.1991.6.4.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altucci L, Rossin A, Raffelsberger W, Reitmair A, Chomienne C, Gronemeyer H. Retinoic acid-induced apoptosis in leukemia cells is mediated by paracrine action of tumor-selective death ligand trail. Nat. Med. 2001;7:680–686. doi: 10.1038/89050. doi:10.1038/89050 [DOI] [PubMed] [Google Scholar]
- Arnould C, Philippe C, Bourdon V, Gregoire M.J, Berger R, Jonveaux P. The signal transducer and activator of transcription STAT5b gene is a new partner of retinoic acid receptor alpha in acute promyelocytic-like leukaemia. Hum. Mol. Genet. 1999;8:1741–1749. doi: 10.1093/hmg/8.9.1741. doi:10.1093/hmg/8.9.1741 [DOI] [PubMed] [Google Scholar]
- Bennett J.M, Catovsky D, Daniel M.T, Flandrin G, Galton D.A, Gralnick H.R, Sultan C. Proposals for the classification of the acute leukaemias. French–American–British (FAB) cooperative group. Br. J. Haematol. 1976;33:451–458. doi: 10.1111/j.1365-2141.1976.tb03563.x. [DOI] [PubMed] [Google Scholar]
- Bennett J.M, Catovsky D, Daniel M.T, Flandrin G, Galton D.A, Gralnick H.R, Sultan C. A variant form of hypergranular promyelocytic leukaemia (M3) Br. J. Haematol. 1980;44:169–170. doi: 10.1111/j.1365-2141.1980.tb01195.x. [DOI] [PubMed] [Google Scholar]
- Bennett J.M, Catovsky D, Daniel M.T, Flandrin G, Galton D.A, Gralnick H.R, Sultan C. Proposed revised criteria for the classification of acute myeloid leukemia. A report of the French–American–British cooperative group. Ann. Intern. Med. 1985;103:620–625. doi: 10.7326/0003-4819-103-4-620. [DOI] [PubMed] [Google Scholar]
- Bernard J, Mathe G, Boulay J, Ceoard B, Chome J. Acute promyelocytic leukemia: a study made on 20 cases. Schweiz Med. Wochenschr. 1959;89:604–608. [PubMed] [Google Scholar]
- Bernard J, Weil M, Boiron M, Jacquillat C, Flandrin G, Gemon M.F. Acute promyelocytic leukemia: results of treatment by daunorubicin. Blood. 1973;41:489–496. [PubMed] [Google Scholar]
- Bernardi R, Pandolfi P.P. Role of PML and the PML–nuclear body in the control of programmed cell death. Oncogene. 2003;22:9048–9057. doi: 10.1038/sj.onc.1207106. doi:10.1038/sj.onc.1207106 [DOI] [PubMed] [Google Scholar]
- Breitman T.R, Selonick S.E, Collins S.J. Induction of differentiation of the human promyelocytic leukemia cell line (HL-60) by retinoic acid. Proc. Natl Acad. Sci. USA. 1980;77:2936–2940. doi: 10.1073/pnas.77.5.2936. doi:10.1073/pnas.77.5.2936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitman T.R, Collins S.J, Keene B.R. Terminal differentiation of human promyelocytic leukemic cells in primary culture in response to retinoic acid. Blood. 1981;57:1000–1004. [PubMed] [Google Scholar]
- Brown D, Kogan S, Lagasse E, Weissman I, Alcalay M, Pelicci P.G, Atwater S, Bishop J.M. A PMLRARα transgene initiates murine acute promyelocytic leukemia. Proc. Natl Acad. Sci. USA. 1997;94:2551–2556. doi: 10.1073/pnas.94.6.2551. doi:10.1073/pnas.94.6.2551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caen, J., Mathe, G., Xuan Chat, L. & Bernard, J. 1957 Etude de la fibrinolyse au cours des hemopathies malignes. In Transcripts of the Sixth Congress of the European Society of Hematology, Copenhagen, pp. 502–506.
- Castaigne S, Chomienne C, Daniel M.T, Ballerini P, Berger R, Fenaux P, Degos L. All-trans retinoic acid as a differentiation therapy for acute promyelocytic leukemia. I. Clinical results. Blood. 1990;76:1704–1709. [PubMed] [Google Scholar]
- Chan K.W, Steinherz P.G, Miller D.R. Acute promyelocytic leukemia in children. Med. Pediatr. Oncol. 1981;9:5–15. doi: 10.1002/mpo.2950090103. [DOI] [PubMed] [Google Scholar]
- Chen S.J, Zelent A, Tong J.H, Yu H.Q, Wang Z.Y, Derre J, Berger R, Waxman S, Chen Z. Rearrangements of the retinoic acid receptor alpha and promyelocytic leukemia zinc finger genes resulting from t(11;17)(q23;q21) in a patient with acute promyelocytic leukemia. J. Clin. Invest. 1993a;91:2260–2267. doi: 10.1172/JCI116453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Brand N.J, Chen A, Chen S.J, Tong J.H, Wang Z.Y, Waxman S, Zelent A. Fusion between a novel kruppel-like zinc finger gene and the retinoic acid receptor-alpha locus due to a variant t(11;17) translocation associated with acute promyelocytic leukaemia. EMBO J. 1993b;12:1161–1167. doi: 10.1002/j.1460-2075.1993.tb05757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G.Q, et al. In vitro studies on cellular and molecular mechanisms of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia: As2O3 induces nb4 cell apoptosis with downregulation of Bcl-2 expression and modulation of PML–RARα/PML proteins. Blood. 1996;88:1052–1061. [PubMed] [Google Scholar]
- Chen G.Q, et al. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): I. As2O3 exerts dose-dependent dual effects on APL cells. Blood. 1997;89:3345–3353. [PubMed] [Google Scholar]
- Chen Z, Chen G.Q, Shen Z.X, Chen S.J, Wang Z.Y. Treatment of acute promyelocytic leukemia with arsenic compounds: in vitro and in vivo studies. Semin. Hematol. 2001;38:26–36. doi: 10.1053/shem.2001.20863. doi:10.1053/shem.2001.20863 [DOI] [PubMed] [Google Scholar]
- Cordonnier C, et al. Acute promyelocytic leukemia in 57 previously untreated patients. Cancer. 1985;55:18–25. doi: 10.1002/1097-0142(19850101)55:1<18::aid-cncr2820550104>3.0.co;2-b. doi:10.1002/1097-0142(19850101)55:1<18::AID-CNCR2820550104>3.0.CO;2-B [DOI] [PubMed] [Google Scholar]
- Corey S.J, Locker J, Oliveri D.R, Shekhter-Levin S, Redner R.L, Penchansky L, Gollin S.M. A non-classical translocation involving 17q12 (retinoic acid receptor alpha) in acute promyelocytic leukemia (APML) with atypical features. Leukemia. 1994;8:1350–1353. [PubMed] [Google Scholar]
- Degos L. The history of acute promyelocytic leukaemia. Br. J. Haematol. 2003;122:539–553. doi: 10.1046/j.1365-2141.2003.04460.x. doi:10.1046/j.1365-2141.2003.04460.x [DOI] [PubMed] [Google Scholar]
- Dong S, et al. Amino-terminal protein–protein interaction motif (POZ-domain) is responsible for activities of the promyelocytic leukemia zinc finger-retinoic acid receptor-alpha fusion protein. Proc. Natl Acad. Sci. USA. 1996;93:3624–3629. doi: 10.1073/pnas.93.8.3624. doi:10.1073/pnas.93.8.3624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapkin R.L, Gee T.S, Dowling M.D, Arlin Z, McKenzie S, Kempin S, Clarkson B. Prophylactic heparin therapy in acute promyelocytic leukemia. Cancer. 1978;41:2484–2490. doi: 10.1002/1097-0142(197806)41:6<2484::aid-cncr2820410659>3.0.co;2-#. doi:10.1002/1097-0142(197806)41:6<2484::AID-CNCR2820410659>3.0.CO;2-# [DOI] [PubMed] [Google Scholar]
- Elliott S, et al. Proof of differentiative mode of action of all-trans retinoic acid in acute promyelocytic leukemia using X-linked clonal analysis. Blood. 1992;79:1916–1919. [PubMed] [Google Scholar]
- Fenaux P, et al. Long-term follow-up confirms the benefit of all-trans retinoic acid in acute promyelocytic leukemia. Leukemia. 2000;14:1371–1377. doi: 10.1038/sj.leu.2401859. doi:10.1038/sj.leu.2401859 [DOI] [PubMed] [Google Scholar]
- Fibach E, Hayashi M, Sachs L. Control of normal differentiation of myeloid leukemic cells to macrophages and granulocytes. Proc. Natl Acad. Sci. USA. 1973;70:343–346. doi: 10.1073/pnas.70.2.343. doi:10.1073/pnas.70.2.343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianni M, Koken M.H, Chelbi-Alix M.K, Benoit G, Lanotte M, Chen Z, de The H. Combined arsenic and retinoic acid treatment enhances differentiation and apoptosis in arsenic-resistant NB4 cells. Blood. 1998;91:4300–4310. [PubMed] [Google Scholar]
- Gianni M, Ponzanelli I, Mologni L, Reichert U, Rambaldi A, Terao M, Garattini E. Retinoid-dependent growth inhibition, differentiation and apoptosis in acute promyelocytic leukemia cells. Expression and activation of caspases. Cell Death Differ. 2000;7:447–460. doi: 10.1038/sj.cdd.4400673. doi:10.1038/sj.cdd.4400673 [DOI] [PubMed] [Google Scholar]
- Grisolano J.L, Wesselschmidt R.L, Pelicci P.G, Ley T.J. Altered myeloid development and acute leukemia in transgenic mice expressing PML–RARα under control of cathepsin G regulatory sequences. Blood. 1997;89:376–387. [PubMed] [Google Scholar]
- Guillemin M.C, et al. In vivo activation of cAMP signaling induces growth arrest and differentiation in acute promyelocytic leukemia. J. Exp. Med. 2002;196:1373–1380. doi: 10.1084/jem.20021129. doi:10.1084/jem.20021129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa F, Privalsky M.L. Phosphorylation of PML by mitogen-activated protein kinases plays a key role in arsenic trioxide-mediated apoptosis. Cancer Cell. 2004;5:389–401. doi: 10.1016/s1535-6108(04)00082-0. doi:10.1016/S1535-6108(04)00082-0 [DOI] [PubMed] [Google Scholar]
- He L.Z, Tribioli C, Rivi R, Peruzzi D, Pelicci P.G, Soares V, Cattoretti G, Pandolfi P.P. Acute leukemia with promyelocytic features in PML/RARα transgenic mice. Proc. Natl Acad. Sci. USA. 1997;94:5302–5307. doi: 10.1073/pnas.94.10.5302. doi:10.1073/pnas.94.10.5302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillestad L.K. Acute promyelocytic leukemia. Acta Med. Scand. 1957;159:189–194. [PubMed] [Google Scholar]
- Huang M.E, Ye Y.C, Chen S.R, Chai J.R, Lu J.X, Zhoa L, Gu L.J, Wang Z.Y. Use of all-trans retinoic acid in the treatment of acute promyelocytic leukemia. Blood. 1988;72:567–572. [PubMed] [Google Scholar]
- Huang X.J, Wiernik P.H, Klein R.S, Gallagher R.E. Arsenic trioxide induces apoptosis of myeloid leukemia cells by activation of caspases. Med. Oncol. 1999;16:58–64. doi: 10.1007/BF02787360. [DOI] [PubMed] [Google Scholar]
- Jemal A, Murray T, Ward E, Samuels A, Tiwari R.C, Ghafoor A, Feuer E.J, Thun M.J. Cancer statistics, 2005. CA Cancer J. Clin. 2005;55:10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- Jensen K, Shiels C, Freemont P.S. PML protein isoforms and the RBCC/TRIM motif. Oncogene. 2001;20:7223–7233. doi: 10.1038/sj.onc.1204765. doi:10.1038/sj.onc.1204765 [DOI] [PubMed] [Google Scholar]
- Jing Y, Wang L, Xia L, Chen G.Q, Chen Z, Miller W.H, Waxman S. Combined effect of all-trans retinoic acid and arsenic trioxide in acute promyelocytic leukemia cells in vitro and in vivo. Blood. 2001;97:264–269. doi: 10.1182/blood.v97.1.264. doi:10.1182/blood.V97.1.264 [DOI] [PubMed] [Google Scholar]
- Kamashev D, Vitoux D, de The H. PML–RARA–RXR oligomers mediate retinoid and rexinoid/cAMP cross-talk in acute promyelocytic leukemia cell differentiation. J. Exp. Med. 2004;199:1163–1174. doi: 10.1084/jem.20032226. doi:10.1084/jem.20032226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarjian H.M, et al. Acute promyelocytic leukemia. M. D. Anderson hospital experience. Am. J. Med. 1986;80:789–797. doi: 10.1016/0002-9343(86)90617-0. doi:10.1016/0002-9343(86)90617-0 [DOI] [PubMed] [Google Scholar]
- Kelly L.M, Kutok J.L, Williams I.R, Boulton C.L, Amaral S.M, Curley D.P, Ley T.J, Gilliland D.G. PML/RARα and FLT3-ITD induce an APL-like disease in a mouse model. Proc. Natl Acad. Sci. USA. 2002;99:8283–8288. doi: 10.1073/pnas.122233699. doi:10.1073/pnas.122233699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kini A.R, Peterson L.A, Tallman M.S, Lingen M.W. Angiogenesis in acute promyelocytic leukemia: induction by vascular endothelial growth factor and inhibition by all-trans retinoic acid. Blood. 2001;97:3919–3924. doi: 10.1182/blood.v97.12.3919. doi:10.1182/blood.V97.12.3919 [DOI] [PubMed] [Google Scholar]
- Kogan S.C, Hong S.H, Shultz D.B, Privalsky M.L, Bishop J.M. Leukemia initiated by PMLRARα: the PML domain plays a critical role while retinoic acid-mediated transactivation is dispensable. Blood. 2000;95:1541–1550. [PubMed] [Google Scholar]
- Kogan S.C, Brown D.E, Shultz D.B, Truong B.T, Lallemand-Breitenbach V, Guillemin M.C, Lagasse E, Weissman I.L, Bishop J.M. BCL-2 cooperates with promyelocytic leukemia retinoic acid receptor alpha chimeric protein (PMLRARα) to block neutrophil differentiation and initiate acute leukemia. J. Exp. Med. 2001;193:531–543. doi: 10.1084/jem.193.4.531. doi:10.1084/jem.193.4.531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lallemand-Breitenbach V, Guillemin M.C, Janin A, Daniel M.T, Degos L, Kogan S.C, Michael Bishop J, de The H. Retinoic acid and arsenic synergize to eradicate leukemic cells in a mouse model of acute promyelocytic leukemia. J. Exp. Med. 1999;189:1043–1052. doi: 10.1084/jem.189.7.1043. doi:10.1084/jem.189.7.1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lallemand-Breitenbach V, et al. Role of promyelocytic leukemia (PML) sumolation in nuclear body formation, 11s proteasome recruitment, and As2O3-induced PML or PML/retinoic acid receptor α degradation. J. Exp. Med. 2001;193:1361–1371. doi: 10.1084/jem.193.12.1361. doi:10.1084/jem.193.12.1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane A.A, Ley T.J. Neutrophil elastase cleaves PML–RARα and is important for the development of acute promyelocytic leukemia in mice. Cell. 2003;115:305–318. doi: 10.1016/s0092-8674(03)00852-3. doi:10.1016/S0092-8674(03)00852-3 [DOI] [PubMed] [Google Scholar]
- Li S.Z. People's Medical Publishing House; Beijing, China: 1982. The compendium of materia medica (originally published in the Ming Dynasty of China, 1578) [Google Scholar]
- Licht J.D, Shaknovich R, English M.A, Melnick A, Li J.Y, Reddy J.C, Dong S, Chen S.J, Zelent A, Waxman S. Reduced and altered DNA-binding and transcriptional properties of the PLZF-retinoic acid receptor-alpha chimera generated in t(11;17)-associated acute promyelocytic leukemia. Oncogene. 1996;12:323–336. [PubMed] [Google Scholar]
- Lin H.K, Bergmann S, Pandolfi P.P. Cytoplasmic PML function in TGF-beta signalling. Nature. 2004;431:205–211. doi: 10.1038/nature02783. doi:10.1038/nature02783 [DOI] [PubMed] [Google Scholar]
- Liu Y.F, Zhu Y.M, Shi Z.Z, Li J.M, Wang L, Chen Y, Shen Z.X, Hu J. Long-term follow-up confirms the benefit of all-trans retinoic acid (ATRA) and arsenic trioxide (As2O3) as front line therapy for newly diagnosed acute promyelocytic leukemia (APL) Blood. 2006;108:170a. [Google Scholar]
- Liu T.X, et al. Gene expression networks underlying retinoic acid-induced differentiation of acute promyelocytic leukemia cells. Blood. 2000;96:1496–1504. [PubMed] [Google Scholar]
- Lu D.P, Qiu J.Y, Jiang B, Wang Q, Liu K.Y, Liu Y.R, Chen S.S. Tetra-arsenic tetra-sulfide for the treatment of acute promyelocytic leukemia: a pilot report. Blood. 2002;99:3136–3143. doi: 10.1182/blood.v99.9.3136. doi:10.1182/blood.V99.9.3136 [DOI] [PubMed] [Google Scholar]
- Marill J, Idres N, Capron C.C, Nguyen E, Chabot G.G. Retinoic acid metabolism and mechanism of action: a review. Curr. Drug Metab. 2003;4:1–10. doi: 10.2174/1389200033336900. doi:10.2174/1389200033336900 [DOI] [PubMed] [Google Scholar]
- Martin S.J, Bradley J.G, Cotter T.G. HL-60 cells induced to differentiate towards neutrophils subsequently die via apoptosis. Clin. Exp. Immunol. 1990;79:448–453. doi: 10.1111/j.1365-2249.1990.tb08110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna R.W, Parkin J, Bloomfield C.D, Sundberg R.D, Brunning R.D. Acute promyelocytic leukaemia: a study of 39 cases with identification of a hyperbasophilic microgranular variant. Br. J. Haematol. 1982;50:201–214. doi: 10.1111/j.1365-2141.1982.tb01910.x. [DOI] [PubMed] [Google Scholar]
- Melnick A, Licht J.D. Deconstructing a disease: RARα, its fusion partners, and their roles in the pathogenesis of acute promyelocytic leukemia. Blood. 1999;93:3167–3215. [PubMed] [Google Scholar]
- Miller W.H, Jr, Schipper H.M, Lee J.S, Singer J, Waxman S. Mechanisms of action of arsenic trioxide. Cancer Res. 2002;62:3893–3903. [PubMed] [Google Scholar]
- Muller S, Matunis M.J, Dejean A. Conjugation with the ubiquitin-related modifier SUMO-1 regulates the partitioning of PML within the nucleus. EMBO J. 1998;17:61–70. doi: 10.1093/emboj/17.1.61. doi:10.1093/emboj/17.1.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nervi C, et al. Caspases mediate retinoic acid-induced degradation of the acute promyelocytic leukemia PML/RARα fusion protein. Blood. 1998;92:2244–2251. [PubMed] [Google Scholar]
- Niu C, et al. Studies on treatment of acute promyelocytic leukemia with arsenic trioxide: remission induction, follow-up, and molecular monitoring in 11 newly diagnosed and 47 relapsed acute promyelocytic leukemia patients. Blood. 1999;94:3315–3324. [PubMed] [Google Scholar]
- Paran M, Sachs L, Barak Y, Resnitzky P. In vitro induction of granulocyte differentiation in hematopoietic cells from leukemic and non-leukemic patients. Proc. Natl Acad. Sci. USA. 1970;67:1542–1549. doi: 10.1073/pnas.67.3.1542. doi:10.1073/pnas.67.3.1542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin D.M, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J. Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- Quignon F, De B.F, Koken M, Feunteun J, Ameisen J.C, de The H. PML induces a novel caspase-independent death process. Nat. Genet. 1998;20:259–265. doi: 10.1038/3068. doi:10.1038/3068 [DOI] [PubMed] [Google Scholar]
- Raelson J.V, Nervi C, Rosenauer A, Benedetti L, Monczak Y, Pearson M, Pelicci P.G, Miller W.J. The PML/RARα oncoprotein is a direct molecular target of retinoic acid in acute promyelocytic leukemia cells. Blood. 1996;88:2826–2832. [PubMed] [Google Scholar]
- Raffoux E, et al. Combined treatment with arsenic trioxide and all-trans-retinoic acid in patients with relapsed acute promyelocytic leukemia. J. Clin. Oncol. 2003;21:2326–2334. doi: 10.1200/JCO.2003.01.149. doi:10.1200/JCO.2003.01.149 [DOI] [PubMed] [Google Scholar]
- Rego E.M, He L.Z, Warrell R.P, Jr, Wang Z.G, Pandolfi P.P. Retinoic acid (RA) and As2O3 treatment in transgenic models of acute promyelocytic leukemia (APL) unravel the distinct nature of the leukemogenic process induced by the PML–RARα and PLZF–RARα oncoproteins. Proc. Natl Acad. Sci. USA. 2000;97:10 173–10 178. doi: 10.1073/pnas.180290497. doi:10.1073/pnas.180290497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocca B, Morosetti R, Habib A, Maggiano N, Zassadowski F, Ciabattoni G, Chomienne C, Papp B, Ranelletti F.O. Cyclooxygenase-1, but not -2, is upregulated in NB4 leukemic cells and human primary promyelocytic blasts during differentiation. Leukemia. 2004;18:1373–1379. doi: 10.1038/sj.leu.2403407. doi:10.1038/sj.leu.2403407 [DOI] [PubMed] [Google Scholar]
- Rowley J.D, Golomb H.M, Dougherty C. 15/17 translocation, a consistent chromosomal change in acute promyelocytic leukaemia. Lancet. 1977;1:549–550. doi: 10.1016/s0140-6736(77)91415-5. doi:10.1016/S0140-6736(77)91415-5 [DOI] [PubMed] [Google Scholar]
- Ruchaud S, Duprez E, Gendron M.C, Houge G, Genieser H.G, Jastorff B, Doskeland S.O, Lanotte M. Two distinctly regulated events, priming and triggering, during retinoid-induced maturation and resistance of NB4 promyelocytic leukemia cell line. Proc. Natl Acad. Sci. USA. 1994;91:8428–8432. doi: 10.1073/pnas.91.18.8428. doi:10.1073/pnas.91.18.8428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runde V, Aul C, Sudhoff T, Heyll A, Schneider W. Retinoic acid in the treatment of acute promyelocytic leukemia: inefficacy of the 13-cis isomer and induction of complete remission by the all-trans isomer complicated by thromboembolic events. Ann. Hematol. 1992;64:270–272. doi: 10.1007/BF01695469. doi:10.1007/BF01695469 [DOI] [PubMed] [Google Scholar]
- Sachs L. Control of normal cell differentiation and the phenotypic reversion of malignancy in myeloid leukaemia. Nature. 1978a;274:535–539. doi: 10.1038/274535a0. doi:10.1038/274535a0 [DOI] [PubMed] [Google Scholar]
- Sachs L. The differentiation of myeloid leukaemia cells: new possibilities for therapy. Br. J. Haematol. 1978b;40:509–517. doi: 10.1111/j.1365-2141.1978.tb05826.x. [DOI] [PubMed] [Google Scholar]
- Salomoni P, Pandolfi P.P. The role of PML in tumor suppression. Cell. 2002;108:165–170. doi: 10.1016/s0092-8674(02)00626-8. doi:10.1016/S0092-8674(02)00626-8 [DOI] [PubMed] [Google Scholar]
- Sanz M.A, et al. Acute promyelocytic leukemia. Therapy results and prognostic factors. Cancer. 1988;61:7–13. doi: 10.1002/1097-0142(19880101)61:1<7::aid-cncr2820610103>3.0.co;2-6. doi:10.1002/1097-0142(19880101)61:1<7::AID-CNCR2820610103>3.0.CO;2-6 [DOI] [PubMed] [Google Scholar]
- Shen Z.X, et al. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL). II. Clinical efficacy and pharmacokinetics in relapsed patients. Blood. 1997;89:3354–3360. [PubMed] [Google Scholar]
- Shen Z.X, et al. All-trans retinoic acid/As2O3 combination yields a high quality remission and survival in newly diagnosed acute promyelocytic leukemia. Proc. Natl Acad. Sci. USA. 2004;101:5328–5335. doi: 10.1073/pnas.0400053101. doi:10.1073/pnas.0400053101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih L.Y, Kuo M.C, Liang D.C, Huang C.F, Lin T.L, Wu J.H, Wang P.N, Dunn P, Lai C.L. Internal tandem duplication and ASP835 mutations of the FMS-like tyrosine kinase 3 (FLT3) gene in acute promyelocytic leukemia. Cancer. 2003;98:1206–1216. doi: 10.1002/cncr.11636. doi:10.1002/cncr.11636 [DOI] [PubMed] [Google Scholar]
- Slack J.L, Waxman S, Tricot G, Tallman M.S, Bloomfield C.D. Advances in the management of acute promyelocytic leukemia and other hematologic malignancies with arsenic trioxide. Oncologist. 2002;7:1–13. doi: 10.1634/theoncologist.7-suppl_1-1. doi:10.1634/theoncologist.7-suppl_1-1 [DOI] [PubMed] [Google Scholar]
- Sohal J, et al. A model of APL with FLT3 mutation is responsive to retinoic acid and a receptor tyrosine kinase inhibitor, SU11657. Blood. 2003;101:3188–3197. doi: 10.1182/blood-2002-06-1800. doi:10.1182/blood-2002-06-1800 [DOI] [PubMed] [Google Scholar]
- Soignet S.L, et al. Complete remission after treatment of acute promyelocytic leukemia with arsenic trioxide. N. Engl. J. Med. 1998;339:1341–1348. doi: 10.1056/NEJM199811053391901. doi:10.1056/NEJM199811053391901 [DOI] [PubMed] [Google Scholar]
- Soignet S.L, et al. United States multicenter study of arsenic trioxide in relapsed acute promyelocytic leukemia. J. Clin. Oncol. 2001;19:3852–3860. doi: 10.1200/JCO.2001.19.18.3852. [DOI] [PubMed] [Google Scholar]
- Sun H.D, Ma L, Hu X.C, Zhang T.D. Ai-lin 1 treated 32 cases of acute promyelocytic leukemia. Chin. J. Integr. Chin. West Med. 1992;12:170–171. [Google Scholar]
- Tallman M.S, et al. All-trans-retinoic acid in acute promyelocytic leukemia. N. Engl. J. Med. 1997;337:1021–1028. doi: 10.1056/NEJM199710093371501. doi:10.1056/NEJM199710093371501 [DOI] [PubMed] [Google Scholar]
- Tallman M.S, Nabhan C, Feusner J.H, Rowe J.M. Acute promyelocytic leukemia: evolving therapeutic strategies. Blood. 2002;99:759–767. doi: 10.1182/blood.v99.3.759. doi:10.1182/blood.V99.3.759 [DOI] [PubMed] [Google Scholar]
- vom Baur E, et al. Differential ligand-dependent interactions between the AF-2 activating domain of nuclear receptors and the putative transcriptional intermediary factors mSUG1 and TIF1. EMBO J. 1996;15:110–124. [PMC free article] [PubMed] [Google Scholar]
- Wang Z.Y. Ham-Wasserman lecture: treatment of acute leukemia by inducing differentiation and apoptosis. Hematology (Am. Soc. Hematol. Educ. Program) 2003:1–13. doi: 10.1182/asheducation-2003.1.1. [DOI] [PubMed] [Google Scholar]
- Warrell R.P, et al. Differentiation therapy of acute promyelocytic leukemia with tretinoin (all-trans-retinoic acid) N. Engl. J. Med. 1991;324:1385–1393. doi: 10.1056/NEJM199105163242002. [DOI] [PubMed] [Google Scholar]
- Warrell R.P, de The H, Wang Z.Y, Degos L. Acute promyelocytic leukemia. N. Engl. J. Med. 1993;329:177–189. doi: 10.1056/NEJM199307153290307. doi:10.1056/NEJM199307153290307 [DOI] [PubMed] [Google Scholar]
- Wells R.A, Hummel J.L, De K.A, Zipursky A, Kirby M, Dube I, Kamel-Reid S. A new variant translocation in acute promyelocytic leukaemia: molecular characterization and clinical correlation. Leukemia. 1996;10:735–740. [PubMed] [Google Scholar]
- Xiao L, Wang K, Teng Y, Zhang J. Component plane presentation integrated self-organizing map for microarray data analysis. FEBS Lett. 2003;538:117–124. doi: 10.1016/s0014-5793(03)00156-x. doi:10.1016/S0014-5793(03)00156-X [DOI] [PubMed] [Google Scholar]
- Yang C, Zhang X. Incidence survey of leukemia in China. Chin. Med. Sci. J. 1991;6:65–70. [PubMed] [Google Scholar]
- Zheng P.Z, et al. Systems analysis of transcriptome and proteome in retinoic acid/arsenic trioxide-induced cell differentiation/apoptosis of promyelocytic leukemia. Proc. Natl Acad. Sci. USA. 2005;102:7653–7658. doi: 10.1073/pnas.0502825102. doi:10.1073/pnas.0502825102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G.B, Zhao W.L, Wang Z.Y, Chen S.J, Chen Z. Retinoic acid and arsenic for treating acute promyelocytic leukemia. PLoS Med. 2005;2:33–38. doi: 10.1371/journal.pmed.0020012. doi:10.1371/journal.pmed.0020012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Koken M.H, Quignon F, Chelbi-Alix M.K, Degos L, Wang Z.Y, Chen Z, de The H. Arsenic-induced PML targeting onto nuclear bodies: implications for the treatment of acute promyelocytic leukemia. Proc. Natl Acad. Sci. USA. 1997;94:3978–3983. doi: 10.1073/pnas.94.8.3978. doi:10.1073/pnas.94.8.3978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, et al. Tissue factors on acute promyelocytic leukemia and endothelial cells are differently regulated by retinoic acid, arsenic trioxide and chemotherapeutic agents. Leukemia. 1999a;13:1062–1070. doi: 10.1038/sj.leu.2401448. doi:10.1038/sj/leu/2401448 [DOI] [PubMed] [Google Scholar]
- Zhu X.H, et al. Apoptosis and growth inhibition in malignant lymphocytes after treatment with arsenic trioxide at clinically achievable concentrations. J. Natl Cancer Inst. 1999b;91:772–778. doi: 10.1093/jnci/91.9.772. doi:10.1093/jnci/91.9.772 [DOI] [PubMed] [Google Scholar]
- Zhu J, Lallemand-Breitenbach V, de The H. Pathways of retinoic acid- or arsenic trioxide-induced PML/RARα catabolism: role of oncogene degradation in disease remission. Oncogene. 2001;20:7257–7265. doi: 10.1038/sj.onc.1204852. doi:10.1038/sj.onc.1204852 [DOI] [PubMed] [Google Scholar]
- Zhu J, Chen Z, Lallemand-Breitenbach V, de The H. How acute promyelocytic leukaemia revived arsenic. Nat. Rev. Cancer. 2002a;2:705–713. doi: 10.1038/nrc887. doi:10.1038/nrc887 [DOI] [PubMed] [Google Scholar]
- Zhu Q, et al. Synergic effects of arsenic trioxide and cAMP during acute promyelocytic leukemia cell maturation subtends a novel signaling cross-talk. Blood. 2002b;99:1014–1022. doi:10.1182/blood.V99.7.2562 [PubMed] [Google Scholar]
- Zhu J, Zhou J, Peres L, Riaucoux F, Honore N, Kogan S, de The H. A sumoylation site in PML/RARA is essential for leukemic transformation. Cancer Cell. 2005;7:143–153. doi: 10.1016/j.ccr.2005.01.005. doi:10.1016/j.ccr.2005.01.005 [DOI] [PubMed] [Google Scholar]